Abstract
Background
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer with very high prevalence in southern China. Phosphatase and tensin homolog (PTEN), a tumor suppressor, was reported to be downregulated in NPC patients and correlated with pathological grade and clinical stage of NPC.
Material/Methods
Luciferase reporter assay, qPCR, and Western blot analysis were used to determine if PTEN is a target of miR-152. The function of miR-152 in cell apoptosis and cell proliferation was examined as well. Tissue samples from NPC patients were also analyzed for PTEN and miR-152 expressions.
Results
Reporter assay indicated miR-152 targets the 3′UTR of PTEN mRNA to inhibit PTEN expression. Transfection of the NPC-derived cell line with miR-152 mimic confirmed these findings. Overexpression of miRNA-152 inhibits apoptosis induced by Cisplatin in NPC cancer cells in vitro. Moreover, overexpression miR-152 also promotes NPC cancer cell invasion and proliferation. Samples from EBV-negative NPC patients demonstrated the down-regulated level of PTEN may be related with overexpression of miR-152.
Conclusions
The miR-152 targets PETN to inhibit cell apoptosis and promote cancer cell proliferation and migration in NPC development.
MeSH Keywords: Apoptosis, MicroRNAs, Nasopharyngeal Neoplasms, PTEN Phosphohydrolase
Background
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer with very high prevalence in southern China [1]. Globally, NPC demonstrates a well-defined distribution [2]. NPC has a very low prevalence rate in Europe (annual incidence rate below 1 per 100 000), but it is highly prevalent in South-East Asia and China [1,3]. Although genetic susceptibility, environmental factors, and Epstein–Barr virus (EBV) latent infection have been considered as key factors in occurrence of NPC, the molecular mechanism of NPC development and progression is still largely unclear [4].
MicroRNAs (miRNAs) are a class of non-coding short RNA molecules that negatively regulate gene expression at the mRNA level [5]. There may be multiple targets of a single miRNA, and abnormal expression of miRNA expression during the development of cancer and in progression of a variety diseases has been documented [6].
PTEN is a phosphoinositide phosphatase, originally identified as a multifunctional tumor suppressor frequently lost in various human cancers [7]. Previous studies reported that PTEN was downregulated in NPC patients as shown by immunohistochemistry assay, and it is correlated with pathological grade and clinical stage of NPC [8,9]. A study identified microRNA-144 as a negative regulator for PTEN to promote cell proliferation, migration, and invasion in nasopharyngeal carcinoma, suggesting a significant role of microRNAs during the prognosis of NPC [1]. However, it has been unknown if there are any other microRNAs also responsible for regulating PTEN in NPC.
Because PTEN down-regulation by miRNA has been reported, during the screening for other functional miRNA targeting PTEN, we identified MicroRNA-152 (miR-152) as a potential regulator for PTEN expression. Further analysis indicated that the phosphatase and tensin homolog could be the putative target of miR-152 and this was further verified in an in vitro study. Moreover, overexpression of miRNA-152 can promote the cell survival and inhibit apoptosis in vitro. Samples from EBV-negative NPC patients demonstrated that the down-regulation of PTEN may be related with overexpression of miR-152. Taken together, our data provide valuable information for understanding NPC development and progression.
Material and Methods
Ethics statement, patients, and samples
Surgical tissue specimens from 4 patients with primary nasopharyngeal who underwent tumor resection at Zhengzhou Central Hospital affiliated with Zhengzhou University were prospectively obtained for this study by following Institutional Review Board guidelines. This study was reviewed and approved by the Research Ethics Board of Zhengzhou Central Hospital affiliated with Zhengzhou University. All participating subjects were formally informed about the purpose of using their samples in this study and a letter of consent was signed by every subject involved. Patients involved in this study were selected according to the following criteria: (1) diagnosis of the primary nasopharyngeal carcinoma based on clinical and histological features; (2) the tumor tissue is surgically resectable; (3) Karnofsky performance status scale ≥70; (4) the samples were EBV-negative. The EBV antibody test was used to determine if the patients were infected by EBV; this was done using the Human Epstein-Barr virus antibody (IgG) ELISA Kit (CUSABIO and CusAB China, Wuhan, Hubei Province, China), following the manufacturer’s instructions.
Cells, miR-152 mimics and chemicals
Human nasopharyngeal carcinoma cell line CNE2Z (EBV-negative) and HEK293T cells were purchased from Shanghai Bioleaf Biotech Co. Ltd (Shanghai, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco). The putative miRNA targeting for PTEN was screened by using TargetScan (http://www.targetscan.org/, Release 7.0, August 2015). The MISSION® microRNA Mimic hsa-miR-152 and the scramble control of microRNA mimic were commercially purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Transfection of CNE2Z and HEK293T cells with plasmid DNA or microRNA mimics was conducted by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the instructions of the manufacturer.
Reporter plasmid and luciferase-based miRNA functional assay
Human PTEN 3′UTR reporter plasmid psiCHECK2-PTEN-3′UTR (Addgene plasmid # 50936) was purchased from Addgene (Cambridge, MA). Luciferase activities in the HEK293T cell transfected with psiCHECK2-PTEN-3′UTR along with miR-152 or scramble control were measured by using Dual-Glo® Luciferase Assay System (Promega) following the instructions of the manufacturer. The luminescence signal of luciferase was measured with a VICTOR3™ Multilabel Counter (Perkin-Elmer, Waltham, MA). Relative percentages of luciferase activity were calculated by comparing them with scramble control transfected HEK293T cells.
Reverse transcription and Real-Time PCR (qPCR)
TRIzol Reagent (Invitrogen) was used for total RNAs isolation from in vitro cultured cells and samples obtained from patients. The cDNA generation for quantification of miRNAs expression was conducted by using the Hairpin-it™ miRNA RT-PCR Quantitation kit (GenePharma, Shanghai, China) and ABM hsa-miR-152 Primers (Applied Biological Materials Inc, Richmond, BC, Canada) according to manufacturer’s instructions. The cDNA of in vitro cultured cells were obtained via reverse transcription by AMV reverse transcriptase (Promega). A combination of oligo dT and random hexamer was used for cDNA synthesis. The real-time PCR(qPCR) detection with SYBR Green Mix (Life technologies) for the targeting genes and miR-152 expression were described previously [10,11]. Transcripts level of human RPL32 and U6 were also monitored from the same sample to serve as reference genes for normalization. The relative gene expression was quantified by the 2−ΔΔCT method, as previously described [12]. Primers used in this study are listed in Table 1.
Table 1.
Primers and their sequence used in this study.
| Primer name | Sequence |
|---|---|
| PTEN -F1 | ACACTCCAGCTGGGATCTGTAAGAGAA |
| PTEN-R1 | TGGTGTCGTGGAGTCG |
| PRL32-F1 | CAACTGGCCATCAGAGTCAC |
| RPL32-R1 | GTGCACATGAGCTGCCTACT |
Flow cytometry based cell apoptosis assay
The CNE2Z cells were transfected with miR-152 mimic or scramble control for 24 h, then treated with Cisplatin (Sigma-Aldrich) at the concentration of 20 μM for another 24 h. Then the cells were trypsinized and fixed with 4% paraformaldehyde solution (Santa Cruz Biotech, Santa Cruz, CA) and permeabilized by PBS containing 1% TritonX100 (Sigma-Aldrich). A total of 1×106 CNE2Z cells were stained with FITC-labeled Annexin V (Sigma-Aldrich) and propidium iodide (Sigma-Aldrich). The stained cells were analyzed via flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA) for apoptosis analysis.
Western blotting
The SDS-PAGE and Western blot analyses were conducted as previously described [11,13]. Briefly, after denatured proteins were transferred to a PVDF membrane, the membrane was blocked and probed by rabbit anti-PTEN antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Specific reactions between different antibodies and corresponding proteins were detected by using goat anti-rabbit conjugated with horseradish peroxidase (Sigma, St. Louis, MO) and revealed by a chemiluminescence substrate (Thermo Fisher Scientific, Waltham, MA). The membrane was also probed with anti-Tubulin antibody (Santa Cruz) to normalize the total protein loading. The chemiluminescence signal was digitally recorded and analyzed by the ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA) with Quantity One Program (Version 4.6, Bio-Rad).
Transwell cell invasion assay
To evaluated the CNE2Z cell invasion capability transfection of miR-152 mimic or scramble control, the Transwell cell invasion assay was conducted as previously described, with modifications [14]. Briefly, 12 h before miRNA mimic transfection, the cell culture medium was discarded and replaced with serum-free DMEM for miRNA transfection. After 24 h, cells were trypsinized and stained with trypan blue for cell counting. Then, a total of 100 uL cell suspension medium was added into a Transwell chamber and cultured for another 48 h, after which the chambers were stained with hematoxylin. Six microscopic fields were randomly selected from each chamber and captured for quantification of cell numbers.
Clonogenic cell survival assay
Clonogenic cell survival assay was conducted as previously described, with modifications [15]. Briefly, miR-152 mimic or scramble control transfected cells were trypsinized and counted for viable cells. A total of 1×104 cells were seeded into 100-mm cell culture dishes and maintained for 1 week, after which the cell colonies were stained with gentian violet for imaging.
Statistical analysis
The statistical analysis was conducted by using SPSS Version 16.0 (SPSS, Chicago, IL). Differences in indicators between treatment samples (e.g., luciferase activity, PTEN mRNA level, and apoptosis percentage) were assessed by use of the t test. A 2-tailed P-value of less than 0.05 was considered significant.
Results
The expression of PTEN was regulated by miR-152
The gene PTEN is considered as a tumor suppressor gene via the action of its phosphatase protein product [16]. PTEN is involved in the regulation of the cell cycle, preventing cells from over-proliferation, as well as cell apoptosis [16,17], and reports also confirmed the downregulation of PTEN in NPC [8,9]. While little is known about the role of miRNA during the downregulation of PTEN in NPC, a screening of putative miRNAs targeting PTEN mRNA was conducted and the miR-152 was identified as a potential regulator (Figure 1A).
Figure 1.
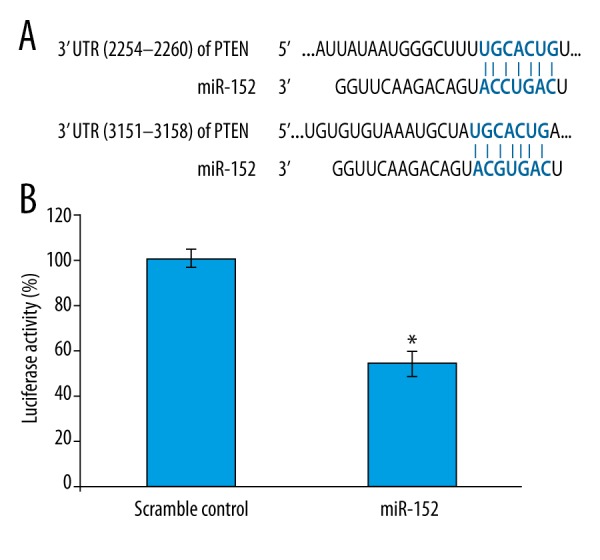
miR152 inhibits PTEN expression via targeting 3′UTR of PTEN mRNA in HEK293T cells. (A) Alignment of putative binding sites of miR-152 with PTEN 3′UTR. (B) Luciferase reporter assay for miR-152 inhibition of PTEN mRNA. The PTEN reporter plasmid was co-transfected with miR-152 mimic or scramble control miRNA to HEK293T cell for 24 h, then the cells were monitored for luciferase activity. For the qualification of luciferase activity, 3 repeated experiments were conducted. Significant differences between different groups are shown by “*” to indicate a significant difference (P<0.05).
To determine if PTEN is targeted by miR-152, the luciferase reporter vector containing PTEN 3′UTR was utilized to check whether overexpression of miR-152 could inhibit luciferase activity. Figure 1B compares the HEK293T cells transfected with scramble control and PTEN3′UTR reporter transfected cells, showing that expression of luciferase was reduced to only 0.4-fold in miR-152 transfected cells, suggesting that the binding of miR-152 to 3′UTR of PTEN mRNA downregulated luciferase activity. Taken together, these data suggested miR-152 could be a potential regulator for PTEN mRNA via targeting its 3′UTR region.
To further confirm the above findings, we also tested the endogenous PTEN mRNA level by qPCR in NPC cell line CNE2Z cells and a EBV-negative NPC cell line [18,19]. As shown in Figure 2A, compared with MOCK-treated cell, transfection of scramble control miRNA had minimal effect on PTEN mRNA level. However, a significant reduction of PTEN mRNA was observed when transfected with miR152-mimic (Figure 2A). Moreover, Western blot analysis for PTEN protein also demonstrated a significant reduction of PTEN protein and confirmed our observation in qPCR (Figure 2B).
Figure 2.
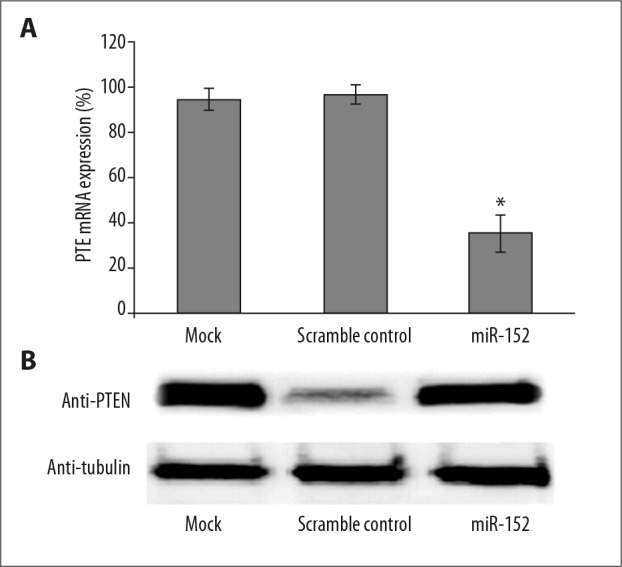
miR152 inhibits PTEN expression in the NPC cell line. (A) Human nasopharyngeal carcinoma cell line CNE2Z (EBV-negative) were transfected with either miR162 mimic or scramble control or left untreated for 48 h, then cells were subjected to RNA isolation and qPCR detection for PTEN transcripts. (B) Western blot for CNE2Z cells transfected with miR-152 mimic or scramble control. At 48 h after transfection, cell lysates were subjected to SDS-PAGE and Western blot analysis with anti-PTEN antibody. Significant differences between different groups are shown by “*” to indicate a significant difference (P<0.01).
Transfection of miR-152 inhibits cisplatin-induced apoptosis
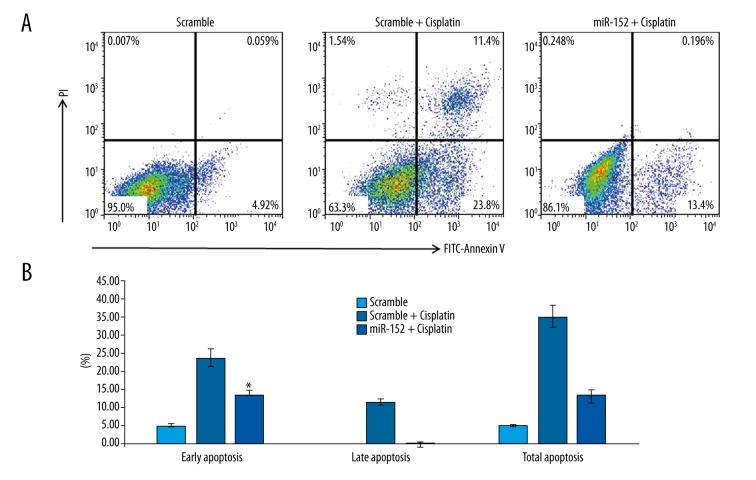
It has been demonstrated that overexpression of PTEN leads to apoptosis [17]; therefore, we expected that inhibition of PTEN via miR-152 would inhibit apoptosis induced by chemotherapy. To validate our speculations, cisplatin, a widely used chemotherapy drug, was used to induce apoptosis of CNE2Z cells. After transfection with scramble control and miR-152 to CNE2Z cells and then treated the cells with cisplatin, the apoptosis status of transfected cells was analyzed by flow cytometry. When cells were transfected with scramble, exposing cells to cisplatin increased early apoptosis and late apoptosis increased drastically to 23.8% and 11.4%, respectively (Figure 3A). However, with the transfection of miR-152 to inhibit the expression of PTEN, cisplatin-induced early and late apoptosis were reduced to 13.4% and 0.196%, respectively (Figure 3A), which suggests a protective role of miR-152 in NPC-derived CNE2Z in response to chemotherapy drugs. Moreover, by repeating the same experiment at least 3 times, the statistical analysis yielded similar results (Figure 3B), which confirmed our previous findings.
Figure 3.
miR152-mediated PTEN down-regulation inhibited apoptosis induced by cisplatin. (A) Cells were transfected with miR-152 mimic or scramble control, followed by treatment with cisplatin, then the cells were fixed and stained with Annexin V and PI for FCM-based apoptosis analysis. (B) The quantification of cell apoptosis. Three individual experiments were repeated for each group. Significant differences between different groups are shown by “*” to indicate a significant difference (P<0.01).
Inhibition of PTEN expression via miR-152 promotes NPC cell invasion and proliferation
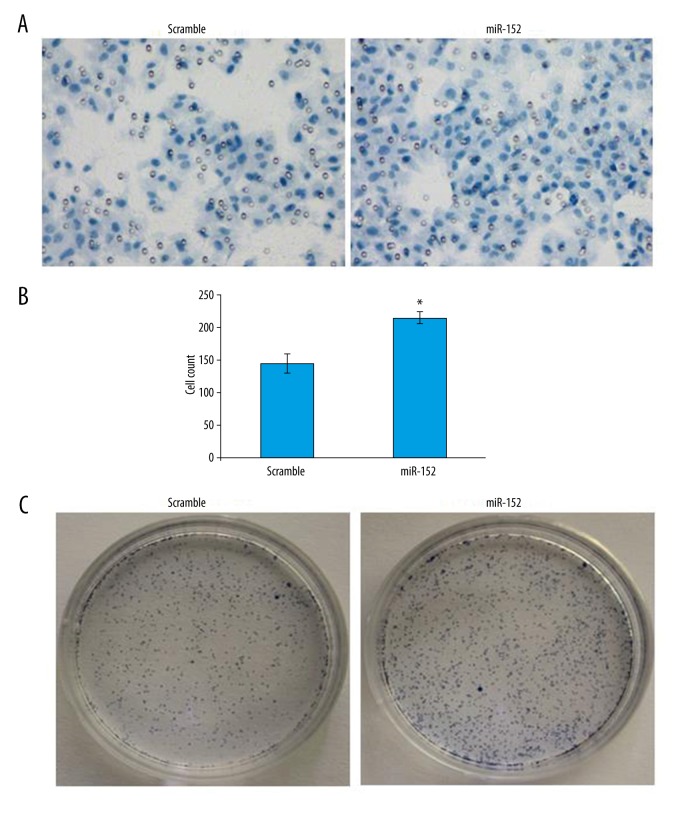
PTEN has been found to play an important role in regulating cell proliferation, cell growth, cell migration, genomic stability, and stem cell self-renewal [20]; therefore, it would be useful to know if inhibition of PTEN via miR-152 can affect cell invasion ability and clone formation ability, since invasiveness of transformed cells is a necessary step for tumor progression [21]. In the cell invasion assay, cell staining results demonstrated a stronger invasion of miR-152-transfected cells (Figure 4A). Statistical analysis demonstrated cell counts were increased from 147 per field to more than 200 (Figure 4B). Moreover, in the clonogenic cell survival assay, which is alternative way to evaluate cell proliferation ability, we observed very strong increase in colonies formed by CNE2Z cells with miR-152 mimic transfection compared with cells transfected with scramble control (Figure 4C). Taken together, these data demonstrated inhibition of PTEN via miR-152 mimic can promote cell invasion and proliferation ability of CNZ2Z cells.
Figure 4.
Transfection of miR-152 in NPC cells promotes its invasion and clone-forming ability. (A) Cell invasion assay image for scramble control or miR152 mimic-transfected CNE2Z cells. (B) Quantification of cell invasion results between scramble control and miR-152 mimic-transfected CNE2Z cells indicated the significant enhancement of cell invasion ability. Error bars represent standard errors of 3 repeated experiments. (C) Clonogenic cell survival assay indicated miR-152 transfection significantly increased the colony-formation ability of CNE2Z cells. Significant differences between groups are shown by “*”, which indicates P<0.05.
The miR-152 overexpression may contribute to PTEN down-regulation in NPC patients
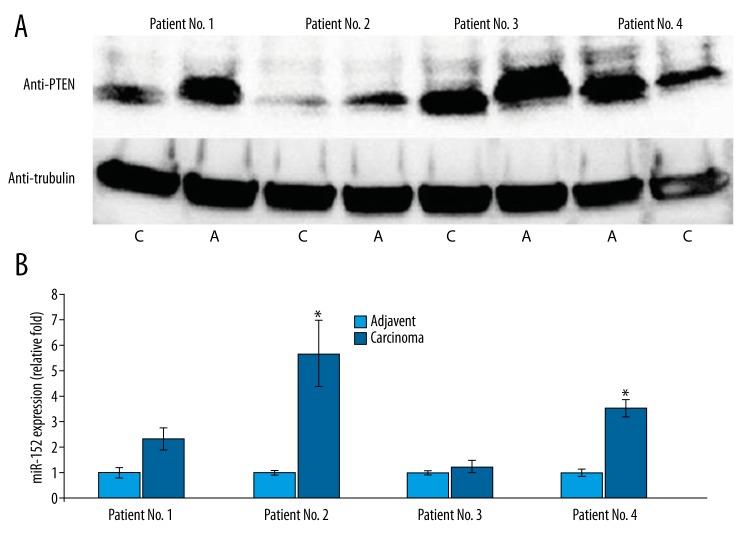
Because the above data demonstrate that miR-152-mediated inhibition of PTEN expression can result in apoptosis induction resistance and promote cancer cell invasion and proliferation of NPC cells, it is necessary to confirm these findings in vivo. Therefore, 4 patients admitted to our hospital who were undergoing surgery to remove NPC tissues and who met our study inclusion criteria agreed to donate their tissues sample for this study. Since the CNZ2Z cells are EBV-negative, to avoiding the interference of EBV in this study, the patients enrolled in this study had to be EBV-negative as well. The adjacent healthy tissues were also collected during the surgery to serve as a control. We first checked the PTEN expression level from these samples via Western blot analysis. As shown in Figure 5B, although the baseline PTEN expression in adjacent tissues was different among the 4 patients, we saw a relative reduction of PTEN level in corresponding cancer tissues, which is consistent with previous reports of down-regulation of PTEN in NPC patients [8,9]. The different baseline level may be due to the different progression stage of NPC in each patient as well as the individual differences or genetic difference among these patients. Moreover, we also measured the expression of miR-152 level from each sample separately. The expression level of miR-152 in adjacent healthy tissues was set as 1-fold for each patient and the experiment was repeated 3 times. As demonstrated in Figure 5B, tumor tissue samples from the patients No. 2 and No. 4 demonstrated a significant increase of miR-152 level, while a slight up-regulation of miR-152 observed in patient No. 1 (Figure 5B). However, for patient No. 3, no significant difference was observed for miR-152 level between adjacent healthy tissues and tumor tissue. Taken together, our study results demonstrate that miR-152 can target PTEN to inhibit chemotherapy-induced apoptosis, as well as promoting cancer cell proliferation and cell invasion.
Figure 5.
The PTEN expression in samples from NPC patients was significantly down-regulated and correlated with up-regulation of miR-152. (A) Western blot for PTEN expression of samples from NPC patients (C – cancer tissues; A – adjuvant healthy tissues). (B) The qPCR result for the expression of miR-152 of samples from NPC patients. The experiment for each sample was repeated at least 3 times. Significant differences between cancer tissues and normal tissues are shown by “*” to indicate a significant difference (P<0.05).
Discussion
Micro-RNAs (miRNAs) have been identified as a novel post-transcription mechanism for regulating gene expression [5]. It has been demonstrate that miRNAs are involved in regulation of many biological functions of cells, including proliferation, migration, and differentiation [5]. We found that miR-152 is a potential regulator for the gene PTEN to protect cancer cells via antagonizing apoptosis induction, as well as promoting cell proliferation and invasion. Moreover, analysis of 4 NPC tumor samples showed that 3 of them up-regulated miR-152, which is also correlated with down-regulation of PTEN protein level.
Based on our recent literature search, miR-152 was previously considered as the only functional microRNA generated from precursor [35]. However, a current database (Target Scan) lists 2 microRNA species – miR-152-3p and miR-152-5p – as mature microRNAs generated by miR-152 precursor. miR-152 is now classified as new miR-152-3p (we used miR-152 in this study for consistency). However, the function of miR-152-5p is still unknown.
Previous reports suggested that miR-152 may be a tumor-suppressing miRNA in certain types of cancers. A report based on prostate cancer suggested that miR-152 targets 3′ UTR of TGF-α and functions as a suppressor of tumor growth [22]. Another report demonstrated that epigenetic silencing by DNA hypermethylation was observed for miR-152 in endometrial cancer, and restoration of miR-152 expression in endometrial cancer cell lines inhibited tumor cell growth both in vitro and in vivo [23]. However, in our study, we found that miR-152 promoted cancer cell growth, enhanced cell invasion, and protect cancer cells from apoptosis induction in the NPC cancer cell line. It is possible that a single miRNA may play different role in different cancers because the targets of a single miRNA are multiple. Colony stimulating factor-1 [24], matrix metalloproteinase 3 [25], Kruppel-like factor 4 [26], and CaMK II [27] were also suggested as targets for miR-152. However, the exact role of miR-152 in different cancer types still needs further investigation.
PTEN is a critical negative regulator of the PI3K-Akt signaling pathway and is considered to be a tumor suppressor [16,20]. The cellular function of PTEN includes regulation of proliferation, cell growth, migration, genomic stability, and stem cell self-renewal [20]. It has been reported that the activity of PTEN can be regulated in a variety of ways, such as mutations, epigenetic silencing, transcriptional repression, aberrant protein localization, and post-translational modifications [20]. Aberrant PTEN expression or activation has been reported in many cancer types [28,29]. Moreover, miRNAs were also identified as novel regulators for PTEN, and these miRNAs include miR-19a [30], miR-21 [31], miR-26a [32], and miR-214 [33]. It is notable that overexpression of these PTEN-targeting miRNAs targeting was considered as a contributing factor for tumor development in these reports [32]. Results of the present study indicate that miR-152 is a regulator miRNA for PTEN in NPC, suggesting that miR-152 also contributes to the development and progression of NPC.
Conclusions
Our study identified a novel miRNA – miR-152 – as a regulator for PTEN expression in NPC cancer, and miR-152 overexpression was demonstrated to be involved in apoptosis resistance, cancer cell invasion, and proliferation. We hope our findings will contribute to a better understanding of the progression of NPC.
Footnotes
Disclosure of conflict of interest
None.
Source of support: Departmental sources
References
- 1.Zhang LY, Ho-Fun Lee V, Wong AM, et al. MicroRNA-144 promotes cell proliferation, migration and invasion in nasopharyngeal carcinoma through repression of PTEN. Carcinogenesis. 2013;34:454–63. doi: 10.1093/carcin/bgs346. [DOI] [PubMed] [Google Scholar]
- 2.Afqir S, Ismaili N, Errihani H. Concurrent chemoradiotherapy in the management of advanced nasopharyngeal carcinoma: Current status. J Cancer Res Ther. 2009;5:3–7. doi: 10.4103/0973-1482.48763. [DOI] [PubMed] [Google Scholar]
- 3.Licitra L, Bernier J, Cvitkovic E, et al. Cancer of the nasopharynx. Crit Rev Oncol Hematol. 2003;45:199–213. doi: 10.1016/s1040-8428(01)00210-4. [DOI] [PubMed] [Google Scholar]
- 4.Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–55. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Yang H, Huo X. [Expression and significance of PTEN in nasopharyngeal carcinoma]. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2004;18:658–59. [in Chinese] [PubMed] [Google Scholar]
- 9.Pedrero JM, Carracedo DG, Pinto CM, et al. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer. 2005;114:242–48. doi: 10.1002/ijc.20711. [DOI] [PubMed] [Google Scholar]
- 10.Patel D, Opriessnig T, Stein DA, et al. Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication. Antiviral Res. 2008;77:95–107. doi: 10.1016/j.antiviral.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel D, Nan Y, Shen M, et al. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J Virol. 2010;84:11045–55. doi: 10.1128/JVI.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Nan Y, Wang R, Shen M, et al. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology. 2012;432:261–70. doi: 10.1016/j.virol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall J. Transwell((R)) invasion assays. Methods Mol Biol. 2011;769:97–110. doi: 10.1007/978-1-61779-207-6_8. [DOI] [PubMed] [Google Scholar]
- 15.Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Methods Mol Med. 2005;110:21–28. doi: 10.1385/1-59259-869-2:021. [DOI] [PubMed] [Google Scholar]
- 16.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10(10):RA235–41. [PubMed] [Google Scholar]
- 17.Hao LS, Zhang XL, An JY, et al. Adenoviral transduction of PTEN induces apoptosis of cultured hepatic stellate cells. Chin Med J (Engl) 2009;122:2907–11. [PubMed] [Google Scholar]
- 18.Sun Y, Hegamyer G, Cheng YJ, et al. An infrequent point mutation of the p53 gene in human nasopharyngeal carcinoma. Proc Natl Acad Sci USA. 1992;89:6516–20. doi: 10.1073/pnas.89.14.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivachandran N, Thawe NN, Frappier L. Epstein-Barr virus nuclear antigen 1 replication and segregation functions in nasopharyngeal carcinoma cell lines. J Virol. 2011;85:10425–30. doi: 10.1128/JVI.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Jiang X. Post-translational regulation of PTEN. Oncogene. 2008;27:5454–63. doi: 10.1038/onc.2008.242. [DOI] [PubMed] [Google Scholar]
- 21.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–47. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C, Li J, Ding Q, et al. miR-152 controls migration and invasive potential by targeting TGFalpha in prostate cancer cell lines. Prostate. 2013;73:1082–89. doi: 10.1002/pros.22656. [DOI] [PubMed] [Google Scholar]
- 23.Tsuruta T, Kozaki K, Uesugi A, et al. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011;71:6450–62. doi: 10.1158/0008-5472.CAN-11-0364. [DOI] [PubMed] [Google Scholar]
- 24.Woo HH, Laszlo CF, Greco S, Chambers SK. Regulation of colony stimulating factor-1 expression and ovarian cancer cell behavior in vitro by miR-128 and miR-152. Mol Cancer. 2012;11:58. doi: 10.1186/1476-4598-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Chopp M, Lu Y, et al. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329:146–54. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Yao Y, Wang P, et al. MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Kruppel-like factor 4. Cancer Lett. 2014;355:85–95. doi: 10.1016/j.canlet.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Tian Y, Ding Y, et al. MiR-152 may silence translation of CaMK II and induce spontaneous immune tolerance in mouse liver transplantation. PLoS One. 2014;9:e105096. doi: 10.1371/journal.pone.0105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–47. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 30.Dou L, Meng X, Sui X, et al. MiR-19a regulates PTEN expression to mediate glycogen synthesis in hepatocytes. Sci Rep. 2015;5:11602. doi: 10.1038/srep11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi L, Bart J, Tan LP, et al. Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC Cancer. 2009;9:163. doi: 10.1186/1471-2407-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse JT, Brennan C, Hambardzumyan D, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–37. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzenbach H, Milde-Langosch K, Steinbach B, et al. Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res Treat. 2012;134:933–41. doi: 10.1007/s10549-012-1988-6. [DOI] [PubMed] [Google Scholar]