Abstract
When studying the pathological mechanisms of epilepsy, there are a seemingly endless number of approaches from the ultrastructural level—receptor expression by EM—to the behavioral level—comorbid depression in behaving animals. Epilepsy is characterized as a disorder of recurrent seizures, which are defined as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain” (Fisher et al., 2005). Such abnormal activity typically does not occur in a single isolated neuron; rather, it results from pathological activity in large groups—or circuits—of neurons. Here we choose to focus on two aspects of aberrant circuits in temporal lobe epilepsy: their organization and potential mechanisms to control these pathological circuits. We also look at two scales: microcircuits, ie, the relationship between individual neurons or small groups of similar neurons, and macrocircuits, ie, the organization of large-scale brain regions. We begin by summarizing the large body of literature that describes the stereotypical anatomical changes in the temporal lobe—ie, the anatomical basis of alterations in microcircuitry. We then offer a brief introduction to graph theory and describe how this type of mathematical analysis, in combination with computational neuroscience techniques and using parameters obtained from experimental data, can be used to postulate how microcircuit alterations may lead to seizures. We then zoom out and look at the changes which are seen over large whole-brain networks in patients and animal models, and finally we look to the future.
Keywords: Circuit reorganization, Neuronal network, Graph theory, Optogenetics, Seizure control
1 ORGANIZATION AND REORGANIZATION OF MICROCIRCUITS: ANATOMICAL CHANGES IN TEMPORAL LOBE EPILEPSY
Temporal lobe epilepsy (TLE) is the most common subtype of epilepsy in human patients (Wiebe, 2000). Unlike many other forms of human epilepsies, TLE results in stereotyped pathological changes that can be examined not only in human tissue but in an array of animal models of this disease (Kandratavicius et al., 2014; Levesque et al., 2015). To understand a network pathology such as epilepsy, a good starting point is to attempt to understand any anatomical microcircuit alterations that may occur between neurons. These anatomical changes have been studied in detail in both humans and animal models of TLE and will be summarized here.
In humans, TLE is most commonly associated with a pathology termed hippocampal sclerosis or Ammon's horn sclerosis which was described in 1880 by Sommer and has been studied with interest ever since (Blüumcke et al., 2002; Jasper and Kershman, 1941; Sano and Malamud, 1953). The most obvious change that occurs with this sclerosis, or scarring, is cell loss. Specifically, there is significant pyramidal cell loss in the hippocampal subfields CA1 and CA3, with lesser amounts of cell loss in CA2 (Fig. 1A and B; De Lanerolle et al., 1989; Steve et al., 2014). In the dentate hilus, many excitatory mossy cells are lost (Blüumcke et al., 2000). Interneurons undergo cell death as well, the specifics of which will be discussed later. Histopathological changes in the granule cell layer of the dentate gyrus range from mild changes such as granule cell layer dispersion to severe changes such as significant cell loss (Blüumcke et al., 2002; De Lanerolle et al., 1989; Thom et al., 2002). Cell death is accompanied by gliosis, specifically proliferation and hypertrophy of astrocytes, which indeed form the substrate of the scar (Binder and Steinhauser, 2006; Blüumcke et al., 1999; Mitchell et al., 1999). This astrocytic hypertrophy and proliferation may indeed play a role in network hyperexcitability, but will not be discussed further in this chapter (Allam et al., 2012; Ullah et al., 2009; Wilcox et al., 2015).
FIG. 1.
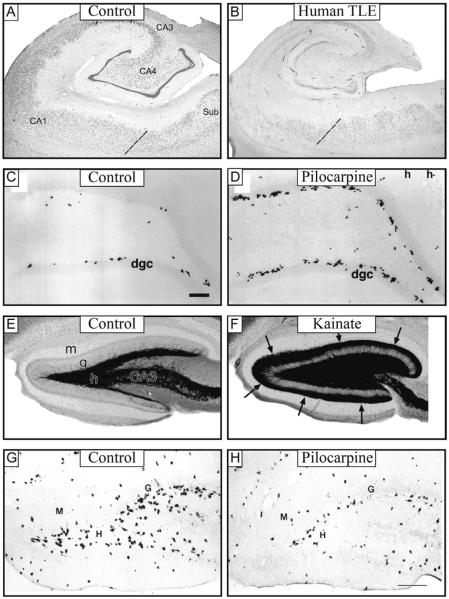
Anatomical changes to the microcircuitry of the hippocampus in temporal lobe epilepsy. (A and B) Nissl-stained sections of the hippocampal formation from an autopsy subject, ie, control (A) and a patient with severe hippocampal sclerosis (B) demonstrating severe cell loss in most cell layers, with relative sparing of CA2, distal CA1, and complete sparing of the subiculum. Dashed lines indicate border between CA1 and subiculum. (C and D) BrdU labeling reveals an increase in the number of newborn neurons at the border of the hilus and granule cell layer after pilocarpine-treated rats (D) compared to controls (C). (E and F) Timm staining, which selectively labels mossy fibers, reveals significant mossy fiber sprouting into the molecular layer in epileptic kainate-treated rats (F, arrows) but not in control animals (E). (G and H) The number of GAD mRNA-containing neurons in the hilus is reduced in pilocarpine-treated (H) when compared to control (G) rats. Sub, subiculum; H, Hilus; dgc, dentate granule cell layer; M, molecular layer; G, granule cell layer.
Panels (A and B): adapted from Andrioli, A., et al., 2007. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience 149 (1), 131–143, (G and H) adapted from Obenaus, A., Esclapez, M., Houser, C.R., 1993. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J. Neurosci. 13 (10), 4470–4485; panels (C and D): adapted from Buckmaster, P.S., Dudek, F.E., 1997. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 77 (5), 2685–2696; and panels (E and F): adapted from Parent, J.M., et al., 1997. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 17 (10), 3727–3738.
Along with cell death comes the birth of new cells. Although the vast majority of neurogenesis occurs in the embryonic brain, it has now been well demonstrated that neurogenesis does occur in a few select areas of the adult brain, including the granule cell layer of dentate gyrus (Kaplan and Hinds, 1977; Toni and Schinder, 2015). Newly born granule cells are functionally incorporated into the hippocampal network and modulate strong inhibition onto mature granule cells (Drew et al., 2016; Van Praag et al., 2002). In animal models of TLE, however, the rate of neurogenesis of granule cells is increased (Fig. 1C and D; Parent and Kron, 2010; Parent et al., 1997). Some of these newborn cells may appear in abnormal locations such as the hilus and the inner molecular layer, or display aberrant morphology such as basal dendrites which project into the hilus (Botterill et al., 2015; Kron et al., 2010; Parent et al., 1997; Spigelman et al., 1998; Walter et al., 2007).
Another anatomical hallmark of TLE is mossy fiber sprouting (Buckmaster, 2012). Mossy fibers, which are the axons of granule cells, normally project to mossy cells and interneurons in the dentate hilus and to dendrites of CA3 pyramidal cells and interneurons in the stratum lucidum. However, in TLE, these axons sprout new processes which create aberrant granule cell to granule cell connections (Fig. 1E and F). Early descriptions of mossy fiber were made human TLE specimens (Scheibel et al., 1974). Subsequently, this phenomenon has been described in many animal models of TLE (reviewed in detail in Buckmaster, 2012). Adult granule cells sprout most commonly to the inner molecular layer of the dentate gyrus to synapse upon other granule cells. They also form new synapses onto granule cells in the granule cell layer, ectopic granule cells in hilus, aberrant granule cell basal dendrites in the hilus, hilar cells, and pyramidal cells of CA2 and CA3 (Haussler et al., 2016; Parent et al., 1997). Finally, connections between sprouted mossy fibers and interneurons have been observed, but the functional consequence of this finding is controversial (Buckmaster, 2012; Wenzel et al., 2000). Electrophysiological evidence has showed that these sprouted mossy fibers form functional synapses onto other granule cells, leading to an aberrant, recurrent excitatory circuit which at least theoretically could lead to runaway excitation and seizure-like activity (Scharfman et al., 2003). Interestingly, mossy fiber sprouting is so robust that fiber-tracking MRI sequences can be used to detect this abnormality in surgically resected human hippocampi (Modo et al., 2016). GABAergic cells have also been shown to sprout axons in human TLE and pilocarpine-treated mice (Buckmaster and Wen, 2011; De Lanerolle et al., 2010; Mathern et al., 1995; Zhang et al., 2009).
Abnormally sprouted mossy fibers are not the only recurrent excitatory fibers to play a role in the microcircuit of TLE. Pyramidal cells in CA3 are well known to exhibit recurrent excitatory to excitatory connections with each other, even in control situations (Le Duigou et al., 2014), although the frequency of such connections is relatively low (estimated to be 2–3% from paired recordings). Additionally, CA3 pyramidal cell axons have also been demonstrated to sprout significantly in epileptic rats, forming denser recurrent CA3-to-CA3 excitatory connections and more extensive Schaffer collateral projections to CA1 (Siddiqui and Joseph, 2005). In contrast to CA3, the CA1 pyramidal cell axons do not directly project to other CA1 pyramidal cells under control conditions. However, after pilocarpine injections, significant CA1 pyramidal cell to pyramidal cell connections were found via tracing with Fluoro-Gold (Long et al., 2014). Finally, mossy cells, excitatory neurons of the hilus, provide excitatory connections to dentate granule cells. These cells are famous for their selective vulnerability to cell death in TLE (Blüumcke et al., 2000; Ratzliff et al., 2002). However, whether their loss contributes to hyperexcitability is still unclear, with one line of evidence supporting the idea that it is the surviving mossy cells that play a role in hyperexcitability due to altered intrinsic and synaptic physiologic properties (Howard et al., 2007; Ratzliff, 2004).
Cell death in TLE is not limited to excitatory neurons: GABAergic neurons are also vulnerable to excitotoxicity (Fig. 1G and H). There are reduced numbers of cells expressing glutamic acid decarboxylase mRNA in epileptic animals (Dinocourt et al., 2003; Obenaus et al., 1993). Unlike principal cells which are defined mainly by their location, characterizing the different subtypes of interneurons is an arduous task. The classification of interneurons rests on axonal innervation patterns of the subcellular domains of postsynaptic cells (eg, basket cells innervate the perisomatic region), as well as on other properties such as location, protein or neuropeptide expression, intrinsic physiology, distribution of axonal arborization, and others (Freund and Buzsáki, 1996; Klausberger et al., 2003; Somogyi and Klausberger, 2005). The loss and alteration of function of hippocampal interneurons in epilepsy has been reviewed elsewhere (Liu et al., 2014). However, as we are focusing here on anatomical changes to the microcircuit, we have summarized the work on interneuronal cell loss, by subtype, in various models of TLE and present it in Table 1.
Table 1.
A Summary of Interneuronal Subtypes Which Are Known to Be Lost or Reduced in Human TLE or Various Animal Models of TLE
| Cell Type: Marker Expression | Hippocampal Subfield | Cell Type | Epilepsy Model | Reference |
|---|---|---|---|---|
| Calbindin | CA1 | Not described | Mouse kainate | Bouilleret et al. (2000) |
| Rat pilocarpine | André et al. (2001) | |||
| Dentate gyrus | Not described | Mouse kainate | Bouilleret et al. (2000) | |
| Calretinin | CA1 | Not described | Rat pilocarpine | André et al. (2001) |
| CA3 | Not described | Human TLE | Toth et al. (2010) | |
| Rat kainate | Magloczky and Freund (1993) | |||
| Rat pilocarpine | André et al. (2001) | |||
| Dentate gyrus | Not described | Human TLE | Toth et al. (2010) | |
| Rat kainate | Magloczky and Freund (1993) | |||
| Rat pilocarpine | André et al. (2001) | |||
| Cholecystokinin | CA1 | Basket cells | Mouse pilocarpine | Wyeth et al. (2010) |
| Dentate gyrus | Presumed basket cells | Rat kindling | Sayin et al. (2003) | |
| GAT-1 | Dentate gyrus | Presumed axo-axonic cells | Rat kindling | Sayin et al. (2003) |
| Neuropeptide Y | CA1 | Not described | Kainate-induced acute seizures | Kuruba et al. (2011) |
| Mouse kainate | Marx et al. (2013) | |||
| Ivy cells | Rat pilocarpine | Orban-Kis et al. (2015) | ||
| CA3 | Not described | Kainate-induced acute seizures | Kuruba et al. (2011) | |
| Dentate gyrus | Not described | Epilepsy caused by electrically induced status in rats | Huusko et al. (2015) | |
| Sun et al. (2007) | ||||
| Human TLE | De Lanerolle et al. (1989) | |||
| Mathern et al. (1995) | ||||
| Sundstrom et al. (2001) | ||||
| Kainate-induced acute seizures | Kuruba et al. (2011) | |||
| Rat pilocarpine | Long et al. (2011) | |||
| Not basket cells | Rat kainate | Sperk et al. (1992) | ||
| Parvalbumin | CA1 | Axo-axonic cells | Rat pilocarpine | Dinocourt et al. (2003) |
| Basket cells | Epilepsy caused by electrically induced status in rats | Huusko et al. (2015) | ||
| Not described | Human TLE | Andrioli et al. (2007) | ||
| Kainate-induced acute seizures | Kuruba et al. (2011) | |||
| Mouse kainate | Bouilleret et al. (2000) | |||
| Marx et al. (2013) | ||||
| Rat pilocarpine | André et al. (2001) | |||
| CA2 | Not described | Human TLE | Andrioli et al. (2007) | |
| CA3 | Basket cells | Epilepsy caused by electrically induced status in rats | Huusko et al. (2015) | |
| Not described | Human TLE | Andrioli et al. (2007) | ||
| Kainate-induced acute seizures | Kuruba et al. (2011) | |||
| Mouse kainate | Marx et al. (2013) | |||
| Dentate gyrus | Basket cells | Epilepsy caused by electrically induced status in rats | Huusko et al. (2015) | |
| Axo-axonic cells | Epilepsy caused by electrically induced status in rats | Gorter et al. (2001) | ||
| Not described | Human TLE | Andrioli et al. (2007) | ||
| Sloviter et al. (1991) | ||||
| Wittner et al. (2001) | ||||
| Kainate-induced acute seizures | Kuruba et al. (2011) | |||
| Mouse kainate | Bouilleret et al. (2000) | |||
| Marx et al. (2013) | ||||
| Rat pilocarpine | André et al. (2001) | |||
| Kobayashi and Buckmaster (2003) | ||||
| Long et al. (2011) | ||||
| Parvalbumin+somatostatin | CA1 | O-LM cells | Rat pilocarpine | Orban-Kis et al. (2015) |
| Somatostatin | CA1 | Not described | Rat pilocarpine | Long et al. (2011) |
| O-LM cells | Dinocourt et al. (2003) | |||
| CA3 | Not described | Rat pilocarpine | Long et al. (2011) | |
| Dentate gyrus | Not described | Epilepsy caused by electrically induced status in rats | Gorter et al. (2001) | |
| Sun et al. (2007) | ||||
| Human TLE | De Lanerolle et al. (1989) | |||
| Mathern et al. (1995) | ||||
| Sundstrom et al. (2001) | ||||
| Mouse kainate | Buckmaster and Dudek (1997) | |||
| Rat kainate | Buckmaster and Jongen-Rêlo (1999) | |||
| Magloczky and Freund (1993) | ||||
| Sperk et al. (1992) | ||||
| Rat pilocarpine | Kobayashi and Buckmaster (2003) | |||
| Long et al. (2011) |
As evidenced by the volume of studies included in Table 1, a great deal of research has been done regarding the (usually partial) demise of interneurons in TLE. It should therefore be noted that of the interneurons lost in the dentate gyrus, the vast majority are hilar interneurons (Buckmaster and Jongen-Rêlo, 1999). A wrinkle in the interpretation of these studies is that cells are often identified by staining for cellular markers, and the loss of marker-stained cells could be subsequent to changed expression of that marker rather than cell death. This was postulated when the loss of parvalbumin-expressing cells, which constitute somatically projecting basket cells, axon initial segment-projecting chandelier cells, and dendritically innervating bistratified cells, was not accompanied by a loss of GABAergic synapses onto the somata or axon initial segments of dentate granule cells in human TLE specimens, and putative basket cell contacts were shown to be preserved in the dentate gyrus of rat pilocarpine model (Obenaus et al., 1993; Wittner et al., 2001). A further study of parvalbumin immunoreactivity in human TLE specimens, performed at the light and electron microscopic levels, revealed that parvalbumin-containing interneurons in CA1, most of which are basket cells and axo-axonic cells, are preserved as long as the CA1 pyramidal cells (their targets) do not degenerate. This study suggested that interneurons which initially express parvalbumin may survive even if parvalbumin immunoreactivity is lost. Additionally, despite many reports of parvalbumin cell loss, in the mouse pilocarpine model, cholecystokinin-expressing basket cells were lost, but parvalbumin-expressing basket cells were specifically conserved (Wyeth et al., 2010). Therefore, the specifics of which interneurons are lost, which are merely changed, and the functional consequences thereof are complicated questions which still remain to be fully answered.
Although TLE is predominantly a pathology of the hippocampus, other mesial temporal structures such as the entorhinal cortex and the amygdala have been implicated in humans and animal models. Recently, recurrent excitatory connections have been demonstrated between principal cells in layer II of the medial entorhinal cortex after kainate-induced seizures (Armstrong et al., 2015). Cell loss in layer III of entorhinal cortex has also been demonstrated (Siddiqui and Joseph, 2005). The amygdala, another adjacent limbic structure, which is often removed at surgery for TLE in humans, has also been implicated with anatomical changes, as it undergoes cell loss as well (Blümcke et al., 2002). Exploring details of microcircuitry in these extrahippocampal regions of the temporal lobe may prove to be fruitful in the future.
In addition to the anatomical changes in TLE described earlier, there are some features of normal hippocampal microcircuitry that predispose this region to seizure susceptibility. For example, recurrent CA3-to-CA3 pyramidal cell connections create a network which can generate bursts and high-frequency oscillations, which have both been correlated with hyperexcitability (reviewed in detail in Le Duigou et al., 2014). More precisely, it has been shown that pyramidal cells in the CA3a subregion (ie, closer to CA1) have more recurrent synapses and play a role as “pacemakers” in the hippocampus (Wittner and Miles, 2007). Recently, Cajal–Retzius cells, an excitatory neuronal subtype believed to be common in early development but degenerate before adulthood, have shown to be functional and present in the adult hippocampus and may even be increased in number in epilepsy (Anstotz et al., 2016; Quattrocolo and Maccaferri, 2014; Thom et al., 2002). While all of the targets of these intriguing cells have not been elucidated, it is quite plausible that they contact other excitatory cells and form an excitatory loop, which could contribute to hippocampal seizure susceptibility. Finally, recent studies have shown that there are distinct subpopulations of CA1 pyramidal cells that form precise and discrete local microcircuits with interneurons and have different susceptibility to modulatory signals such as endocannabinoids (Lee et al., 2014; Maroso et al., 2016).
We have limited our discussion here to anatomical changes seen in TLE. The organization, structure, and function of microcircuits can also be affected by many other alterations that are known to occur in the hippocampus in TLE, including but not limited to changes in neurotransmitter receptor signaling (ie, changes in the expression of glutamate or GABA receptors), changes in modulatory signaling (ie, cannabinoids, neurosteroids), a wide variety of changes in synaptic physiology, channelopathies, the role of different types of interneurons in normal and pathological oscillations, and changes in the ultrastructure of synapses (Dalby and Mody, 2001; Ferando and Mody, 2012; Grabenstatter et al., 2012; Houser, 2014; Mathern et al., 1998; Soltesz et al., 2015; Wolfart and Laker, 2015). These types of changes have been well documented but are beyond the scope of this review.
2 FROM ORGANIZATION TO CONTROL OF NEURONAL CIRCUITS: INTRODUCTION TO GRAPH THEORY
Before we discuss how the above anatomical changes might lead to hyperexcitability or seizures, we pause for a brief background on graph theory. Pioneered by Euler in the 18th century, it has gained a recent resurgence in popularity, in large part due to the important work of Watts and Strogatz who demonstrated its usefulness in describing systems as diverse as neural networks of Caenorhabditis elegans, power grids, and the interconnectedness of film actors (Watts and Strogatz, 1998).
Graph theory uses the concepts of nodes and edges to represent elements in a network and the connections among them. This mathematical analysis is perfectly suited for the study of one of the most complex biological systems in existence, the mammalian brain. The application of graph theory to neural systems is intuitive: on the microscale, nodes represent neurons and edges represent synapses; on a larger scale, nodes represent brain regions and edges represent white matter tracts. This type of analysis, along with computational modeling techniques, can be applied to systematically examine individual alterations observed experimentally in epilepsy.
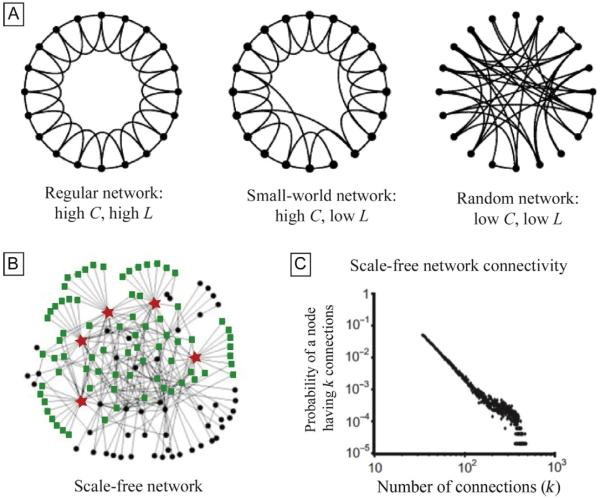
One of the early results of graph theoretical analysis of neural systems was that many neuronal networks appear to display properties typical of the so-called small-world networks. The simplest way to describe the small-world network is through the use of two main parameters: the clustering coefficient C (representing the density of local connections) and the path length L (the average distance between two connected cells) (Watts and Strogatz, 1998). The small-world network is characterized by a high C, due to the high number of local interactions, and a small L, indicating a short average path length between two connected neurons (Fig. 2A). A network with small-world organization can better support high levels of local and global synchrony seen in real neuronal networks, yet it requires many long-range connections.
FIG. 2.
Introduction to graph theory. (A) Illustration of the progression from a regular network to a small-world network to a random network. A regular network has a high number of local connections and no long-distance connections, resulting in a high clustering coefficient (C) and a high path length (L). By replacing a few local connections with long-distance connections, the overall shortest path length (L) is dropped significantly, and yet a high C remains, creating a small-world network. In a random network, the small probability of local connections and the low number of randomly created long distance connections result in low C and low D. (B) A graphical representation of a scale-free network demonstrates a small number of highly connected nodes or hubs, represented by stars. These five hubs make primary connections with 60% of the rest of the nodes in the graph (rectangles). This is in contrast to an exponential network (not shown), in which the five most highly connected nodes only primarily contact 27% of all nodes in the graph. (C) A log–log graph demonstrates the power law relationship between the probability of a node having k connections (y-axis) and the number of connections in the graph (x-axis).
Panel (A): adapted from Watts, D.J., Strogatz, S.H., 1998. Collective dynamics of “small-world” networks. Nature 393 (6684), 440–442; panel (B): adapted from Albert, R., Jeong, H., Barabasi, A.L., 2000. Error and attack tolerance of complex networks. Nature 406, 378–382; and panel (C): adapted from Morgan, R.J., Soltesz, I., 2008. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc. Natl. Acad. Sci. U.S.A. 105 (16), 6179–6184.
Subsequently, a related, important concept in network organization was introduced, the scale-free network (Albert et al., 2000; Barabasi and Albert, 1999). In scale-free networks, nodes on a graph have highly differing degrees of connectivity which are distributed according to a power law: a few nodes (which are called hubs) possess a high degree of connectivity, whereas the majority of nodes are only sparsely connected to others (Fig. 2B and C). The origin of the scale-free nature of a network lies in two important characteristics, both relevant to neuronal networks as they develop embryologically. The first is that these networks can grow: ie, they can start with a few nodes, and then new nodes are added. The second is that when new nodes are added, they are more likely to be connected to highly connected nodes (referred to as the “rich gets richer” rule). In neuronal networks, the presence of hub cells has been shown to increase firing (Grinstein and Linsker, 2005; Morgan and Soltesz, 2008). Additionally, scale-free networks exhibit a high degree of efficiency required for neuronal networks (Buzsáki et al., 2004) and also show a high degree of robustness, limiting the vulnerability of the network when some of the nodes are damaged (Albert et al., 2000). It is likely that real neuronal networks exhibit some properties of both scale-free and small-world networks, as they are not mutually exclusive and both exhibit high C and low L (Dorogovtsev et al., 2002; Lin and Zhang, 2014). Indeed, the degree of small worldness expressed by a scale-free network may affect the amount of neuronal synchrony (Massobrio et al., 2015).
3 BEGINNING TO CONTROL MICROCIRCUITS: USING GRAPH THEORY TO CONTROL CIRCUITS IN SILICO
We will now focus our attention on studies that use computational techniques to apply graph theory as a technique in understanding how experimentally demonstrated changes in microcircuitry contribute to network hyperexcitability. TLE development is most often characterized by three different stages: (1) an initial precipitating event, (2) a period of epileptogenesis, and (3) recurrent spontaneous seizures. Most of the anatomical and physiological changes occur during the period of epileptogenesis. One issue with attempting to interpret experimental results is that in TLE, a plethora of changes occur simultaneously during epileptogenesis. Therefore, it is difficult to prove which alterations may be epileptogenic, which may be compensatory, and which may in fact be protective against seizures. Computational modeling, based fundamentally on graph theory, offers a potential solution to this as each variable can be tested individually. Once crucial epileptogenic changes are identified, variables of the in silico models can then be adjusted to control the circuit and bring it back to a healthy state.
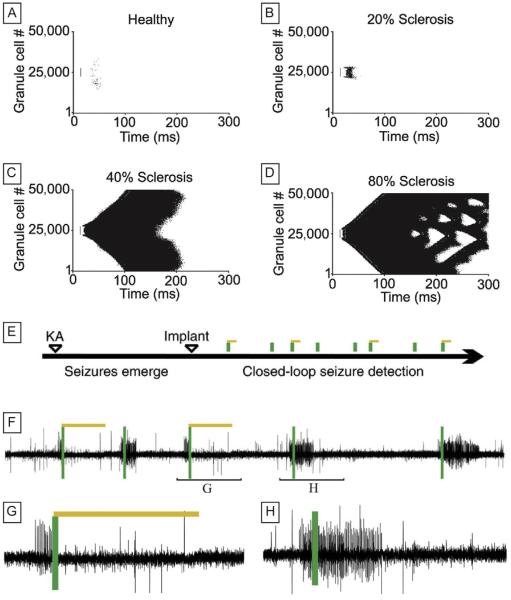
The dentate gyrus is an area which undergoes drastic alterations in its microcircuitry (reviewed earlier, but also see Tejada and Roque, 2014). Mossy fiber sprouting and hilar cell loss are the two most characteristic hallmarks of TLE in the dentate, and yet there has been great controversy regarding the functional significance of each (Bernard et al., 1998; Buckmaster, 2012; Ratzliff et al., 2002; Sloviter, 1991). Therefore, a model of the dentate gyrus was created to determine whether sprouting and cell loss could affect network excitability (Santhakumar et al., 2005). This biophysically realistic model demonstrated that the dentate gyrus shows a small-world organization and that gradually increasing neuronal cell loss and mossy fiber sprouting led to an increase in small worldness and, therefore, an increase in network excitability. The initial model consisted of 500 neurons, and a later study expanded upon this work to create a network of 50,000 realistic cells as well as a structural model of 1,000,000 cells (Dyhrfjeld-Johnsen et al., 2007). These studies demonstrated that the survival of only a small fraction (20%) of hilar cells was able to sustain network hyperexcitability, and that mossy fiber sprouting played a crucial role in this hyperexcitability. In both the 500-cell and 50,000-cell biophysically realistic models, minimal mossy fiber sprouting resulted in spread of seizure-like events and boosted the network excitability, and increasing levels of mossy fiber sprouting and hilar cell loss contributed to further pathological activity (Fig. 3; Dyhrfjeld-Johnsen et al., 2007; Santhakumar et al., 2005). Other studies have likewise presented similar findings that mossy fiber sprouting and hilar cell loss are correlated with seizure frequency (Howard et al., 2007; Lytton et al., 1998). In addition, such studies have shown that a combination of sodium channel mutations (also known to occur in TLE) and mossy fiber sprouting leads to even higher levels of network excitability (Thomas et al., 2010), and that structural alterations to the dendritic tree known to occur in granule cells actually reduce their excitability and thus are protective against mossy fiber sprouting-induced hyperexcitability (Tejada et al., 2012). Interestingly, pharmacological blockade of mossy fiber sprouting reportedly does not prevent epilepsy, emphasizing the multifactorial nature of seizure generation (Buckmaster and Lew, 2011; Heng et al., 2013).
FIG. 3.
Control of the microcircuit: computational and experimental examples. (A–D) Effects of the sclerosis-related topological changes on granule cell activity in a functional model network. Raster plots of the first 300 ms of action potential discharges of granule cells in a large-scale functional model network at increasing degrees of sclerosis (ie, hilar cell loss and mossy fiber sprouting). The most pronounced hyperactivity was observed at submaximal (80%) sclerosis. Small vertical line indicates time of simulated stimulation and which granule cells were stimulated. (E–H) Closed-loop optogenetic control of temporal lobe seizures in epileptic mice. (E) Experimental timeline. KA: unilateral hippocampal injection of kainic acid. Implant: optrode implantation. (F–H) Example electrographic seizures. Green (gray in the print version) bars represent a detected seizure, and yellow (light gray in the print version) bars represent the closed-loop activation of amber light (589 nm) in order to activate halorhodopsin in principal cells (note that, after detection of a seizure, the light was either switched on or it was not switched on in a random fashion, with the latter serving as internal control). Also note that in G, when light is activated, the seizure is terminated, but that in H, without amber light, the seizure continues.
Panels (A–D): adapted from Dyhrfjeld-Johnsen, J., et al., 2007. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J. Neurophysiol. 97 (2), 1566–1587 and panels (E–H): adapted from Krook-Magnuson, E., et al., 2013. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 4, 1376.
As the exact microcircuitry pattern of mossy fiber sprouting and incorporation of newborn granule cells is unknown, logic would dictate that the pattern of new connectivity should be considered carefully when testing the effects of such changes on network activity. Given the great interest in scale-free networks and the role that hubs play in this type of system, hub neurons were incorporated into the network and its effect on hyperexcitability was studied. The addition of just a few highly connected hubs led to powerful increases in network excitability, emphasizing the importance of examining precise details of network microcircuitry (Morgan and Soltesz, 2008). They hypothesized that granule cells with aberrant basal dendrites might be the underlying biological substrate for hub cells. Since that time, real hub neurons have been demonstrated in developing hippocampal brain slices, providing the first experimental evidence for hub cells and supporting the theory that the hippocampus may be organized into a scale-free network (Bonifazi et al., 2009). The authors were able to selectively stimulate either weakly connected neurons or hub neurons and measure network activity with calcium imaging. As predicted, only hub cell activation resulted in large-scale network synchronization, emphasizing the functional importance of these hubs. Mathematical modeling of scale-free networks has shown that hub cells arise early during the growth of a network, and indeed genetic studies have shown that a small group of cells born early in the embryonic stage survive into adulthood and exhibit high levels of connectivity (Marissal et al., 2012; Picardo et al., 2011). Removing hub cells experimentally therefore represents a future possibility for exerting control on an excitable network.
A combination of experimental and computational techniques can also be used to analyze the importance of specific microcircuits. Two-photon calcium imaging was used to record activity from many individual neurons at once in hippocampal slices from either control or pilocarpine-treated epileptic mice, and synchronous network events were detected, at much greater frequency in the epileptic slices (Feldt Muldoon et al., 2013). Surprisingly, the activity of individual neurons in the slice was not always correlated to the network activity. Therefore, a clustering analysis derived from the graph theory was used to detect groups of neurons which exhibit temporally correlated activity. It was determined that the neuronal clusters in epileptic slices are much more spatially localized than the control slices, despite the fact that the synchronous network events seen in both groups of slices appear similar. An important conclusion of this study is that in epileptic networks events that appear similar at macroscopic (eg, EEG) level are actually highly variable at a microscopic level. Moreover, this work demonstrates that it is possible to distinguish between physiological and epileptic circuits using a computational approach, emphasizing the value of computational neuroscience in studying brain networks.
A different approach to using computational modeling to investigate mechanisms of hyperexcitability relies on starting with healthy neuronal networks and then controlling various circuit parameters until hyperexcitability occurs. In a two-dimensional simplified, 1000-neuron model that was created to adhere to small-world network characteristics, the addition of new “granule cells” can be performed while still maintaining a small-world network. If, however, the initial network is more random, the addition of granule cells leads to synchronous bursting and high firing rates (Schneider-Mizell et al., 2010). Another study generated small-world networks resembling either CA1 or CA3 networks as seen in a hippocampal slice. Changing various network parameters, such as synaptic strength and network connectivity, allowed the authors to determine how the network moved from a “normal” network to a bursing or seizing network (Netoff et al., 2004). The networks based on CA3 connectivity were more likely to burst than the networks based on CA1, as expected from the known properties of these hippocampal subfields in vitro.
Overall computational studies showing that neuronal networks behave similarly to other large-scale networks suggest that the connectivity of the brain is built to guarantee the most energetically efficient information flow between different areas. The presence of hub cells also suggests that alterations occurring in these few highly connected neurons (or indeed the addition of new hub cells in epileptic states) may have critical consequences in the network dynamics resulting in the development of neurological disorders. The relationship between experimental data and computational modeling is a two-way street: experimental findings form the basic building blocks of computational modeling, which in turn can lead to a greater understanding of experimental data. Computational models can easily be manipulated and individual parameters controlled, giving hints as to what changes can eliminate or reduce hyperexcitability. These answers will then drive future experiments, and eventually future treatments as well.
4 FURTHER CONTROL OF MICROCIRCUITS: CAN WE LEARN TO CONTROL A PATHOLOGICAL CIRCUIT IN ORDER TO TREAT EPILEPSY?
Ultimately, the goal of all research into epileptic circuits is to understand this pathology in order to develop better treatments for patients with TLE. Once details of epileptic circuits are known at small and large scales, controlling the circuit may become possible. There are a wide variety of techniques other than anatomy or electrophysiology which may be useful for continuing to helping to map out these circuits including an innovative type of high-resolution microscopy called STORM (Dani et al., 2010; Dudok et al., 2015), calcium imaging (Feldt Muldoon et al., 2013; Lillis et al., 2015), voltage-sensitive dyes (Takano and Coulter, 2012), multielectrode arrays, and optogenetic techniques (Bui et al., 2015). Other techniques which may allow control of pathologically hyperexcitable networks either in animal models or patients include cell transplant (Henderson et al., 2014), designer receptors exclusively activated by designer drugs, aka DREADDs (reviewed in Krook-Magnuson and Soltesz, 2015), responsive neurostimulation, and deep brain stimulation (Bui et al., 2015).
Although controlling epileptic circuits may seem like a futuristic concept, the advent of optogenetics has placed this possibility within reach. This technique relies on the expression of light-induced opsins that can either depolarize (to activate) or hyperpolarize (to silence) neurons in response to light. Genetic or viral targeting of opsin expression allows selective targeting of specific neuronal subpopulations which can then be modulated on a millisecond timescale (reviewed in Bernstein and Boyden, 2011; Krook-Magnuson and Soltesz, 2015; Yizhar et al., 2011). In 2009, a proof-of-concept study was published to demonstrate that epileptiform events in the hippocampus could be controlled with optogenetic manipulation in vitro. Optical silencing of CA1 or CA3 pyramidal cells, accomplished by activating an inhibitory opsin, was able to suppress epileptiform bursting in slices (Tønnesen et al., 2009). Since then, there have been great technological efforts to harness the power of optogenetics in order to detect and stop seizures in vivo. In this regard, a recent study used a mouse kainate model of TLE, in which the convulsant was injected unilaterally, to test the effect of on-demand optogenetics on spontaneous seizure activity in chronic TLE (Krook-Magnuson et al., 2013). The authors developed a novel, closed-loop system which allowed temporally precise opsin activation only when seizure detection had occurred. They first expressed an inhibitory opsin (halorhodopsin) in excitatory principal cells in the CA1 area of the hippocampus which, upon turning on the laser, led to hyperpolarization and effective silencing of CA1 pyramidal cells, successfully terminating the seizures. As a complementary approach, they expressed the excitatory opsin channelrhodopsin in parvalbumin-expressing interneurons. Parvalbumin-expressing interneurons, although they represent less than 3% of the neuronal population in CA1 (Bezaire and Soltesz, 2013), were chosen as a target because they give rise to powerful inhibition onto CA1 pyramidal cell somata and axon initial segments. On-demand channelrhodopsin-induced activation of parvalbumin-expressing interneurons was also able to halt seizure activity in the epileptic mice. Surprisingly, seizure control was even obtained when the light was targeted to the hippocampus contralateral to the site of kainate injection, suggesting that seizures can be controlled far from the site of insult. Therefore, a follow-up study was performed which demonstrated that temporal lobe seizures could be terminated by light-mediated modulation of a very distant target: the cerebellum. Moreover, by specifically targeting the vermis of the cerebellum, they were also able to decrease both seizure duration and seizure frequency (Krook-Magnuson et al., 2014). Although this chapter is focused on TLE, it is worth noting that closed-loop optogenetic control of seizures has been successfully implemented in other seizure models as well. Cortical stroke-induced chronic seizures can be stopped by optogenetic silencing of thalamocortical neurons, and tetanus toxin-induced neocortical seizures can be controlled by on-demand silencing of pyramidal cells (Paz et al., 2012; Wykes et al., 2012).
Another method that has been used to optogenetically control seizures lies in restoring lost safety mechanisms that exist in the healthy hippocampus. The “dentate gate” theory postulates that the dentate gyrus, which receives much of the incoming neural information to the hippocampus, normally acts as a checkpoint and can shut down incoming excitability. However, in epilepsy, this gate breaks down and allows pathological epileptiform activity to pass through to the rest of the hippocampus (Heinemann et al., 1992; Lothman et al., 1992). A recent in vivo study has not only lent support to this theory but has demonstrated that the restoration of the dentate gate through optogenetic hyperpolarization of granule cells stops spontaneous seizures in epileptic mice (Krook-Magnuson et al., 2015). Taken together, these optogenetic studies prove that inhibition or excitation of small but specific populations of cells can control seizures in vivo in animal models. Moreover, the effect of modulation of distant areas on temporal lobe seizures may indicate the presence of target circuits that could be exploited to obtain seizure suppression in TLE.
5 NETWORK ORGANIZATION AT THE MACROCIRCUIT LEVEL: APPLICATIONS OF GRAPH THEORY AT A LARGER SCALE
Although most of the pathological changes in TLE occur in the hippocampus and adjacent structures, this disease affects extratemporal structures as well, as evidenced by the facts that many patients with TLE do not have a complete remission of their seizures after TLE and also that many patients with TLE suffer from psychiatric comorbidities (Kandratavicius et al., 2014; Wiebe and England, 2001). Therefore, graph theory analysis has been proposed as a way to understand the changing circuitry of the entire brain that occurs in human epilepsy, with dual goals of understanding the pathophysiology of this disease and of potentially using graph theory to help localize seizure foci and guide clinical treatment (Haneef and Chiang, 2014).
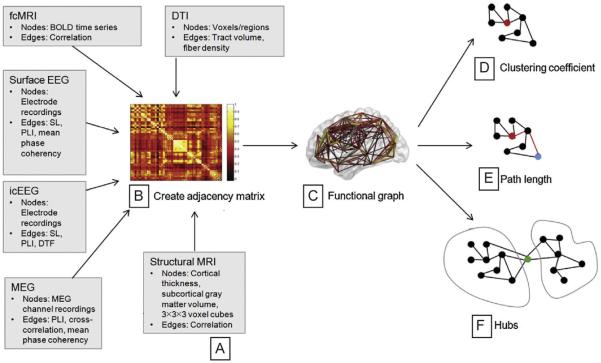
When applying graph theory to the analysis of microcircuits in TLE, it is clear what to use as representation for nodes and edges: neurons and their synaptic connections, which can be quantified with histological and electrophysiological techniques. When zooming out to a larger picture, mapping the whole brain (or the whole temporal lobe) onto a graph is less intuitive, especially in terms of demonstrating and quantifying connections between nodes. Therefore, it is not surprising that a wide variety of methods exist to solve this problem (Fig. 4). Advantages to these techniques include that they can be used to look at the human brain in entirety, that imaging-based techniques and some recording techniques are noninvasive, and that human TLE subjects can be studied without the need to rely on animal models. Using various MRI sequences, nodes can be defined based on known brain areas, and edges are then measured either using diffusion tensor imaging (DTI) to trace axonal connections or using wavelet transforms of functional MRIs to establish activity correlation (Bullmore and Sporns, 2009; Haneef and Chiang, 2014). Correlations of cortical thickness, which can be measured precisely with surface electrodes or MRI, have also been shown as a way to establish connectivity between brain regions, based on the theory that anatomical changes will result from changes in activity (Lerch et al., 2006). Noninvasive EEG can be analyzed with each electrode as a node and the synchronization between leads as the edges. In patients undergoing presurgical evaluation, detailed intracranial recordings are often made using either surface electrodes or depth electrodes (stereo-EEG). Stereo-EEG is a safe and effective technique that is useful for mapping graph structure in TLE given that the hippocampus and amygdala are not located at the surface of the brain (Gonzalez-Martinez et al., 2013). Finally, magnetoencephalography (MEG) has been used successfully to map the global network structure of the human brain (Bartolomei et al., 2006).
FIG. 4.
Graph theoretical analysis of macrocircuits. (A) Various imaging and recording techniques can be used to define the nodes and edges of a large-scale graph. (B and C) An adjacency matrix is created from these nodes and edges, and then converted into a functional graph. (D–F) This graph is then analyzed for small-world characteristics such as clustering coefficient (D) and path length (E), and then for the presence and characteristics of network hubs (F) which would determine the scale-free nature of the network.
Adapted from Haneef, Z., Chiang, S., 2014. Clinical correlates of graph theory findings in temporal lobe epilepsy. Seizure 23 (10), 809–818.
Just as early computational studies of hippocampal microcircuits focused on determining the graph structure of healthy brain networks, early studies of the macrocircuitry of whole human brains were also performed to determine whether this complex system adheres to the principles of mathematically described systems such as small-world or scale-free networks. Wavelet transform correlation analysis performed on fMRI of whole human brain in the awake, resting state, reveals that overall the brain appears to have a small-world, but not scale-free organization (Achard et al., 2006). In this study, as in many whole-brain studies, the nodes are defined as large brain areas such as hippocampus, thalamus, or prefrontal cortex. Many other studies, based on cortical thickness MEG, EEG, MRI, and functional MRI, have also suggested that the brain is a small-world network (He et al., 2007; Stam, 2004; Stam and van Straaten, 2012). On the other hand, the existence of scale-free network characteristics in the healthy human brain is controversial (He et al., 2007; Pritchard et al., 2014).
Several studies have examined network properties in patients, specifically path length (L) and clustering coefficient (C), to investigate whether the small-world architecture is altered by TLE. The most commonly found changes include increased C and increased L in TLE patients as compared to controls (Bartolomei et al., 2013; Horstmann et al., 2010; Ponten et al., 2007). However, other studies have found different and even opposing changes in C and L (Liao et al., 2010; Quraan et al., 2013; Xu et al., 2014). These inconsistent results are likely due to the use of a variety of techniques for creation of the graphs (Horstmann et al., 2010), difference in frequency band for analysis (Horstmann et al., 2010; Quraan et al., 2013; Wilke et al., 2009, 2011), variability among patients, and a low sample number. Additional difficulty with determining MRI-based network analysis is that network characteristics have been shown to change over time in the same patient (Chiang et al., 2016). However, the takeaway message is that TLE does alter the network architecture of the epileptic human brain.
Luckily, there have also been some exciting and promising results from whole-brain network analysis in TLE patients. DTI shows increased connectivity between hippocampus and thalamus in patients with TLE (Dinkelacker et al., 2015). This changed connectivity could certainly be important given that limbic seizures are known to propagate through the thalamus in both human and animal models (Blumenfeld et al., 2004; Guye et al., 2006; Patel et al., 1988; Sloan et al., 2011). Additionally, MEG analysis was able to demonstrate the presence of hub-like regions in both temporal cortex and hippocampus of patients with TLE that do not exist in control subjects, and the importance of hubs to excitability was discussed earlier (Jin et al., 2015).
Recording from large populations of neurons in human studies can also lead to clues about what might start or stop seizures. A computational neuronal population model was developed in order to simulate findings from depth electrode recordings (stereo-EEG) from patients with TLE (Wendling et al., 2005). The model parameters were fit using an evolutionary algorithm to match each patient's EEG at different points around the seizure event, ie, interictal, preonset, onset, and ictal. The authors determined that excitation gradually increased between time points. Slow dendritic inhibition increased between interictal and preonset, dropped drastically at onset, and returned to normal in the ictal state. Finally, fast somatic inhibition increased from preonset to onset and then dropped drastically in ictal state. This study presents the interesting implication that, in the future, if recording techniques can deconvolve subpopulations of neurons, the balance between activity of different neuronal populations may offer another method of seizure prediction, and underlines the fact that seizures are not triggered simply by an increase in excitation or a decrease in inhibition. However, tetrode recordings from epileptic rats have shown that alterations in the firing patterns of putative excitatory and inhibitory neurons in the preictal period are complex and may also depend on that animal's behavioral state (eg, sleep, or sleep-to-wake transitions; Ewell et al., 2015).
As mentioned in Section 1, a well-accepted definition of a seizure is “abnormal excessive or synchronous neuronal activity in the brain” (Fisher et al., 2005). However, analysis of multichannel EEG recordings during seizures does not support such a straightforward interpretation. Ictal events may involve local hypersynchrony, but are not correlated with global hypersynchrony (Schindler et al., 2007). Similar results were obtained when single units were recorded from multielectrode arrays in patients undergoing invasive monitoring for surgical evaluation. Surprisingly, even at the local level, there was great variability in spike timing and spike rate between single units rather than massive hypersynchrony, even in the epileptogenic zone (Truccolo et al., 2011). These results corroborate the experimental findings of Feldt Muldoon et al. which suggested that there is no complete synchrony among all neurons during network events resembling seizures in slices from epileptic animals (Feldt Muldoon et al., 2013).
Finally, there has been some interest in using graph theory to analyze individual patients' networks in an attempt to guide treatment by helping to define the epileptogenic zone. When the strength of connectivity was measured in patients with TLE using functional connectivity of the stereo-EEG electrodes, patients with lower mean and standard deviation of connectivity strength had a significantly better outcome after temporal lobectomy (Antony et al., 2013). When the graph characteristics of intracranial EEG were analyzed, it turns out that the node with the highest “outdegree,” ie, highest number of outgoing connections, was always within the clinically determined epileptogenic zone that ended up being resected at surgery (van Mierlo et al., 2014). Additionally, another similar study revealed that patients who became seizure free after surgery underwent resections that included areas of especially active nodes, especially as measured in the gamma frequency (Wilke et al., 2009, 2011). Therefore, the presence of one or several highly connected hubs may signal an epileptogenic zone.
The final goal of all epilepsy research is to create new treatments for patients with epilepsy. Whether optogenetic closed-loop technology, developed to control microcircuits in animal models, will be directly applied to humans remains to be seen. Translating these exciting results to TLE patients will require the conquering of major technological and ethical hurdles (for discussion, see Bui et al., 2015). However, knowledge of the mechanisms and locations by which control may be obtained could allow translational researchers and clinicians to improve currently approved technology in the meantime. For example, responsive neurostimulation is a closed-loop technology which has recently been approved for patients with epilepsy, by which an electrode is implanted into the seizure focus (Heck et al., 2014). The electrode is capable of recording and stimulating: seizures are detected with customizable seizure detection algorithms, and then the electrodes stimulate the area to abort the seizure. Having a better idea of the circuitry involved in controlling seizures in animal models may allow for additional anatomical locations for electrode targeting as well as improved seizure detection algorithms.
6 CONCLUSIONS
In summary, computational science and experimental techniques offer complementary techniques to allow researchers to understand brain dynamics in normal and pathological conditions. Many anatomical details about circuit reorganization in epilepsy have already been demonstrated, and these predictions can offer the possibility of expanding this knowledge further. As computational models grow in size, accuracy, and complexity, they will produce more reliable predictions as to what variables are epileptogenic, and these predictions can then be experimentally validated. This cycle of experimenting, modeling, and experimenting again is particularly relevant in the epilepsy field, given the varied and complex anatomical and physiological changes that alter the hippocampal network during epileptogenesis.
ACKNOWLEDGMENTS
The work was funded by the US National Institutes of Health grants NS35915 and NS94668 (to I.S.), R25NS065741-04S1 (to A.A.), and the National Aeronautics and Space Administration grant NSCOR NNX10AD59G (to I.S.).
REFERENCES
- Achard S, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26(1):63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R, Jeong H, Barabasi AL. Error and attack tolerance of complex networks. Nature. 2000;406:378–382. doi: 10.1038/35019019. [DOI] [PubMed] [Google Scholar]
- Allam SL, et al. A computational model to investigate astrocytic glutamate uptake influence on synaptic transmission and neuronal spiking. Front. Comput. Neurosci. 2012;6:1–16. doi: 10.3389/fncom.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André V, et al. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11(4):452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Andrioli A, et al. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149(1):131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Anstotz M, et al. Developmental profile, morphology, and synaptic connectivity of Cajal-Retzius cells in the postnatal mouse hippocampus. Cereb. Cortex. 2016;26:855–872. doi: 10.1093/cercor/bhv271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AR, et al. Functional connectivity estimated from intracranial EEG predicts surgical outcome in intractable temporal lobe epilepsy. PLoS One. 2013;8(10):e77916. doi: 10.1371/journal.pone.0077916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, et al. Target-selectivity of parvalbumin-positive interneurons in layer II of medial entorhinal cortex in normal and epileptic animals. Hippocampus. 2015;26(6):779–793. doi: 10.1002/hipo.22559. epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi AL, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, et al. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin. Neurophysiol. 2006;117(9):2039–2049. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, et al. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin. Neurophysiol. 2013;124(12):2345–2353. doi: 10.1016/j.clinph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Bernard C, et al. Interneurones are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res. 1998;32(1–2):93–103. doi: 10.1016/s0920-1211(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011;15(12):592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezaire MJ, Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23(9):751–785. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54(5):358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Blüumcke I, et al. Cellular pathology of hilar neurons in Ammon's horn sclerosis. J. Comp. Neurol. 1999;414(4):437–453. doi: 10.1002/(sici)1096-9861(19991129)414:4<437::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Blüumcke I, et al. Loss of hilar mossy cells in Ammon's horn sclerosis. Epilepsia. 2000;41(Suppl. 6):S174–S180. doi: 10.1111/j.1528-1157.2000.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Blüumcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002;12(2):199–211. doi: 10.1111/j.1750-3639.2002.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb. Cortex. 2004;14(8):892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326(5958):1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Botterill JJ, et al. Aberrant hippocampal neurogenesis after limbic kindling: relationship to BDNF and hippocampal-dependent memory. Epilepsy Behav. 2015;47:83–92. doi: 10.1016/j.yebeh.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, et al. Early loss of interneurons and delayed subunit-specific changes in GABA A-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, et al., editors. Jasper's Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US); Bethesda, MD: 2012. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 1997;77(5):2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J. Neurosci. 1999;19(21):9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 2011;31(6):2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52(11):2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui AD, Alexander A, Soltesz I. Neuroscientist. 2015. Seizing control: from current treatments to optogenetic interventions in epilepsy; p. 1073858415619600. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, et al. Interneuron diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27(4):186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Chiang S, et al. Time-dependence of graph theory metrics in functional connectivity analysis. Neuroimage. 2016;125:601–615. doi: 10.1016/j.neuroimage.2015.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO, Mody I. The process of epileptogenesis: a pathophysiological approach. Curr. Opin. Neurol. 2001;14(2):187–192. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Dani A, et al. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68(5):843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lanerolle NC, et al. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- De Lanerolle NC, Lee TS, Spencer DD. Histopathology of human epilepsy. Epilepsia. 2010;51(Suppl. 5):37. [Google Scholar]
- Dinkelacker V, et al. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: enhanced connectivity per hippocampal voxel. Epilepsia. 2015;56(8):1217–1226. doi: 10.1111/epi.13051. [DOI] [PubMed] [Google Scholar]
- Dinocourt C, et al. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J. Comp. Neurol. 2003;459(4):407–425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- Dorogovtsev SN, Goltsev AV, Mendes JFF. Pseudofractal scale-free web. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002;65(6):1–4. doi: 10.1103/PhysRevE.65.066122. [DOI] [PubMed] [Google Scholar]
- Drew LJ, et al. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus. 2016;26(6):763–778. doi: 10.1002/hipo.22557. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok B, et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci. 2015;18(1):75–86. doi: 10.1038/nn.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, et al. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J. Neurophysiol. 2007;97(2):1566–1587. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- Ewell LA, et al. Brain state is a major factor in preseizure hippocampal network activity and influences success of seizure intervention. J. Neurosci. 2015;35:15635–15648. doi: 10.1523/JNEUROSCI.5112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt Muldoon S, Soltesz I, Cossart R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. Proc. Natl. Acad. Sci. U.S.A. 2013;110(9):3567–3572. doi: 10.1073/pnas.1216958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 2012;53(Suppl. 9):89–101. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- Fisher RS, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez J, et al. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013;54(2):323–330. doi: 10.1111/j.1528-1167.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- Gorter JA, et al. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur. J. Neurosci. 2001;13(4):657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Russek SJ, Brooks-Kayal AR. Molecular pathways controlling inhibitory receptor expression. Epilepsia. 2012;53(Suppl. 9):71–78. doi: 10.1111/epi.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein G, Linsker R. Synchronous neural activity in scale-free network models versus random network models. Proc. Natl. Acad. Sci. U.S.A. 2005;102(28):9948–9953. doi: 10.1073/pnas.0504127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(7):1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Chiang S. Clinical correlates of graph theory findings in temporal lobe epilepsy. Seizure. 2014;23(10):809–818. doi: 10.1016/j.seizure.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler U, et al. Mossy fiber sprouting and pyramidal cell dispersion in the hippocampal CA2 region in a mouse model of temporal lobe epilepsy. Hippocampus. 2016;26(5):577–588. doi: 10.1002/hipo.22543. [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex. 2007;17(10):2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- Heck CN, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, et al. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Henderson KW, et al. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J. Neurosci. 2014;34(40):13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng K, Haney MM, Buckmaster PS. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013;54(9):1535–1541. doi: 10.1111/epi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann MT, et al. State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin. Neurophysiol. 2010;121(2):172–185. doi: 10.1016/j.clinph.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Houser CR. Do structural changes in GABA Neurons give rise to the epileptic state. In: Scharfman HE, Buckmaster PS, editors. Issues in Clinical Epileptology: A View from the Bench. Springer, Dordrecht, Heidelberg; New York, London: 2014. pp. 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AL, et al. Opposing modifications in intrinsic currents and synaptic inputs in post-traumatic mossy cells: evidence for single-cell homeostasis in a hyperexcitable network. J. Neurophysiol. 2007;97(3):2394–2409. doi: 10.1152/jn.00509.2006. [DOI] [PubMed] [Google Scholar]
- Huusko N, Romer C, Ndode-Ekane XE, Lukasiuk K, Pitkanen A. Loss of hippocampal internerons and epileptogenesis: a comparison of two animal models of acquired epilepsy. Brain Struct. Funct. 2015;220(1):153–191. doi: 10.1007/s00429-013-0644-1. [DOI] [PubMed] [Google Scholar]
- Jasper H, Kershman J. Electroencephalographic classification of the epilepsies. Arch. Neurol. Psychiatr. 1941;45(6):903–943. [Google Scholar]
- Jin S-H, Jeong W, Chung CK. Mesial temporal lobe epilepsy with hippocampal sclerosis is a network disorder with altered cortical hubs. Epilepsia. 2015;56(5):772–779. doi: 10.1111/epi.12966. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, et al. Animal models of epilepsy: use and limitations. Neuropsychiatr. Dis. Treat. 2014;10:1693–1705. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Hinds J. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Klausberger T, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J. Neurosci. 2003;23(6):2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 2010;30(6):2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat. Neurosci. 2015;18(3):331–338. doi: 10.1038/nn.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, et al. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, et al. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. 2014;1(1):1–27. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, et al. In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 2015;593(10):2379–2388. doi: 10.1113/JP270056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruba R, et al. Differential susceptibility of interneurons expressing neuropeptide Y or parvalbumin in the aged hippocampus to acute seizure activity. PLoS One. 2011;6(9):e24493. doi: 10.1371/journal.pone.0024493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duigou C, et al. Recurrent synapses and circuits in the CA3 region of the hippocampus: an associative network. Front. Cell. Neurosci. 2014;7:1–13. doi: 10.3389/fncel.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, et al. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82(5):1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Levesque M, Avoli M, Bernard C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J. Neurosci. Methods. 2015;260:45–52. doi: 10.1016/j.jneumeth.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis KP, et al. Evolution of network synchronization during early epileptogenesis parallels synaptic circuit alterations. J. Neurosci. 2015;35(27):9920–9934. doi: 10.1523/JNEUROSCI.4007-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang Z. Controlling the efficiency of trapping in a scale-free small-world network. Sci. Rep. 2014;4:6274. doi: 10.1038/srep06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-Q, et al. Dysfunction of hippocampal interneurons in epilepsy. Neurosci. Bull. 2014;30(6):985–998. doi: 10.1007/s12264-014-1478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, et al. Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCl-pilocarpine. Int. J. Neurosci. 2011;121(2):69–85. doi: 10.3109/00207454.2010.530007. [DOI] [PubMed] [Google Scholar]
- Long L-L, et al. Aberrant neuronal synaptic connectivity in CA1 area of the hippocampus from pilocarpine-induced epileptic rats observed by fluorogold. Int. J. Clin. Exp. Med. 2014;7(9):2687–2695. [PMC free article] [PubMed] [Google Scholar]
- Lothman E, Stringer J, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- Lytton WW, Hellman KM, Sutula TP. Computer models of hippocampal circuit changes of the kindling model of epilepsy. Artif. Intell. Med. 1998;13(1–2):81–97. doi: 10.1016/s0933-3657(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience. 1993;56(2):317–336. doi: 10.1016/0306-4522(93)90334-c. [DOI] [PubMed] [Google Scholar]
- Marissal T, et al. Pioneer glutamatergic cells develop into a morpho-functionally distinct population in the juvenile CA3 hippocampus. Nat. Commun. 2012;3:1316. doi: 10.1038/ncomms2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, et al. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx M, Haas CA, Häussler U. Differential vulnerability of interneurons in the epileptic hippocampus. Front. Cell. Neurosci. 2013;7:167. doi: 10.3389/fncel.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massobrio P, Pasquale V, Martinoia S. Self-organized criticality in cortical assemblies occurs in concurrent scale-free and small-world networks. Sci. Rep. 2015;5:10578. doi: 10.1038/srep10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, et al. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J. Neurosci. 1995;15(5 Pt. 2):3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, et al. Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol. Dis. 1998;5(3):151–176. doi: 10.1006/nbdi.1998.0200. [DOI] [PubMed] [Google Scholar]
- Mitchell L, et al. Anterior temporal abnormality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology. 1999;52(2):327–336. doi: 10.1212/wnl.52.2.327. [DOI] [PubMed] [Google Scholar]
- Modo M, et al. Detection of aberrant hippocampal mossy fiber connections: ex vivo mesoscale diffusion MRI and microtractography with histological validation in a patient with uncontrolled temporal lobe epilepsy. Hum. Brain Mapp. 2016;37:780–795. doi: 10.1002/hbm.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc. Natl. Acad. Sci. U.S.A. 2008;105(16):6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, et al. Epilepsy in small-world networks. J. Neurosci. 2004;24(37):8075–8083. doi: 10.1523/JNEUROSCI.1509-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J. Neurosci. 1993;13(10):4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban-Kis K, Szabadi T, Szilagyi T. The loss of Ivy cells and the hippocampal input modulatory O-LM cells contribute to the emergence of hyperexcitability in the hippocampus. Rom. J. Morphol. Embryol. 2015;56(1):155–161. [PubMed] [Google Scholar]
- Parent JM, Kron MM. Neurogenesis and epilepsy. Epilepsia. 2010;51(Suppl. 5):45. [Google Scholar]
- Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Millan MH, Meldrum BS. Decrease in excitatory transmission within the lateral habenula and the mediodorsal thalamus protects against limbic seizures in rats. Exp. Neurol. 1988;101(1):63–74. doi: 10.1016/0014-4886(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Paz JT, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2012;16(1):64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo MA, et al. Pioneer GABA cells comprise a subpopulation of hub neurons in the developing hippocampus. Neuron. 2011;71(4):695–709. doi: 10.1016/j.neuron.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin. Neurophysiol. 2007;118(4):918–927. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, et al. Functional brain networks formed using cross-sample entropy are scale free. Brain Connect. 2014;4(6):454–464. doi: 10.1089/brain.2013.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocolo G, Maccaferri G. Optogenetic activation of Cajal-Retzius cells reveals their glutamatergic output and a novel feedforward circuit in the developing mouse hippocampus. J. Neurosci. 2014;34(39):13018–13032. doi: 10.1523/JNEUROSCI.1407-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraan MA, et al. Altered resting state brain dynamics in temporal lobe epilepsy can be observed in spectral power, functional connectivity and graph theory metrics. PLoS One. 2013;8(7):e68609. doi: 10.1371/journal.pone.0068609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff A.d.H. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J. Neurosci. 2004;24(9):2259–2269. doi: 10.1523/JNEUROSCI.5191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff AH, et al. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 2002;25(3):140–144. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- Sano K, Malamud N. Clinical significance of sclerosis of the cornu ammonis: ictal psychic phenomena. AMA Arch. Neurol. Psychiatr. 1953;70(1):40–53. doi: 10.1001/archneurpsyc.1953.02320310046003. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J. Neurophysiol. 2005;93(1):437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Sayin U, et al. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J. Neurosci. 2003;23(7):2759–2768. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, et al. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J. Neurophysiol. 2003;90(4):2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Crandall PH, Scheibel AB. The hippocampal-dentate complex in temporal lobe epilepsy: a Golgi study. Epilepsia. 1974;15:55–79. doi: 10.1111/j.1528-1157.1974.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Schindler K, et al. Assessing seizure dynamics by analysing the correlation structure of multichannel intracranial EEG. Brain. 2007;130:65–77. doi: 10.1093/brain/awl304. [DOI] [PubMed] [Google Scholar]
- Schneider-Mizell CM, et al. From network structure to network reorganization: implications for adult neurogenesis. Phys. Biol. 2010;7:046008. doi: 10.1088/1478-3975/7/4/046008. [DOI] [PubMed] [Google Scholar]
- Siddiqui AH, Joseph SA. CA3 axonal sprouting in kainate-induced chronic epilepsy. Brain Res. 2005;1066(1–2):129–146. doi: 10.1016/j.brainres.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Zhang D, Bertram EH. Increased GABAergic inhibition in the midline thalamus affects signaling and seizure spread in the hippocampus-prefrontal cortex pathway. Epilepsia. 2011;52(3):523–530. doi: 10.1111/j.1528-1167.2010.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1(1):41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, et al. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J. Comp. Neurol. 1991;308(3):381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- Soltesz I, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat. Rev. Neurosci. 2015;16(5):264–277. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005;562(Pt. 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, et al. Functional changes in neuropeptide Y- and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience. 1992;50(4):831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- Spigelman I, et al. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86(1):109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Functional connectivity patterns of human magnetoencephalographic recordings: a “small-world” network? Neurosci. Lett. 2004;355(1–2):25–28. doi: 10.1016/j.neulet.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Straaten ECW. The organization of physiological brain networks. Clin. Neurophysiol. 2012;123(6):1067–1087. doi: 10.1016/j.clinph.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Steve TA, Jirsch JD, Gross DW. Quantification of subfield pathology in hippocampal sclerosis: a systematic review and meta-analysis. Epilepsy Res. 2014;108(8):1279–1285. doi: 10.1016/j.eplepsyres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Sun C, et al. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulated-based animal model of temporal lobe epilepsy. J. Comp. Neurol. 2007;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom LE, et al. Somatostatin- and neuropeptide Y-synthesizing neurones in the fascia dentata of humans with temporal lobe epilepsy. Brain. 2001;124:688–697. doi: 10.1093/brain/124.4.688. [DOI] [PubMed] [Google Scholar]
- Takano H, Coulter DA. Imaging of hippocampal circuits in epilepsy. In: Noebels JL, et al., editors. Jasper's Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US); Bethesda, MD: 2012. [PubMed] [Google Scholar]
- Tejada J, Roque AC. Computational models of dentate gyrus with epilepsy-induced morphological alterations in granule cells. Epilepsy Behav. 2014;38:63–70. doi: 10.1016/j.yebeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Tejada J, et al. Morphological alterations in newly born dentate gyrus granule cells that emerge after status epilepticus contribute to make them less excitable. PLoS One. 2012;7(7):e40726. doi: 10.1371/journal.pone.0040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J. Neuropathol. Exp. Neurol. 2002;61(6):510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Reid CA, Petrou S. Mossy fiber sprouting interacts with sodium channel mutations to increase dentate gyrus excitability. Epilepsia. 2010;51(1):136–145. doi: 10.1111/j.1528-1167.2009.02202.x. [DOI] [PubMed] [Google Scholar]
- Toni N, Schinder AF. Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015;8(1):a018903. doi: 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnesen J, et al. Optogenetic control of epileptiform activity. Proc. Natl. Acad. Sci. U.S.A. 2009;106(29):12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, et al. Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain. 2010;133(9):2763–2777. doi: 10.1093/brain/awq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, et al. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011;14(5):635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah G, et al. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states. II. Network and glial dynamics. J. Comput. Neurosci. 2009;26(2):171–183. doi: 10.1007/s10827-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mierlo P, et al. Functional brain connectivity from EEG in epilepsy: seizure prediction and epileptogenic focus localization. Prog. Neurobiol. 2014;121:19–35. doi: 10.1016/j.pneurobio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C, et al. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 2007;27(28):7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wendling F, Hernandez A, Bellanger J-J. Interictal to ictal transition in human temporal lobe epilepsy: insights from a computational model of intracerebral EEG. J. Clin. Neurophysiol. 2005;22(5):343–356. [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, et al. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10(3):244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wiebe S. Epidemiology of temporal lobe epilepsy. Can. J. Neurol. Sci. 2000;27(Suppl. 1):S6–S10. doi: 10.1017/s0317167100000561. discussion S20–S21. [DOI] [PubMed] [Google Scholar]
- Wiebe S, England TN. A randomized, controlled trial of surgery. N. Engl. J. Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, et al. Altered structure and function of astrocytes following status epilepticus. Epilepsy Behav. 2015;49:17–19. doi: 10.1016/j.yebeh.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C, Worrell GA, He B. Analysis of epileptogenic network properties during ictal activity. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009:2220–2223. doi: 10.1109/IEMBS.2009.5334866. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C, Worrell G, He B. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia. 2011;52(1):84–93. doi: 10.1111/j.1528-1167.2010.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittner L, Miles R. Factors defining a pacemaker region for synchrony in the hippocampus. J. Physiol. 2007;584(3):867–883. doi: 10.1113/jphysiol.2007.138131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittner L, et al. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience. 2001;108(4):587–600. doi: 10.1016/s0306-4522(01)00446-8. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Laker D. Homeostasis or channelopathy? Acquired cell type-specific ion channel changes in temporal lobe epilepsy and their antiepileptic potential. Front. Physiol. 2015;6:168. doi: 10.3389/fphys.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth MS, et al. Selective reduction of CCK-positive basket cell innervation in a model of temporal lobe epilepsy. J. Neurosci. 2010;30(26):8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes RC, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci. Transl. Med. 2012;4(161):161ra152. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, et al. Disrupted topological properties of brain white matter networks in left temporal lobe epilepsy: a diffusion tensor imaging study. Neuroscience. 2014;279:155–167. doi: 10.1016/j.neuroscience.2014.08.040. [DOI] [PubMed] [Google Scholar]
- Yizhar O, et al. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J. Neurosci. 2009;29(45):14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]