Abstract
IMPORTANCE
Evidence about the efficacy of laparoscopic resection of rectal cancer is incomplete, particularly for patients with more advanced-stage disease.
OBJECTIVE
To determine whether laparoscopic resection is noninferior to open resection, as determined by gross pathologic and histologic evaluation of the resected proctectomy specimen.
DESIGN, SETTING, AND PARTICIPANTS
A multicenter, balanced, noninferiority, randomized trial enrolled patients between October 2008 and September 2013. The trial was conducted by credentialed surgeons from 35 institutions in the United States and Canada. A total of 486 patients with clinical stage II or III rectal cancer within 12 cm of the anal verge were randomized after completion of neoadjuvant therapy to laparoscopic or open resection.
INTERVENTIONS
Standard laparoscopic and open approaches were performed by the credentialed surgeons.
MAIN OUTCOMES AND MEASURES
The primary outcome assessing efficacy was a composite of circumferential radial margin greater than 1 mm, distal margin without tumor, and completeness of total mesorectal excision. A 6%noninferiority margin was chosen according to clinical relevance estimation.
RESULTS
Two hundred forty patients with laparoscopic resection and 222 with open resection were evaluable for analysis of the 486 enrolled. Successful resection occurred in 81.7%of laparoscopic resection cases (95%CI, 76.8%–86.6%) and 86.9%of open resection cases (95%CI, 82.5%–91.4%) and did not support noninferiority (difference, −5.3%; 1-sided 95%CI, −10.8%to ∞; P for noninferiority = .41). Patients underwent low anterior resection (76.7%) or abdominoperineal resection (23.3%). Conversion to open resection occurred in 11.3%of patients. Operative time was significantly longer for laparoscopic resection (mean, 266.2 vs 220.6 minutes; mean difference, 45.5 minutes; 95%CI, 27.7–63.4; P < .001). Length of stay (7.3 vs 7.0 days; mean difference, 0.3 days; 95%CI, −0.6 to 1.1), readmission within 30 days (3.3%vs 4.1%; difference, −0.7%; 95%CI, −4.2%to 2.7%), and severe complications (22.5%vs 22.1%; difference, 0.4%; 95%CI, −4.2%to 2.7%) did not differ significantly. Quality of the total mesorectal excision specimen in 462 operated and analyzed surgeries was complete (77%) and nearly complete (16.5%) in 93.5%of the cases. Negative circumferential radial margin was observed in 90% of the overall group (87.9% laparoscopic resection and 92.3%open resection; P = .11). Distal margin result was negative in more than 98%of patients irrespective of type of surgery (P = .91).
CONCLUSIONS AND RELEVANCE
Among patients with stage II or III rectal cancer, the use of laparoscopic resection compared with open resection failed to meet the criterion for noninferiority for pathologic outcomes. Pending clinical oncologic outcomes, the findings do not support the use of laparoscopic resection in these patients.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00726622
Treatment of curable, locally advanced (stage II or III) rectal cancer relies on surgical resection as the core feature of a multimodality treatment process.1,2 Surgical resection remains the most important treatment modality for rectal cancer in terms of a curative resection, staging, prognosis, and subsequent therapeutic decisions.3 Surgical integrity of the specimen and tumor pathologic staging is the most important prognostic factor in development of recurrent rectal cancer.4 Total mesorectal excision completeness has become a marker for a good surgical technique and predicts the likelihood of local recurrence of the cancer in the pelvis.5,6
Laparoscopic treatment of rectal cancer must achieve at least equivalent results in comparison with open laparotomy and total mesorectal excision before being considered an acceptable alternative to open resection. The current body of level 1 evidence (meta-analysis) calls for additional large randomized trials to provide data for combinedanalysis.7–9 Most of the trials reported to date have come from single institutions or have not limited stage of rectal cancer to curable, locally advanced disease (stage II and III) treated uniformly with neoadjuvant therapy.10–15
The primary aim of the current study was to determine whether laparoscopic resection for rectal cancer is noninferior to open resection according to the primary outcome of a composite pathology-based end point of total mesorectal excision completeness and negative distal and circumferential radial margin results. Secondary aims included assessment of disease-free survival and rate of local recurrence, as well as quality of life and patient-related benefit for laparoscopic resection.
Methods
Study Design and Oversight
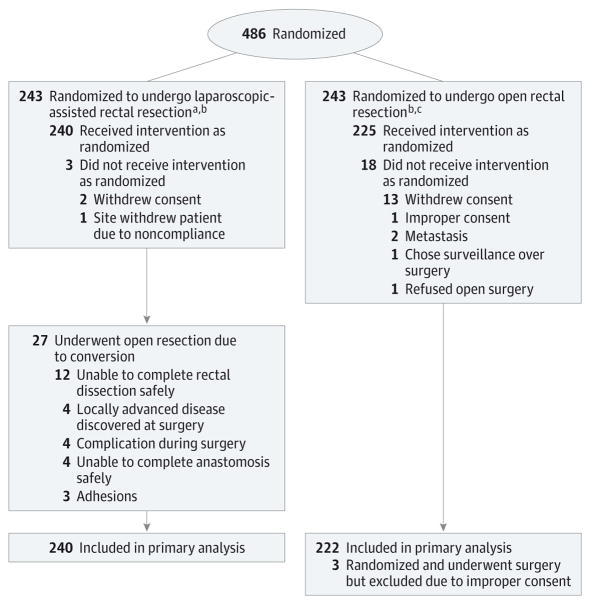
This was a multicenter balanced randomized trial conducted in the United States and Canada (Figure) (protocol in Supplement 1). Eligible patients were aged 18 years or older, had a body mass index of 34 or less, had an Eastern Cooperative Oncology Group performance score less than 3, and had histologically proven adenocarcinoma of the rectum at or below 12 cm above the anal verge (by rigid proctoscopy), with clinical stage II, IIIA, IIIB (T3N0M0, TanyN1 or 2, M0, and no T4) determined by rectal cancer protocol magnetic resonance imaging or transrectal ultrasonography. Clinical staging (including carcinoembryonic antigen levels, liver function tests, and computed tomography of chest, abdomen, and pelvis) was performed before neoadjuvant therapy. Race/ ethnicity was self-reported by patients in accordance with fixed categories and collected to determine generalizability of the conclusions. All patients were to have completed fluorouracil-based chemoradiotherapy or radiotherapy alone, according to institution-specific protocols (registered with the study), and the operation was to have been performed within 4 to 12 weeks of the final radiation treatment. Exclusion criteria were as follows: a history of invasive pelvic malignancy within 5 years, psychiatric or addictive disorders that affected compliance to the protocol, severe incapacitating disease (American Society of Anesthesiologists classification IV or V), systemic disease that would preclude use of a laparoscopic approach (eg, cardiovascular, renal, hepatic), or conditions that would limit the success of laparoscopic resection (multiple previous laparotomies or severe adhesions).
Figure. Flow of Patients Through the ACOSOG Z6051 Randomized Clinical Trial.
Data on assessment of eligibility were not collected. Of the 486 patients randomized, 5 patients’ data could not be used in any analysis (including demographics). Of these 5 patients 1 was allocated to receive the laparoscopic-assisted resection and 4 were allocated to receive the open rectal resection. The patient allocated to the laparoscopic-assisted resection refused to participate after randomization and refused to have any data used. This patient did not receive surgery. The 4 patients allocated to open rectal resection did not receive proper consent; hence, no data could be used. Of those 4 patients, 3 went on to receive the allocated intervention per protocol.
aPatients found to be ineligible after randomization (n = 10): Pregnancy test not conducted before neoadjuvant therapy (n = 3), no transrectal ultrasound/magnetic resonance imaging (TRUS/MRI) conducted before the start of neoadjuvant therapy (n = 2), no computed tomography (CT) scan of the abdomen and pelvis before neoadjuvant therapy (n = 1), liver metastasis (n = 1), liver metastasis and stage I (n = 2), and lower lobe metastasis (n = 1).
bOne patient from the laparoscopic arm and 4 patients from the open resection arm are not shown in Table 1 because of patient refusal or improper consent.
cPatients found to be ineligible after randomization (n = 18): Consent after registration (n = 2), consent after registration and CT scan of the abdomen and pelvis after neoadjuvant therapy and TRUS/MRI not conducted (n = 1), consent after registration and stage I (n = 1), CT scan of the abdomen and pelvis after neoadjuvant therapy (n = 2), metastatic adenocarcinoma (n = 1), pregnancy test not conducted (n = 3), pregnancy test not conducted and body mass index higher than 34 (n = 1), severe dysplasia (n = 1), stage I (n = 1), TRUS/MRI not conducted (n = 4), and TRUS/MRI not conducted and stage I (n = 1).
Surgeons were credentialed before patient enrollment (requirements in Appendix B in Supplement 2). The study protocol was approved by the individual participating institutions’ review boards, as well as the central institutional review board for the National Cancer Institute. All participants gave written informed consent before study enrollment.
Interventions
Standard laparoscopic and open approaches were used according top references of the individual surgeons. The hand-assisted approach was used by inserting a hand-access port at the beginning of the operation to facilitate dissection in the upper abdomen during the operation. The number and pattern of laparoscopic or robotic ports were left to the preference of the surgeon. In the open resection arm, there were no mandates on the use of drains, wound protectors, or adhesion barriers. The hybrid technique in the open resection arm potentially allowed a smaller wound after mobilization of the proximal colon and vessel ligation laparoscopically and limitation of the incision to below the umbilicus. The entire pelvic dissection was accomplished with open technique and hand instruments and hand retraction. The laparoscopic resection pelvic dissection of the rectum could be performed only with laparoscopic instruments under the pneumoperitoneum in the laparoscopic resection cases. Abdominal wound closure, venous thrombolic event prophylaxis, bowel preparation, and postoperative antibiotics were per the standard processes of the institution.
Surgeons were instructed to perform proximal ligation of the feeding vessels (inferior mesenteric artery and inferior mesenteric vein), usually at the aorta and the inferior border of the pancreas, respectively. They were to mobilize the splenic flexure of the colon for all cases and to standardize the mesenteric resection proximal to the tumor. The surgical dissection plane in the pelvis was identified in the areolar tissue plane outside the visceral fascia of the mesorectum at the level of the sacral promontory. Medial-to-lateral or lateral-to-medial technique was used according to surgeon preference or the needs of the case. The mesorectal mobilization in the plane outside the mesorectum was performed with sharp or energy dissection and carried well below the site of the tumor in the bowel. This dissection allowed a right-angled transection of the rectum and mesentery 5 cm below the tumor for upper rectal cancers and low enough to remove the entire mesorectum for middle and low rectal lesions. The sharp division of Waldeyer fascia, where it reflected onto the posterior surface of the mesorectum from the presacral fascia, was needed to reach the low rectum at the upper end of the anal canal. Distal margin was determined to be adequate if the line of transection was 5 cm below the tumor for upper rectal lesions, if it was 2 cm below the line of transection for middle rectal lesions, and if the frozen or fixed section of the distal margin was tumor free (>1 mm) for low rectal lesions.
The need for abdominoperineal resection and removal of the sphincter with colostomy construction was determined by the features of the tumor in the pretreatment evaluation (tumor not separable from pelvic floor structures or external sphincter muscle). This pretreatment plan may have been modified at the discretion of the surgeon if the tumor response was so complete that an ultra low coloanal anastomosis could be accomplished with negative distal margin results. A change to an abdominoperineal resection with colostomy was based on intraoperative findings that suggested the possibility of positive radial margin results or was made if a sphincter-sparing technique was not possible because of factors such as blood supply and length of the proximal colon not allowing the distal rectum to be reached for anastomosis. The need to change to an abdominoperineal resection approach should not have been due to the inability to complete the procedure by the laparoscopic resection approach because conversion to open resection was considered the fallback in those circumstances.
Outcomes
The primary outcome was a composite of distal margin (>1mm between the closest tumor to the cut edge of the tissue), circumferential radial margin (>1mm between the deepest extent of tumor invasion into the mesorectal fat and the inked surface on the fixed specimen), and total mesorectal excision quality (complete: smooth surface of mesorectal fascia with all fat contained in the enveloping fascia to a level 5 cm below the tumor for tumor-specific total mesorectal excision for upper rectal cancer, or the entire mesorectal envelope present for low rectal cancer; or nearly complete: the mesorectal envelope was intact except for defects no more than 5mm deep, with no loss of mesorectal fat). Pathologists received the specimens to ink the surface of the rectum to facilitate the determination of circumferential radial margin and to grade the completeness of the total mesorectal excision specimen. All 3 of the parameters (distal margin, circumferential radial margin, and total mesorectal excision quality) must have been achieved for the surgery to be considered a success. The primary outcome was modified during the trial (Appendix C in Supplement 2).
Secondary outcomes included disease-free survival and rate of local recurrence, as well as quality of life and patient-related benefit for laparoscopic resection (blood loss, length of stay, and pain medicine use). Of the secondary outcomes, only the patient related benefit data are complete for reporting; all other outcomes are still being collected. Patients were assessed for complications at discharge from the hospital and at 4 to 6 weeks postoperatively. Complications were classified by the Clavien-Dindo method, and the arms were compared by number of grade III to V occurrences.16 Adverse events were collected and graded by MedDRA(Medical Dictionary for Regulatory Activities) code as related or not related to the technique.
The primary analysis was a modified intent-to-treat, ie, patients who were randomized to the laparoscopic procedure but during the operation were converted to the open procedure were included in the laparoscopic arm for analysis, and any patients who were randomized but then canceled or were withdrawn before surgery on-trial were excluded from analyses. An additional analysis of the primary end point according to protocol guidelines was conducted, ie, only patients who received the intervention they were assigned to were included in the analysis. An audit of the surgery and pathology was conducted (Appendix D in Supplement 2).
Randomization was performed centrally. Through the use of a minimization algorithm, laparoscopic-assisted or open rectal resection was assigned to minimize imbalance with respect to the following stratification variables: surgeon, site of primary tumor (high, middle, or low rectum according to the subclassification of the 12 cm of rectum into equal thirds), and planned operative procedure (low anterior resection with anastomosis or abdominoperineal resection with colostomy). No blinding of interventions was conducted.
Statistical Analysis
Assuming a baseline rate of 90%oncologic success (circumferential radial margin results negative, distal margin results negative, and total mesorectal excision complete or nearly complete) for the open resection arm, the sample size of 480 patients (240 per arm) provided 80%power to declare noninferiority if oncologic success rates were truly identical, using a 1-sided z score with α = .10 for falsely declaring noninferiority when the true oncologic success rate for laparoscopic resection was 84%. Calculations were based on a 2-sample binomial noninferiority calculation, performed with EAST version 4.0, with a 90% success rate for the control group and a 6%noninferiority margin, chosen according to clinical relevance estimation from medical oncology trials. A single interim analysis for futility for the primary end point was planned and conducted after 240 patients were accrued, using an O’Brien-Fleming stopping boundary. A z score of −1.145 was the final cutoff for noninferiority. All categorical comparisons outside of the primary end point and oncologic comparison of circumferential radial margin, distal margin, and total mesorectal excision were 2-sided and conducted with the χ2 test; continuous comparisons were conducted with the Wilcoxon rank sum test. Data lock was performed in October 2014 after data cleaning was completed. The analysis was generated with SAS version 9.3.
Results
Between October 2008 and September 2013, 486 patients were randomized (Figure). Five patients were excluded from analyses because of improper enrollment: 4 were registered before signing consent (open resection arm), and 1 patient refused to provide data and consent was withdrawn (laparoscopic resection arm). Characteristics of the 481 patients available for resection are shown in Table 1. Four hundred sixty-two patients (240 laparoscopic resection and 222 open resection) were evaluable for analysis of surgical and patient-related outcomes. Patients underwent low anterior resection (76.7%) or abdominoperineal resection (23.3%) of the rectum.
Table 1.
Demographic and Clinical Characteristicsa
| Laparoscopic Resection (n = 242) | Open Resection (n = 239) | |
|---|---|---|
| Male sex | 156 (64.5) | 158 (66.1) |
| Age, mean (SD), y | 57.7 (11.5) | 57.2 (12.1) |
| Race, No. (%) | ||
| White | 200 (82.6) | 207 (86.6) |
| Black or African American | 21 (8.7) | 11 (4.6) |
| Asian | 11 (4.5) | 11 (4.6) |
| American Indian or Alaska Native | 4 (1.7) | 1 (0.4) |
| Native Hawaiian/Pacific Islander | 1 (0.4) | 1 (0.4) |
| Unknown | 5 (2.1) | 8 (3.3) |
| BMI, mean (SD)b | 26.4 (4.0) | 26.8 (4.2) |
| Planned operative procedure, No. (%) | ||
| Abdominal perineal resection | 55 (22.7) | 57 (23.8) |
| Low anterior resection | 187 (77.3) | 182 (76.2) |
| Location of tumor in rectum, No. (%) | ||
| High | 33 (13.6) | 28 (11.7) |
| Middle | 85 (35.1) | 95 (39.7) |
| Low | 124 (51.2) | 116 (48.5) |
| Tumor distance from anal verge, mean (SD), cm | 6.1 (3.1) | 6.3 (3.0) |
| Tumor size, largest dimension, mean (SD), cm | 4.2 (2.2) | 4.3 (2.0) |
| ECOG Zubrod performance score, No. (%)c | ||
| 0–1 | 238 (98.8) | 233 (97.5) |
| ≥2 | 3 (1.2) | 6 (2.5) |
| Preoperative clinical staged | ||
| I | 2 (0.8) | 3 (1.3) |
| IIA | 99 (40.9) | 92 (38.5) |
| IIIA | 11 (4.5) | 11 (4.6) |
| IIIB | 114 (47.1) | 114 (47.7) |
| IIIC | 16 (6.6) | 19 (7.9) |
| Previous therapy received, No. (%) | ||
| Chemotherapy + radiatione | 227 (95.0) | 217 (91.2) |
| Radiation alone | 8 (3.3) | 13 (5.5) |
| Chemotherapyf | 4 (1.7) | 8 (3.4) |
| Unknowng | 3 | 1 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; ECOG, Eastern Cooperative Oncology Group.
One patient from the laparoscopic arm and 4 patients from the open resection arm are not shown because of patient refusal or improper consent.
One patient with BMI >34 (45.3) was considered ineligible.
One laparoscopic resection patient was missing the score; it was not assessed by the enrolling physician, but the site did state that the patient met criteria for eligibility but could not provide a definite value of 0, 1, or 2.
Patients with stage I tumors (n = 5) were considered ineligible but still evaluable for analysis.
All patients received fluorouracil, except for 1 patient in the laparoscopic resection arm who received oxiliplatin plus radiotherapy. Five patients in the laparoscopic resection arm and 7 in the open resection arm received oxiliplatin in addition to fluorouracil and radiotherapy.
Fluorouracil delivered.
Patient sites had documentation that patients received neoadjuvant therapy but were unable to document the types of therapy received.
Quality of the total mesorectal excision specimen in 462 operated and analyzed surgeries was complete (77.1%) and nearly complete (16.5%) in 93.5%of the cases (Table 2). Negative circumferential radial margin result was observed in 90% of the overall group (87.9% laparoscopic resection and 92.3% open resection; P = .11). Distal margin result was negative in more than 98% of patients irrespective of type of surgery (P = .91) (Table 2). Overall surgical success, measured by a negative distal and circumferential radial margin result and complete total mesorectal excision, was higher in the open resection arm (86.9%) vs laparoscopic resection arm (81.7%).
Table 2.
Surgical Success Outcomes
| Laparoscopic Resection (n = 240) | Open Resection (n = 222) | Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Composite Outcome, No. (%) | ||||
| Total mesorectal excision complete | ||||
| CRM ≤1 mm, DM(+) | 1 (0.4) | 0 | ||
| CRM ≤1 mm, DM(−) | 16 (6.7) | 14 (6.3) | ||
| CRM >1 mm, DM(+) | 2 (0.8) | 3 (1.4) | ||
| CRM >1 mm, DM(−) | 156 (65.0) | 164 (73.9) | ||
| Total mesorectal excision nearly complete | ||||
| CRM ≤1 mm, DM(+) | 0 | 1 (0.5) | ||
| CRM ≤1 mm, DM(−) | 6 (2.5) | 0 | ||
| CRM >1 mm, DM(−) | 40 (16.7) | 29 (13.1) | ||
| Total mesorectal excision incomplete | ||||
| CRM ≤1 mm, DM(−) | 6 (2.5) | 2 (0.9) | ||
| CRM >1 mm, DM(+) | 1 (0.4) | 0 | ||
| CRM >1 mm, DM(−) | 12 (5.0) | 9 (4.1) | ||
| Percentage (95% CI)a | ||||
| CRM >1 mm or distance = NA | 87.9 (83.8 to 92.0) | 92.3 (88.8 to 95.8) | −4.4 (−9.8 to 0.98) | .11b |
| Distal margin negative | 98.3 (96.7 to 99.95) | 98.2 (96.5 to 99.95) | −0.1 (−2.3 to 2.5) | .91b |
| Complete or nearly complete total mesorectal excision | 92.1 (88.7 to 95.5) | 95.1 (92.2 to 97.9) | −3.0 (−7.4 to 1.5) | .20b |
| Successful resectiond | ||||
| Modified intent to treat | 81.7 (76.8 to 86.6) | 86.9 (82.5 to 91.4) | −5.3 (−10.8 to ∞)c | .41 |
| Per protocole | 81.7 (76.5 to 86.9) | 86.9 (82.5 to 91.4) | −5.3 (−11.0 to ∞)c | .41 |
Abbreviations: CRM, circumferential radial margin; DM, distal margin; NA, not applicable; + sign, distal margin positive (<1mm clear); − sign, distal margin negative (≥1mm clear).
All CIs are 2-sided 95%CI unless specifically noted.
χ2 Test statistic P value (2-sided).
z Statistic P value for noninferiority, H0: P1 − P2 ≤ margin; Ha: P1 − P2 > margin (margin = .06), 1-sided 95%CI. P1 indicates probability of success for patients randomized to laparoscopic resection arm, and P2 indicates probability of success for patients randomized to the open resection arm.
Defined as all composite end points met.
Per protocol includes only patients who received the intervention they were randomized to receive (n = 435: 213 in the laparascopic arm and 222 in the open resection arm).
For the modified intent-to-treat population, the 1-sided 95% CI for the difference in rates was −10.8% to ∞, demonstrating that a 6%or greater decrease in the rate of successful resection could not be excluded. The per-protocol analysis had similar findings, with P for noninferiority = .41 and a 1-sided 95%CI of −11.0%to ∞.
Conversion of laparoscopic to an open procedure was required for 11% of the laparoscopic resection patients. A plan for a sphincter-sparing low anterior resection in laparoscopic resection patients was changed to abdominoperineal resection in 2.7% of cases (77.3% planned and 74.6% accomplished). Open resection never required switching from sphincter-sparing to abdominoperineal resection. A diverting ileostomy was used in the majority of low anterior resection cases. Only 3.5%of the entire study group (N = 6 laparoscopic resection; N = 10 open resection) did not receive a stoma of some kind, and there was no difference in stoma use between laparoscopic and open resection.
Operative time was significantly longer for laparoscopic resection (mean, 266.2 vs 220.6 minutes; mean difference, 45.5 minutes; 95% CI, 27.7 to 63.4; P<.001). Length of stay (7.3 vs 7.0 days; mean difference, 0.3 days; 95% CI, −0.6 to 1.1) and read mission within 30 days (3.3% vs 4.1%; difference, −0.7%; 95% CI, −4.2% to 2.7%) were not significantly different. Operative outcomes are shown in Table 3.
Table 3.
Secondary Surgery and Pathology Outcomes
| Laparoscopic Resection (n = 240) | Open Resection (n = 222) | P Value | |
|---|---|---|---|
| Surgical approach, No. (%) | |||
| Low anterior resection | 69 (28.8) | 73 (32.9) | .34 |
| Low anterior resection + coloanal anastomosis | 110 (45.8) | 96 (43.2) | |
| Abdominal perineal resection | 58 (24.2) | 47 (21.2) | |
| Low Hartmann | 1 (0.4) | 0 | |
| Total proctocolectomy | 2 (0.8) | 6 (2.7) | |
| Surgical approach for laparoscopic arm, No. (%) | |||
| Laparoscopic | 165 (68.8) | ||
| Hand assisted | 41 (17.1) | ||
| Robotic assisted | 34 (14.2) | ||
| Ostomy created at the resection, No. (%) | |||
| Colostomy construction | 63 (26.3) | 47 (21.2) | .25 |
| Ileostomy | 171 (71.3) | 165 (74.3) | |
| Sphincter preservation planned before surgery, No. (%) | 191 (79.6) | 174 (78.4) | .75 |
| Surgical approach, No. (%) | |||
| Low anterior resection | 68 (35.6) | 71 (40.8) | .35 |
| Low anterior resection + coloanal anastomosis | 109 (57.1) | 92 (52.9) | |
| Abdominal perineal resection | 11 (5.8) | 6 (3.4) | |
| Low Hartmann | 1 (0.5) | 0 | |
| Total proctocolectomy | 2 (1.0) | 5 (2.9) | |
| Margins examined by frozen section, No. (%) | 51 (21.3) | 55 (24.8) | .37 |
| Rectum intact, No. (%) | 203 (84.6) | 201 (90.5) | .05 |
| Perioperative and postoperative outcomes | |||
| Open-to-close operative time, mean (SD), min | 266.2 (101.9) | 220.6 (92.4) | <.001 |
| Total estimated blood loss, mL | |||
| Mean (SD) | 256.1 (305.8) | 318.4 (331.7) | .004 |
| Median (IQR) | 150 (100–300) | 200 (100–400) | |
| Final incision length, mean (SD), cm | 7.0 (5.7) | 16.5 (8.4) | <.001 |
| Length of hospital stay, mean (SD), d | 7.3 (5.4) | 7.0 (3.4) | .10 |
| Intensive care unit stay, d | |||
| Mean (SD) | 0.7 (3.5) | 0.4 (1.3) | .93 |
| Median (IQ) | 0 (0–0) | 0 (0–0) | |
| Days receiving parenteral narcotics, mean (SD) | 4.2 (3.9) | 4.2 (2.8) | .09 |
| Days receiving oral analgesics | |||
| Mean (SD) | 5.3 (8.1) | 5.7 (9.9) | .21 |
| Median (IQR) | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | |
| First postsurgery bowel movement, median (range), d | 2.0 (0–15.0) | 3.0 (0–12.0) | .03 |
| First postsurgery flatus, median (range), d | 2.0 (0–15.0) | 2.0 (0–10.0) | .07 |
| Total length of resected sample, mean (SD), cm | 28.9 (10.8) | 29.5 (11.0) | .33 |
| Distance to nearest radial margin, mean (SD), mm | 10.5 (9.2) | 12.8 (11.2) | .03 |
| Distance to radial margin, No. (%) | |||
| ≤1 mm | 29 (12.1) | 17 (7.7) | .11 |
| >1 mm | 211 (87.9) | 205 (92.3) | |
| Distance to distal margin, mean (SD), cm | 3.2 (2.6) | 3.1 (1.9) | .82 |
| No. of lymph nodes examined, mean (SD) | 17.9 (10.1) | 16.5 (8.4) | .22 |
| No. of positive lymph nodes | |||
| Mean (SD) | 0.8 (2.1) | 1.1 (3.0) | .32 |
| Median (IQR) | 0 (0–1.0) | 0 (0–1.0) | |
| Stage, No. (%)a | |||
| 0 | 55 (23.0) | 43 (19.4) | .26 |
| I | 76 (31.8) | 68 (30.6) | |
| IIA | 46 (19.2) | 45 (20.3) | |
| IIB | 1 (0.4) | 5 (2.3) | |
| IIIA | 14 (5.9) | 11 (5.0) | |
| IIIB | 30 (12.6) | 37 (16.7) | |
| IIIC | 16 (6.7) | 17 (7.7) | |
| IV | 1 (0.4) | 3 (1.4) | |
| Complete pathologic response, No. (%) | 70 (29.2) | 50 (22.5) | .10 |
| Tumor size, No. | 170 | 169b | |
| Mean (SD) | 2.3 (1.8) | 2.4 (1.7) | .58 |
| Histologic grade, differentiated, No. (%) | |||
| Well | 19 (11.2) | 15 (8.8) | .35 |
| Moderately | 131 (77.5) | 135 (78.9) | |
| Poorly | 18 (10.7) | 16 (9.4) | |
| Undifferentiated | 1 (0.6) | 5 (2.9) | |
| Missingc | 1 | 1 | |
Abbreviation: IQR, interquartile range.
A laparoscopic resection patient was missing stage data.
Tumor size was missing for 3 patients in the open resection arm. The reasons were as follows (as stated by the sites after query): tumor was multifocal and microscopic and thus overall measurement was unobtainable (n = 1); unable to perform accurate measure of tumor size (n = 1); and scattered microscopic foci (n = 1).
Histologic grade was missing for 1 patient in the laparoscopic resection arm (the site stated “not done”) and for 1 patient in the open resection arm (the site stated “unknown”).
There were no significant differences in length of specimen removed (Table 3). A review of the results from the top 10 accruing surgeons, who were responsible for 271 of the patients enrolled (laparoscopic resection = 137; open resection = 134), revealed that the successful operation rate for laparoscopic resection was lower than or the same as open resection for 8 of the 10 surgeons. Only 2 surgeons had better results for laparoscopic resection (by 1 patient each). Complications occurred in 57.1% of patients after laparoscopic resection and 58.1% after open resection (Table 4). Severe complications (Clavien-Dindo class 3 to 5) occurred in 22.5%of the laparoscopic arm and 22.1% of the open arm (difference, 0.4%; 95%CI, −4.2%to 2.7%).
Table 4.
Intraoperative and Postoperative Complications
| No. (%)
|
P Value | ||
|---|---|---|---|
| Laparoscopic Resection (n = 240) | Open Resection (n = 222) | ||
| Complications (intraoperative and postoperative)a | 137 (57.1) | 129 (58.1) | .93 |
| Total intraoperative complicationsb | 26 (10.8) | 17 (7.7) | |
| Rectum | 10 (4.2) | 3 (1.4) | .26 |
| Colon | 3 (1.3) | 0 | |
| Small bowel NOS | 0 | 1 (0.4) | |
| Ureter | 1 (0.4) | 0 | |
| Bladder | 1 (0.4) | 0 | |
| Spleen | 0 | 3 (1.4) | |
| Hemorrhage/bleeding associated with surgery | 8 (3.3) | 8 (3.6) | |
| Otherc | 5 (2.1) | 4 (1.8) | |
| Maximum grade of postoperative complications, Clavien-Dindod | 129 (53.8) | 120 (54.1) | |
| 3 | 46 (19.2) | 42 (18.9) | .46 |
| 4 | 6 (2.5) | 5 (2.2) | |
| 5 | 2 (0.8) | 2 (0.9) | |
| Anastomotic leak during postoperative periode | 5 (2.1) | 5 (2.3) | |
| 30-Day mortalityf | 2 (0.8) | 2 (0.9) | .95 |
| Rehospitalization (within 30 d from surgery) | 8 (3.3) | 9 (4.1) | .81 |
| Reoperation | 12 (5.0) | 5 (2.3) | .20 |
| Days to reoperation | |||
| Mean | 8.9 | 8.0 | |
| Median (range) | 8.5 (1–18) | 7.0 (3–20) | |
| Missing data | 7 | 8 | |
| Reason for reoperation | |||
| Anastomotic leak | 2 (0.8) | 3 (1.4) | |
| Evacuation of hematoma | 1 | 0 | |
| Exploratory laparotomy, abdominal washout | 1 | 0 | |
| Herniation of small bowel | 1 | 0 | |
| Ileostomy revision, small bowel obstruction | 1 | 0 | |
| Ileus | 1 | 0 | |
| Partial pancreatectomy with splenic preservation | 1 | 0 | |
| Perineal wound debridement | 1 | 0 | |
| Rectal bleed | 1 | 0 | |
| Small-bowel obstruction | 1 | 1 | |
| Tracheostomy for respiratory failure | 1 | 0 | |
| Revision of medication catheter | 0 | 1 | |
Abbreviation: NOS, not otherwise specified
Any event during intraoperative complication or adverse event during perioperative period (1–2 weeks postsurgery) regardless of grade or relationship counts as an event; 1 event per patient.
Intraoperative complications from the rectal surgery form; maximum 1 event per patient.
On the laparoscopic resection arm: distal stapler (n = 1), pancreas (n = 1), prostate (n = 1), and vagina (n = 1). On the open resection arm: perforation, genitourinary: urethra (n = 1), nerves, peripheral (n = 1), urethra (n = 1), leak; gastrointestinal: NOS (n = 1), leak; and gastrointestinal: rectum (n = 1).
Postoperative complications from perioperative form, any grade regardless of relationship; maximum 1 event per patient.
On the laparoscopic resection arm: gastrointestinal: rectum (n = 4); genitourinary: stoma (n = 1). On the open resection arm: gastrointestinal: rectum (n = 5). Clavien-Dindo.16
On the laparoscopic resection arm: cardiac ischemia/infarction (possibly related; occurred on day 44 after surgery) and gastrointestinal–other (definitely related). On the open resection arm: aspiration (possibly related) and thrombosis/thrombus/embolism (possibly related).
Discussion
In this multicenter, prospective, randomized, controlled trial of patients with stage II or III rectal cancer at or below 12 cm above the anal verge, all of whom underwent neoadjuvant therapy by protocol design, we found that laparoscopic resection failed to meet the criterion for noninferiority for pathologic outcomes compared with open resection and was thus potentially inferior. The end point comparing gross and histologic assessment of the resected proctectomy specimen specifically used clear distal and radial margins and completeness of the total mesorectal excision specimen as a combined assessment of optimal surgery, which has been shown in other trials to be associated with long-term oncologic outcome.17
Failure to reject inferiority of the laparoscopic resection in the treatment of rectal cancer according to oncologic parameters was not the anticipated outcome of this study. This group of highly motivated, credentialed, expert laparoscopic rectal surgeons was ideal to test this hypothesis. Most of the surgeons were from institutions that participated in the Clinical Outcomes of Surgical Therapy Trial for laparoscopic treatment of colon cancer.18,19 The technique itself, along with the current methodology available, must be questioned if motivated experts cannot produce a quality specimen defined by this novel combined metric. The learning curve cannot be invoked to explain our results because conversion rates were reasonable (11%), and every participating surgeon passed a credentialing process (Appendixes C and E in Supplement 2). The random audit of laparoscopic videos carried out in the first 100 laparoscopic cases was confirmatory for expertise in technique used throughout the trial. Total blinded review of all photographs of total mesorectal excision specimens also pointed toward a very low failure rate in the laparoscopic cases. This study has one of the highest rates of complete total mesorectal excision (entire group 93.6% successful total mesorectal excision) (Table 2) in the literature and speaks to the quality of surgery performed.7
Use of pathologic oncologic markers related to quality of the rectal specimen is a unique methodology. Quality of the rectal specimen is especially relevant when a new surgical technique (laparoscopy) is compared with an already existing one (open). The time required to follow up for long-term end points such as overall survival sometimes encourages the specialty to bypass scientific analysis of the new technique (for example, in laparoscopic cholecystectomy); our endpoint was intended to remove this barrier. This study’s use of a novel composite measure (complete or nearly complete total mesorectal excision, negative circumferential radial margin result, and negative distal margin result) as a surrogate for oncologic outcome is also unique. Rectal cancer is especially suited to this form of surrogate evaluation because assessment of the total mesorectal excision specimen can be standardized and it is a direct result of oncologic technique. Moreover, circumferential radial margins are reported routinely, and the distal margin is documentable. All of these factors reflect surgical judgment and technique of the surgeon as the rectum is dissected in the confines of the bony pelvis. Quirke and colleagues4 correlated the plane of dissection during rectal resection with oncologic survival and recurrence. Violation of the peritonealized posterior surface of the mesorectum can be correlated within complete removal of locally malignant tissue and increased local and distant recurrences.4 Additionally, exposure of tumor at the circumferential radial margin carries an extremely high risk of local recurrence, though it may be less after neoadjuvant therapy. Individuals who have undergone a complete total mesorectal excision and still have tumor at the inked margin are at high risk for local and distant recurrence. As a result, a positive circumferential radial margin result in the setting of complete total mesorectal excision has become a biologic marker for abadprognosis.6 We therefore used a novel composite measure of resection quality; however, its effect as a prognostic indicator for long-term outcomes for this group of patients may require further evaluation after our follow-up for the secondary end point of survival and recurrence.
One explanation for our findings is that proctectomy is challenging at baseline, and it can be even more difficult to work in the deep pelvis with in-line rigid instruments from angles that require complicated maneuvers to reach the extremes of the pelvis. It is possible that modification of instruments or a different platform such as robotics will improve efficacy of minimally invasive techniques. The skill of the operating surgeon is critical to the success of the procedure. This reason alone was impetus to credential our participating surgeons. A critical question is whether this technique, even if found to be definitively noninferior, would be transferable to the general surgeon or colorectal surgeon who does not routinely use minimally invasive rectal surgery in his or her practice. Another secondary outcome of the trial was to determine which instrumentation correlated with failure or success in the technique. Wristed instruments may provide the needed control in the deep pelvis. Placement of instruments in line with side walls of the pelvis and remote control of these instruments provides ergonomic feasibility to perform minimally invasive resection. These are characteristics of the existing robotic platform, but limitations still exist in the setting of challenging pelvic cases. Data are becoming available regarding the use of robotic pelvic dissection, but multicenter randomized trials do not yet exist, to our knowledge.11
The current literature contains a number of reports from randomized trials and meta-analyses of prospective and retrospective trials comparing laparoscopic and open resection of rectal cancer. The CLASICC trial had only a small subset of patients with rectal cancer and noticed an increase in circumferential radial margin cancer positivity in the low anterior laparoscopic resection group. However, long-term follow-up of the CLASICC trial reported in 2013 suggested that long-term local and distant recurrence for rectal cancer treated laparoscopically was the same as for open treatment.15 The COREAN trial compared laparoscopic and open resection of 340 neoadjuvant treated patients with stage II and III mid to low rectal cancer. Their recent 2014 report of long-term follow-up and the earlier (2010) short-term outcomes showed no difference in long-term outcome or quality of the oncologic resection (circumferential radial margin, total mesorectal excision completeness, lymph node evaluation, and complication rate).13,20 However, the COREAN trial was carried out in 3 tertiary referral hospitals by a limited number of surgeons. A recent meta-analysis by Arezzo et al7 included 8 randomized controlled trials and 19 prospective or retrospective studies with 2659 and 8202 patients, respectively. Their analysis end points included positive circumferential radial margin result (primary end point) and positive distal margin, lymph node harvest, total mesorectal excision completeness, R0 resection, and local recurrence results (secondary end points). Patients with positive circumferential radial margin results were similar with respect to laparoscopic resection and open resection (10.3% and 11.6%). Total mesorectal excision completeness was 85% overall: 85% and 86% for laparoscopic resection and open resection patients, respectively, in the subgroup with cancer within 12 cm of the anal verge. Local recurrence was 3.5% and 5.6% for laparoscopic resection and open resection patients, respectively, with cancer within 12 cm of the anal verge. All of these findings are similar to those of our study. Their conclusion was that a good-quality randomized, clinical trial was needed to answer the oncologic question. In all of the comparisons, the potential for diminished outcome with laparoscopic resection compared with open resection was observed.8–10,12
The Colorectal Cancer Laparoscopic or Open Resection II (COLOR II) trial included 1044 patients with stage I to II rectal cancer within 15 cm of the anal verge, randomized 2:1 laparoscopic to open resection. Neoadjuvant therapy was used in only 59% of patients, and 30% of patients had clinical stage I disease (vs 1% for the current study).14 Only 29% of patients in COLOR II had tumors in the low rectum (vs 51% in the current study). Pathologic complete response occurred in 8% to 10% of patients in COLORII and 23% and 19% in the current study. Total mesorectal excision completeness was 92% and 94% compared with 92% and 95% in the current study. Distal margin results were all negative in COLOR II compared with 98% in the current study. The most notable difference was circumferential radial margin result positivity: 10% for laparoscopic and open in COLOR II and 12% and 7.7% in the current study. The circumferential radial margin positivity rate for COLORII in the low rectum open arm was 22% and only 9% in the laparoscopic arm. Three-year local recurrence was 5% in the COLOR II patients. The difference in stage of disease, tumor height, and use of neoadjuvant chemotherapy make it difficult to compare these studies. The general conclusion from these reports is that laparoscopic resection of rectal cancer is safe and feasible, but the oncologic efficacy has not been definitively established.
Conclusions
Among patients with stage II or III rectal cancer, the use of laparoscopic resection compared with open resection failed to meet the criterion for noninferiority for pathologic outcomes. Pending clinical oncologic outcomes, the findings do not support the use of laparoscopic resection in these patients.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by a grant from the National Cancer Institute, awarded to the American College of Surgeons Oncology Group (ACOSOG). The American Society of Colon and Rectal Surgeons provided support for the comparison of the quality-of-life survey results between the laparoscopic and open resection arms. The Society of American Gastrointestinal and Endoscopic Surgeons provided support for the evaluation of instrumentation in the laparoscopic resection–treated patients. In addition, the Covidien Company provided unrestricted research support to the ACOSOG infrastructure for the trial.
Study Group Members
Advocate Lutheran General Hospital, Park Ridge, IL (Leela Prasad); Allegheny General Hospital, Pittsburgh, PA (James McCormick); Baylor University Medical Center, Dallas, TX (James W. Fleshman); Boone Hospital Center, Columbia, MO (Walter Peters); Cleveland Clinic Foundation, Cleveland, OH (Luca Stocchi); Cleveland Clinic–Weston, Weston, FL (Steven Wexner); Columbia University Medical Center, New York, NY (Daniel Feingold); Duke University Medical Center, Durham, NC (Linda Farkas); Franciscan Saint Francis Health–Mooresville, Mooresville, IN (Dipen C. Maun); Indiana University Hospital/Melvin and Bren Simon Cancer Center, Indianapolis (Virgilio George); Integris Cancer Institute of Oklahoma, Oklahoma City (Chris M. Davis); Indiana University Health North Hospital, Carmel (Virgilio George); John B. Amos Cancer Center, Columbus, GA (William Taylor); John F. Kennedy Medical Center, Edison, NJ (Bertram Chinn); John Muir Medical Center, Walnut Creek, CA (Samuel C. Oommen); John Muir Medical Center–Concord Campus, Concord, CA (Samuel C. Oommen); Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA (Maher Abbas); Lahey Hospital and Medical Center, Burlington, MA (Peter Marcello); Lankenau Medical Center, Wynnewood, PA (John Marks); MD Anderson Cancer Center, Houston, TX (George Chang); Mayo Clinic, Rochester, MN (David Larson); Mayo Clinic, Scottsdale, AZ (Tonia Young-Fadok); Memorial Sloan Kettering Cancer Center, New York, NY (Martin Weiser); NorthShore University Health System–Evanston Hospital, Evanston, IL (Marc Singer); Northwestern University, Chicago, IL (Amy Halverson); Ochsner Medical Center Jefferson, New Orleans, LA (David Margolin); Providence Portland Medical Center, Portland, OR (Mark Whiteford); Rush University Medical Center, Chicago, IL (Marc Brand, Theodore Saclarides); Saint Joseph’s Healthcare Charlton Campus, Hamilton, Ontario, Canada (Mehran Anvari); Saint Paul’s Hospital, Vancouver, British Columbia, Canada (P. Phang); Sidney and Lois Eskenazi Hospital, Indianapolis, IN (Virgilio George); Spectrum Health–Blodgett Campus, Grand Rapids, MI (Rebecca Hoedema); State University of New York Upstate Medical University, Syracuse (Jiri Bem); Stony Brook University Medical Center, Stony Brook, NY (Roberto Bergamaschi); Tampa General Hospital, Tampa, FL (Jorge Marcet); University of Chicago, Chicago, IL (Alessandro Fichera, Mitchell Posner); University of Iowa Hospitals and Clinics, Iowa City (John Byrn); University of Pittsburgh Cancer Institute (UPCI), Pittsburgh, PA (Herbert Zeh); Vanderbilt University/Ingram Cancer Center, Nashville, TN (Alan Herline); Washington University School of Medicine, Saint Louis, MO (Elisa Birnbaum, James Fleshman, Matthew Mutch, Paul Wise); Western Pennsylvania Hospital, Pittsburgh (James McCormick).
Author Contributions
Dr Sargent and Ms Branda had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fleshman, Sargent, Boller, George, Abbas, Chang, Herline, Wexner, Margolin, Marcello, Posner, Read, Monson, Wren, Nelson.
Acquisition, analysis, or interpretation of data: Fleshman, Branda, Sargent, George, Peters, Maun, Chang, Herline, Fichera, Mutch, Wexner, Whiteford, Marks, Birnbaum, Margolin, Larson, Marcello, Monson, Pisters.
Drafting of the manuscript: Fleshman, Sargent, George, Abbas, Herline, Fichera, Mutch, Larson.
Critical revision of the manuscript for important intellectual content: Fleshman, Branda, Sargent, Boller, Abbas, Peters, Maun, Chang, Wexner, Whiteford, Marks, Birnbaum, Margolin, Larson, Marcello, Posner, Read, Monson, Wren, Pisters, Nelson.
Statistical analysis: Branda, Sargent, Boller, Marks.
Obtained funding: Fleshman, Herline, Nelson.
Administrative, technical, or material support: Sargent, Boller, Whiteford, Marks, Margolin, Larson, Posner, Read, Wren, Pisters, Nelson.
Study supervision: Fleshman, Sargent, George, Herline, Wexner, Marks, Monson, Nelson.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Branda reports receiving research support from Covidien unrelated to this work. Dr Peters reports receiving personal fees from Ethicon EndoSurgery unrelated to this work. Dr Change reports serving on the scientific advisory board for Johnson & Johnson. Dr Wexner reports owning stock options in Asana Medical, LifeBond, and Renew Medical; consulting for Brace Pharmaceuticals, CareFusion, Edwards LifeSciences, Incontinence Devices Inc, Karl Storz Endoscopy America, LifeBond, Mederi Therapeutics, Medtronic, and Novadaq; and receiving inventor’s income from Covidien, Karl Storz Endoscopy America, and Unique Surgical Innovations. Dr Marcello reports receiving honoraria from Applied Medical, Covidien, and Olympus for activities unrelated to this work. Dr Wren reports having a consulting relationship with Intuitive Surgical Inc, manufacturer of the only surgical robotic system commercially available used for minimally invasive rectal cancer surgery; this relationship was not in place during the conduct of the research reported herein. Dr Nelson reports receiving royalties for a licensed patent on a robotic stapling device. No other disclosures were reported.
Role of the Funder/Sponsor
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The study was monitored through the National Cancer Institute (NCI) and Clinical Trials Support Unit with ACOSOG (now part of Alliance).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Enrolling institutions received per capita funding from the US NCI according to accrual to the protocol.
Additional Contributions
The Steering Committee provided support and guidance during the entire process and deserves special acknowledgment: Maher Abbas, MD (Cleveland Clinic Abu Dhabi), Megan Branda, MS (Alliance Statistics and Data Center, Mayo Clinic), George Chang, MD (MD Anderson Cancer Center), Alessandro Fichera, MD (University of Chicago), Alan Herline, MD (Vanderbilt University School of Medicine), Matthew Mutch, MD (Washington University in St Louis), John Marks, MD (Lankenau Hospital), Peter Marcello, MD (Lahey Clinic), David Margolin, MD (Ochsner Clinic), John Monson, MD (University of Rochester), Peter W. T. Pisters, MD (MD Anderson Cancer Center), Mark Whiteford, MD (The Oregon Clinic, Oregon Health & Science University), Thomas Read, MD (Lahey Clinic), Steven Wexner, MD (Cleveland Clinic–Weston), Sherry M. Wren, MD (Stanford University), and Heidi Nelson, MD (Mayo Clinic). We would also like to thank our data quality specialist Pam Fain Pribyl, BA (Mayo Clinic), and analyst Xiomara Carrero, BS (Mayo Clinic). All trial participants contributed to study design and participated in numerous meetings at American College of Surgeons Oncology Group Trial development meetings. None of these individuals received compensation for their role in this study.
References
- 1.Dorrance HR, Docherty GM, O’Dwyer PJ. Effect of surgeon specialty interest on patient outcome after potentially curative colorectal cancer surgery. Dis Colon Rectum. 2000;43(4):492–498. doi: 10.1007/BF02237192. [DOI] [PubMed] [Google Scholar]
- 2.Porter GA, Soskolne CL, Yakimets WW, Newman SC. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227(2):157–167. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monson JR, Weiser MR, Buie WD, et al. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56(5):535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 4.Quirke P, Steele R, Monson J, et al. MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parfitt JR, Driman DK. The total mesorectal excision specimen for rectal cancer: a review of its pathological assessment. J Clin Pathol. 2007;60(8):849–855. doi: 10.1136/jcp.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 7.Arezzo A, Passera R, Salvai A, et al. Laparoscopy for rectal cancer is oncologically adequate: a systematic review and meta-analysis of the literature. Surg Endosc. 2015;29(2):334–348. doi: 10.1007/s00464-014-3686-4. [DOI] [PubMed] [Google Scholar]
- 8.Sajid MS, Ahamd A, Miles WF, Baig MK. Systematic review of oncological outcomes following laparoscopic vs open total mesorectal excision. World J Gastrointest Endosc. 2014;6(5):209–219. doi: 10.4253/wjge.v6.i5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad NZ, Racheva G, Elmusharaf H. A systematic review and meta-analysis of randomized and non-randomized studies comparing laparoscopic and open abdominoperineal resection for rectal cancer. Colorectal Dis. 2013;15(3):269–277. doi: 10.1111/codi.12007. [DOI] [PubMed] [Google Scholar]
- 10.Trastulli S, Cirocchi R, Listorti C, et al. Laparoscopic vs open resection for rectal cancer: a meta-analysis of randomized clinical trials. Colorectal Dis. 2012;14(6):e277–e296. doi: 10.1111/j.1463-1318.2012.02985.x. [DOI] [PubMed] [Google Scholar]
- 11.Xiong B, Ma L, Zhang C, Cheng Y. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res. 2014;188(2):404–414. doi: 10.1016/j.jss.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Martel G, Crawford A, Barkun JS, et al. Expert opinion on laparoscopic surgery for colorectal cancer parallels evidence from a cumulative meta-analysis of randomized controlled trials. PLoS One. 2012;7(4):e35292. doi: 10.1371/journal.pone.0035292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11(7):637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 14.van der Pas MH, Haglind E, Cuesta MA, et al. Colorectal Cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 15.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(1):75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen. J Clin Oncol. 2002;20(7):1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 19.Kuhry E, Schwenk W, Gaupset R, et al. Long-term outcome of laparoscopic surgery for colorectal cancer: a Cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34(6):498–504. doi: 10.1016/j.ctrv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15(7):767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.