Abstract
Background
Exercise alleviates pain and it is a central component of treatment strategy for chronic pain in clinical setting. However, little is known about mechanism of this exercise-induced hypoalgesia. The mesolimbic dopaminergic network plays a role in positive emotions to rewards including motivation and pleasure. Pain negatively modulates these emotions, but appropriate exercise is considered to activate the dopaminergic network. We investigated possible involvement of this network as a mechanism of exercise-induced hypoalgesia.
Methods
In the present study, we developed a protocol of treadmill exercise, which was able to recover pain threshold under partial sciatic nerve ligation in mice, and investigated involvement of the dopaminergic reward network in exercise-induced hypoalgesia. To temporally suppress a neural activation during exercise, a genetically modified inhibitory G-protein-coupled receptor, hM4Di, was specifically expressed on dopaminergic pathway from the ventral tegmental area to the nucleus accumbens.
Results
The chemogenetic-specific neural suppression by Gi-DREADD system dramatically offset the effect of exercise-induced hypoalgesia in transgenic mice with hM4Di expressed on the ventral tegmental area dopamine neurons. Additionally, anti-exercise-induced hypoalgesia effect was significantly observed under the suppression of neurons projecting out of the ventral tegmental area to the nucleus accumbens as well.
Conclusion
Our findings suggest that the dopaminergic pathway from the ventral tegmental area to the nucleus accumbens is involved in the anti-nociception under low-intensity exercise under a neuropathic pain-like state.
Keywords: Chronic pain, mesolimbic dopaminergic network, exercise-induced hypoalgesia, treadmill exercise, DREADD, brain reward system, ventral tegmental area, nucleus accumbens
Background
Exercise-induced hypoalgesia (EIH) is well known as a phenomenon that exercise alleviates pain in a clinical setting.1,2 Despite this clinical evidence, exercise is painful in some patients and they report that physical activity and exercise worsen their symptoms. Daenen et al.1 described this negative phenomenon as dysfunctional EIH, but the cause was still unknown. In a meta-analytic review, Kelly et al.2 also referred to the narrow therapeutic window of exercise for chronic pain patients. They reported that, while EIH had a fixed effect in healthy participants, an optimal dose of exercise to achieve an anti-nociception could not be determined in chronic pain patients. While regular exercise programs have beneficial effects for chronic pain, both the magnitude and direction of the effect are highly variable for several kinds of exercise and depend on the chronic pain condition as well as the intensity of the exercise.2
Perhaps the most plausible mechanism of EIH involves the endogenous opioid system. β-endorphins are released by adequate exercise, which reduce the sensitivity to pain.3–6 In addition, results in animals show that non-opioid systems (e.g., endocannabinoid,7,8 neurotransmitters such as serotonin and norepinephrine9–12) may also play a role. In the present study, we focused on the dopaminergic system as a novel mechanism of EIH and hypothesized that dopaminergic activation is upstream of anti-nociceptive mechanisms reported previously. Dopamine neurons that project from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) are a key component of the brain reward system.13 These neurons react to a reward stimulus and generate positive emotions including motivation and pleasure. However, non-responders who do not receive an anti-nociception from exercise may feel negative, rather than positive, emotions. We assumed that EIH might depend on the activation of dopamine neurons.
Activation of the dopaminergic system is unlikely to occur in people with chronic pain.14,15 In animal experiments, neuropathic pain induced by partial sciatic nerve ligation (PSNL) causes a negative functional plasticity in the mesolimbic dopaminergic system.16 We previously reported that sustained pain stimuli reduced both µ-opioid receptor function in the VTA and dopamine release in the NAc, resulting in the suppression of reward effects of systemic morphine under the chronic pain state.17
Exercise may be able to reverse this plasticity in the dopaminergic system. Six weeks of wheel running increased tyrosine hydroxylase (TH) levels in the VTA, increased delta opioid receptor in the NAc shell, and reduced levels of dopamine receptor 2 in the NAc core in healthy rats.18 However, little is known about the role of dopaminergic activation in the anti-nociception by exercise in chronic pain. Therefore, we produced an exercise protocol by which chronic pain-model mice could recover their sensitivity to pain and tested whether the specific suppression of dopamine neurons can reverse the anti-nociception due to exercise.
Method
Animals
The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals, Hoshi University, as adopted by the Committee on Animal Research of Hoshi University, which is accredited by the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was approved by the Animal Research Committee of Hoshi University. Every effort was made to minimize the numbers and any suffering of animals used in the following experiments. B6.SJL-Slc6a3tm1.1(cre)Bkmn/J (DAT-Cre) mice with an internal ribosome entry site-linked Cre recombinase gene inserted just downstream of the translational stop codon of the endogenous dopamine transporter gene (Slc6a3; DAT) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6J (Jackson Laboratory) mice and DAT-Cre mice were fed ad libitum and housed at a room temperature of 24 ± 1℃ with a 12-h light–dark cycle (light on 8:00 am to 8:00 pm). Seven- to 13-week-old male mice were used in all experiments.
Neuropathic pain model
We produced a PSNL model by tying a tight ligature with 8-0 silk suture around approximately half the diameter of the sciatic nerve on the right side of mice under anesthesia with 3% isoflurane as described previously.19 In sham-operated mice, the nerve was exposed without ligation. This model may mimic important characteristics of chronic neuropathic pain in patients following peripheral nerve injury.
Treadmill exercise
Half of the nerve-ligated mice and sham-operated mice ran for 60 min every evening, five days per week, for one or two weeks. Running sessions were performed on a four-lane motorized treadmill equipped with electric shock (Treadmill for Rats and Mice Model MK-680 S; Muromachi Kikai Co., Ltd). Current was not used to avoid inflicting pain. The treadmill was set at an inclination of 0°. All mice were acclimated to the treadmill belt for 10 min before starting locomotion. The running speed was accelerated at 0.5 m/s2, and the maximal speed was either 6 m/min or 12 m/min.
Thermal hyperalgesia and tactile allodynia
To quantify the sensitivity to thermal or tactile stimulation after PSNL, paw withdrawal was measured using a thermal stimulus apparatus or von Frey filaments, respectively. The measurements were performed approximately 15 h after a running session. When a measurement was planned on an exercise day, mice performed the treadmill exercise after the measurement performed 15 h after the previous run.
To assess the sensitivity to thermal stimulation, each of the hind paws of mice was tested individually using a thermal stimulus apparatus (model 33 Analgesia Meter; IITC Inc./Life Science Instruments, Woodland Hills, CA, USA). The intensity of the thermal stimulus was adjusted to achieve an average baseline paw-withdrawal latency of approximately 8 to 10 s in naive mice. Only quick hind paw movements (with or without licking of the hind paws) away from the stimulus were considered to be a withdrawal response. Paw movements associated with locomotion or weight-shifting were not counted as a response. The paws were measured alternating between the left and right with an interval of more than 3 min between measurements. The latency of paw withdrawal after the thermal stimulus was determined as the average of three trials per paw on each side. Before the behavioral responses to the thermal stimulus were tested, mice were habituated for 1 h in an acrylic cylinder (15 cm high and 8 cm in diameter). Under these conditions, the latency of paw withdrawal in response to a thermal stimulus was tested before PSNL, and on the days indicated in the experimental protocols.
To quantify the sensitivity to a tactile stimulus, paw withdrawal in response to a tactile stimulus was measured using von Frey filaments (North Coast Medical, Inc., Morgan Hill, CA, USA) with a bending force of 0.02 g or 0.16 g as described previously.20 The von Frey filament was applied to the plantar surface of the hind paw for 3 s, and this was repeated three times at intervals of at least 5 s. Each of the hind paws was tested individually. Paw withdrawal in response to a tactile stimulus was evaluated by scoring as follows: 0, no response; 1, a slow and slight withdrawal response; 2, a slow and prolonged flexion withdrawal response (sustained lifting of the paw) to the stimulus; 3, a quick withdrawal response away from the stimulus without flinching or licking; 4, an intense withdrawal response away from the stimulus with brisk flinching and/or licking. We refer to the evaluated number as the allodynia score in response to a tactile stimulus. The allodynia score was determined as the average of two trials per paw on each side. Paw movements associated with locomotion or weight-shifting were not counted as a response. The paws were measured alternating between left and right with an interval of more than 3 min between measurements. Before the behavioral responses to a tactile stimulus were tested, mice were habituated for 1 h on an elevated nylon mesh floor. Under these conditions, the allodynia score was measured before PSNL, and on the days indicated in the experimental protocols.
Temporal suppression of specific neural pathway
Designer Receptors Exclusively Activated by Designer Drugs (DREADD) is a novel method for activating or suppressing specific neural cells. This chemical-genetic approach does not require the placement of an optical fiber on the top of the head and connecting it to an optical device, as in optogenetics.21 This would be advantageous for continuous neural suppression during exercise. hM4Di is a suppressive G-protein-coupled receptor and clozapine N-oxide (CNO) can continuously activate it for many hours after administration.22–24 Intraperitoneal injection of 1 mg/kg CNO is sufficient to activate the Gi-DREADD system in the central nervous system.25 We used this system and suppressed specific dopamine neurons projecting from the VTA to the NAc only during exercise, and not while measuring pain thresholds. In the pharmacogenetic experiments, mice received intraperitoneal injections of either CNO or equivalent vehicle solution 60 min before every running session. Mice in the CNO group received an injection of 1 mg/kg CNO, which was first dissolved to 10 mg/ml in double-distilled water, and then adjusted to 0.1 mg/ml with natural saline. Mice in the vehicle group received an injection of 10 ml/kg saline. CNO was purchased from Abcam plc.
Viral constructs
To achieve cell-type-specific hM4Di expression, we used a Cre-inducible recombinant Adeno Associated Virus serotype 10 (AAV10) vector carrying the hM4Di gene under the human Synapsin 1 promoter (AAV10-hM4Di). The hM4Di-mCherry coding sequence was inserted between incompatible loxP and lox2272 sites in a reverse orientation for Cre-dependent expression. In DAT-Cre transgenic mice, AAV-hM4Di was microinjected into the VTA to express hM4Di receptors selectively in DAT-positive neurons.
For neural pathway-specific Cre recombinase expression, we used a lentiviral vector for neuron-specific retrograde gene transfer (NeuRet) carrying the Cre recombinase gene under the Murine Stem Cell Virus promoter (NeuRet-Cre). Cre recombinase sequence was cloned into the lentiviral vector carrying a PolyA-tail to stabilize mRNA. In C57BL6/J mice, NeuRet-Cre was microinjected into the NAc to express Cre recombinase selectively in neurons projecting to the NAc, including VTA dopamine neurons. To achieve specific hM4Di expression in the neural pathway projecting from the VTA to the NAc, AAV-hM4Di was microinjected into the VTA after a sufficient interval (five weeks or more) from the microinjection of NeuRet-Cre.
Viral microinjections
More than two weeks before the treadmill exercise, DAT-Cre mice and C57/BL mice in the pharmacogenetic experiments underwent stereotactic surgery for viral microinfusion of AAV-hM4Di into the VTA. Mice were anesthetized with 3% isoflurane and placed in a stereotaxic apparatus. The skull was exposed and holes were drilled in the skull bilaterally above the VTA (anteroposterior = −2.5, mediolateral = ± 1.3, ventral = −4.8 mm from bregma, and angle = 10°) according to an atlas of the mouse brain (Franklin and Paxinos, 1997). AAV (1.0 µl/side) was microinjected at a rate of 0.25 µl/min (4 min). The needle was left in place after micro injection. More than five weeks before the microinjection of AAV, C57/BL mice received a microinjection of the lentivirus (NeuRet-Cre) (1.0 µl/side) into the bilateral NAc (anteroposterior = + 1.4, mediolateral = ± 1.5, ventral = −3.6 mm from bregma, and angle = 10°).
Immunohistochemistry
Mice were deeply anesthetized by the inhalation of 3% isoflurane with oxygen and perfused intracardially by saline with 0.6% heparin followed by freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer. After perfusion, the brain was quickly removed, and thick coronal sections that included the NAc and VTA were obtained using Brain Blocker (Neuroscience Inc.). The brain sections were fixed in 4% paraformaldehyde for 2 h, and then permeated with 20% sucrose in 0.1 M phosphate buffer for 1 day and 30% sucrose in 0.1 M phosphate-buffered saline for two days with agitation. The brain sections were frozen in O.C.T. compound (Sakura Finetek, Tokyo, Japan). Transverse sections were cut with a cryostat (Leica CM1510; Leica Microsystems, Heidelberg, Germany) at a thickness of 10 – 15 µm.
Brain sections that included the NAc or VTA were incubated in 10% normal horse serum or 3% or 10% normal goat serum in 0.01 M phosphate-buffered saline for 1 h at room temperature, and then incubated with the following primary antibodies for 24–48 h: anti-mCherry (1:1000, Abcam plc., Cambridge, UK), anti-TH (mouse monoclonal, 1:4500, ImmunoStar, Inc., WI, USA), and anti-dopamine transporter (DAT) (rat monoclonal, 1:3000, Millipore Co, CA, USA). The antibody was rinsed and incubated with an appropriate secondary antibody conjugated with Alexa Fluor® 488 and/or 546 (Molecular Probes, Inc., Eugene, OR, USA) for 2 h at room temperature. The slides were cover-slipped with Dako Fluorescent mounting medium (Dako, Denmark). Fluorescence of immunolabeling was detected using light microscope (BX61; Olympus, Tokyo, Japan) and photographed with a digital camera (CoolSNAP HQ; Olympus), and confocal scanning laser microscope (FV1200; Olympus).
Statistical analysis
Data are expressed as the mean with SEM. Equality of group variances was tested by Brown–Forsythe test. The statistical significance of differences between groups was assessed with two-way analysis of variance followed by Sidak’s multiple comparisons test for repeated measured data. All statistical analyses were performed with Prism version 6.0f (GraphPad Software).
Results
Effect of low-speed treadmill exercise (6 m/min) on neuropathic pain-like allodynia
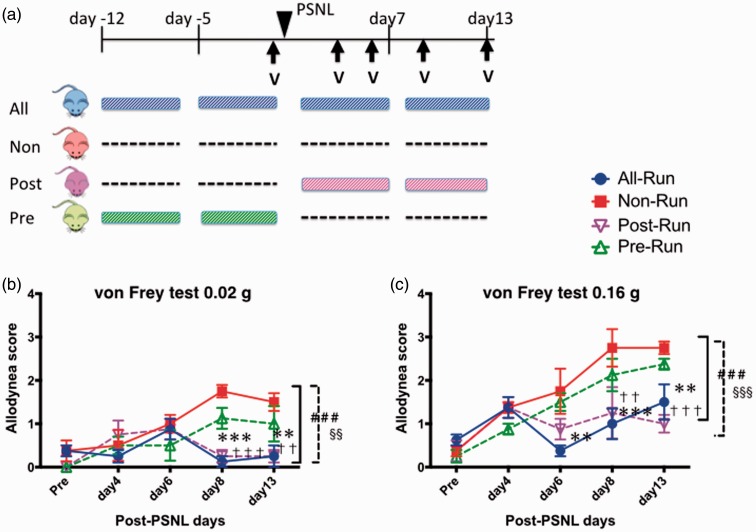
The allodynia score gradually increased in the non-run and pre-run groups, but not in the all-run and post-run groups (Figure 1). There were significant differences in the allodynia score between the all-run and non-run groups and between the post-run and non-run groups both on 0.02 g and 0.16 g von Frey test (F(3, 60) = 8.30, 9.39, p < 0.001). The scores on day 8 and 13 in the all-run and the post-run groups were significantly lower than those in the non-run group (p < 0.001). All mice seemed to run normally under ligation of the right sciatic nerve. The allodynia score in the pre-run group tended to be lower than that in the non-run group, but this difference was not significant.
Figure 1.
Effect of low-speed treadmill exercise (6 m/min) on pain threshold. (a) Schedule of experimental protocol. Effect of low-speed treadmill exercise (6 m/min) was tested under neuropathic pain-like state. PSNL was performed on all mice in four groups. All-run group (blue) exercised for four weeks, whereas non-run group (red) did no exercise. Post-run group (green) exercised for two weeks after the PSNL, whereas pre-run group (purple) did for two weeks just before the PSNL. (b, c) Change of pain threshold due to the exercise, measured by von Frey test (0.02 g (b) and 0.16 g (c)). Each point represents the mean ± SEM of four samples. ###p < 0.001, **p < 0.01, ***p < 0.001; all-run vs. non-run. §§p < 0.01, §§§p < 0.001, ††p < 0.01, †††p < 0.001; post-run vs. non-run. PSNL: partial sciatic nerve ligation; V: Von Frey test.
Effect of high-speed treadmill exercise (12 m/min) on neuropathic pain-like allodynia
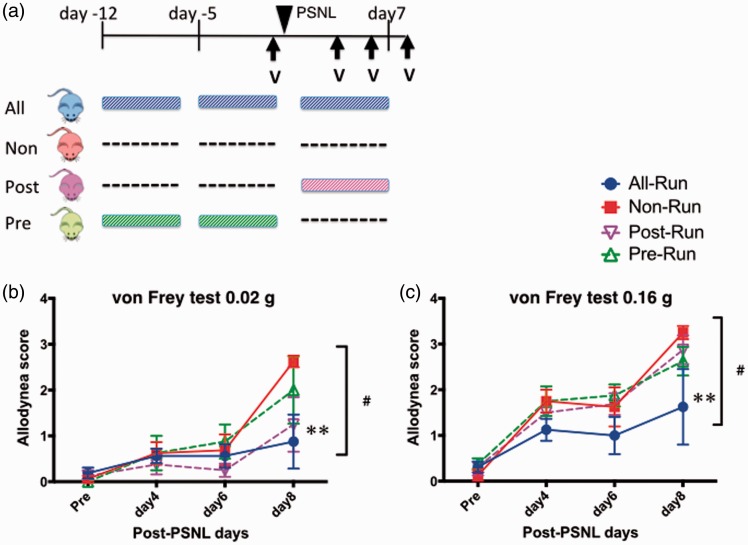
The allodynia score in the all-run group was significantly lower than that in the non-run group on the 0.16 g von Frey test (F(3, 48) = 3.87, p < 0.05, Figure 2). There was no significant difference between the post-run and non-run groups, unlike with low-speed treadmill exercise (6 m/min). Furthermore, we had to stop the experiment at 10 days after PSNL because a few mice in the post-run group stopped running. Instead, they sat on the conveyer belt of the treadmill and remained there for several minutes. This resistance to the protocol started in the second week of exercise and all of the mice had run as usual until that day. On the other hand, the mice in the all-run group continued running on the treadmill run at high speed as well as at low speed. In the pre-run group, there was no problem in the treadmill exercise performed before PSNL.
Figure 2.
Effect of high-speed treadmill exercise (12 m/min) on pain threshold. (a) Schedule of experimental protocol. Effect of high-speed treadmill exercise (6 m/min) was tested under neuropathic pain-like state. PSNL was performed on all mice in four groups. All-run group (blue) exercised for three weeks, whereas non-run group (red) did no exercise. Post-run group (green) exercised for just a week after the PSNL, whereas pre-run group (purple) did for two weeks before the PSNL. (b, c) Change of pain threshold due to the exercise, measured by von Frey test (0.02 g (b) and 0.16 g (c)). Each point represents the mean ± SEM of four samples. #p < 0.05, **p < 0.01; all-run vs. non-run. PSNL: partial sciatic nerve ligation; V: Von Frey test.
Development of EIH model (6 m/min)
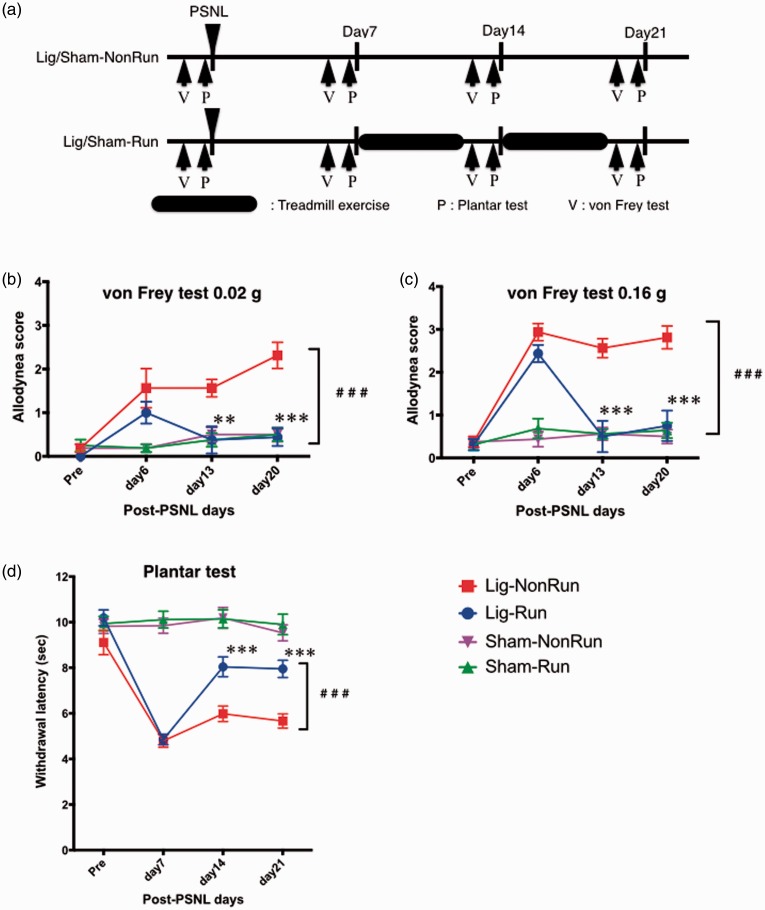
We observed a significant improvement in pain thresholds after treadmill exercise in the lig-run group mice in the 0.02 g, the 0.16 g von Frey test, and the plantar test (F(3, 111) = 25.21, 56.13, F(3, 171) = 89.45, p < 0.001, Figure 3). On the other hand, a high allodynia score and the low withdrawal latency were observed from days 6 to 21 in the von Frey test and the plantar test in the lig-nonrun group. Both the allodynia score and the withdrawal latency were significantly recovered at days 13 and 20 and days 14 and 21, respectively, in the lig-run group compared to the lig-nonrun group. There was no change in the pain threshold for three weeks after the sham operation in both the sham-run and sham-nonrun groups.
Figure 3.
Pain threshold recovery under neuropathic pain-like state due to low-speed treadmill exercise (6 m/min): Exercise-induced hypoalgesia (EIH) model. (a) Schedule of experimental protocol. Treadmill exercise was performed at speed of 6 m/min for two weeks from seven days after PSNL in lig-run group (blue) and sham-run group (green), while lig-nonrun group (red) and sham-nonrun group (purple) did no exercise. (b-d) Change of pain threshold due to the exercise, measured by von Frey test (0.02 g (b) and 0.16 g (d)) and plantar test (d). Each point represents the mean ± SEM of 8 or 12 samples. ###p < 0.001, **p < 0.01, ***p < 0.001; lig-run vs. lig-non-run. PSNL: partial sciatic nerve ligation; P: planter test, V: Von Frey test.
Influence of inhibiting VTA dopamine cells via DREADD-mill system on EIH
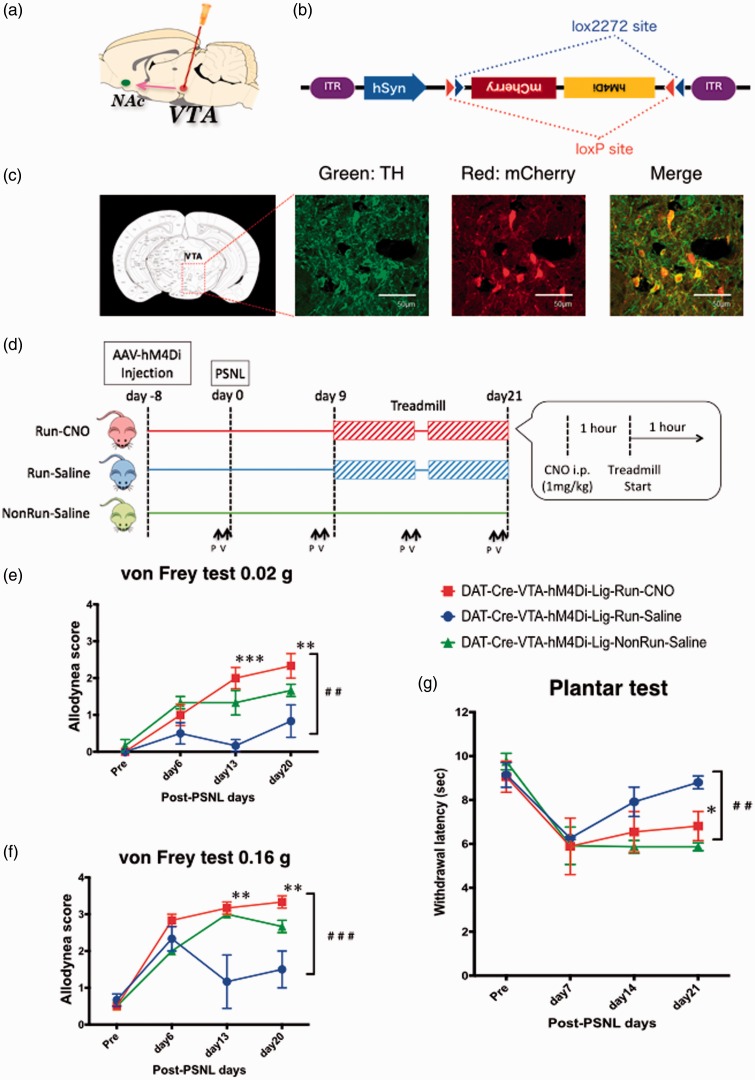
DAT-Cre mice have DAT-positive neurons that express Cre enzyme. Therefore, in these animals, the Cre enzyme is only expressed on dopamine neurons since DAT has a specific function of dopamine re-uptake from a synaptic cleft and a DAT-positive neuron is a dopamine neuron. AAV-hM4Di has two kinds of lox sequences that act as a FLEX switch with the Cre enzyme to express hM4Di (Figure 4(b)). Most dopamine cells exist in the VTA and the substantia nigra in the ventral midbrain.26 It is well known that the dopamine neurons in the lateral VTA terminate in the NAc shell and code value signals.27 We induced hM4Di specifically into the dopamine neurons in the lateral VTA and suppressed their neural activities during the treadmill exercise in the DREADD-mill test. As shown in Figure 4(a), we microinjected AAV-hM4Di into the VTA of DAT-Cre mice at 17 days before the first day of treadmill exercise because at least 14 days is required for hM4Di expression via cre-lox recombination. We confirmed the expression of hM4Di in dopamine neurons by colocalization of TH and mCherry on double-stained brain slices that included the VTA area (Figure 4(c)). All of the mice started the treadmill exercise on the ninth day after PSNL. As shown in Figure 4(d), they started running an hour after the intraperitoneal injection of CNO or saline to allow time for sufficient neural suppression due to the Gi-DREADD system. Despite treadmill exercise, the run-CNO group mice showed high allodynia score and low withdrawal latency every day after PSNL (Figure 4(e) to (g)). On the other hand, the run-saline group mice showed improvements in both the allodynia score and the withdrawal latency after exercise. As a result, there were significant differences in the von Frey and plantar tests between the run-CNO and run-saline groups (F(2, 24) = 15.68, 12.84, and 10.76, p < 0.001).
Figure 4.
Effect of specific pharmacogenetic suppression of VTA dopamine neural activity on EIH. (a) Injection site for AAV-hM4Di in VTA. (b) Schematic construct of AAV10-hM4Di. (c) Specific expression of the AVV-derived transgenes in the VTA of DAT-Cre/TG mice. Fluorescent immunostaining revealed mCherry-labeled hM4Di receptors (red) on TH-labeled dopamine neurons (green). Yellow in the merged picture represents colocalization of hM4Di and TH. (d) Schedule of the experimental protocol. AAV-hM4Di was injected at VTA in two weeks before the PSNL, subsequently the hM4Di expression induced by FLEX switch system was restricted to DAT positive cells. Treadmill exercise (6 m/min) was performed for two weeks from nine days after PSNL. CNO was injected an hour before the exercise everyday (1 mg/kg, i.p.). (e-g) Anti-EIH effect due to temporally suppressed activity of VTA dopamine neuron induced by Gi-DREADD system, measured by von Frey test (0.02 g (e) and 0.16 g (f)) and plantar test (g). Each point represents the mean ± SEM of three samples. ##p < 0.01, ###p < 0.001, *p < 0.05, **p < 0.01, ***p < 0.001. VTA: ventral tegmental area; NAc: nucleus accumbens; PSNL: partial sciatic nerve ligation; P: planter test, V: Von Frey test.
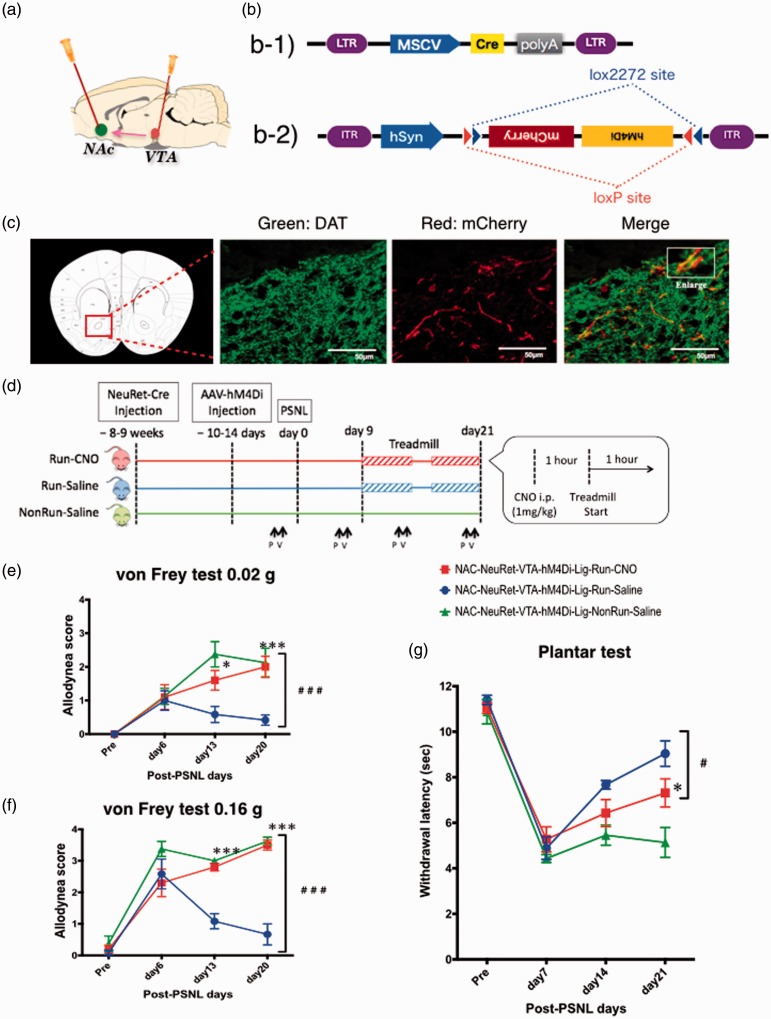
Influence of inactivation of VTA-NAc neurons via NeuRet-mill system on EIH
The VTA dopamine neurons suppressed in the DREADD-mill experiment terminate not only in the NAc but also in the amygdala, the hippocampus, the cingulate gyrus, and the prefrontal cortex.28 To focus on the neural pathway from the VTA to the NAc, we planned an experiment with NeuRet-mill (Figure 5). As shown in Figure 5(a) and (b), NeuRet-Cre is capable of neuron-specific retrograde introduction of the gene sequence of the Cre enzyme. In the present study, the Cre enzyme was expressed in all kinds of neurons terminating in the NAc, including dopamine neurons in the VTA, after the microinjection of NeuRet-Cre into the NAc. Additionally, the second microinjection of AAV-hM4Di into the VTA induced the specific expression of hM4Di on VTA neurons that terminate in the NAc. To allow for sufficient Cre enzyme expression via retrograde neural infection, we allowed for an interval of six to seven weeks between the microinjection of NeuRet-Cre into the bilateral NAc and the microinjection of AAV-hM4Di into the bilateral VTA. We confirmed the expression of hM4Di in dopamine neurons by colocalization of DAT and mCherry on double-stained brain slices that included the NAc area (Figure 5(c)). AAV-hM4Di was injected at least two weeks before the treadmill exercise (Figure 5(d)). The subsequent schedule was the same as that in the DREADD-mill experiment. Despite the treadmill exercise, the run-CNO group showed a high allodynia score and low withdrawal latency every day after PSNL (Figure 5(e) to (g)). On the other hand, the run-saline group showed improvements in both the allodynia score and the withdrawal latency after exercise. As a result, there were significant differences in the results of the von Frey and plantar tests between the run-CNO and run-saline groups (F(2, 48) = 13.88, 32.10, and 13.98, p < 0.001).
Figure 5.
Effect of specific pharmacogenetic suppression of VTA-NAc neural pathway on EIH. (a) Injection site for NeuRet-Cre in NAc and for AAV-hM4Di in VTA. (b) Schematic construct of NeuRet-Cre (b-1) and AAV-hM4Di (b-2). (c) Specific expression of the AVV-derived transgenes in the NAc after NeuRet-Cre microinjection. Fluorescent immunostaining revealed mCherry-labeled hM4Di receptors (red) on DAT-labeled dopamine neurons (green). (d) Schedule of the experimental protocol. NeuRet-Cre was injected at NAc in nine weeks before the PSNL for enough expressions of Cre recombinase. AAV-hM4Di was injected at VTA in two weeks before the PSNL, subsequently the hM4Di expression induced by FLEX switch system was restricted to the initially infected cells. Treadmill exercise (6 m/min) was performed for two weeks from nine days after PSNL. CNO was injected an hour before the exercise everyday (1 mg/kg, i.p.). (e-g) Anti-EIH effect due to temporally suppression of the VTA dopamine neuron induced by Gi-DREADD system, measured by von Frey test (0.02 g (e) and 0.16 g (f)) and plantar test (g). Each point represents the mean ± SEM of four to six samples. ##p < 0.01, ###p < 0.001, ***p < 0.001. VTA: ventral tegmental area; NAc: nucleus accumbens; PSNL: partial sciatic nerve ligation; P: Planter test, V: Von Frey test.
Discussion
In the present study, we investigated possible involvement of mesolimbic dopaminergic network in EIH. To find a well-designed condition for EIH, two different speeds of treadmill run were tested at first. Pain threshold was measured at least 15 h after the treadmill exercise, rather than immediately after treadmill running. We needed to demonstrate a long-term analgesic effect due to exercise, since short-term analgesia would not be helpful for chronic pain patients in a clinical situation. With low-speed treadmill exercise (low-TE), the pain threshold was significantly reduced in both the all-run and post-run groups, indicating that exercise after PSNL was important for the anti-nociception.
With high-speed treadmill exercise (high-TE), unlike low-TE, there was no difference in the pain threshold between the post-run and non-run groups. Although the difference in the mechanism between high-TE and low-TE under the present condition remained unclear, these results indicate that the EIH effect may require an adequate intensity of exercise for each individual.
To clarify the role of the dopaminergic pathway from the VTA to the NAc in the mechanism of EIH with treadmill exercise, we performed two chemogenetic experiments: “DREADD-mill” and “NeuRet-mill.” In the DREADD-mill test, the specific inhibition of dopamine neurons by the CNO administration dramatically suppressed the recovery of the pain threshold compared to that in the run-saline group. The NeuRet-mill test was performed to specify the neural pathway from the VTA to the NAc and the CNO dramatically suppressed the recovery of the pain threshold as well. These comparable findings suggest that the VTA-NAc dopaminergic pathway is strongly associated with the mechanism of EIH.
In general, the dopaminergic pathway is associated with positive emotions including euphoria and motivation29 and the positive emotions may mask the sensation of pain.30 In the present study, we clearly demonstrated that exercise could exert a number of health benefits that can promote positive well-being mental states and relieve pain by the mesolimbic dopaminergic activation. Although more multiangle researches must be performed in future studies, it is likely that the potential of exercise in supporting mental health as well as pain relief is promising along with the recovery from the plasticity in the dopaminergic system under severe pain. However, in the clinical setting, we should consider as mentioned that exercise does not always relieve pain. One possibility for the reason why exercise cannot activate the brain’s pleasure circuit in some cases is that a well-balanced exercise, not too much exercise, is crucial for overall health and best brain performance. Therefore, we hypothesize that adequate exercise may reduce mental stress as well as pain associated with the brain’s dopamine circuit.
On the other hand, the present study has several limitations. The mechanism of EIH that follows dopaminergic activation is still unknown. We assume that it involves indirect mechanisms through the pain matrix, which is the pain-related brain network. One of these is the enhancement of analgesic mechanisms, including the activation of endopioid, endocannabinoid, and the descending suppression systems. Another is the inhibition of the pain-enhancement system in relation to chronic stress, anxiety, and fear. Positive emotions induced by exercise may cause relative suppression of the brain system related to negative emotions.
While we have demonstrated an analgesic effect at least 15 h after treadmill exercise, the reason why EIH lasts for many hours is also a subject for future investigations. Exercise may have a capability of lasting recovery from the negative neural functions induced by persistent pain. Such continuous plasticity may involve an epigenetic mechanism.
An excitatory impulse from peripheral neurons due to muscle motion and haptic stimuli may activate the dopaminergic system through the brain networks associated with neurological inputs to the thalamus. One possibility is that top-down signals from the motor cortex, like other stimuli, may spread into several brain networks including the dopaminergic system. On the other hand, some neurons may respond to an increase in systemic oxygen delivery corresponding to the local oxygen demands of muscle tissues. At present, we have no significant evidence regarding how to achieve dopaminergic activation, other than the many studies which have shown that this can be done through exercise.31 More studies in the field of neuroscience will be needed to identify the mechanism.
In conclusion, we found that the dopaminergic pathway from the VTA to the NAc is involved in the anti-nociception under low-intensity exercise under a neuropathic pain-like state.
Author Contributions
KW, ES, NK, and Minoru N designed research. KW, TK, YH, Michiko N, RK, HN and MW performed research. SK, KK, and AY contributed reagents/analytic tools. KW and HM analyzed data. KW and Minoru N wrote the paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Minoru Narita is supported by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018, S1411019. Kenta Wakaizumi is supported as a research assistant in part by a Grant-in-Aid for the Program for Leading Graduate School for “Science for Development of Super Mature Society” from the Ministry of Education, Culture, Sport, Science, and Technology in Japan.
References
- 1.Daenen L, Varkey E, Kellmann M, et al. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin J Pain 2015; 31: 108–114. [DOI] [PubMed] [Google Scholar]
- 2.Kelly MN, Roger BF, Joseph LR. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012; 13: 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise: an update. Sports Med 1997; 24: 8–16. [DOI] [PubMed] [Google Scholar]
- 4.Paulev PE, Thorboll JE, Nielsen U, et al. Opioid involvement in the perception of pain due to endurance exercise in trained man. Jpn J Physiol 1989; 38: 507–517. [DOI] [PubMed] [Google Scholar]
- 5.Pertovaara A, Kemppainen P, Johansson G, et al. Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res 1982; 251: 83–92. [DOI] [PubMed] [Google Scholar]
- 6.Schobel HP, Handwerker HO, Schmieder RE, et al. Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensive vs. borderline hypertensive men. J Auton Nerv Syst 1998; 69: 49–55. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med 2004; 38: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raichlen DA, Foster AD, Seillier A, et al. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol 2013; 113: 869–875. [DOI] [PubMed] [Google Scholar]
- 9.Bobinski F, Ferreira TA, Córdova MM, et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 2015; 156: 2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol 1997; 7: 820–825. [DOI] [PubMed] [Google Scholar]
- 11.Korb A, Bonetti LV, Da Silva SA, et al. Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem Res 2010; 35: 380–389. [DOI] [PubMed] [Google Scholar]
- 12.De Souza GG, Duarte ID, De Castro Perez A. Differential involvement of central and peripheral α2 adrenoreceptors in the antinociception induced by aerobic and resistance exercise. Anesth Analg 2013; 116: 703–711. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res 2000; 126: 263–285. [DOI] [PubMed] [Google Scholar]
- 14.Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015; 87: 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vachon-Presseau E, Centeno MV, Ren W, et al. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 2016; 95: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niikura K, Narita M, Butelman ER, et al. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci 2010; 31: 299–305. [DOI] [PubMed] [Google Scholar]
- 17.Narita M1, Nakamura A, Ozaki M, et al. Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology 2008; 33: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood BN, Foley TE, Lea TV, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res 2011; 217: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 1998; 76: 215–222. [DOI] [PubMed] [Google Scholar]
- 20.Narita M, Usui A, Narita M, et al. Protease-activated receptor-1 and platelet-derived growth factor in spinal cord neurons are implicated in neuropathic pain after nerve injury. J Neurosci 2005; 25: 10000–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogan SC, Roth BL. Remote Control of Neuronal Signaling. Pharmacol Rev 2011; 63: 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander GM, Rogan SC, Abbas AI, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009; 63: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armbruster BN, Li X, Pausch MH, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 2007; 104: 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner DM, Meltzer HY, Veinbergs I, et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology (Berl) 2004; 177: 207–216. [DOI] [PubMed] [Google Scholar]
- 25.Chang WH, Lin SK, Lane HY, et al. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 1998; 22: 723–739. [DOI] [PubMed] [Google Scholar]
- 26.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: An update. Trends Neurosci 2007; 30: 194–202. [DOI] [PubMed] [Google Scholar]
- 27.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68: 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malenka RC, Nestler EJ and Hyman SE. Chapter 6: Widely projecting systems: monoamines, acetylcholine, and orexin. In: Sydor A and Brown RY (eds) Molecular neuropharmacology. A foundation for clinical neuroscience (2nd ed.). New York, NY: McGraw-Hill Medical, 2009, pp.147–148, 154–157.
- 29.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology 2009; 56: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vachon-Presseau E, Centeno MV, Ren W, et al. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 2016; 95: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis 2003; 13: 1–14. [DOI] [PubMed] [Google Scholar]