Abstract
Background
The long-term survival benefit of lobectomy over sublobar resection for early-stage non-small cell lung cancer must be weighed against a potentially increased risk of perioperative mortality. The objective of the current study was to create a risk score to identify patients with favorable short-term outcomes following lobectomy.
Methods
The 2005–2012 American College of Surgeons National Surgical Quality Improvement Program database was queried for patients undergoing a lobectomy or sublobar resection (either segmentectomy or wedge resection) for lung cancer. A multivariable logistic regression model was utilized to determine factors associated with 30-day mortality among the lobectomy group and to develop an associated risk score to predict perioperative mortality.
Results
Of the 5,749 patients who met study criteria, 4,424 (77%) underwent lobectomy, 1,098 (19%) underwent wedge resection, and 227 (4%) underwent segmentectomy. Age, chronic obstructive pulmonary disease, previous cerebrovascular event, functional status, recent smoking status, and surgical approach (minimally invasive versus open) were utilized to develop the risk score. Patients with a risk score of 5 or lower had no significant difference in perioperative mortality by surgical procedure. Patients with a risk score greater than 5 had significantly higher perioperative mortality after lobectomy (4.9%) as compared to segmentectomy (3.6%) or wedge resection (0.8%, p < 0.01).
Conclusions
In this study, we have developed a risk model that predicts relative operative mortality from a sublobar resection as compared to a lobectomy. Among patients with a risk score of 5 or less, lobectomy confers no additional perioperative risk over sublobar resection.
Lung cancer is the leading cause of cancer-related death in the United States [1]. Recommended treatment for early-stage non-small cell lung cancer (NSCLC) consists of an anatomic lobectomy and mediastinal lymph node dissection or sampling in patients with suitable anatomy and adequate physiologic reserve [2, 3]. Unfortunately, many of the disease processes and environmental risk factors that predispose a patient to lung cancer are associated with other health issues that may place them at an increased risk for perioperative morbidity and mortality.
Previous studies have demonstrated that less-extensive surgical resections such as segmentectomy or wedge resection can reduce perioperative mortality, and these sublobar resections are often recommended for small tumors, elderly patients, and patients with poor preoperative pulmonary function [4–9]. Among most patients with early stage disease, however, the benefit in short-term mortality associated with a less-extensive resection may be offset by inferior oncologic outcomes[10–12]. Despite this, it remains unclear which patients should receive a less-extensive resection because of their increased risk of perioperative mortality.
Currently, there are limited data available to clinicians to help select which extent of resection is optimal for a particular patient based on their comorbidities, age, and overall health status. The available data lack the granularity to aid in risk prediction, and clinicians must rely on subjective assessment of perioperative risks. We hypothesized that there are patients for whom the short-term risk of lobectomy is not significantly increased over sublobar resection, and thus these patients may be more appropriately directed towards lobectomy.
Material and Methods
National Surgical Quality Improvement Program
The American College of Surgeons’ (ACS’s) National Surgical Quality Improvement Program (NSQIP) is an outcomes-based program designed to improve the quality of surgical care provided in the United States. Data are collected and validated from more than 700 participating centers in order to provide feedback on hospital performance [13]. These data are available in a de-identified fashion, and include patient characteristics, operative characteristics, and short-term outcomes for a variety of surgical procedures. The Duke University institutional review board determined this study was exempt from review prior to data analysis.
Patient Population
The 2005–2012 ACS NSQIP participant user files were queried for patients 18 years of age or older who underwent wedge resection (current procedural terminology [CPT] codes: 32500, 32505, 32657, 32666), segmentectomy (CPT codes: 32484, 32669), or lobectomy (CPT codes: 32480, 32663) for a primary malignancy of the lung (International Statistical Classification of Disease Ninth Edition codes 162.3, 162.4, 162.5, 162.8, and 162.9). Patients were excluded if they had disseminated cancer or underwent emergency surgery. As stage of disease is not available in the ACS NSQIP, patients who received induction chemotherapy or radiation were also excluded in an attempt to limit the analysis to patients with clinical early stage disease. Patients with an American Society of Anesthesiologists (ASA) class of 4 or 5 were also excluded because by definition these patients either have a severe systemic disease that is a constant threat to life or are moribund patients not expected to survive without surgery, and it was therefore felt that these patients did not match the clinical scenario of interest where surgeons were trying to balance short- and long-term risks.
Variables
Variables included in the analysis included patient characteristics (age, sex, race, ASA class, and body mass index [kg/m2]) and comorbidities (diabetes, chronic obstructive pulmonary disorder [COPD], history of cerebrovascular accident [CVA] or transient ischemic attack [TIA], cardiac history [including congestive heart failure within 30 days of surgery, history of myocardial infarction within 6 months of surgery, previous percutaneous coronary intervention, previous cardiac surgery, or history of angina within one month of surgery], history of shortness of breath [defined as having shortness of breath upon moderate exertion or at rest], dependent functional status [defined as requiring assistance to complete activities of daily living], treatment with steroids within 30 days of surgery, smoking history within the past year, preoperative albumin, >10% weight loss in past year, and ASA class). Operative characteristics (operative time and use of a video-assisted thoracoscopic approach) and short-term outcomes (superficial wound infection [defined as either purulent drainage, a positive culture, localized inflammation requiring opening of incision, or diagnosis of surgical site infection afflicting only the skin and subcutaneous tissue occurring within 30 days of the operation], deep wound infection [defined similarly to a superficial wound infection but involving the fascial and muscle layers], organ space infection [defined as purulence, a positive culture of the organ/space, an abscess, or a diagnosis of an organ space infection by a physician occurring within 30 days of the operation], wound dehiscence, urinary tract infection [defined as symptomatic bacteriuria or symptoms along with a positive urinalysis], pneumonia [requiring both positive radiologic findings and either specific symptoms or a positive culture], failure to wean from a ventilator for > 48 hours, postoperative transfusion requirement, cerebrovascular accident, myocardial infarction, pulmonary embolism, renal failure [either a rise in creatinine of >2 mg/dL from prior to the operation or a new requirement for dialysis], sepsis [defined as systemic inflammatory response syndrome along with either a presumed or proven source of infection], reintubation, reoperation, and 30-day readmission) were also included. Postoperative length of stay and 30-day or in-hospital mortality were also identified (defined hereafter as operative mortality). A morbidity composite including organ space infection, wound dehiscence, failure to wean from the ventilator, cerebrovascular accident, cardiac arrest, myocardial infarction, reintubation, reoperation, and renal failure was also compiled. Exact definitions of variables used in this analysis can be found in the ACS-NSQIP User Guide [14].
Statistical Analysis
Patients were initially grouped by the type of lung resection performed (wedge versus segmentectomy versus lobectomy) and compared for baseline characteristics, operative characteristics, and short-term outcomes. Continuous variables were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test or Fisher’s exact test as appropriate.
In order to create a risk model for early mortality following lobectomy, only patients who received a lobectomy were selected. A logistic regression model was created in order to determine patient characteristics associated with increased odds of operative mortality following lobectomy. Variables included in the model were based on clinical relevance and included age, diabetes, COPD, history of CVA/TIA, cardiac history, shortness of breath, dependent functional status, steroid therapy within 30 days of surgery, smoking status within past year, preoperative albumin, weight loss greater than 10% in past 6 months, ASA class, and use of a minimally invasive approach. All continuous variables were categorized in order to aid in the development of the risk score. Cutoffs for this categorization were determined by inspecting the association between the continuous variable and the odds of operative mortality. Final variables were selected utilizing backward stepwise variable selection by the Akaike information criterion. The c-statistic was calculated in order to estimate model discrimination. Model validation was also performed using bootstrap resampling.
The regression coefficients of the variables retained in the model were then divided by the minimum absolute value of all the regression coefficients to determine the score associated with each variable. The scores for each patient were then summed together to determine an individual risk score. Among the entire cohort, three logistic regression models were created with the procedure and risk score cutoff as predictors and operative mortality as an outcome, with an interaction term between the two predictors, to determine a score at which there was a significant interaction between the risk score and procedure type with regard to their association with perioperative mortality. The three models including the cutoffs >5 versus ≤5, >6 versus ≤6, and >7 versus ≤7. Short-term outcomes including operative mortality, the morbidity composite, pneumonia, failure to wean from the ventilator within 48 hours, and reintubation were then compared by procedure received for patients with a risk score both above and below the risk score for which there was a significant interaction with procedure type. All statistical analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value of 0.05 was used to define statistical significance.
Results
A total of 5,749 patients met study criteria, of which 4,424 (77.0%) underwent lobectomy, 227 (3.9%) underwent segmentectomy, and 1,098 (19.1%) underwent wedge resection. The median age of the cohort was 69 years (interquartile range [IQR], 62 to 75) and 54.1% (n = 3,100) were female. Median postoperative length of stay was 5 days (IQR, 4 to 7), and 8.4% (n = 484) of the cohort experienced the morbidity composite. Operative mortality was 1.8% (n = 103).
Compared with those who underwent sublobar resection, patients who received lobectomy tended to be younger, were less likely to carry a diagnosis of COPD or be treated with steroids within 30 days of the operative procedure, and were more likely to have been a smoker within the past year (Table 1). Median operative time was significantly longer for patients receiving lobectomy (161 minutes) compared with patients receiving segmentectomy (135 minutes) or wedge resection (99 minutes, p < 0.01, Table 2). The majority of wedge resections were performed thoracoscopically (n = 716, 65.2%) compared with only 42.2% (n = 1,868) for lobectomies and 37.4% (n = 85) for segmentectomies.
Table 1.
Baseline Patient Demographics and Comorbidities by Operative Intervention
| Variable | Overall | Lobectomy | Segmentectomy | Wedge Resection | p |
|---|---|---|---|---|---|
| N | 5,749 | 4424 (77.0%) | 227 (3.9%) | 1098 (19.1%) | |
| Age, y | 69 (62, 75) | 69 (61, 75) | 70 (63, 75) | 71 (64, 76) | <0.01 |
| Age | <0.01 | ||||
| <65 | 1,899 (33.0%) | 1,529 (34.6%) | 68 (30.0%) | 302 (27.5%) | |
| 65–80 | 3,303 (57.5%) | 2,499 (56.5%) | 138 (60.8%) | 666 (60.7%) | |
| >80 | 547 (9.5%) | 396 (9.0%) | 21 (9.3%) | 130 (11.8%) | |
| Female | 3,100 (54.1%) | 2,381 (54.0%) | 107 (47.1%) | 612 (55.9%) | 0.05 |
| Race | 0.61 | ||||
| White | 4,446 (77.3%) | 3,409 (77.1%) | 175 (77.1%) | 862 (78.5%) | |
| Black | 308 (5.4%) | 236 (5.3%) | 12 (5.3%) | 60 (5.5%) | |
| Hispanic | 218 (3.8%) | 174 (3.9%) | 12 (5.3%) | 32 (2.9%) | |
| Other/unknown | 777 (13.5%) | 605 (13.7%) | 28 (12.3%) | 144 (13.1%) | |
| Body mass index (kg/m2) | 26.6 (23.5, 30.6) | 27 (23, 31) | 27 (24, 31) | 27 (23, 30) | 0.56 |
| Diabetes | 828 (14.4%) | 632 (14.3%) | 28 (12.3%) | 168 (15.3%) | 0.46 |
| Chronic obstructive pulmonary disease | 1,543 (26.8%) | 1,116 (25.2%) | 65 (28.6%) | 362 (33.0%) | <0.01 |
| History of CVA/TIA | 260 (4.5%) | 200 (4.5%) | 9 (4%) | 51 (4.6%) | 0.90 |
| Cardiac history | 470 (8.2%) | 351 (7.9%) | 20 (8.8%) | 99 (9%) | 0.47 |
| Shortness of breath | <0.01 | ||||
| No | 4,293 (74.7%) | 3,352 (75.8%) | 178 (78.4%) | 763 (69.5%) | |
| With exertion | 1,355 (23.6%) | 1,004 (22.7%) | 46 (20.3%) | 305 (27.8%) | |
| At rest | 101 (1.8%) | 68 (1.5%) | 3 (1.3%) | 30 (2.7%) | |
| Dependent functional status | 74 (1.3%) | 54 (1.2%) | 2 (0.9%) | 18 (1.6%) | 0.49 |
| Steroid treatment (30-day) | 215 (3.7%) | 143 (3.2%) | 13 (5.7%) | 59 (5.4%) | <0.01 |
| Smoking history (past year) | 1,986 (34.5%) | 1,595 (36.1%) | 70 (30.8%) | 321 (29.2%) | <0.01 |
| Albumin (g/dL) | 4.1 (3.8, 4.3) | 4 (4, 4) | 4 (4, 4) | 4 (4, 4) | 0.83 |
| Weight loss >10% in past 6 months | 172 (3%) | 138 (3.1%) | 4 (1.8%) | 30 (2.7%) | 0.48 |
| ASA class | 0.21 | ||||
| 1 or 2 | 1,255 (21.9%) | 986 (22.3%) | 51 (22.5%) | 218 (19.9%) | |
| 3 | 4,488 (78.1%) | 3,433 (77.7%) | 176 (77.5%) | 879 (80.1%) |
Categorical values are presented as frequency (percentage) and continuous variables are presented as median (interquartile range).
ASA = American Society of Anesthesiologists; CVA = cerebrovascular accident; TIA = transient ischemic attack.
Table 2.
Operative Characteristics and Outcomes by Operative Intervention
| Variable | Overall | Lobectomy | Segmentectomy | Wedge Resection | p |
|---|---|---|---|---|---|
| N | 5,749 | 4,424 (77.0%) | 227 (3.9%) | 1098 (19.1%) | |
| Operative time (min) | 150 (108, 205) | 162 (121, 217) | 135 (98, 186) | 99 (70, 147) | <0.01 |
| VATS | 2,669 (46.4%) | 1,868 (42.2%) | 85 (37.4%) | 716 (65.2%) | <0.01 |
| Superficial infection | 62 (1.1%) | 49 (1.1%) | 3 (1.3%) | 10 (0.9%) | 0.74 |
| Deep wound infection | 11 (0.2%) | 8 (0.2%) | 1 (0.4%) | 2 (0.2%) | 0.52 |
| Organ space infection | 32 (0.6%) | 29 (0.7%) | 0 (0%) | 3 (0.3%) | 0.26 |
| Dehiscence | 3 (0.1%) | 1 (0%) | 0 (0%) | 2 (0.2%) | 0.21 |
| Urinary tract infection | 124 (2.2%) | 100 (2.3%) | 3 (1.3%) | 21 (1.9%) | 0.63 |
| Pneumonia | 346 (6%) | 293 (6.6%) | 13 (5.7%) | 40 (3.6%) | <0.01 |
| Failure to wean from ventilator | 151 (2.6%) | 125 (2.8%) | 6 (2.6%) | 20 (1.8%) | 0.18 |
| Bleeding requiring transfusion | 234 (4.1%) | 196 (4.4%) | 15 (6.6%) | 23 (2.1%) | <0.01 |
| Postop CVA | 29 (0.5%) | 22 (0.5%) | 2 (0.9%) | 5 (0.5%) | 0.55 |
| Postop MI | 25 (0.4%) | 19 (0.4%) | 2 (0.9%) | 4 (0.4%) | 0.45 |
| Pulmonary embolism | 42 (0.7%) | 35 (0.8%) | 2 (0.9%) | 5 (0.5%) | 0.47 |
| Renal failure | 46 (0.8%) | 38 (0.9%) | 1 (0.4%) | 7 (0.6%) | 0.79 |
| Sepsis | 175 (3.0%) | 149 (3.4%) | 8 (3.5%) | 18 (1.6%) | 0.01 |
| Reintubation | 208 (3.6%) | 166 (3.8%) | 9 (4%) | 33 (3%) | 0.48 |
| Reoperation | 241 (4.2%) | 193 (4.4%) | 9 (4%) | 39 (3.6%) | 0.48 |
| Readmission (30-day) | 274 (8.2%) | 204 (8.0%) | 12 (7.2%) | 58 (9.5%) | 0.45 |
| Postop length of stay (days) | 5 (4, 7) | 5 (4, 8) | 5 (3, 7) | 4 (3, 6) | <0.01 |
| Morbidity composite | 484 (8.4%) | 385 (8.7%) | 20 (8.8%) | 79 (7.2%) | 0.27 |
| Mortality | 103 (1.8%) | 87 (2.0%) | 4 (1.8%) | 12 (1.1%) | 0.14 |
Categorical values are presented as frequency (percentage) and continuous variables are presented as median (interquartile range).
CVA = cerebrovascular accident; MI = myocardial infarction; VATS = video-assisted thoracoscopic surgery.
Patients who underwent lobectomy or segmentectomy were more likely to develop postoperative pneumonia (6.6% and 5.7%, respectively) than patients who received wedge resection (3.6%, p < 0.01). Patients who received lobectomy or segmentectomy also had longer median postoperative length of stays (5 days for both) compared with patients who received wedge resection (4 days, p < 0.01). There were no significant differences in either the morbidity composite or operative mortality between groups, with a major morbidity rate of 8.7% for the lobectomy group, 8.8% for the segmentectomy group, and 7.2% for the wedge resection group (p = 0.27). The mortality rate was 2.0% in the lobectomy group, 1.8% in the segmentectomy group, and 1.1% in the wedge resection group (p = 0.14).
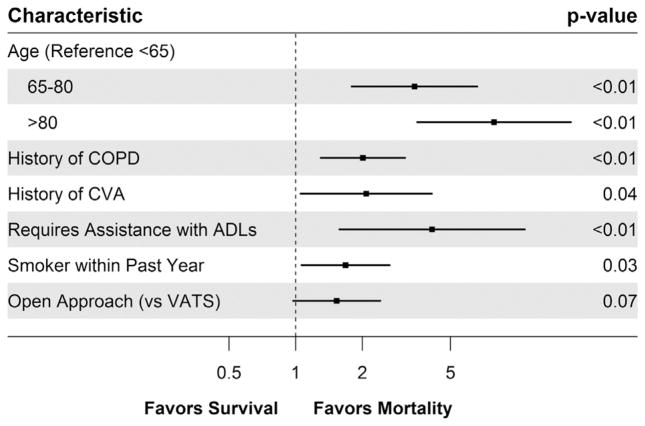
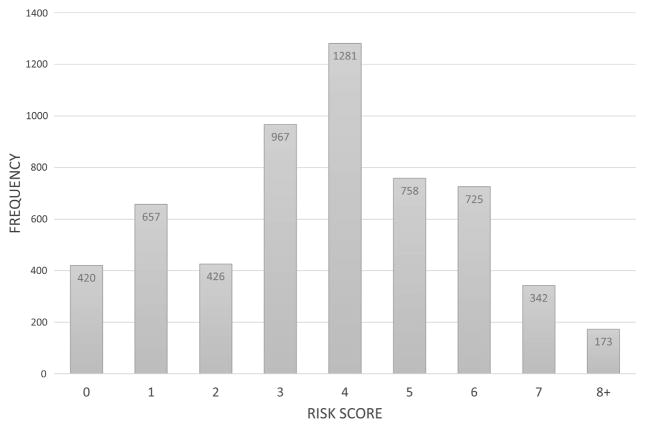
A multivariable logistic regression model was then created in order to determine patient and operative characteristics associated with operative mortality following lobectomy. In order to aid in the eventual development of the risk score, age was categorized as less than 65 years, 65 to 80 years, and greater than 80 years. Albumin was categorized as having a low preoperative albumin (<4.0 g/dL) versus having a normal preoperative albumin (≥4.0 g/dL). Following backward variable selection, age, COPD, a history of a CVA, dependent functional status, a history of smoking within the past year, and the use of an open approach all remained in the model (Fig 1; c-statistic, 0.72). Following the division of the coefficient of each variable by the lowest coefficient, and rounding to the nearest whole number, a risk score was developed (Table 3; c-statistic, 0.68). The median risk score for the population was 4 (IQR, 2 to 5; Fig 2). The c-statistic after bootstrap validation remained at 0.68.
Fig 1.
Patient and operative characteristics associated with operative mortality following lobectomy for lung cancer. (ADL = activities of daily living; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; VATS = video-assisted thoracoscopic surgery.)
Table 3.
Patient and Operative Characteristics Utilized for the Risk Score Along With Their Associated Score
| Patient Characteristic | Risk Score |
|---|---|
| Age 65–80 y | 3 |
| Age >80 y | 5 |
| History of chronic obstructive pulmonary disease | 2 |
| History of cerebrovascular accident or transient ischemic attack | 2 |
| Dependent functional status | 3 |
| History of smoking within past year | 1 |
| Open surgical approach | 1 |
Fig 2.
Variation in risk score among patients.
Multiple regression models were then created in order to determine the risk score at which there was a significant interaction between the risk score and the type of procedure (wedge resection, segmentectomy, and lobectomy) with regards to their association with mortality. A significant interaction was found by dividing patients with a risk score greater than 5 versus 5 or lower. When investigating outcomes by this threshold, patients with a risk score of 5 or lower were found to have no significant difference in operative mortality, major complication, or failure to wean from ventilator within 48 hours by operative procedure; among patients with a risk score greater than 5, however, the incidence of these complications was significantly higher for patients undergoing a more extensive procedure (Table 4).
Table 4.
Comparison of Outcomes by Surgical Procedure and Risk Score
| Group | Wedge Resection | Segmentectomy | Lobectomy | p |
|---|---|---|---|---|
| Number of patients | ||||
| Score ≤5 | 835 | 172 | 3502 | |
| Score >5 | 263 | 55 | 922 | |
| Operative mortality | ||||
| Score ≤5 | 1.2% | 1.2% | 1.2% | 0.99 |
| Score >5 | 0.8% | 3.6% | 4.9% | <0.01 |
| Major complication | ||||
| Score ≤5 | 6.7% | 8.1% | 7.2% | 0.74 |
| Score >5 | 8.7% | 10.9% | 14.4% | 0.04 |
| Pneumonia | ||||
| Score ≤5 | 3.0% | 5.2% | 5.1% | 0.03 |
| Score >5 | 5.7% | 7.3% | 12.6% | <0.01 |
| Failure to wean from ventilator >48 h | ||||
| Score ≤5 | 1.6% | 2.3% | 1.9% | 0.67 |
| Score >5 | 2.7% | 3.6% | 6.4% | 0.04 |
| Reintubation | ||||
| Score ≤5 | 2.8% | 4.1% | 2.8% | 0.75 |
| Score >5 | 3.8% | 3.6% | 7.5% | 0.07 |
Comment
The optimal surgical management of early stage NSCLC remains a matter of controversy. In an otherwise fit patient, lobectomy provides an adequate oncologic resection while maintaining sufficient pulmonary reserve [10]. Unfortunately, a substantial number of patients present with significant comorbidities, and this increases both their risk of perioperative morbidity and mortality as well as their competing risk of death from other etiologies; these potentially negate the benefit of a more extensive resection. Therefore, patient selection, especially in the setting of comorbid conditions, remains a difficult issue for surgeons. In this study, we developed a risk score that may be used to select patients for whom a sublobar resection does not lower their short-term operative risks. Patients with a risk score of less than or equal to 5 had no significant difference in perioperative morbidity or mortality by type of surgical resection, whether it was a wedge resection, segmentectomy, or lobectomy. Therefore, these patients would likely receive no short-term benefit from receiving a sublobar resection. If long-term survival is anticipated, these patients should undergo a lobectomy.
The most important finding of our study is the demonstration that certain patients can be selected who receive no short-term benefit from avoiding lobectomy in terms of morbidity or mortality. Using the risk score derived in this study, a patient with a risk score of five or less has similar rates of operative mortality, major complications, and prolonged ventilation regardless of the operative procedure used. Unfortunately, as this database does not track long-term or oncologic outcomes, we cannot determine among patients with a risk score of 6 or greater how a less-extensive resection would affect their long-term outcomes, and therefore we cannot comment on whether these patients should undergo a lobectomy or a sublobar resection.
An important point that should be made is that the use of this risk score rests on the assumption that lobectomy provides a better long-term oncologic outcome than sublobar resection—a point that is debated, especially for patients with very early disease. Multiple studies have demonstrated no long-term benefit from a lobectomy for patients with stage 1A (≤2 cm tumor with no nodal or metastatic involvement) disease as compared to a sub-lobar resection, and currently the National Comprehensive Cancer Network guidelines state that a sublobar resection may be appropriate in many of these patients [2, 15–17]. Other studies have contested these findings, however [10–12]. A recent publication by Speicher and colleagues found that among a national cohort of patients with stage 1A disease, even after adjustment, a sublobar resection was associated with significantly worse survival as compared with a lobectomy [11]. There is currently an ongoing prospective randomized controlled trial being performed by the Cancer and Lymphoma Group B (CALGB 140503) that will provide important insight into this question [18].
Although limited by a smaller sample size, our mortality model among patients receiving a lobectomy in the ACS NSQIP database demonstrated some similarities to the model developed by the Society of Thoracic Surgeons (STS) [19]. Similar to the findings of Kozower and colleagues, we found age and functional status (defined by the Zubrod score in the STS risk model) to be significantly associated with operative mortality. Interestingly, whereas we also found cerebrovascular disease, smoking status, and the use of an open procedure to be significantly associated with increased mortality, these findings were not found in the STS risk model for mortality, but only for major morbidity. This may be a function of different patient populations, but it may also be due to the larger number of variables included in the STS model, which may have reduced the power to detect this association. Lastly, although the STS model did not specifically investigate COPD, they did demonstrate a significant inverse correlation between pulmonary function tests (forced expiratory volume in one second) and mortality, which is likely a more specific measurement of lung function than a binary diagnosis of COPD.
There are limitations of this study. First and foremost, as a retrospective analysis of a multi-institutional de-identified database, there may be bias regarding patient selection for operative intervention. Nevertheless, we chose to use this database as it has granular data regarding patient baseline comorbidities. Second, our inability to determine stage and pathologic diagnosis means that some patients may have had higher-stage disease, and some patients in this sample may have had small cell lung cancer. By excluding patients who received neoadjuvant therapy, however, these patients likely make up a very small segment of our final sample. Furthermore, stage of disease and pathologic diagnosis should not have an effect on short-term outcomes, and therefore it would not have affected our findings. Third, there are important preoperative and postoperative factors that are not included in this data set which may be useful in this risk score (such as pulmonary function testing), or may be substantial causes of morbidity in this patient population (such as prolonged air leak). It may be useful in the future to re-perform this analysis in a data set such as the STS General Thoracic Surgery Database, in which these variables are available. Furthermore, this data set does not record long-term outcomes such as long-term need for oxygen, or long-term functional limitations, which may be important outcomes to consider when selecting the appropriate operation.
Fourth, there are likely patients in the lobectomy group for whom a less extensive resection may not have been technically feasible based on tumor location. Fifth, there were very few patients who underwent segmentectomy, reducing the power available to analyze this population. Lastly, although the ACS NSQIP database includes patients from a large number of hospitals, many hospitals, including many high-volume cardiothoracic centers, do not provide their data to this database, and furthermore those that do only provide a subset of their procedures, which recent data has shown may not provide the most accurate findings [20]. Therefore, validating our findings in a sample such as the STS database may be useful.
In conclusion, utilizing the ACS NSQIP database, we have developed a risk score that may be used to select patients for which lobectomy and sublobar resections have similar perioperative risks. As there are data from other studies demonstrating that sublobar resection is associated with increased locoregional recurrence and worse survival, patients with a risk score of 5 or less should be strongly considered for a lobectomy in order to optimize long-term oncologic outcomes. Future studies are necessary in order to validate our findings.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Drs Brian C Gulack, Babatunde A Yerokun, and Matthew G Hartwig are supported by the NIH funded Cardiothoracic Surgery Trials Network, 5U01HL088953-05.
Footnotes
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
Dr D’Amico discloses a financial relationship with Scanlan.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) NCCN Clinical practice guidelines in oncology. [Accessed Jan 3, 2016];Non-small cell lung cancer version 3.2016. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 3.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6 2015. JNCCN. 2015;13:515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 4.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non–small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 5.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non–small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006;82:408–16. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg. 2010;90:1651–6. doi: 10.1016/j.athoracsur.2010.06.090. [DOI] [PubMed] [Google Scholar]
- 7.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–700. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 8.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–45. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 9.Linden PA, D’Amico TA, Perry Y, et al. Quantifying the safety benefits of wedge resection: a Society of Thoracic Surgery database propensity-matched analysis. Ann Thorac Surg. 2014;98:1705–11. doi: 10.1016/j.athoracsur.2014.06.017. discussion 1711–1712. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 622–623. [DOI] [PubMed] [Google Scholar]
- 11.Speicher PJ, Gu L, Gulack BC, et al. Sublobar resection for clinical stage IA non–small-cell lung cancer in the United States. Clin Lung Cancer. 2016;17:47–55. doi: 10.1016/j.cllc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the National Cancer Data Base. J Thorac Oncol. 2015;10:1625–33. doi: 10.1097/JTO.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Surgeons. [Accessed Sep 13, 2014];NSQIP history. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/history.
- 14.American College of Surgeons. [Accessed Mar 27, 2016];ACS NSQIP participant use data file. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use.
- 15.McGuire AL, Hopman WM, Petsikas D, Reid K. Outcomes: wedge resection versus lobectomy for non-small cell lung cancer at the Cancer Centre of Southeastern Ontario 1998–2009. Can J Surg. 2013;56:E165–70. doi: 10.1503/cjs.006311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–4. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 17.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011;139:491–6. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 18.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5:1583–93. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 19.Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–81. doi: 10.1016/j.athoracsur.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 20.Allen MS, Blackmon S, Nichols FC, Cassivi SD, Shen KR, Wigle DA. Comparison of two national databases for general thoracic surgery. Ann Thorac Surg. 2015;100:1155–62. doi: 10.1016/j.athoracsur.2015.05.031. [DOI] [PubMed] [Google Scholar]