Summary
Despite advances in assisted reproduction techniques, the poor quality and failures in embryo in vitro development remain as drawbacks resulting in low pregnancy rate. Mouse embryonic fibroblasts (MEFs) have been widely used to support embryonic stem cells. Mesenchymal cells (MSCs) have also been shown to release bioactive factors. In the present study we have evaluated the ability of MSCs and MEFs to support early development of mouse embryos. The embryos were cultivated alone or in coculture with inactivated MSC or MEF for 4 days. After 4 days in culture the percentage of blastocyst formation in coculture with MSC (91.7±4.3%) or MEF (95.1±3.3%) was higher than in the control group (72.2±9.0%). We did not observe any difference in proliferation or apoptosis. However, the blastocysts cocultured with MSC or MEF presented a significantly higher number of cells within the inner cell mass per embryo when compared to controls. The MSC and MEF groups presented also a higher cell number and diameter when compared to the CTRL. In summary, our data indicate that coculture with MSC or MEF improve early embryonic development and quality in vitro.
Keywords: In vitro embryonic development, coculture, mesenchymal stem cells, murine embryonic fibroblast
1. Introduction
Infertility is a critical component of reproductive health, affecting 50 million couples worldwide (Mascarenhas et al. 2012). Assisted reproductive technologies (ART) have helped many infertile couples, and ART babies comprise 1.5% of all births in the United States (Sunderam et al. 2014). Despite the undeniable improvement in the field, the efficacy of in vitro fertilization (IVF) procedures remains low and even when two or three embryos are transferred, the pregnancy rate is around 30% per IVF treatment cycle (Choi et al. 2013; Kupka et al. 2014). In addition, multiple gestation results in a much higher incidence of health complications for mothers and their babies (Ajduk and Zernicka-Goetz 2013). This low pregnancy efficacy and high number of transferred embryos required could likely be overcome by improving the quality of in vitro treatment of the embryos. In addition to its impact on human reproduction, improvement in the application of ART to animal species would also benefit assisted breeding programs in livestock.
The majority of human preimplatation embryos are morphologically variable, with unevenly sized cells frequently displaying cytoplasmic blebs of varying sizes (Hardy and Spanos 2002). The poor development and implantation could be due to several factors, such as chromosomal abnormalities (Jamieson et al. 1994; Munne et al. 1995), inadequate nuclear or cytoplasmic maturation during oogenesis (Moor et al. 1998), poor embryonic-maternal dialogue or a suboptimal culture environment (Bavister 1995). The culture medium must contain the necessary components to support the embryo development and these molecules should pass through the pellucid zone, a highly porous glycoprotein membrane. Pellucid zone permeability appears to be independent of the developmental stage of the embryo (Turner and Horobin 1997). Several different protocols have been designed to optimize development rate and quality of the embryos in vitro. The main goal is to optimize culture condition to increase the chances of the embryo to achieve implantation and a better outcome of the infertility treatment. One approach is to simulate the maternal environment using various coculture conditions with somatic cells (Cordova et al. 2014; Duszewska et al. 2000; Goovaerts et al. 2011; Kervancioglu et al. 1997). Despite studies showing that coculture with somatic cells can improve embryonic development, culture conditions are not completely effective to support early in vitro development in any species without altering normal embryonic development (Watson et al. 2004).
Stem cells are characterized by their ability to differentiate into many lineage-specific cell types and are being used for tissue engineering applications (Bernardo et al. 2012; Bianco et al. 2001; Jasmin et al. 2012; Moraes et al. 2012). Bone marrow mesenchymal stem cells (MSC) have been widely used for cell therapy due to their unique properties of releasing bioactive factors and supporting cell survival and growth (Caplan 2009; Uccelli et al. 2008). In addition, mouse embryonic fibroblast (MEF) has been widely used as a feeder layer to support embryonic stem cells due to their release of important bioactive factors which maintain the stem cells in an undifferentiated state (Bryja et al. 2006; Lim and Bodnar 2002).
Based on the release of factors from these cells that would be expected to improve the number and quality of embryos produced in vitro, we hypothesized that MSC and MEF could be used as a feeder layer to support early embryonic development. Here we chose a mouse model to develop our experimental design since there are ethical conflicts for studies with human embryos. Thus, the goal of this study was to assess the impact of coculture of embryos with MSC and MEF. For these studies, we used a simple coculture system with very low numbers of embryos in each culture to directly evaluate embryo growth and viability.
2. Material and Methods
2.1. Animals
Experiments were performed on adult C57BL/6 mice (8–10 weeks old) and Wistar rats (10–12 weeks old). All experiments were performed in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23), and were approved by the Committee for the Use of Experimental Animals of Universidade Federal de Juiz de Fora, MG, Brazil (Protocol nº 080/2012).
2.2. Isolation and Culture of Bone Marrow Mesenchymal Cells
Bone marrow cells were obtained from tibias and femurs of rats. The bones were isolated, epiphyses were removed and individually inserted in 1 mL pipette tips inside 15 mL tubes. The bones were centrifuged at 300 × g for 1 min and the pellets suspended in Dulbecco’s modified Eagle’s high glucose medium (DMEM; Invitrogen Inc., Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Invitrogen Inc. Sao Paulo, SP, Brazil), 2 mM l-glutamine (Invitrogen), 100 U/mL penicillin (Sigma-Aldrich Co., St. Louis, MO, USA), and 100 μg/mL streptomycin (Sigma-Aldrich). The cells were plated in culture dishes with supplemented DMEM and maintained in 5% CO2 atmosphere at 37°C. After 48–72 hrs of culture the medium was aspirated to remove non-adherent cells and the adherent cells were grown to confluence before each passage. Medium was replaced three times a week and all experiments were performed on second or third passage cells. The cell cycle was inactivated by 10 μg/mL Mitomycin C (Sigma-Aldrich) incubation for 2–3 hours; inactivated MSC are termed iMSC.
In a previous study from our group using the same protocol described here, we observed that, when treated appropriately, the MSC were able to differentiate into cells recognizable with markers for chondrocytes, osteocytes and adipocytes, confirming their stem cell potential (Jasmin et al. 2011).
2.3. Isolation and culture of Mouse Embryonic Fibroblasts
Pregnant female mice were euthanized 13 days after mating for embryo acquisition. The head and the viscera were removed and the remaining tissues was minced and digested in 0.05% trypsin/EDTA (Invitrogen) by manual pipetting. This solution was inactivated with fetal bovine serum, centrifuged 300 × g for 3 min and the pellets suspended in supplemented DMEM. The cells were plated in culture dishes with supplemented DMEM and maintained in 5% CO2 atmosphere at 37°C. The adherent cells were grown to confluence before each passage. Medium was replaced three times a week and all experiments were performed on second or third passage cells. The cell cycle was inactivated by 10 μg/mL Mitomycin C (Sigma-Aldrich) incubation for 2–3 hours; these cells are termed iMEF.
2.4. Embryo Acquisition and Cultivation
Female mice were caged overnight with males at a 2:1 ratio. The females with confirmed plugs were euthanized 39–40 hours after mating to collect 2-cell stage embryos. The uterine tube and the distal portion of the uterine horn were isolated in phosphate-buffered saline (PBS). The embryos were flushed into 37 ºC heated EmbryoMax M2 medium (Millipore, Billerica, MA, USA) and after three washes morphologically normal embryos were randomly cultivated in 60 μL drops of EmbryoMax KSOM medium without supplements (Millipore) under mineral oil in specified experimental conditions. After 2 days 50% KSOM media was replaced for all groups. Twenty four hours before embryo acquisition, we thawed and plated 104 iMSC or iMEF in the cultivation drops with supplemented DMEM. Approximately one hour before the cocultures we washed the cells three times with PSB and incubated them with the embryo media (KSOM) to acclimatize. The cells and embryos where maintained in incubator at 37ºC and 5% CO2 in high relative humidity. Embryonic development was evaluated daily for 4 days after establishing the cocultures (~ 138 hours after mating) and cleavage, morulae and blastocyst formation were measured. We compared the following groups: CTRL - cultured in control culture medium; iMSC - cocultured with inactivated MSCs; iMEF - cocultured with inactivated MEFs; and 2d iMSC+CTRL - cocultured with inactivated MSCs for the first 2 days followed by culture in CTRL condition for the next 2 days.
The groups were separated in two experiments. In the first experiment we performed the CTRL, iMSC and iMEF treatments with 3–5 embryos in each group with 12 repetitions (n=50 for CTRL, n=43 for iMSC and n=48 for iMEF groups). For the second experiment we compared the CTRL and 2d iMSC+CTRL groups with 3–5 embryos per treatment with 5 repetitions (n=20 for CTRL, n=20 for 2d iMSC groups).
2.5. Immunocytochemistry and TUNEL Assay
After 3 days in culture (~114 hours after mating) the blastocysts were fixed for 30 minutes in 4% paraformaldehyde for immunocytochemistry and TUNEL assay. The embryos were acquired in a separate experiment from those described above (subsection 2.4). For immunocytochemistry the embryos were washed three times with PBS with 0.1% Triton X-100, incubated with 5% normal goat serum (Sigma-Aldrich) in PBS for 30 min, and then incubated with the primary antibody overnight at 4°C. The embryos were then incubated with the secondary antibody and mounted with VectaShield (Vector Laboratories Inc., Burlingame, CA, USA). Immunostaining with anti-Ki67 (1:400, rabbit IgG, Abcam Inc., Cambridge, MA, USA) and anti-Oct4 (1:400, rabbit IgG, Cell Signaling, Danvers, MA, USA) were used to detect proliferation and inner cell mass, respectively. The secondary antibody used in this study was Cy3-conjugated goat-anti-rabbit IgG (1:1,000; Jackson ImmunoResearch Inc., West Grove, PA, USA).
For TUNEL assays the blastocysts were permeabilized by performing two washes with PBS 0.2% Triton X-100 before exposure to 50 μL of TdT reaction mix for 1 hour at 37 ºC in a humidified chamber. We used the DeadEnd™ Fluorometric TUNEL system (Promega Corporation, Madison, WI, USA) following manufacturer’s recommendations.
Cell nuclei were counterstained with 0.1% 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). In addition, we used the DAPI staining to count the total number of cells and to measure the blastocyst diameter. The photomicrographs were obtained using a Zeiss LSM 510 Duo confocal microscope (Zeiss, GmbH, Germany). Quantifications were performed using AxioVision 4.3 software (Zeiss). The count was performed by observation of serial confocal optical sections of labeled embryos. The total number of TUNEL-, Ki67 and Oct 4-positive cells was divided by the total number of DAPI-stained cells to obtain the percentage data. For diameter measurements, a line was traced along the long axis of the blastocysts side after merging all confocal sections followed by a second line perpendicular to the first. Diameters reported are the means of these two diameters. In blastocysts undergoing hatching, we did not measure the protrusion of trophectoderm cells.
2.6. Statistical Analysis
The statistical significance was evaluated by non-parametric Kruskal-Wallis test with Dunns post-test for comparison among multiple groups and t-test for comparison between two groups. All calculations were done using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and p<0.05 was considered as statistically significant. The data are presented as means and the error bars represent the standard errors of the means.
3. Results
3.1. Embryo Development in Vitro
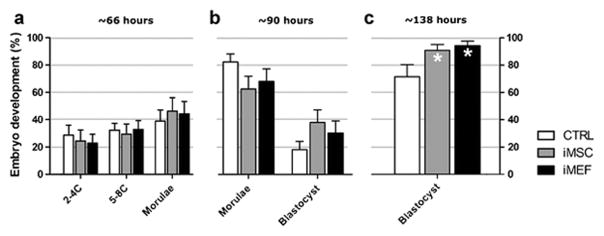
The analysis showed that the embryos cocultured with iMSC or iMEF developed more rapidly when compared with the control group. One day after culture bega (~66 hours after mating) the groups showed similar development as follow: 2–4 cells (28.7 ±7.2, 24.3±8.1 and 22.8±6.5 % for CTRL, iMSC and iMEF respectively); 5–8C (34.6±4.5, 29.3±7.5 and 32.9±6.3%) and morulae (36.7±7.5, 46.3±9.8 and 44.3±9.0%). However, a difference in development began to be apparent at ~90 hour after mating, with fewer morulae (81.8±5.8, 62.2±9.2 and 67.7±8.9% for CTRL, iMSC and iMEF respectively) and more blastocysts (18.2±5.8, 37.8±9.2 and 30.2±8.6%) in the coculture groups (Figure 1A–B), and the difference was statistically significant at the fourth day in culture (~138 hours after mating) where we observed a higher percentage of blastocysts in coculture with iMEF (95.1±3.3%) or iMSC (91.7±4.3%) when compared to the CTRL group (72.2±9.0%) (Figure 1C).
Figure 1. Embryonic development at in culture and coculture.
Embryos were cultivated in CTRL condition or in coculture with iMSC or iMEF. The development stage was evaluated at approximately (A) 66, (B) 90 and (C) 138 hours after mating. n=50 for CTRL, n=43 for iMSC and n=48 for iMEF groups. Group abbreviations: control condition (CTRL); cocultured with inactivated mesenchymal cells (iMSC); cocultured with inactivated murine embryonic fibroblasts (iMEF). *P<0.05
We also analyzed the effect of withdrawing iMSC on the second day of culture (2d iMSC+CTRL) when the majority of the embryos are in the morulae stage. Although rate of blastocyst formation was very similar to the continuous coculture, we did not observe statistical difference between CTRL (71. 7±10.7%) and their respective iMSC (95.0±5.0%) groups despite a clear tendency (p=0.08) for such improvement in cocultures as illustrated in Figure 2. However a statistical difference was observed when we evaluated the rate of hatched blastocysts [56.7±8.1% in CTRL and 90.0±6.1% in 2d iMSC group (p=0.03)].
Figure 2. Evaluation of embryonic development at reduced period in coculture.
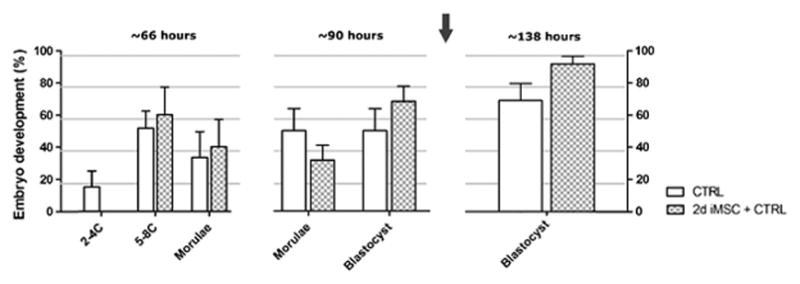
Embryos were cultivated alone or in coculture with iMSC for 2 days and afterward transferred to CTRL culture system. The arrow indicates the time point of culture condition modification. n=20 for CTRL, n=20 for 2d iMSC groups. Group abbreviations: control condition (CTRL); cocultured with MSCs inactivated for the first 2 days followed by culture in CTRL condition for the next 2 days (2d iMSC+CTRL). P>0.05
3.2. Total cell Number and Blastocyst Diameter
In the present study we calculated the total number of cells in the blastocyst and the blastocyst diameter using DAPI staining to perform these evaluations (Figure 3). We observed that the blastocysts co-cultivated with iMSC and iMEF presented a higher number of cells (70.9±2.5 and 74.5±2.7, respectively) and a larger diameter (133.7±2.5 and 139±2.3 μm, respectively) than those cultivated in control condition (cell number: 60.3±2.1 and diameter: 123.8±2.0 μm).
Figure 3. Cell number and blastocyst diameter of embryos.
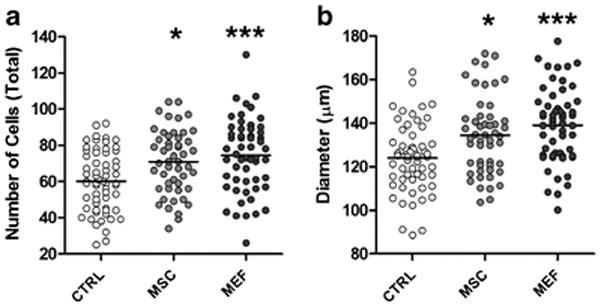
cultivated in CTRL condition or in coculture with iMSC or iMEF for 3 days (~114 hours after mating). (A) Total cell number per embryo, assessed by observation of serial confocal optical sections of embryos stained with DAPI. (B) Blastocyst diameter. Both experiments were performed in same structures and the total number was n=61 for CTRL, n=50 for iMSC and n=56 for iMEF groups. Group abbreviations: control condition (CTRL); cocultured with inactivated mesenchymal cells (iMSC); cocultured with inactivated murine embryonic fibroblasts (iMEF). *P<0.05 and ***P<0.001
3.2. TUNEL and Immunostaining Analyses
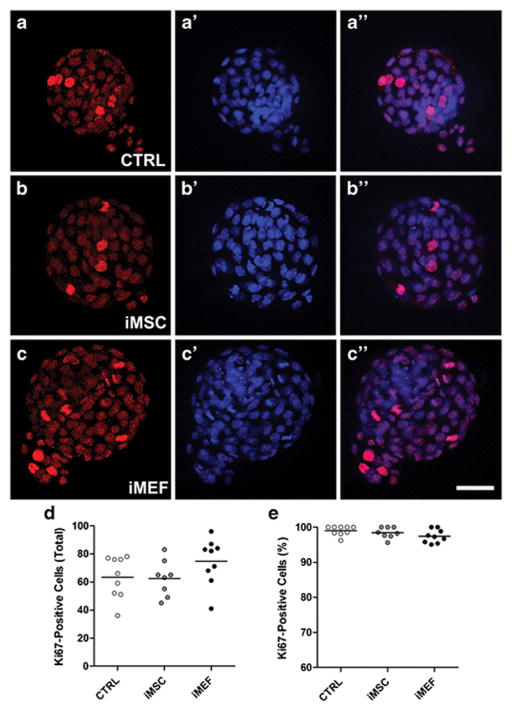
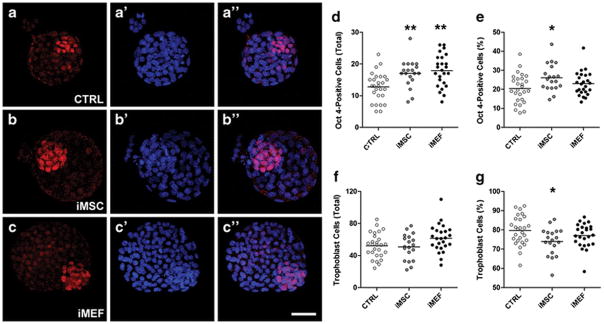
Blastocysts fixed after 3 days in culture were used to quantify the number of cell undergoing apoptosis by TUNEL assay and the proliferation rate, the cell number in the inner cell mass and the number of cells in the trophoblast by immunostaining. We did not observe any difference in the number of apoptotic cells among the groups (Figure 4) or in the proliferation rates among the different experimental conditions (Figure 5). However, it was interesting to notice a significant difference in inner cell mass among the groups (Figure 6). The iMSC (17.0±1.0) and iMEF (17.9±1.0) groups presented a higher absolute number of Oct 4-positive cells per embryo when compared to control group (12.7±0.9, Figure 6D). However, when we analyzed the percentage of Oct 4-positive cells we noticed that only iMSC (26.1±1.6%) treatment was different from control treatment (20.4±1.5%, Figure 6E). For trophoblast evaluation we did not detect differences among the groups in total number of trophoblasts per embryo (Figure 6F) but we did observe a slightly lower percentage of trophoblast cells when we compared iMSC (73.9±1.6%) with CTRL group (79.6±1.5%) as shown in Figure 6G.
Figure 4. Apoptosis analysis in CTRL or co-cultivated embryos for 3 days (~114 hours after mating).
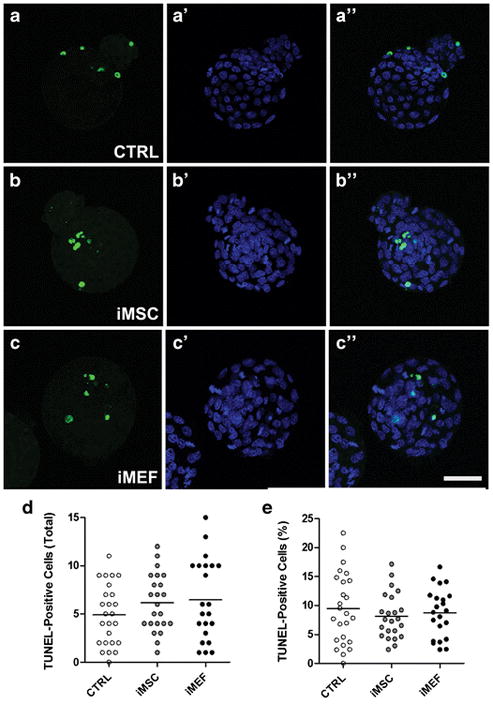
TUNEL-positive cells shown in green and nuclei counterstained with DAPI (blue). Representative sequential images from (A-A″) CTRL, (B-B″) iMSC and (C-C″) iMEF groups. (D) Number of TUNEL-positive cells per embryo. (E) Percentage of apoptotic cells per embryo. n=25 for CTRL, n=23 for iMSC and n=22 for iMEF groups. Group abbreviations: control condition (CTRL); cocultured with inactivated mesenchymal cells (iMSC); cocultured with inactivated murine embryonic fibroblasts (iMEF).
Figure 5. Proliferation analysis after 3 days (~114 hours after mating) in vitro.
Photomicrographs showing Ki67-positive (red) and nuclei labeled with DAPI (blue) of embryos in the 3 different experimental conditions. Representative sequential images from (A-A″) CTRL, (B-B″) iMSC and (C-C″) iMEF groups. (D) Number of proliferating cells per embryo. (E) Percentage of Ki67-positive cells per embryo. n=9 for CTRL, n=8 for iMSC and n=9 for iMEF groups. Group abbreviations: control condition (CTRL); cocultured with inactivated mesenchymal cells (iMSC); cocultured with inactivated murine embryonic fibroblasts (iMEF).
Figure 6. Immunostaining for inner cell mass and trophoblast in embryos cultivated in CTRL condition or with cells for 3 days (~114 hours after mating).
The antibody anti-Oct 4 (in red) was used to differentiate inner cell mass from trophoblast by exclusion since all cells were counterstained with DAPI (in blue) as represented in the sequential images (A-A″) CTRL, (B-B″) iMSC and (C-C″) iMEF groups. (D) The absolute number of Oct 4-positive cells per embryo and (E) the percentage of Oct 4-positive cells. (F) Total number of trophoblast and (G) percentage of trophoblast. n=27 for CTRL, n=19 for iMSC and n=25 for iMEF groups. Group abbreviations: control condition (CTRL); cocultured with inactivated mesenchymal cells (iMSC); cocultured with inactivated murine embryonic fibroblasts (iMEF). *P<0.05 and **P<0.01
4. Discussion
The post fertilization period is a critical time affecting blastocyst quality and implantation efficiency. It is well known that embryos produced in vitro have a lower quality when compared to those produced in vivo. Such difference has been attributed to many factors including intrinsic genetic abnormalities, damage during collection and manipulations of the gametes. However, culture conditions also represent crucial parameters that can and should be carefully optimized (Gelber et al. 2011). Inadequate culture conditions do not allow the embryo to preserve its homeostasis, resulting in short-term alterations in morphology, cell proliferation, apoptosis and metabolism. Over the longer term, adverse effects include reduced pregnancy rate, increased abortion risk, congenital abnormalities, postnatal death and diseases in adulthood (Hentemann et al. 2011).
Several authors have already described the beneficial effects of coculture on embryo development in vitro (Cordova et al. 2014; Duszewska et al. 2000; Goovaerts et al. 2011; Kervancioglu et al. 1997). However, the culture conditions have not been completely optimized to support early in vitro development in any species (Watson et al. 2004). In the present study, we observed that both types of cells, MSC and MEF, improved mouse embryo development in vitro. An improvement in mouse embryo development after MEF coculture was also observed in a study where the authors used MEF and human embryonic fibroblast to prevent the effect of in vitro visible light exposure (Nematollahi-mahani et al. 2009). We are not aware of any other study evaluating the direct effect of MSC in in vitro embryo development. However, there is one study using a porcine model which evaluated the effect of MSC conditioned medium (Park et al. 2013) and another in bovine cells evaluating the effect of embryonic stem cell conditioned medium on embryo development in vitro (Kim et al. 2011). Both studies are from the same group and results were similar, with addition of 10% stem cell conditioned medium improving embryonic development when compared to control group cultivated without FBS; however, no significant difference was observed when compared to treatment with 10% FBS.
Although we did not detect an effect of 2d iMSC on blastocyst formation, the number of hatched blastocysts was significantly higher. This result reveals that brief coculture with MSC can exert a long-term effect on embryonic development. The hatching process is critical for embryonic implantation and has been associated with subsequent viable pregnancy, whereas delayed implantation has been associated with a high incidence of abortion (Hammadeh et al. 2011). In the clinic, embryos can be transferred within 2–5 days after initial cultivation (Maxwell et al. 2015; Sunderam et al. 2014), thus the positive effect of shorter-term coculture with MSC is quite relevant.
During preimplantation development the embryo undergoes cell division, apoptosis and differentiation. A correlation has been found between blastocyst quality score and the proportion of embryos developing to the blastocyst stage, rate of development, metabolism, cell number in the blastocyst, and incidence of cell division and cell death (Gelber et al. 2011; Kim et al. 2011; Matsuura et al. 2010; Polisseni et al. 2010). In our study we observed a higher number of cells per embryo and larger blastocyst diameter after coculture with iMSC or iMEF when compared to controls. However, when we evaluated the number of cells from ICM and trophoblast we noticed that only ICM had a higher number of cells, indicating a specific proliferation in this area. The ICM consists of cells that will give rise to the future embryo body and some extra-embryonic tissues. Therefore, it is not surprising that the size of ICM, measured either as the cell number or as the area, has been found to be an important indicator of viability and implantation success (Ajduk and Zernicka-Goetz 2013; Gardner et al. 2000; Lane and Gardner 1997; Richter et al. 2001). We also analyzed the proliferation and apoptosis rate but we did not observe significant differences among the groups. Since this evaluation was performed in the whole embryo it is possible that we missed specific changes in ICM and trophoblast regions. The choice for embryo-transfer number is based on patient characteristics and embryo quality, indicating that the quality of the embryos is more important than the quantity in ART. In USA, the average number of embryos transferred per procedure varied from 2.0 among women aged <35 years (range: 1.5 to 2.2) to 2.3 among women aged 35–40 years (range: 1.8 to 2.8), and 2.9 among women aged >40 years (range: 2.1 to 4.0) (Sunderam et al. 2014). Multiple birthrates for transfers of two embryos to patients <35 years, 35–37 years, and 38–40 years were 40%, 33%, and 28%, respectively (Wright et al. 2008). Thus numerous publications have investigated the practice of elective single-embryo transfer since it has been advocated as the only effective means to avoid multiple pregnancy in IVF cycles (“Elective single-embryo transfer” 2012).
The analyses performed in the present study revealed superior development of embryos co-cultivated with iMSC or iMEF. It has been described that cocultured cells release a complex mixture of many different growth factors into the embryo culture media, which is believed to be the primary reason behind the improved embryonic development associated with coculture (Richter 2008). In the reproductive tract the preimplantation embryos develop in the absence of direct cell contact in a free-floating way, nevertheless they are dependent on released factors by oviduct, uterus luminal and by themselves (Besenfelder et al. 2012; Mishra and Seshagiri 2000; Seshagiri et al. 2002), including factors as IGF-I and -II, TGFα, TGFβ, IL-1b, EGF, SCF and LIF among others (Hardy and Spanos 2002). It is well known that both MSC and MEF cells release bioactive factors supporting embryonic growth and it is likely that these factors positively impacted early embryo development as shown here. Several growth factors are produced by MSC such as TGFb1, HGF, IL-10, PGE2, IDO, HLA-G5, IL-6, VEGF and IGF-I (Caplan 2009; Uccelli et al. 2008) which can induce cell proliferation (Cheng and Yau 2008) and maintenance of bone marrow stem cells in an undifferentiated state (Gronthos and Simmons 1996). In addition, Lim and Bodnar (2002) identified 136 unique protein species in conditioned medium from MEF which included some that are known to participate in cell growth and differentiation, extracellular matrix formation and remodeling (Lim and Bodnar 2002). The proteomic analysis has revealed the complexity of the environment provided by the feeder cells.
Another important characteristic of MSC is their high antioxidant capacity as shown by different studies (Cho et al. 2012; Kemp et al. 2010). Physiological levels of reactive oxygen species (ROS) such as H2O2 and superoxide are generally produced during the normal metabolism of mammalian embryos however it seems likely that embryo cultured in vitro may be exposure to oxidative stress for which their defense mechanisms are insufficient to protect their cellular structures. This so-called “oxidative stress” situation critically threatens embryonic development and accounts, amongst the most important causes of retarded embryonic development, compromised viability and embryo arrest in several species (Ali et al. 2003; Guerin et al. 2001; Hosseini et al. 2011). To modulate extracellular ROS media can be supplemented with extra-cellular enzymatic antioxidants such as superoxide dismutase (Nonogaki et al. 1991). It has been shown that MSC can secrete superoxide dismutase which is a major antioxidant defense that protects tissues within the body from oxidative stress (Cho et al. 2012; Kemp et al. 2010).
Despite many years of testing coculture to improve in vitro embryos development it is still not exactly clear what these cells provide for them. For human embryos several feeder cell types have been clinically tested such as bovine uterine fibroblasts, human and bovine oviductal cells, Vero cells, cumulus cells, fetal skin fibroblasts, ovarian cancer cells, Buffalo rat liver cells and endometrial cells (Kattal et al. 2008). Due to the unique properties of MSC in releasing growth, antioxidant and immunomodularory factors together with simple isolation methods of these cells and the possibility to use human cells either autologous or allogeneic in ART, it seems likely that MSC is could be a preferable feeder cell type than MEF or other somatic cells.
Comparing our data with other studies in the literature we have observed that a large variation in blastocyst formation rate has been reported. Different mouse strains, culture medium and number of embryos per microdrop appear to be largely responsible for this variation. In a recent study using coculture of activated macrophages to improve embryonic development, authors reported a blastocyst formation rate of 76.9 % in control condition (Lee et al. 2015). In another recent study testing the effect of cyclopamine, reported blastocyst formation rates were 53.6 and 57.1% in their control groups (Liu et al. 2014). In addition other authors have reported a high rate of embryo development (more than 90% blastocyst formation) (Biggers et al. 2005; Gelber et al. 2011). In our study we observed that 72.2% of the embryos turned into blastocysts in control condition, a smaller percentage than in these last two cited studies. However, both authors cultivated more than twice as many embryos per microdrop than we used in our study, which could explain the difference. Using a protocol similar to ours, Matsuura et. al. (2010) observed that the developmental rate was proportional to the number of cultivated embryos per microdrop (75% for 10 embryos, 46% for 4–6 embryos and less than 30% for 1 embryo) (Matsuura et al. 2010). According to previous reports the concentration and production of autocrine and/or paracrine factors enhance mouse embryo development and thereby grouped embryos develop better than individually cultivated embryos (Contramaestre et al. 2008). Thus, based on the knowledge that ART protocols commonly cultivate the embryos individually (De Vos et al. 2015), we hypothesize that coculture with MSC or MEF could improve the development of these embryos and due to the previously described MSC proprieties they could be a better type for ART than MEF. Further studies are required to critically evaluate the conditioned medium in the cocultures and thereby potentially identify embryotrophic factors that can supply adjunctive supplements and improve embryogenesis.
In summary, this study brings a novel coculture system that may provide an improvement in quality of in vitro embryo cultures in particular for protocols culturing single or few embryos per drop.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; BPD-00021-12, REDE26/11 and REDE 31/11) and DECIT/SCTIE/MS through CNPq and supported by FAPERJ.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors’ Contributions
JJ accomplished all the experiments and performed the statistical analysis. VMP conceived of the study, participated in its design and coordination and helped to draft the manuscript. DCS conceived of the study, participated in its design and coordination and helped to draft the manuscript. RMO conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript
Contributor Information
Vera Maria Peters, Email: peters.vera@ufjf.edu.br.
David C Spray, Email: david.spray@einstein.yu.edu.
Rosalia Mendez-Otero, Email: rmotero@biof.ufrj.br.
References
- Ajduk A, Zernicka-Goetz M. Quality control of embryo development. Molecular aspects of medicine. 2013;345:903–918. doi: 10.1016/j.mam.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;593–4:939–949. doi: 10.1016/s0093-691x(02)01125-1. [DOI] [PubMed] [Google Scholar]
- Bavister BD. Culture of preimplantation embryos: facts and artifacts. Human reproduction update. 1995;12:91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant. 2012;472:164–171. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- Besenfelder U, Havlicek V, Brem G. Role of the oviduct in early embryo development. Reproduction in domestic animals = Zuchthygiene. 2012;47(Suppl 4):156–163. doi: 10.1111/j.1439-0531.2012.02070.x. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;193:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod. 2005;2012:3376–3384. doi: 10.1093/humrep/dei228. [DOI] [PubMed] [Google Scholar]
- Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nature protocols. 2006;14:2082–2087. doi: 10.1038/nprot.2006.355. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;2172:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg. 2008;202:94–101. doi: 10.1053/j.semtcvs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Cho KA, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell biology international. 2012;3612:1267–1274. doi: 10.1042/CBI20110634. [DOI] [PubMed] [Google Scholar]
- Choi YM, Chun SS, Han HD, Hwang JH, Hwang KJ, Kang IS, Kim DW, Kim KC, Kim T, Kwon HC, Lee WD, Lee JH, Lee KS, Lee GH, Lee SH, Lee YI, Min EG, Moon HS, Moon SY, Roh SI, Yoon TK. Current status of assisted reproductive technology in Korea, 2009. Obstetrics & gynecology science. 2013;566:353–361. doi: 10.5468/ogs.2013.56.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contramaestre AP, Sifontes F, Marin R, Camejo MI. Secretion of stem cell factor and granulocyte-macrophage colony-stimulating factor by mouse embryos in culture: influence of group culture. Zygote. 2008;164:297–301. doi: 10.1017/S0967199408004760. [DOI] [PubMed] [Google Scholar]
- Cordova A, Perreau C, Uzbekova S, Ponsart C, Locatelli Y, Mermillod P. Development rate and gene expression of IVP bovine embryos cocultured with bovine oviduct epithelial cells at early or late stage of preimplantation development. Theriogenology. 2014;819:1163–1173. doi: 10.1016/j.theriogenology.2014.01.012. [DOI] [PubMed] [Google Scholar]
- De Vos A, Janssens R, Van de Velde H, Haentjens P, Bonduelle M, Tournaye H, Verheyen G. The type of culture medium and the duration of in vitro culture do not influence birthweight of ART singletons. Hum Reprod. 2015;301:20–27. doi: 10.1093/humrep/deu286. [DOI] [PubMed] [Google Scholar]
- Duszewska AM, Reklewski Z, Pienkowski M, Karasiewicz J, Modlinski JA. Development of bovine embryos on Vero/BRL cell monolayers (mixed co-culture) Theriogenology. 2000;548:1239–1247. doi: 10.1016/s0093-691x(00)00430-1. [DOI] [PubMed] [Google Scholar]
- Elective single-embryo transfer. Fertility and sterility. 2012;974:835–842. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertility and sterility. 2000;736:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- Gelber K, Tamura AN, Alarcon VB, Marikawa Y. A potential use of embryonic stem cell medium for the in vitro culture of preimplantation embryos. J Assist Reprod Genet. 2011;288:659–668. doi: 10.1007/s10815-011-9587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goovaerts IG, Leroy JL, Rizos D, Bermejo-Alvarez P, Gutierrez-Adan A, Jorssen EP, Bols PE. Single in vitro bovine embryo production: coculture with autologous cumulus cells, developmental competence, embryo quality and gene expression profiles. Theriogenology. 2011;767:1293–1303. doi: 10.1016/j.theriogenology.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Simmons PJ. The biology and application of human bone marrow stromal cell precursors. J Hematother. 1996;51:15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Human reproduction update. 2001;72:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hammadeh ME, Fischer-Hammadeh C, Ali KR. Assisted hatching in assisted reproduction: a state of the art. Journal of assisted reproduction and genetics. 2011;282:119–128. doi: 10.1007/s10815-010-9495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;1722:221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril. 2011;954:1291–1294. doi: 10.1016/j.fertnstert.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Hosseini SO, Aghaee F, Hosseini SM, Hajian M, Forouzanfar M, Noorbakhshnia M, Gourabi H, Shahverdi AH, Dizaj AVT, Nasr-Esfahani MH. Effect of culture condition and cell-permeable superoxide dismutase on levels of reactive oxygen species (ROS) production in “in vitro” produced sheep embryos. Small Ruminant Res. 2011;971–3:88–93. [Google Scholar]
- Jamieson ME, Coutts JR, Connor JM. The chromosome constitution of human preimplantation embryos fertilized in vitro. Hum Reprod. 1994;94:709–715. doi: 10.1093/oxfordjournals.humrep.a138575. [DOI] [PubMed] [Google Scholar]
- Jasmin, Jelicks LA, Koba W, Tanowitz HB, Mendez-Otero R, Campos de Carvalho AC, Spray DC. Mesenchymal bone marrow cell therapy in a mouse model of chagas disease. Where do the cells go? PLoS Negl Trop Dis. 2012;612:e1971. doi: 10.1371/journal.pntd.0001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin, Torres AL, Nunes H, Passipieri J, Jelicks L, Gasparetto E, Spray D, Campos de Carvalho A, Mendez-Otero R. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. Journal of nanobiotechnology. 2011;91:4. doi: 10.1186/1477-3155-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattal N, Cohen J, Barmat LI. Role of coculture in human in vitro fertilization: a meta-analysis. Fertility and sterility. 2008;904:1069–1076. doi: 10.1016/j.fertnstert.2007.07.1349. [DOI] [PubMed] [Google Scholar]
- Kemp K, Hares K, Mallam E, Heesom KJ, Scolding N, Wilkins A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010;1146:1569–1580. doi: 10.1111/j.1471-4159.2009.06553.x. [DOI] [PubMed] [Google Scholar]
- Kervancioglu ME, Saridogan E, Atasu T, Camlibel T, Demircan A, Sarikamis B, Djahanbakhch O. Human Fallopian tube epithelial cell co-culture increases fertilization rates in male factor infertility but not in tubal or unexplained infertility. Hum Reprod. 1997;126:1253–1258. doi: 10.1093/humrep/12.6.1253. [DOI] [PubMed] [Google Scholar]
- Kim EY, Lee JB, Park HY, Jeong CJ, Riu KZ, Park SP. The use of embryonic stem cell derived bioactive material as a new protein supplement for the in vitro culture of bovine embryos. The Journal of reproduction and development. 2011;573:346–354. doi: 10.1262/jrd.10-113a. [DOI] [PubMed] [Google Scholar]
- Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, Calhaz-Jorge C, De Geyter C, Goossens V. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHREdagger. Hum Reprod. 2014;2910:2099–2113. [Google Scholar]
- Lane M, Gardner DK. Differential regulation of mouse embryo development and viability by amino acids. Journal of reproduction and fertility. 1997;1091:153–164. doi: 10.1530/jrf.0.1090153. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim J, Kim SH, Kang HG, Jun JH. Effects of Coculture With Immune Cells on the Developmental Competence of Mouse Preimplantation Embryos in Vitro and in Utero. Reprod Sci. 2015 doi: 10.1177/1933719115574342. [DOI] [PubMed] [Google Scholar]
- Lim JW, Bodnar A. Proteome analysis of conditioned medium from mouse embryonic fibroblast feeder layers which support the growth of human embryonic stem cells. Proteomics. 2002;29:1187–1203. doi: 10.1002/1615-9861(200209)2:9<1187::AID-PROT1187>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei Z, Huang Y, Bai C, Zan L, Li G. Cyclopamine did not affect mouse oocyte maturation in vitro but decreased early embryonic development. Animal science journal = Nihon chikusan Gakkaiho. 2014;859:840–847. doi: 10.1111/asj.12220. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;912:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Hayashi N, Kuroda Y, Takiue C, Hirata R, Takenami M, Aoi Y, Yoshioka N, Habara T, Mukaida T, Naruse K. Improved development of mouse and human embryos using a tilting embryo culture system. Reproductive biomedicine online. 2010;203:358–364. doi: 10.1016/j.rbmo.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Maxwell SM, Melzer-Ross K, McCulloh DH, Grifo JA. A comparison of pregnancy outcomes between day 3 and day 5/6 embryo transfers: does day of embryo transfer really make a difference? Journal of assisted reproduction and genetics. 2015;322:249–254. doi: 10.1007/s10815-014-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Seshagiri PB. Heparin binding-epidermal growth factor improves blastocyst hatching and trophoblast outgrowth in the golden hamster. Reproductive biomedicine online. 2000;13:87–95. doi: 10.1016/s1472-6483(10)61945-1. [DOI] [PubMed] [Google Scholar]
- Moor RM, Dai Y, Lee C, Fulka J., Jr Oocyte maturation and embryonic failure. Human reproduction update. 1998;43:223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- Moraes L, Vasconcelos-dos-Santos A, Santana FC, Godoy MA, Rosado-de-Castro PH, Jasmin, Azevedo-Pereira RL, Cintra WM, Gasparetto EL, Santiago MF, Mendez-Otero R. Neuroprotective effects and magnetic resonance imaging of mesenchymal stem cells labeled with SPION in a rat model of Huntington’s disease. Stem Cell Res. 2012;92:143–155. doi: 10.1016/j.scr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertility and sterility. 1995;642:382–391. [PubMed] [Google Scholar]
- Nematollahi-mahani SN, Pahang H, Moshkdanian G, Nematollahi-mahani A. Effect of embryonic fibroblast cell co-culture on development of mouse embryos following exposure to visible light. Journal of assisted reproduction and genetics. 2009;262–3:129–135. doi: 10.1007/s10815-008-9290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Protection from Oxidative Stress by Thioredoxin and Superoxide-Dismutase of Mouse Embryos Fertilized Invitro. Human Reproduction. 1991;69:1305–1310. doi: 10.1093/oxfordjournals.humrep.a137532. [DOI] [PubMed] [Google Scholar]
- Park HY, Kim EY, Lee SE, Choi HY, Moon JJ, Park MJ, Son YJ, Lee JB, Jeong CJ, Lee DS, Riu KJ, Park SP. Effect of human adipose tissue-derived mesenchymal-stem-cell bioactive materials on porcine embryo development. Molecular reproduction and development. 2013;8012:1035–1047. doi: 10.1002/mrd.22270. [DOI] [PubMed] [Google Scholar]
- Polisseni J, Sa WF, de Guerra MO, Machado MA, Serapiao RV, Carvalho BC, Camargo LS, Peters VM. Post-biopsy bovine embryo viability and whole genome amplification in preimplantation genetic diagnosis. Fertility and sterility. 2010;933:783–788. doi: 10.1016/j.fertnstert.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Current opinion in obstetrics & gynecology. 2008;203:292–304. doi: 10.1097/GCO.0b013e3282fe743b. [DOI] [PubMed] [Google Scholar]
- Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertility and sterility. 2001;766:1157–1167. doi: 10.1016/s0015-0282(01)02870-9. [DOI] [PubMed] [Google Scholar]
- Seshagiri PB, Mishra A, Ramesh G, Rao RP. Regulation of peri-attachment embryo development in the golden hamster: role of growth factors. Journal of reproductive immunology. 2002;531–2:203–213. doi: 10.1016/s0165-0378(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance--United States, 2011. MMWR Surveill Summ. 2014;6310:1–28. [PubMed] [Google Scholar]
- Turner K, Horobin RW. Permeability of the mouse zona pellucida: a structure-staining-correlation model using coloured probes. J Reprod Fertil. 1997;1112:259–265. doi: 10.1530/jrf.0.1110259. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;89:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Natale DR, Barcroft LC. Molecular regulation of blastocyst formation. Anim Reprod Sci. 2004;82–83:583–592. doi: 10.1016/j.anireprosci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance--United States, 2005. MMWR Surveill Summ. 2008;575:1–23. [PubMed] [Google Scholar]