Abstract
BACKGROUND
Splenic angioembolization (SAE) is increasingly used in the management of splenic injuries in adults, although its value in pediatric trauma is unclear. We sought to assess outcomes related to splenectomy vs SAE.
METHODS
The National Trauma Data Bank was queried for patients 0 to 15 years of age from 2007 to 2011. Subgroup analysis of splenectomy vs SAE was performed for high-grade injuries using propensity analysis and inverse probability weighting.
RESULTS
Of 11,694 children presenting with splenic trauma, over 90% were treated nonoperatively. Adjusted analysis of high-grade injuries included 265 children who underwent splenectomy and 199 who underwent SAE. The Injury Severity Score, number of transfusions, and complications rates were not significantly different between the 2 groups. Overall adjusted mortality for children with high-grade injuries was 13.4% following splenectomy and 10.0% following SAE (P = .31)
CONCLUSION
Patients undergoing SAE for high-grade splenic trauma have comparable morbidity and mortality with splenectomy.
Keywords: Angioembolization, Pediatric trauma, Splenic trauma, Splenectomy
The spleen is a commonly injured organ in children with blunt abdominal trauma.1 For several decades, nonoperative management of most children with blunt splenic injury has been considered the standard of care.2–5 Such an approach has obviated the need for splenectomy in over 90% of pediatric patients who sustain blunt splenic injury, avoiding the risk of overwhelming postsplenectomy sepsis.6,7 Moreover, splenic preservation after blunt injury has been associated with reductions in blood transfusion requirement, healthcare resource utilization, and postinjury mortality rates.8,9
Splenic angioembolization (SAE) has emerged as an alternative to splenectomy for patients with blunt splenic injury who have failed or who are anticipated to fail nonoperative management. Although successful use in adult patients with splenic injury has been widely demonstrated, descriptions of its application in children have been limited to small, single-center case series.10–15 The objective of our study was to use a large, national dataset to define current treatment strategies and outcomes of children with splenic trauma, and compare the outcomes of SAE with splenectomy.
Methods
Data source
The National Trauma Data Bank is a database maintained by the American College of Surgeons and contains adult and pediatric data from over 900 US trauma centers. Data are organized in de-identified Research Datasets for use by investigators.
Study population
The Duke University Institutional Review Board determined this study exempt from review. The National Trauma Data Bank (version 7.2) was queried for all children aged 0 to 15 years who presented from 2007 to 2011 with a splenic injury (International Classification of Disease [ICD-9] code 865). Patients were then categorized by management strategy as follows: nonoperative, splenic repair (ICD-9 code 41.95), splenectomy (ICD-9 code 41.5), or embolization (ICD-9 codes 38.91, 39.79, 39.77, and 88.47). For the adjusted analysis, only patients with an Abbreviated Injury Score (AIS) for a grade IV or V splenic injury were included, as these patients have a greater likelihood of failing nonoperative management.1
Statistical analysis
Patient and injury characteristics, transfusion requirements, postprocedural complications, length of stay, and death were described for the overall population and for each treatment modality. Groups were initially compared via unadjusted analysis. Continuous variables were summarized with median and interquartile ranges (IQRs) and compared using the Kruskal–Wallis test. Categorical variables were summarized with frequency and percentages and compared using the Fisher's exact test or chi-square test, as appropriate.
A propensity analysis using inverse probability weighting (IPW) was performed to account for nonrandom treatment selection and to create balanced cohorts based on measured preoperative variables. Multivariable logistic regression was used to calculate the probability of each patient to undergo a specific treatment. Patients were then weighted by the inverse of their probability for a given treatment. IPW is a well-accepted strategy for creating a quasi-experimental design in the setting of an observational study, with good reduction of bias.16–18 Because the goal of IPW is to develop a cohort of patients where clinical equipoise exists for the decision to use either surgical splenectomy or SAE, we employed a trimmed tails methodology to eliminate patients where clinical equipoise may have been lacking. Specifically, we excluded patients with greater than 95% propensity for one treatment to ensure more comparable groups. An adjusted, weighted analysis comparing patient characteristics and outcomes was performed by using the multiplicative inverse of the probability of treatment within our regression model. Variables used for IPW adjustment include age, sex, race, open injury, nonsplenic abdominal AIS, splenic AIS (grade IV vs V), overall thoracic AIS, head injury, shock, Injury Severity Score (ISS), Glasgow Coma Score (GCS), penetrating injury (vs blunt), mechanism of injury, intentional injury, and year of admission. All statistical analyses were performed using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). A P value less than .05 was considered significant.
Results
A total of 11,694 pediatric patients with splenic injury were included for analysis (Fig. 1). Patient demographics and overall injury characteristics are described in Table 1. The median age was 12 years and the majority of injured children were male (71.8%). Patients were more commonly injured in motor vehicle collisions (51.0%) or falls (21.5%). The complication rate was 13.6% and overall mortality was 4%.
Figure 1.
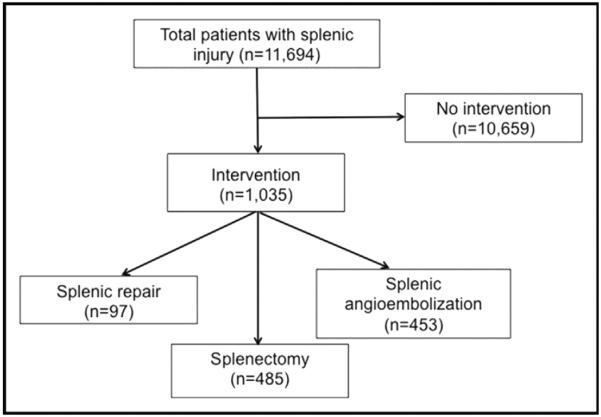
Diagram summarizing the number of patients for each intervention.
Table 1.
Demographics, injury characteristics, and outcomes of all patients presenting with splenic trauma from 2007 to 2011
| Variable | n (%) |
|---|---|
| Median age (years) (IQR) | 12 (7–14) |
| Female | 3,288 (28.2%) |
| Pediatric trauma level | |
| I | 4,967 (42.5%) |
| II | 1,391 (11.9%) |
| Other | 5,336 (45.6%) |
| Race/ethnicity | |
| White | 7,987 (72%) |
| Black | 1,100 (9.9%) |
| Hispanic | 1,325 (11.9%) |
| Other race | 687 (6.2%) |
| Splenic AIS | |
| 2 | 5,269 (45.1%) |
| 3 | 2,073 (17.7%) |
| 4 | 3,330 (28.5%) |
| 5 | 956 (8.2%) |
| ISS | |
| Mild (≤8) | 2,245 (20.1%) |
| Moderate (9–14) | 3,359 (30.1%) |
| Severe (15–24) | 3,002 (26.9%) |
| Extremely severe (≥25) | 2,550 (22.9%) |
| GCS total | |
| 15 | 8,829 (80.5%) |
| 9–14 | 794 (7.2%) |
| ≤8 | 1,347 (12.3%) |
| Mechanism of injury | |
| MVC | 5,961 (51%) |
| Fall | 2,517 (21.5%) |
| Struck | 1,392 (11.9%) |
| Stab | 51 (.4%) |
| GSW | 142 (1.2%) |
| Other | 1,622 (13.9%) |
| Transfusion | |
| RBC | 691 (5.9%) |
| Platelet | 108 (.9%) |
| Complications | |
| None | 4,562 (86.4%) |
| ARF | 22 (.4%) |
| ARDS | 188 (3.6%) |
| Wound infection | 57 (1.1%) |
| DVT | 39 (.7%) |
| PE | 6 (.1%) |
| Pneumonia | 196 (3.7%) |
| Sepsis | 49 (.9%) |
| Median LOS (IQR) | 4 (2–6) |
| Mortality | 472 (4%) |
AIS = Abbreviated Injury Score; ARDS = acute respiratory distress syndrome; ARF = acute renal failure; DVT = deep venous thrombosis; GCS = Glasgow Coma Score; GSW = gunshot wound; IQR = interquartile range; ISS = Injury Severity Score; LOS = length of stay; MVC = motor vehicle collision; PE = pulmonary embolism; RBC = red blood cells.
Of children with blunt splenic injury, 10,659 (91.1%) required no intervention for their splenic injury, 97 (.8%) underwent splenic repair, 453 (3.9%) underwent SAE, and 485 (4.2%) underwent splenectomy (Table 2). Level I pediatric trauma centers performed a greater proportion of embolizations compared with level II centers. Those undergoing splenectomy or SAE had higher median ISS (P < .001). Patients undergoing SAE had lower median GCS (11, IQR 3 to 15), compared with splenectomy (15, IQR 4 to 15), splenic repair (15, IQR 15 to 15), and no intervention (15, IQR 15 to 15) (P < .001). Rates of blood and platelet transfusions were higher in those undergoing SAE and splenectomy (P < .001). Mortality was 15.3% for children who underwent splenectomy and 15.5% for those who underwent SAE, compared with 1% for those undergoing splenic repair and 3.1% for those managed nonoperatively (P < .001).
Table 2.
Univariate analysis comparing injury characteristics and outcomes, stratified by intervention required
| Variable | No intervention (n = 10,659) | Splenic repair (n = 97) | Splenectomy (n = 485) | SAE (n = 453) | P value |
|---|---|---|---|---|---|
| Pediatric trauma Level | <.001 | ||||
| I | 4,633 (43.5%) | 28 (28.9%) | 120 (24.7%) | 186 (41.1%) | |
| II | 1,270 (11.9%) | 16 (16.5%) | 57 (11.8%) | 48 (10.6%) | |
| Other | 4,756 (44.6%) | 53 (54.6%) | 308 (63.5%) | 219 (48.3%) | |
| Age (years) | <.001 | ||||
| <1 | 243 (2.3%) | 4 (4.1%) | 7 (1.4%) | 21 (4.6%) | |
| 1–4 | 1,125 (10.6%) | 11 (11.3%) | 29 (6%) | 32 (7.1%) | |
| 5–9 | 2,729 (25.6%) | 21 (21.6%) | 61 (12.6%) | 72 (15.9%) | |
| 10–15 | 6,562 (61.6%) | 61 (62.9%) | 388 (80%) | 328 (72.4%) | |
| Splenic AIS | <.001 | ||||
| 2 | 4,949 (46.4%) | 37 (38.1%) | 78 (16.1%) | 205 (45.3%) | |
| 3 | 1,955 (18.3%) | 17 (17.5%) | 50 (10.3%) | 51 (11.3%) | |
| 4 | 2,994 (28.1%) | 35 (36.1%) | 162 (33.4%) | 139 (30.7%) | |
| 5 | 713 (6.7%) | 8 (8.2%) | 187 (38.6%) | 49 (10.8%) | |
| Median ISS (IQR) | 13 (9–21) | 16 (10–25) | 26 (17–38) | 29 (17–41) | <.001 |
| ISS | < .001 | ||||
| Mild (≤8) | 2,202 (21.6%) | 11 (12.1%) | 16 (3.5%) | 16 (3.7%) | |
| Moderate (9–14) | 3,254 (32%) | 26 (28.6%) | 42 (9.2%) | 37 (8.7%) | |
| Severe (15–24) | 2,759 (27.1%) | 30 (33%) | 98 (21.5%) | 115 (26.9%) | |
| Extremely severe (≥25) | 1,968 (19.3%) | 24 (26.4%) | 299 (65.7%) | 259 (60.7%) | |
| GCS total | <.001 | ||||
| 15 | 8,285 (83%) | 78 (81.2%) | 278 (60.3%) | 188 (43.8%) | |
| 9–14 | 695 (7%) | 5 (5.2%) | 49 (10.6%) | 45 (10.5%) | |
| ≤8 | 1,004 (10.1%) | 13 (13.5%) | 134 (29.1%) | 196 (45.7%) | |
| Blood transfusions | |||||
| RBC | 425 (4%) | 17 (17.5%) | 116 (23.9%) | 133 (29.4%) | <.001 |
| Platelet | 44 (.4%) | 2 (2.1%) | 31 (6.4%) | 31 (6.8%) | <.001 |
| Complications | |||||
| None | 4,176 (89.4%) | 43 (82.7%) | 172 (64.9%) | 171 (58.6%) | <.001 |
| ARF | 16 (.3%) | 0 (0%) | 2 (.8%) | 4 (1.4%) | .051 |
| ARDS | 115 (2.5%) | 1 (1.9%) | 24 (9.1%) | 48 (16.4%) | <.001 |
| Wound infection | 41 (.9%) | 2 (3.8%) | 10 (3.8%) | 4 (1.4%) | <.001 |
| DVT | 25 (.5%) | 0 (0%) | 6 (2.3%) | 8 (2.7%) | <.001 |
| PE | 4 (.1%) | 0 (0%) | 0 (0%) | 2 (.7%) | .094 |
| Pneumonia | 133 (2.8%) | 1 (1.9%) | 21 (7.9%) | 41 (14%) | <.001 |
| Sepsis | 32 (.7%) | 0 (0%) | 10 (3.8%) | 7 (2.4%) | <.001 |
| Median LOS (IQR) | 4 (2–6) | 7 (5–11) | 6 (4–13) | 7 (4–15) | <.001 |
| Median ICU days (IQR) | 2 (1–4) | 3 (2–5) | 3 (1–7) | 4 (2–9) | <.001 |
| Median ventilator days (IQR) | 2 (1–6) | 2 (1–11) | 2 (1–6) | 4 (2–9) | .11 |
| Mortality | 327 (3.1%) | 1 (1%) | 74 (15.3%) | 70 (15.5%) | <.001 |
AIS = Abbreviated Injury Score; ARDS = acute respiratory distress syndrome; ARF = acute renal failure; DVT = deep venous thrombosis; GCS = Glasgow Coma Score; ICU = intensive care unit; IQR = interquartile range; ISS = Injury Severity Score; LOS = length of stay; PE = pulmonary embolism; RBC = red blood cells; SAE = splenic angioembolization.
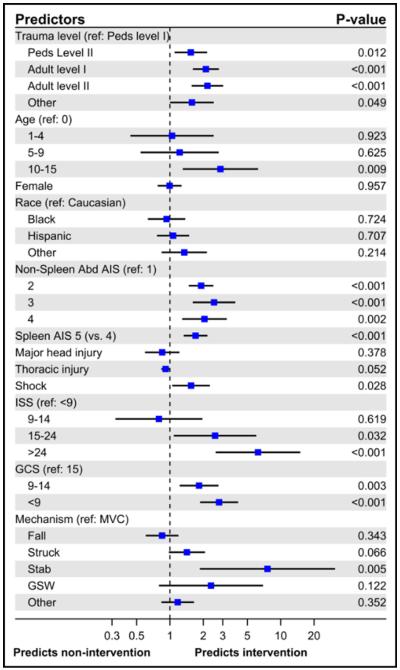
Of children with grades IV and V splenic injuries, 87.6% (n = 4,306) of the children were treated nonoperatively, 358 (7.3%) underwent splenectomy, and 204 (4.1%) underwent SAE (Table 3). The transfusion rates for those managed nonoperatively were lower than those who underwent an intervention. Children who underwent splenectomy or SAE had more complications and a higher mortality rate than those who did not undergo an intervention. Variables predictive of intervention are presented in Fig. 2. Children were more likely to undergo an intervention if they were older, had higher AIS or lower GCS, were stabbed, or presented in shock. Treatment at a level I pediatric trauma center was associated with noninterventional management of high-grade splenic injuries even after adjustment for patient case mix.
Table 3.
Demographics, injury characteristics, and outcomes among children with grade IV and V splenic injury
| Variable | No intervention (n = 4,306) | Splenic repair (n = 49) | Splenectomy (n = 358) | SAE (n = 204) | P value |
|---|---|---|---|---|---|
| Pediatric trauma level | <.001 | ||||
| I | 1,949 (45.3%) | 14 (28.6%) | 96 (26.8%) | 85 (41.7%) | |
| II | 568 (13.2%) | 12 (24.5%) | 44 (12.2%) | 30 (14.7%) | |
| Other | 1,789 (41.5%) | 23 (46.9%) | 218 (60.9%) | 89 (43.6%) | |
| Age (years) | <.001 | ||||
| <1 | 89 (2.1%) | 0 (0%) | 5 (1.4%) | 9 (4.4%) | |
| 1–4 | 374 (8.7%) | 4 (8.2%) | 22 (6.1%) | 10 (4.9%) | |
| 5–9 | 1,102 (25.6%) | 14 (28.6%) | 42 (11.7%) | 27 (13.2%) | |
| 10–15 | 2,741 (63.7%) | 31 (63.3%) | 289 (80.7%) | 158 (77.5%) | |
| Splenic AIS | <.001 | ||||
| 4 | 3,449 (80.1%) | 40 (81.6%) | 159 (44.4%) | 143 (70.1%) | |
| 5 | 857 (19.9%) | 9 (18.4%) | 199 (55.6%) | 61 (29.9%) | |
| Median ISS (IQR) | 16 (9–22) | 17 (16–25) | 26 (20–38) | 27 (17–38) | <.001 |
| ISS | <.001 | ||||
| Mild (≤8) | 205 (5%) | 1 (2.3%) | 5 (1.5%) | 2 (1.1%) | |
| Moderate (9–14) | 1,350 (33%) | 9 (20.5%) | 17 (5.1%) | 10 (5.3%) | |
| Severe (15–24) | 1,566 (38.3%) | 18 (40.9%) | 72 (21.8%) | 67 (35.3%) | |
| Extremely severe (≥25) | 967 (23.7%) | 16 (36.4%) | 237 (71.6%) | 111 (58.4%) | |
| GCS | <.001 | ||||
| 15 | 3,533 (87.4%) | 41 (83.7%) | 210 (61.8%) | 116 (60.1%) | |
| 9–14 | 222 (5.5%) | 2 (4.1%) | 44 (12.9%) | 19 (9.8%) | |
| ≤8 | 286 (7.1%) | 6 (12.2%) | 86 (25.3%) | 58 (30.1%) | |
| Blood transfusions | |||||
| RBC | 167 (3.9%) | 9 (18.4%) | 82 (22.9%) | 55 (27%) | <.001 |
| Platelet | 14 (.4%) | 2 (4.1%) | 23 (6.4%) | 9 (4.4%) | <.001 |
| Complications | |||||
| None | 1,820 (92%) | 19 (70.2%) | 133 (66/8%) | 78 (64.5%) | <.001 |
| ARF | 3 (.2%) | 0 (0%) | 1 (.5%) | 0 (0%) | .47 |
| ARDS | 45 (2.3%) | 0 (0%) | 15 (7.5%) | 22 (18.2%) | <.001 |
| Wound infection | 12 (.6%) | 0 (0%) | 8 (4%) | 1 (.8%) | .001 |
| DVT | 7 (.4%) | 0 (0%) | 5 (2.5%) | 4 (3.3%) | <.001 |
| PE | 1 (.1%) | 0 (0%) | 0 (0%) | 2 (1.7%) | .018 |
| Pneumonia | 38 (1.9%) | 0 (0%) | 17 (8.4%) | 14 (11.6%) | <.001 |
| Sepsis | 10 (.5%) | 0 (0%) | 8 (4%) | 1 (.8%) | <.001 |
| Median LOS (IQR) | 4 (3–6) | 7 (6–9) | 6 (4–12) | 7 (4–12) | <.001 |
| Median ICU days (IQR) | 2 (1–3) | 3 (2–5) | 3 (1–7) | 4 (2–6) | <.001 |
| Median ventilator days (IQR) | 2 (1–6) | 2 (1–2.5) | 2 (1–6) | 5 (2–9) | .001 |
| Mortality | 107 (2.5%) | 1 (2%) | 45 (12.6%) | 18 (8.8%) | <.001 |
AIS = Abbreviated Injury Score; ARDS = acute respiratory distress syndrome; ARF = acute renal failure; DVT = deep venous thrombosis; GCS = Glasgow Coma Score; ICU = intensive care unit; IQR = interquartile range; ISS = Injury Severity Score; LOS = length of stay; PE = pulmonary embolism; RBC = red blood cells; SAE = splenic angioembolization.
Figure 2.
Forest plot demonstrating variables predictive of intervention for children with grade IV or V splenic injury.
Propensity analysis of high-grade splenic injuries
Only patients with grade IV or V splenic injury were included in the propensity analysis, resulting in 265 patients (57.1%) in the splenectomy cohort and 199 (42.9%) in the SAE cohort. Thirty-six patients (18.1%) in the SAE group failed and required splenectomy. Differences in demographics, postprocedural complications, and outcomes before and after IPW adjustments are listed in Table 4. There were no significant differences in age, sex, race, splenic grade, and ISS between the 2 groups following adjustment.
Table 4.
Demographics, injury characteristics, postprocedural complications, and outcomes of splenectomy vs SAE, before and after adjustment with inverse probability weighting
| Unadjusted analysis |
IPW adjusted analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Splenectomy (n = 265) | SAE (n = 199) | P value | Splenectomy (n = 265) | SAE (n = 199) | P value |
| Age (years) | 12.1 ± 3.6 | 11.9 ± 3.8 | .69 | 12.0 ± 3.6 | 12.1 ± 3.7 | .71 |
| Female sex (%) | 29.4 | 30.7 | .78 | 29.0 | 31.4 | .63 |
| Race (%) | .48 | .91 | ||||
| White | 73.6 | 68.8 | 69.6 | 69.9 | ||
| Black | 7.2 | 11.1 | 7.9 | 8.8 | ||
| Hispanic | 13.2 | 13.1 | 13.0 | 13.8 | ||
| Other | 6.0 | 7.0 | 9.5 | 7.4 | ||
| Pediatric trauma level (%) | .001 | .95 | ||||
| I | 24.9 | 41.7 | 31.6 | 31.4 | ||
| II | 12.1 | 8.0 | 10.2 | 11.3 | ||
| Other | 63.0 | 50.3 | 58.2 | 57.3 | ||
| ISS | 30.5 ± 13.7 | 32.4 ± 15 | .15 | 32 ± 14.5 | 31.5 ± 14.1 | .74 |
| GCS | 11.7 ± 5.1 | 10.4 ± 5.4 | .009 | 10.9 ± 5.5 | 11 ± 5.3 | .92 |
| Grade V splenic injury (%) | 54.0 | 37.7 | <.001 | 46.8 | 45.2 | .77 |
| RBC transfusion (%) | 23.0 | 32.2 | .03 | 26.1 | 30.0 | .44 |
| Platelet transfusion (%) | 6.0 | 9.5 | .17 | 7.5 | 9.9 | .46 |
| Complication (%) | ||||||
| None | 66.2 | 57.8 | .15 | 63.3 | 64.4 | .87 |
| ARF | 0 | 1.6 | .16 | 0 | 1 | .16 |
| ARDS | 7.9 | 20.3 | .003 | 7.9 | 15.9 | .056 |
| Wound infection | 4.0 | 3.1 | .71 | 3.8 | 4.7 | .76 |
| DVT | 2.6 | 5.5 | .24 | 4.2 | 5.7 | .68 |
| PE | 0 | 1.6 | .16 | 0 | 1.1 | .18 |
| Pneumonia | 9.9 | 11.7 | .63 | 13.1 | 9.4 | .51 |
| Sepsis | 3.3 | .8 | .13 | 2.7 | .4 | .083 |
| LOS (days) | 9.6 ± 10.6 | 11.6 ± 12.5 | .079 | 9.9 ± 10.6 | 10.9 ± 11.1 | .37 |
| Mortality (%) | 10.9 | 13.1 | .49 | 13.4 | 10.0 | .31 |
Unless otherwise specified, data are presented as mean ± standard deviation.
ARDS = acute respiratory distress syndrome; ARF = acute renal failure; DVT = deep venous thrombosis; GCS = Glasgow Coma Score; IPW = inverse probability weighting; ISS = injury severity score; LOS = length of stay; PE = pulmonary embolism; RBC = red blood cells; SAE = splenic angioembolization.
Children who were managed by splenectomy or SAE had no significant differences in rates of both red blood cell (P = .44) and platelet (P = .46) transfusions following IPW. The most common postprocedural complications were acute respiratory distress syndrome (ARDS) and pneumonia. Rates of ARDS were higher in the SAE group in the unadjusted analysis (P = .003) and trended toward significance following adjustment (P = .058). Before and after IPW, rates of wound infection, pneumonia, and sepsis were not significantly different. Length of stay and number of intensive care unit or ventilator days were not significantly different. In the unadjusted analysis, there was no difference in mortality between children undergoing splenectomy vs SAE (P = .47); this held true after adjustment with a mortality rate of 13.4% in the splenectomy group and 10.0% in the SAE group (P = .31).
Comments
Our analysis represents the largest published description of SAE utilization for blunt splenic injury in pediatric patients, and the largest study to compare the outcomes of such patients with those of patients undergoing splenectomy. Although the role of SAE has been firmly established in adults as a treatment option for those who fail nonoperative management of splenic injury, its utility in the management of pediatric splenic trauma is limited.2–7 Upon analysis of a national database, we found that embolization for high-grade splenic injury has equivalent transfusion rates, morbidity, and mortality compared with splenectomy by adjusted analysis.
Complications following SAE can be quite severe, with rates from 23% to 62% in adults, and include pseudocysts, secondary vascular abnormality, pancreatitis, renal impairment, puncture-site hematomas, ARDS, splenic infarction, and splenic abscess.8–10 In our series, 41.4% of children had a complication, similar to these studies. The most common complications were ARDS (16.4%) and pneumonia (14%). Complications reported in children undergoing SAE include pleural effusions, transient hypertension, puncture-site hematomas, splenic abscesses, infarction, and contrast-induced acute renal insufficiency. Overall morbidity rates vary from 7% to 58%.3,4,11
The overall mortality for those undergoing SAE was 15.5% for all grades and 10% in the adjusted analysis of grades IV and V splenic injury. Vo et al4 reported an overall mortality rate of 22% in pediatric patients undergoing SAE for splenic trauma, but this study included embolization of other organs besides the spleen in the analysis. In comparison, smaller studies with 15 or less patients reported no deaths.2,3 Our study draws from a national database and provides more robust and generalizable estimates of the morbidity and mortality for this procedure.
Based on the results of our study, SAE is equivalent to splenectomy for management of high-grade splenic injuries. Previous studies in children have demonstrated safety of embolization for traumatic splenic injuries, but have not provided direct comparison of two procedures.2–4 In adults, SAE has been demonstrated to improve splenic salvage rates for grade IV and V injuries.12 This point is especially important in the pediatric population, as children have a higher mortality rate from sepsis following splenectomy compared with adults.13–15,19,20 In our series, we do not know if embolization was proximal or distal, but theoretically, proximal splenic embolization should cause less impairment than selective distal embolization, as it allows the spleen to remain at least partially perfused, reducing risk of infection.21–23 A study of computed tomography findings of embolized spleens found that proximal embolization was associated with less frequent and smaller splenic infarcts than distal embolization.24
Limitations inherent to studies based on large database analysis exist. Improper coding may lead to poor recognition of some procedures. In regards to transfusions, the number of units given is unknown, as is the time in relation to the procedure. Indications for embolizationvs splenectomy were not available within the database, nor was the timing of the procedure in relation to the injury. For adult trauma patients, protocols have been developed to determine which patients are eligible for embolization. As the American College of Surgeons move toward establishing pediatric surgical centers of excellence, similar guidelines for children would be of utmost importance.25 With level I pediatric trauma centers having the lowest rates of intervention after adjustment for patient case mix in our study, this variation may suggest non–evidence-based differences in the threshold for intervention that would benefit from further study and standardized indications. Centers have relied on evidence of vascular injury on computed tomography scan, denoted by contrast blush, splenic vascular injuries, American Association for the Surgery of Trauma (AAST) grade III to V injury, and large hemoperitoneum, or active extravasation of contrast as signs that embolization is warranted.2,5,6,8,26–32 We sought to compare outcomes of SAE vs splenectomy in children with isolated splenic injuries; however, the database lacked granularity for direct analysis. Finally, the radiation dosing was not known for embolization procedures in our series. If embolization is to be more widely used for management of traumatic injuries in children, efforts should be made to minimize radiation exposure.
Conclusions
This analysis suggests that the use of SAE in pediatric patients who sustain severe blunt splenic injury is relatively safe. Endovascular intervention is a reasonable, minimally invasive option in high-grade splenic trauma and is comparable with splenectomy in terms of morbidity and mortality.
Acknowledgments
No funding was received for this study.
Footnotes
The authors declare no conflicts of interest.
The National Trauma Data Bank remains the full and exclusive copyrighted property of the American College of Surgeons. The American College of Surgeons is not responsible for claims arising from works based on original data, test, tables, or figures.
References
- 1.Powell M, Courcoulas A, Gardner M, et al. Management of blunt splenic trauma: significant differences between adults and children. Surgery. 1997;122:654–60. doi: 10.1016/s0039-6060(97)90070-2. [DOI] [PubMed] [Google Scholar]
- 2.Gross JL, Woll NL, Hanson CA, et al. Embolization for pediatric blunt splenic injury is an alternative to splenectomy when observation fails. J Trauma Acute Care Surg. 2013;75:421–5. doi: 10.1097/TA.0b013e3182995c70. [DOI] [PubMed] [Google Scholar]
- 3.Kiankhooy A, Sartorelli KH, Vane DW, et al. Angiographic embolization is safe and effective therapy for blunt abdominal solid organ injury in children. J Trauma. 2010;68:526–31. doi: 10.1097/TA.0b013e3181d3e5b7. [DOI] [PubMed] [Google Scholar]
- 4.Vo NJ, Althoen M, Hippe DS, et al. Pediatric abdominal and pelvic trauma: safety and efficacy of arterial embolization. J Vasc Interv Radiol. 2014;25:215–20. doi: 10.1016/j.jvir.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Davis KA, Fabian TC, Croce MA, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008–13. doi: 10.1097/00005373-199806000-00013. discussion, 1013–5. [DOI] [PubMed] [Google Scholar]
- 6.Dent D, Alsabrook G, Erickson BA, et al. Blunt splenic injuries: high nonoperative management rate can be achieved with selective embolization. J Trauma. 2004;56:1063–7. doi: 10.1097/01.ta.0000123037.66867.f2. [DOI] [PubMed] [Google Scholar]
- 7.Sclafani SJ, Shaftan GW, Scalea TM, et al. Nonoperative salvage of computed tomography-diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma. 1995;39:818–25. doi: 10.1097/00005373-199511000-00004. discussion, 826–7. [DOI] [PubMed] [Google Scholar]
- 8.Haan JM, Biffl W, Knudson MM, et al. Splenic embolization revisited: a multicenter review. J Trauma. 2004;56:542–7. doi: 10.1097/01.ta.0000114069.73054.45. [DOI] [PubMed] [Google Scholar]
- 9.Wu SC, Chen RJ, Yang AD, et al. Complications associated with embolization in the treatment of blunt splenic injury. World J Surg. 2008;32:476–82. doi: 10.1007/s00268-007-9322-x. [DOI] [PubMed] [Google Scholar]
- 10.Clancy AA, Tiruta C, Ashman D, et al. The song remains the same although the instruments are changing: complications following selective non-operative management of blunt spleen trauma: a retrospective review of patients at a level I trauma centre from 1996 to 2007. J Trauma Manag Outcomes. 2012;6:4. doi: 10.1186/1752-2897-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekeh AP, McCarthy MC, Woods RJ, et al. Complications arising from splenic embolization after blunt splenic trauma. Am J Surg. 2005;189:335–9. doi: 10.1016/j.amjsurg.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Requarth JA, D'Agostino RB, Jr, Miller PR. Nonoperative management of adult blunt splenic injury with and without splenic artery embolotherapy: a meta-analysis. J Trauma. 2011;71:898–903. doi: 10.1097/TA.0b013e318227ea50. discussion, 903. [DOI] [PubMed] [Google Scholar]
- 13.Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182–6. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 14.Cullingford GL, Watkins DN, Watts AD, et al. Severe late postsplenectomy infection. Br J Surg. 1991;78:716–21. doi: 10.1002/bjs.1800780626. [DOI] [PubMed] [Google Scholar]
- 15.Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. 1991;78:1031–8. doi: 10.1002/bjs.1800780904. [DOI] [PubMed] [Google Scholar]
- 16.Shadish WR, Clark MH, Steiner PM. Can nonrandomized experiments yield accurate answers? A randomized experiment comparing random and nonrandom assignments. J Am Stat Assoc. 2008;103:1334–44. [Google Scholar]
- 17.Kuss O, Legler T, Borgermann J. Treatment effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J Clin Epidemiol. 2001;64:1076–84. doi: 10.1016/j.jclinepi.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–7. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 19.Singer DB. Postsplenectomy sepsis. Perspect Pediatr Pathol. 1973;1:285–311. [PubMed] [Google Scholar]
- 20.Waldron DJ, Harding B, Duignan J. Overwhelming infection occurring in the immediate post-splenectomy period. Br J Clin Pract. 1989;43:421–2. [PubMed] [Google Scholar]
- 21.Anderson JH, VuBan A, Wallace S, et al. Transcatheter splenic arterial occlusion: an experimental study in dogs. Radiology. 1977;125:95–102. doi: 10.1148/125.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Keramidas DC, Kelekis D, Dolatzas T, et al. The collateral arterial network of the spleen following ligation of the splenic artery in traumatic rupture of the spleen; an arteriographic study. Z Kinderchir. 1984;39:50–1. doi: 10.1055/s-2008-1044169. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka H, Kuroda C, Hori S, et al. Splenic embolization for hypersplenism using steel coils. AJR Am J Roentgenol. 1985;144:1269–74. doi: 10.2214/ajr.144.6.1269. [DOI] [PubMed] [Google Scholar]
- 24.Killeen KL, Shanmuganathan K, Boyd-Kranis R, et al. CT findings after embolization for blunt splenic trauma. J Vasc Interv Radiol. 2001;12:209–14. doi: 10.1016/s1051-0443(07)61827-2. [DOI] [PubMed] [Google Scholar]
- 25.Miller PR, Chang MC, Hoth JJ, et al. Prospective trial of angiography and embolization for all grade III to V blunt splenic injuries: nonoperative management success rate is significantly improved. J Am Coll Surg. 2014;218:644–8. doi: 10.1016/j.jamcollsurg.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Haan J, Ilahi ON, Kramer M, et al. Protocol-driven nonoperative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma. 2003;55:317–21. doi: 10.1097/01.ta.0000083336.93868.f7. discussion, 321–2. [DOI] [PubMed] [Google Scholar]
- 27.Haan JM, Bochicchio GV, Kramer N, et al. Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma. 2005;58:492–8. doi: 10.1097/01.ta.0000154575.49388.74. [DOI] [PubMed] [Google Scholar]
- 28.Liu PP, Lee WC, Cheng YF, et al. Use of splenic artery embolization as an adjunct to nonsurgical management of blunt splenic injury. J Trauma. 2004;56:768–72. doi: 10.1097/01.ta.0000129646.14777.ff. discussion, 773. [DOI] [PubMed] [Google Scholar]
- 29.Rajani RR, Claridge JA, Yowler CJ, et al. Improved outcome of adult blunt splenic injury: a cohort analysis. Surgery. 2006;140:625–31. doi: 10.1016/j.surg.2006.07.005. discussion, 631–2. [DOI] [PubMed] [Google Scholar]
- 30.Shanmuganathan K. Multi-detector row CT imaging of blunt abdominal trauma. Semin Ultrasound CT MR. 2004;25:180–204. doi: 10.1016/j.sult.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Shanmuganathan K, Mirvis SE, Boyd-Kranis R, et al. Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217:75–82. doi: 10.1148/radiology.217.1.r00oc0875. [DOI] [PubMed] [Google Scholar]
- 32.Smith HE, Biffl WL, Majercik SD, et al. Splenic artery embolization: have we gone too far? J Trauma. 2006;61:541–4. doi: 10.1097/01.ta.0000235920.92385.2b. discussion, 545–6. [DOI] [PubMed] [Google Scholar]