Abstract
Background
This study aimed to characterize intra-operative electroencephalography (EEG) during moderate hypothermic circulatory arrest (MHCA) with selective antegrade cerebral perfusion (SACP), which has not been described previously.
Methods
This was a single-institution retrospective study of patients undergoing aortic hemiarch replacement using MHCA (temperatures <28°C at circulatory arrest [CA]) and unilateral SACP with EEG monitoring from July 1, 2013 to November 1, 2014. The EEG pattern was determined before and immediately after CA, as well as after establishment of SACP. Patient and procedural characteristics and outcomes were determined and compared after stratification by the presence of ischemic EEG changes.
Results
The study included 71 patients. Before CA, 47 patients (66%) demonstrated a continuous EEG pattern, with or without periodic complexes, and 24 (34%) had a burst suppression EEG pattern. Immediately after CA, abrupt loss of electrocerebral activity occurred in 32 patients (45%), suggestive of cerebral ischemia. Establishment of unilateral SACP rapidly restored electrocerebral activity in all but 2 patients. One patient had persistent loss of left-sided activity, which resolved after transition to bilateral SACP. Another patient had persistent global loss of activity and was placed back on cardiopulmonary bypass for further cooling before reinitiation of CA. No significant differences in characteristics or outcomes were assessed between patients with and without loss of EEG activity.
Conclusions
Nearly half of patients undergoing hemiarch replacement with MHCA/SACP experience abrupt loss of electrocerebral activity after CA is initiated. Although unilateral SACP usually restores prearrest electrocerebral activity, intraoperative EEG may be particularly valuable for the identification of patients with persistent cerebral ischemia even after SACP.
Induced hypothermia has been used for organ protection during aortic arch operations requiring circulatory arrest (CA) ever since the landmark report of Griepp and colleagues [1] in 1975. By cooling the brain and visceral organs, tissue oxygen and metabolic demands are reduced to the extent that the period of ischemia resulting from CA can be better withstood [2–5]. Because the brain is particularly sensitive to transient periods of hypoxia, neuroprotection is the paramount concern during these procedures. The traditional viewpoint has been that maximal cerebral protection is achieved at temperatures sufficient to induce electrocerebral inactivity (ECI) on electroencephalography (EEG), under the presumption that maximal suppression of cerebral metabolic activity is achieved at ECI [3, 6]. As a result, many centers have used intraoperative EEG to allow for the identification of ECI before initiating CA [7–10], which leads to average minimum temperatures of less than 16°C [11, 12].
Although cooling to ECI may maximally reduce cerebral oxygen demand, there are concerns that the extreme temperature reductions required to reach ECI may lead to adverse outcomes related to hypothermia-induced coagulopathy [13, 14], prolonged periods on cardiopulmonary bypass (CPB), or direct hypothermic neuronal injury [15–17]. Furthermore, the introduction of adjunctive cerebral perfusion strategies has allowed continued perfusion and cooling of the brain after systemic CA, which has provided an additional mode of neuroprotection. These factors have led a number of centers to use a strategy of more moderate degrees of systemic hypothermia with adjunctive cerebral perfusion during aortic arch operations [14, 18–22]. Neurologic and survival outcomes have generally been comparable between deep and moderate hypothermia organ-protective strategies, and in the absence of high quality randomized data, uncertainty remains about the optimal degree of hypothermia for these procedures [23].
Starting in July 2013, our institution began to use moderate hypothermic CA (MHCA) with unilateral selective antegrade cerebral perfusion (SACP) as the predominate strategy for organ protection during aortic hemiarch replacement, whereas our previous approach was deep hypothermic CA (DHCA) with cooling to ECI before CA [9, 11]. This change in practice was incited due to the theoretic concerns surrounding DHCA as well as the comparable outcomes obtained from groups using MHCA with adjunctive cerebral perfusion. Although we no longer routinely cool to ECI, we have continued to routinely use intraoperative EEG monitoring. The objective of this study was to characterize EEG activity during MHCA with unilateral SACP to assess the extent to which ischemic EEG changes occur during these procedures.
Material and Methods
Study Design and Patient Selection
This was a retrospective cohort study approved by the Duke University Medical Center Institutional Review Board. Because our institution transitioned to a predominate neuroprotective strategy during hemiarch replacement of MHCA (initiation of CA at systemic temperature <28°C regardless of whether ECI had been reached) with unilateral SACP beginning in July 2013, we identified all patients who underwent this procedure from our prospectively maintained institutional aortic surgery database from July 1, 2013, to November 1, 2014. During this period, EEG monitoring was used in all elective cases and in nonelective cases when available. Nonelective cases in which EEG monitoring was not used were excluded. In addition, only cases in which a neuroprotective strategy of MHCA and unilateral SACP had been used at the outset were included. Patient and procedural characteristics as well as outcomes data were obtained from our database. The Society of Thoracic Surgeons definitions were used to define patient comorbidities and postoperative outcomes [24]. Data relating to EEG reports and tracings were obtained from additional review of the medical record.
EEG Monitoring
EEG monitoring was performed in accordance with our previously described approach [11]. Briefly, the baseline EEG was recorded after anesthetic induction but before induction of cooling. Continuous EEG monitoring was conducted from the onset of CPB, through cooling, CA, and rewarming, until separation from CPB. Unlike our previous approach, the presence of ECI was not required before CA was initiated. Instead, after CA, the surgeon was notified of any EEG changes indicative of cerebral ischemia. If such changes did not resolve after unilateral SACP, additional corrective action was taken.
Conduct of Procedures
Surgical techniques for aortic hemiarch replacement at our institution have been described previously [9, 25]. Unilateral SACP was initiated immediately (≤1 minute) after opening the aortic arch in all cases. Specifically, after clamping the base of the innominate and left common carotid arteries, perfusion was initiated through an 8-mm Dacron (DuPont, Wilmington, DE) side graft attached to the right axillary artery at a flow rate of 5 to 15 mL/kg/min and an inflow temperature of 12°C to a target right radial arterial pressure of 50 to 70 mm Hg, as described [25].
Our approach to anesthesia in this study cohort was modified from our previously described practice [11]. We no longer use total intravenous anesthesia in cases with CA. Instead, anesthesia is induced using a combination of intravenous fentanyl and propofol. Neuromuscular blockade is achieved and maintained using standard nondepolarizing agents at the anesthesiologist’s discretion. Before CPB, anesthesia is maintained with the inhalational anesthetic agent isoflurane titrated to a bispectral index level between 40 and 60. Small boluses of fentanyl are administered as needed. After institution of CPB, isoflurane is administered through the CPB circuit. Isoflurane is discontinued once the patient has been cooled to 28°C and restarted during rewarming. In addition, no anesthetic is provided in the cerebral perfusate during SACP. Other aspects of anesthetic and circulatory support management have not changed significantly from previously described methods [11].
Outcomes and Statistical Analysis
The EEG pattern was assessed before and immediately after CA as well as after establishment of SACP. The primary study outcome was loss of electrocerebral activity, indicative of cerebral ischemia, after the initiation of CA. In addition, an unadjusted comparison of the cohort was performed after stratification by whether loss of electrocerebral activity occurred with CA as a secondary exploratory analysis to investigate whether patient or procedural factors were associated with the occurrence of ischemic EEG changes or whether such changes affected clinical outcomes.
Baseline characteristics, procedural characteristics, and outcomes are summarized using the median and inter-quartile range for continuous variables and counts and percentages for categoric variables. One-way analysis of variance or Wilcoxon rank sum tests were used to compare continuous variables, and Fisher exact tests (cell counts <5) or Pearson χ2 tests were used to compare categoric variables. A p value of less than 0.05 was used to indicate statistical significance. All statistical analyses were performed using R 3.0.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 109 consecutive patients undergoing aortic hemiarch replacement were identified. The lack of EEG monitoring resulted in the exclusion of 27 nonelective patients (24.7%), and another 11 (10.1%) were excluded because an organ-protection strategy of DHCA was pursued at the outset of the procedure at the surgeon’s discretion. DHCA was used instead of MHCA during the study period for situations in which there was (1) unfavorable anatomy for axillary cannulation, in which case DHCA with retrograde cerebral perfusion was used, or (2) anticipation of relatively prolonged periods CA due to disease complexity.
Of the remaining cohort of 71 patients, 47 (66%) demonstrated a continuous EEG pattern, with or without periodic complexes, and 24 (34%) had a burst suppression EEG pattern before CA was initiated. ECI was not reached in any patient before CA. Immediately after CA, an abrupt loss of electrocerebral activity occurred in 32 patients (45%), indicative of cerebral ischemia. In the remaining 39 patients (55%), electrocerebral activity was maintained in the brief period (≤1 minute in both patients who lost and maintained EEG activity) between CA and SACP and persisted once SACP was established. Among the patients who abruptly lost electrocerebral activity after arrest, establishment of unilateral SACP rapidly restored activity in 30 of 32 (94%; Fig 1). However, unilateral SACP did not completely resolve the changes in 2 patients (6%). In 1 patient, unilateral SACP restored right-sided EEG activity, but loss of left-sided electrocerebral activity persisted (Fig 2). In an attempt to ameliorate this, bilateral ACP was initiated to augment perfusion to the left side of the brain, which partially restored left-sided EEG activity. In another patient, SACP did not restore any electrocerebral activity (Fig 3). This patient was therefore placed back on CPB and cooled further before inducing CA a second time.
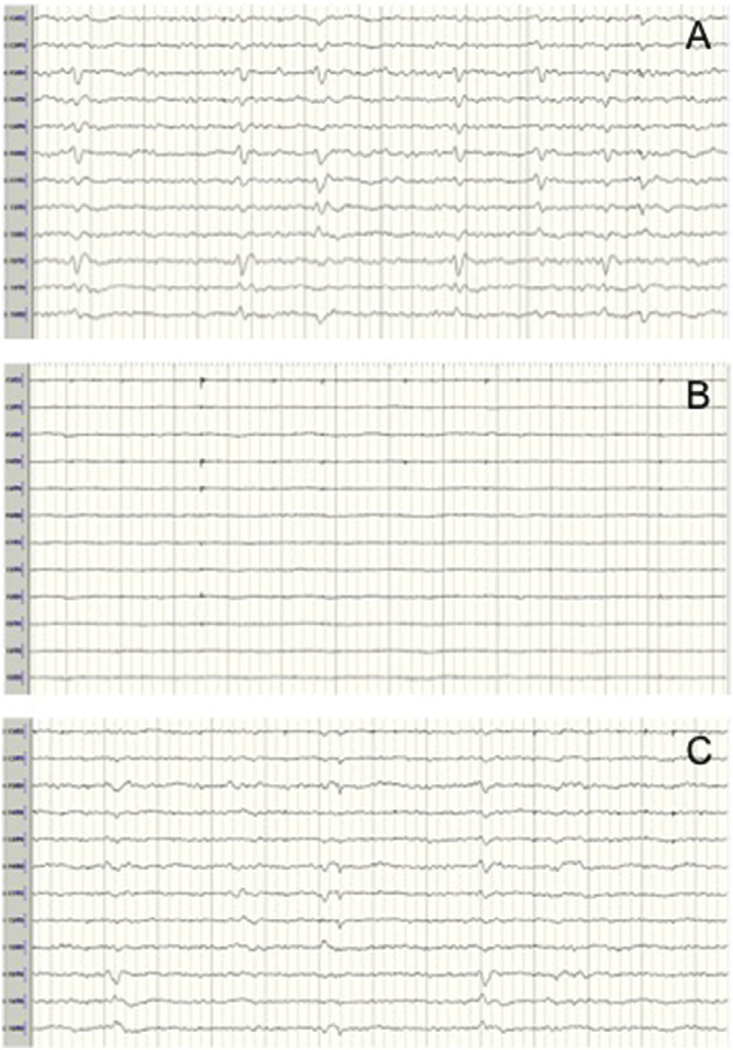
Fig 1.
Restoration of electroencephalography (EEG) activity after circulatory arrest with the establishment of unilateral selective antegrade cerebral perfusion (SACP). (A) EEG before arrest shows burst suppression (nasopharyngeal temperature: 26.6°C). (B) EEG immediately after arrest demonstrates loss of electrocerebral activity. (C) EEG after unilateral SACP shows return of burst suppression pattern.
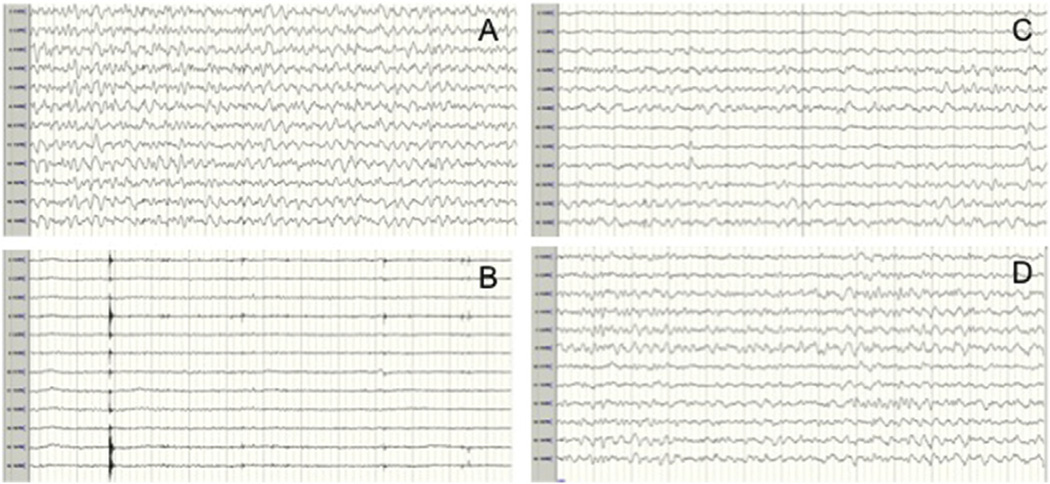
Fig 2.
Persistent loss of left-sided electrocerebral activity after establishment of selective antegrade cerebral perfusion (SACP). (A) Electroencephalogram (EEG) before arrest shows a continuous pattern with diffuse wave slowing (nasopharyngeal temperature: 28.1°C). (B) EEG after arrest demonstrates loss of electrocerebral activity. (C) EEG after unilateral SACP shows persistent loss of electrocerebral activity in the left-sided leads. (D) EEG after transition to bilateral SACP shows partial return of left-sided activity.
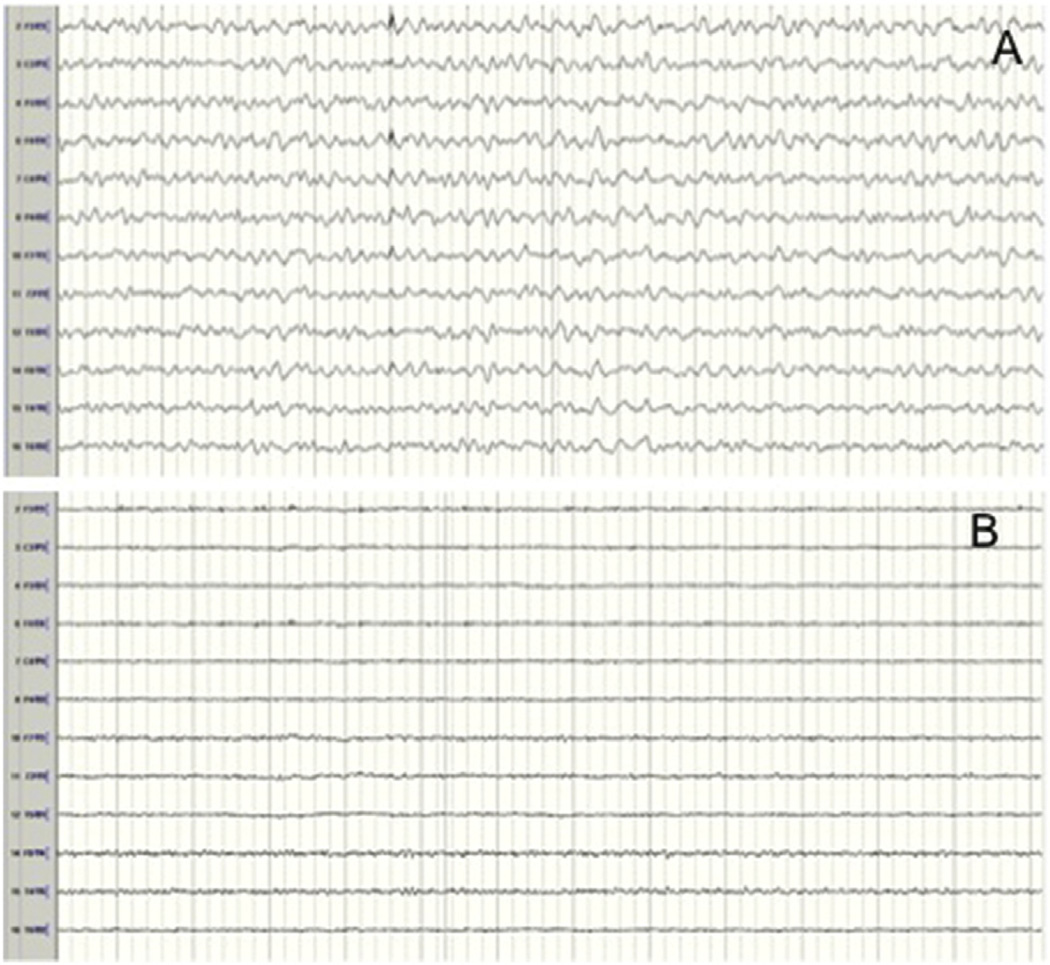
Fig 3.
Persistent global suppression of electrocerebral activity after establishment of selective antegrade cerebral perfusion (SACP). (A) Electroencephalogram (EEG) before shows a continuous pattern with diffuse wave slowing (nasopharyngeal temperature: 27.5°C). (B) EEG after unilateral SACP shows persistent global suppression of EEG activity.
Review of unadjusted baseline characteristics revealed no significant differences between patients who experienced abrupt loss of electrocerebral activity and those who did not (Table 1). With regard to procedural characteristics, more than 90% of cases in the cohort were elective, with a similar breakdown in case status between the groups (Table 2). There were also no statistically significant differences in duration of CA, CPB, SACP, and cooling. Although not statistically significant, there was a trend towards higher nasopharyngeal temperature at the time of arrest in patients who abruptly lost electrocerebral activity upon CA compared with those who did not (27.1°C [interquartile range: 22.2, 27.5] vs 24.5°C [22.1, 27.1], p = 0.14). In addition, there was a trend among patients who lost EEG activity toward a higher proportion having a continuous-wave EEG pattern pre-CA compared with patients who maintained EEG activity (78.1% vs 56.4%, p = 0.08).
Table 1.
Patient Characteristics Subdivided by the Loss or Maintenance of Electroencephalographic Activity Immediately After Circulatory Arrest
| Characteristica | Total (N = 71) |
Loss of EEG Activity (n = 32) |
Maintenance of EEG Activity (n = 39) |
p Value |
|---|---|---|---|---|
| Age, y | 64 (53, 69) | 60 (51, 68) | 64 (54, 73) | 0.24 |
| Male sex | 45 (63) | 20 (63) | 25 (64) | 0.99 |
| White race | 59 (83) | 29 (91) | 30 (77) | 0.20 |
| Body mass index, kg/m2 | 28.5 (25.4, 32.1) | 28.7 (26.6, 32.0) | 28.1 (24.8, 31.9) | 0.54 |
| Hypertension | 54 (76) | 26 (81) | 28 (72) | 0.41 |
| Hyperlipidemia | 46 (64) | 21 (66) | 25 (64) | 0.99 |
| Smoker | 22 (31) | 11 (34) | 11 (28) | 0.61 |
| Diabetes mellitus | 4 (6) | 1 (3) | 3 (8) | 0.62 |
| Coronary artery disease | 17 (24) | 6 (19) | 11 (28) | 0.41 |
| History of stroke/TIA | 4 (6) | 2 (6) | 2 (5) | 0.99 |
| COPD | 10 (14) | 3 (9) | 7 (18) | 0.49 |
| Chronic kidney disease | 5 (7) | 3 (9) | 2 (5) | 0.65 |
| Prior aortic operation | 10 (14) | 2 (6) | 8 (21) | 0.11 |
| Connective tissue disorder | 3 (4) | 1 (3) | 2 (5) | 0.99 |
| Bicuspid aortic valve | 32 (45) | 18 (56) | 14 (36) | 0.10 |
| Type A dissection | 5 (7) | 1 (3) | 4 (10) | 0.37 |
| Acute | 2 (3) | 1 (3) | 1 (3) | 0.99 |
| Chronic | 3 (4) | 0 (0) | 3 (8) | 0.25 |
Continuous data are shown as the median (interquartile range) and categoric data as number (%).
COPD = chronic obstructive pulmonary disease; EEG = electroencephalography; TIA = transient ischemic attack.
Table 2.
Procedural Characteristics Subdivided by the Loss or Maintenance of Electroencephalographic Activity Immediately After Circulatory Arrest
| Characteristica | Total (N = 71) |
Loss of EEG Activity (n = 32) |
Maintenance of EEG Activity (n = 39) |
p Value |
|---|---|---|---|---|
| Redo sternotomy | 11 (15) | 3 (9) | 8 (21) | 0.32 |
| Root replacement | 21 (30) | 10 (31) | 11 (28) | 0.80 |
| Ascending aorta replacement | 51 (72) | 22 (69) | 29 (74) | 0.61 |
| Procedural status | 0.99 | |||
| Elective | 67 (94) | 31 (97) | 36 (92) | |
| Urgent | 3 (4) | 1 (3) | 2 (5) | |
| Emergency | 1 (1) | 0 (0) | 1 (3) | |
| Max aortic diameter, cm | 5.4 (5, 5.9) | 5.5 (5.0, 6.0) | 5.4 (5.1, 5.8) | 0.80 |
| ASA class | 0.85 | |||
| 2 | 20 (28) | 10 (31) | 10 (26) | |
| 3 | 47 (66) | 20 (63) | 27 (69) | |
| 4 | 4 (6) | 2 (6) | 2 (5) | |
| Operative time, min | 309 (265, 341.5) | 306 (262, 342) | 313 (269, 331) | 0.89 |
| Circulatory arrest time, min | 14 (13, 17) | 15 (13, 18) | 14 (12, 16) | 0.15 |
| Cross-clamp time, min | 115 (104, 139.5) | 114 (99, 144) | 117 (108, 135) | 0.80 |
| CPB time, min | 154 (140.5, 177.5) | 151 (138, 183) | 155 (142, 176) | 0.70 |
| SACP time, min | 13 (11, 16) | 14 (11, 17) | 13 (11, 15) | 0.22 |
| Cooling time, min | 54 (47, 65) | 55 (48, 64) | 54 (47, 66) | 0.95 |
| NP temperature at arrest, °C | 25.7 (22.2, 27.5) | 27.2 (22.2, 27.6) | 24.5 (22.1, 27.1) | 0.14 |
| Core temperature at arrest, °C | 27.8 (24.5, 29.0) | 28.0 (24.3, 29.0) | 27.4 (24.7, 29.0) | 0.89 |
| Minimum NP temperature, °C | 20.7 (18.6, 22.7) | 21.3 (19.0, 24.0) | 20.0 (18.6, 21.7) | 0.17 |
| Minimum core temperature, °C | 26.2 (24.0, 28.0) | 27.1 (24,0, 28.0) | 25.8 (24.1, 28.0) | 0.75 |
| Pre-arrest EEG pattern | 0.08 | |||
| Continuous/periodic complexes | 47 (66) | 25 (78) | 22 (56) | |
| Burst suppression/cooling pattern | 24 (34) | 7 (22) | 17 (44) |
Continuous data are shown as the median (interquartile range) and categoric data as number (%).
ASA = American Society of Anesthesiologists; CPB = cardiopulmonary bypass; EEG = electroencephalography; NP = nasopharyngeal; SACP = selective antegrade cerebral perfusion.
In a review of unadjusted clinical outcomes (Table 3), one 30-day death occurred in the group with maintained EEG activity in a patient who had undergone concomitant destination left ventricular assist device placement. After a 3-week hospital course, she died several days after discharge due to power failure of her left ventricular assist device. There were no incidences of postoperative stroke, transient ischemic attack, or permanent mental status change in the cohort. Five patients experienced a transient mental status change postoperatively, with no significant difference in incidence between groups.
Table 3.
Outcomes Subdivided by the Loss or Maintenance of Electroencephalographic Activity Immediately After Circulatory Arrest
| Characteristic | Total (N = 71) No. (%) |
Loss of EEG Activity (n = 32) No. (%) |
Maintenance of EEG Activity (n = 39) No. (%) |
p Value |
|---|---|---|---|---|
| 30-day mortality | 1 (1) | 0 (0) | 1 (2.6) | 0.99 |
| Stroke | 0 (0) | 0 (0) | 0 (0) | NA |
| Transient ischemic attack | 0 (0) | 0 (0) | 0 (0) | NA |
| Mental status change | ||||
| Permanent | 0 (0) | 0 (0) | 0 (0) | NA |
| Transient | 5 (7) | 3 (9) | 2 (5) | 0.65 |
EEG = electroencephalography; NA = not applicable.
Comment
Historically, intraoperative EEG has been used during aortic arch operations with DHCA to identify ECI and thereby ensure maximal cerebral metabolic suppression before initiating CA. However, with an increasing number of centers using MHCA with SACP [19, 26, 27], CA is now routinely being initiated at warmer temperatures with maintained electrocerebral activity. To our knowledge, this is the first study that has characterized EEG during aortic arch operations with MHCA and SACP. We observed that patients cooled to moderate hypothermia had a continuous EEG wave pattern or burst suppression before CA. After CA, 45% of the 71-patient cohort abruptly lost electrocerebral activity, whereas the remainder maintained electrocerebral activity during the short interval between arrest and establishment of SACP. SACP also rapidly restored electrocerebral activity in the large majority of patients who lost activity immediately after CA. However, 2 patients demonstrated persistent loss of activity even after establishment of unilateral SACP, and additional intervention was required to restore electrocerebral activity and ensure neuroprotection.
In 2001 Stecker and colleagues [12] elegantly described how EEG progresses through a predictable series of changes during the process of cooling. First, there is a generalized progressive decrease in the amplitude of continuous EEG waveforms along with the appearance of periodic complexes, followed by the onset of burst suppression before finally reaching ECI. The findings of the present study are consistent with the Stecker study to the extent that a continuous or burst suppression EEG pattern was observed before initiation of CA at moderate degrees of hypothermia. The novel aspects of this study lie in the EEG changes observed after MHCA. The abrupt loss of EEG activity between CA and SACP observed in nearly half the cohort indicates the brain is often transiently at risk of ischemic injury using a MHCA/SACP neuroprotective strategy. Nevertheless, although the small cohort size of this study prevents definitive conclusions on the safety of MHCA/SACP, the clinical outcomes presented here add support to existing literature suggesting that MHCA with SACP is a safe approach for neuroprotection in selected patients who have brief periods of CA, at least with respect to neurologic outcomes, such as stroke, that can be grossly appreciated [14, 18–22]. Importantly, however, there is also increasing recognition that periods of hypothermic CA place patients at risk for loss of higher cognitive functions and other subtler neurocognitive deficits [16, 17]. The transient loss of EEG activity frequently observed with MHCA/SACP raises questions about whether this strategy compared with DHCA places patients at greater risk for neuronal injury and resultant neurocognitive dysfunction, although additional study will be needed to evaluate this possibility.
Perhaps the most alarming finding of this study was that establishment of unilateral SACP did not restore EEG activity in 2 patients who lost activity after CA. There are at least two potential explanations for these occurrences. First, the regional cerebral perfusion provided by unilateral SACP may have been insufficient to meet cerebral metabolic demands. Theoretically, this would be more likely to occur at warmer temperatures [4]. Interestingly, the activity changes refractory to unilateral SACP developed in patients who were on the high end of the spectrum for nasopharyngeal temperature (28.1°C and 27.5°C, respectively) at the time of arrest. Also of note, there was a trend toward higher nasopharyngeal temperature and increased presence of a continuous EEG wave pattern at the time of arrest in patients who lost EEG activity between arrest and SACP compared with those who did not, suggesting that higher metabolic demand may underlie loss of electrocerebral activity upon CA.
A second potential explanation for loss of electrocerebral activity refractory to unilateral SACP is the presence of incomplete communication of the cerebral vasculature. Unfortunately, preoperative or postoperative cerebrovascular imaging was not available for either patient with refractory loss of activity to assess this possibility. However, in the absence of preoperative cerebrovascular imaging, this anecdotal experience suggests that EEG monitoring may be a valuable tool for the intraoperative detection of patients inadequately protected by MHCA/SACP.
Our study has a number of limitations. First, we could only provide characterization of EEG during MHCA/SACP to the level of granularity that could be reliability abstracted from the medical record. A well-designed prospective study could provide information on the precise duration of time between the initiation of unilateral SACP and the return of electrocerebral activity or details on postoperative neurologic examinations and cognitive function. Second, we generally use transcutaneous cerebral oximetry in addition to EEG for cases with HCA. Unfortunately, transcutaneous cerebral oximetry measurements are not accessible in the medical record and therefore could not be correlated to the EEG findings presented. Lastly, it is important to note that this is a selected population of largely elective cases with short periods of CA. Whether similar results would be obtained in nonelective cases or in cases requiring longer periods of arrest is unclear.
In conclusion, the current study suggests that nearly half of patients undergoing aortic hemiarch replacement with MHCA will demonstrate signs on EEG concerning for potential ischemic cerebral injury. Although establishment of unilateral SACP will usually ameliorate these EEG changes, a few patients will experience persistent loss of electrocerebral activity. Further study is needed to determine whether transient loss of electrocerebral activity during MHCA with SACP places patients at risk for adverse neurocognitive outcomes to better delineate the optimal cerebral protection strategy during arch replacement.
Footnotes
Presented at the Poster Session of the Fifty-first Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 24–28, 2015.
References
- 1.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–1063. [PubMed] [Google Scholar]
- 2.Strauch JT, Spielvogel D, Lauten A, et al. Optimal temperature for selective cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:74–82. doi: 10.1016/j.jtcvs.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 3.Mezrow CK, Midulla PS, Sadeghi AM, et al. Evaluation of cerebral metabolism and quantitative electroencephalography after hypothermic circulatory arrest and low-flow cardiopulmonary bypass at different temperatures. J Thorac Cardiovasc Surg. 1994;107:1006–1019. [PubMed] [Google Scholar]
- 4.McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg. 1999;67:1895–1899. doi: 10.1016/s0003-4975(99)00441-5. discussion 1919–21. [DOI] [PubMed] [Google Scholar]
- 5.Khaladj N, Peterss S, Pichlmaier M, et al. The impact of deep and moderate body temperatures on end-organ function during hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2011;40:1492–1499. doi: 10.1016/j.ejcts.2011.03.031. discussion 1499. [DOI] [PubMed] [Google Scholar]
- 6.Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab. 1992;12:877–880. doi: 10.1038/jcbfm.1992.120. [DOI] [PubMed] [Google Scholar]
- 7.Bavaria JE, Pochettino A, Brinster DR, et al. New paradigms and improved results for the surgical treatment of acute type a dissection. Ann Surg. 2001;234:336–342. doi: 10.1097/00000658-200109000-00007. discussion 342–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coselli JS, Crawford ES, Beall AC, Jr, Mizrahi EM, Hess KR, Patel VM. Determination of brain temperatures for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg. 1988;45:638–642. doi: 10.1016/s0003-4975(10)64766-2. [DOI] [PubMed] [Google Scholar]
- 9.Lima B, Williams JB, Bhattacharya SD, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011;77:1438–1444. [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg. 2004;78:1274–1282. doi: 10.1016/j.athoracsur.2004.04.063. discussion 1283–4. [DOI] [PubMed] [Google Scholar]
- 11.James ML, Andersen ND, Swaminathan M, et al. Predictors of electrocerebral inactivity with deep hypothermia. J Thorac Cardiovasc Surg. 2014;147:1002–1007. doi: 10.1016/j.jtcvs.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 13.Livesay JJ, Cooley DA, Reul GJ, et al. Resection of aortic arch aneurysms: a comparison of hypothermic techniques in 60 patients. Ann Thorac Surg. 1983;36:19–28. doi: 10.1016/s0003-4975(10)60643-1. [DOI] [PubMed] [Google Scholar]
- 14.Leshnower BG, Myung RJ, Thourani VH, et al. Hemiarch replacement at 28 degrees C: an analysis of mild and moderate hypothermia in 500 patients. Ann Thorac Surg. 2012;93:1910–1915. doi: 10.1016/j.athoracsur.2012.02.069. discussion 1915–6. [DOI] [PubMed] [Google Scholar]
- 15.Kumral E, Yuksel M, Buket S, Yagdi T, Atay Y, Guzelant A. Neurologic complications after deep hypothermic circulatory arrest: types, predictors, and timing. Tex Heart Inst J. 2001;28:83–88. [PMC free article] [PubMed] [Google Scholar]
- 16.Welz A, Pogarell O, Tatsch K, Schwarz J, Cryssagis K, Reichart B. Surgery of the thoracic aorta using deep hypothermic total circulatory arrest. Are there neurological consequences other than frank cerebral defects? Eur J Cardiothorac Surg. 1997;11:650–656. doi: 10.1016/s1010-7940(96)01129-3. [DOI] [PubMed] [Google Scholar]
- 17.Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg. 1999;117:156–163. doi: 10.1016/s0022-5223(99)70481-2. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg. 2007;133:501–509. doi: 10.1016/j.jtcvs.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg. 2007;31:618–622. doi: 10.1016/j.ejcts.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Urbanski PP, Lenos A, Bougioukakis P, Neophytou I, Zacher M, Diegeler A. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg. 2012;41:185–191. doi: 10.1016/j.ejcts.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski PP. Selective antegrade cerebral perfusion and mild (28 degrees C-30 degrees C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. 2012;144:1042–1049. doi: 10.1016/j.jtcvs.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosyula P, Jassar AS, Menon RS, et al. Moderate versus deep hypothermic circulatory arrest for elective aortic transverse hemiarch reconstruction. Ann Thorac Surg. 2015;99:1511–1517. doi: 10.1016/j.athoracsur.2014.12.067. [DOI] [PubMed] [Google Scholar]
- 23.Englum BR, Andersen ND, Husain AM, Mathew JP, Hughes GC. Degree of hypothermia in aortic arch surgery—optimal temperature for cerebral and spinal protection: deep hypothermia remains the gold standard in the absence of randomized data. Ann Cardiothorac Surg. 2013;2:184–193. doi: 10.3978/j.issn.2225-319X.2013.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Society of Thoracic Surgeons. [Accessed October 26, 2014];Adult cardiac surgery data collection. Available at: http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection. [Google Scholar]
- 25.Ganapathi AM, Hanna JM, Schechter MA, et al. Antegrade versus retrograde cerebral perfusion for hemiarch replacement with deep hypothermic circulatory arrest: does it matter? A propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148:2896–2902. doi: 10.1016/j.jtcvs.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. 1999;67:1874–1878. doi: 10.1016/s0003-4975(99)00411-7. discussion 1891–4. [DOI] [PubMed] [Google Scholar]
- 27.Di Eusanio M, Schepens MA, Morshuis WJ, et al. Brain protection using antegrade selective cerebral perfusion: a multicenter study. Ann Thorac Surg. 2003;76:1181–1188. doi: 10.1016/s0003-4975(03)00824-5. discussion 1188–9. [DOI] [PubMed] [Google Scholar]