Abstract
Extracellular nucleotides regulate a variety of cellular responses involved in inflammation via the activation of P2 receptors. Here, we show that nucleotides regulate TLR2-induced neutrophil migration both in vivo and in vitro. The nucleotide scavenger apyrase inhibited neutrophil recruitment in murine air pouches injected with the TLR2 agonist Pam3CSK4. In agreement, the supernatants of either human primary monocytes or monocytic cells (THP-1 and U937) treated with Pam3CSK4 recruited significantly fewer neutrophils when the former cells were treated in the presence of apyrase. As demonstrated with inhibitory Ab, these supernatants induced neutrophil migration due to IL-8 secretion. In addition, IL-8 secretion was markedly diminished by the non-selective P2 receptor antagonists reactive blue 2 and suramin, and by a selective P2Y6 antagonist, MRS2578. Selective antagonists of P2Y1 (MRS2500) and P2Y11 (NF157) did not affect IL-8 release. The knock-down of either P2Y2 or P2Y6 with specific shRNA diminished IL-8 secretion from Pam3CSK4-treated THP-1 cells. Altogether, these results show that extracellular nucleotides, via P2Y2 and P2Y6 receptors, regulate neutrophil migration by controlling TLR2-induced IL-8 release from human monocytes. In line with our previous work on TLR4, this study further supports the importance of nucleotides in bacterial-induced neutrophil migration.
Keywords: Cell trafficking, Human monocytes, Inflammation, Neutrophils, Pam3CSK4
Introduction
Monocytes play an important role in immune defences against invading bacteria and viruses via TLR activation [1]. TLR recognize highly conserved structural motifs expressed by microbes called PAMP. Monocytes express various TLR including TLR4 (that is activated by LPS, a cell wall component of Gram-negative bacteria), TLR2 (activated by peptidoglycan and lipopeptide, components of Gram-positive bacteria), TLR5 (activated by the bacterial flagellin) and TLR7/8 (activated by single-stranded viral RNA) [2–5]. In response to TLR activation by PAMP, monocytes release various cytokines and chemokines that orchestrate the innate immune responses [6–8]. For example, these cells secrete large amounts of the chemokine IL-8 that plays a major role in neutrophil recruitment at sites of infection [9].
In addition to TLR, monocytes abundantly express P2 receptors that are activated by extracellular nucleotides. P2 receptors are divided into two subfamilies: the G-protein-coupled receptors (P2Y1, 2, 4, 6, 11–14) and the ligand-gated ion channels (P2X1–7). The P2Y subtypes differ in their selectivity toward adenine (ATP, ADP) and uracil nucleotides (UTP, UDP) while all P2X receptors are activated by ATP [10, 11]. Human primary monocytes and monocytic cell lines (THP-1 and U937) express the mRNA of various P2 receptors of which P2X1,7 and P2Y2,6,11 are common to all these cells [12, 13]. There is growing evidence indicating that P2 receptors mediate some important proinflammatory responses activated by the stimulation of monocytes/macrophages with PAMP. Firstly, LPS stimulation of macrophages and microglia is accompanied by the release of ATP. The release of IL-1 from the latter LPS-sitmulated cells was prevented by the addition of a nucleotide scavenger (apyrase) to their medium [14, 15]. In macrophages, extracellular ATP also serves as a secondary stimulus required for LPS-induced production of IL-18 [16, 17]. In addition, we have demonstrated that LPS-induced IL-8 release from human primary monocytes and monocytic THP-1 cells is mediated by an autocrine stimulation of P2Y6 nucleotide receptor [14, 15]. Furthermore, we more recently observed that neutrophil transendothelial migration induced by IL-8 also involved the costimulation of endothelial P2 receptors in vitro [18].
In this work, we show that extracellular nucleotides mediate neutrophil migration induced by TLR1/2 agonist both in vivo and in vitro.
Results
Extracellular nucleotides are involved in Pam3CSK4-induced neutrophil migration in vivo
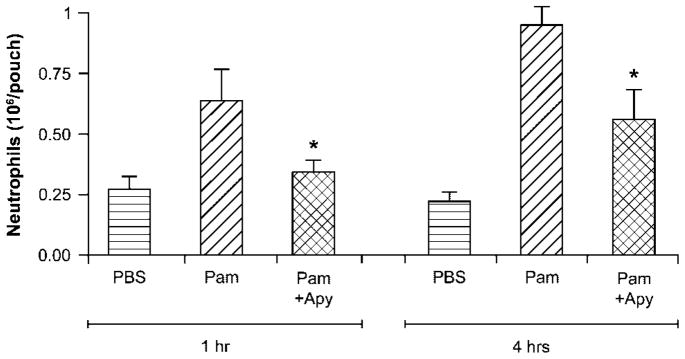
We first investigated whether extracellular nucleotides play a role in TLR2-induced neutrophil migration using the mouse air pouch model. This model allows a precise quantification of migrated neutrophils. As seen in Fig. 1, the administration of the TLR2 agonist Pam3CSK4 in the air pouch elicited neutrophil migration at 4 h, which was markedly decreased by the co-administration of apyrase, a nucleotide scavenger (1.0 × 106±0.09 versus 0.5 × 106±0.13 cells, n = 8, p = 0.03). A similar inhibition of neutrophil migration by apyrase was also observed at 1 h after Pam3CSK4 injection. However, fewer leukocytes were present in the air pouch at this time point (~ .6 × 106±0.33 versus 0.3 × 106±0.08 cells, n = 7, p = 0.03, Fig. 1), which is in agreement with the kinetics of cell migration in the air pouch [19]. These data suggest that nucleotides participate in the regulation of Pam3CSK4-induced neutrophil migration in vivo.
Figure 1.
The depletion of extracellular nucleotides reduces Pam3CSK4-induced neutrophil migration in vivo. Mouse air pouches were raised on the back of female CD-1® mice by subcutaneous injection of sterile air. Six days later, mice were split in three groups and were injected in the air pouches with 1 mL PBS (control) or synthetic tripalmitoylated lipopeptide Pam3CSK4 (Pam, 10 ng), with or without apyrase (Apy, 2 U) in PBS, respectively. The cells (largely neutrophils) accumulated in the pouches were collected 1 and 4 h later and analyzed as described in the Materials and methods. Data show mean+SEM of seven or eight mice per group. *p = 0.03.
Extracellular nucleotides trigger neutrophil migration in vitro by activating IL-8 release
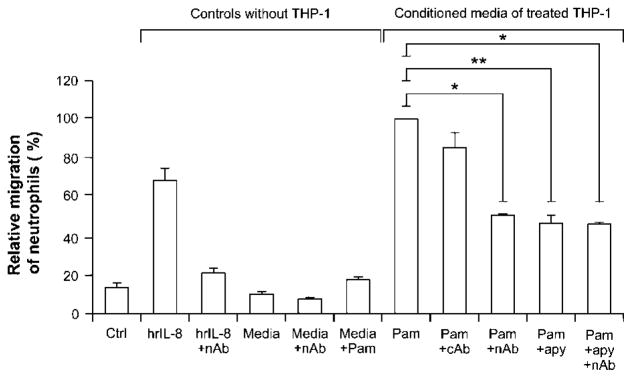
We then tested whether extracellular nucleotides were also involved in Pam3CSK4-induced neutrophil migration using human cells as assessed with the modified Boyden chamber system. Neutrophil migration is regulated by various inflammatory mediators released at sites of infection such as chemokines, which are secreted mainly by monocytes/macrophages and other cells also present in the air pouch [14]. Therefore, we tested whether the inhibition of nucleotide signaling in Pam3CSK4-treated monocytic cells by apyrase would diminish the ability of the supernatants of these cells to attract neutrophils to migrate through a monolayer of endothelial cells, HUVEC. As shown in Fig. 2, the supernatants of the monocytic cells (THP-1 and U937) stimulated in the presence of apyrase recruited significantly fewer neutrophils (60% inhibition) compared to the supernatants of the cells stimulated in the absence of this enzyme.
Figure 2.
Nucleotides trigger neutrophil migration in vitro by controlling IL-8 release in monocytic cells stimulated with Pam3CSK4. Cells and media were prepared as described in the Materials and methods. Briefly, monocytic cells (106/mL) were stimulated for 18 h with Pam3CSK4 (1 μg/mL) in the presence or absence of apyrase (2 U/mL). Prior to transmigration assay, some of these media were incubated overnight at 4°C, with or without IL-8 neutralizing Ab (nAb; MAB208, 10 μg/mL) or with an irrelevant mouse IgG1 Ab (cAb) as a negative control. The transmigration assays with freshly isolated neutrophils were then performed. Monocyte diluent (media: 10 mM HEPES+1 mM sodium pyruvate+10% FBS in RPMI-1640), with or without human recombinant IL-8 (hrIL-8, 2 ng/mL), was used as positive and negative controls, respectively. Data show mean+SEM of three to five independent experiments with neutrophils from different donors. The 100% of migrated neutrophils was set with the media of Pam3CSK4-treated monocytes (Pam) and, depending on the assay, ranged from 0.3 to 0.63 × 106 neutrophils. *p<0.05; **p<0.01.
IL-8 is a major human chemokine for neutrophils that can be responsible for the migration observed here. To verify this hypothesis, the supernatants of stimulated monocytic cells were treated with IL-8 neutralizing Ab before transmigration assays. These Ab markedly diminished the number of neutrophils attracted by the supernatants of Pam3CSK4-stimulated cells (Fig. 2) but did not further reduce neutrophil migration to the supernatants of monocytes stimulated with Pam3CSK4 in the presence of apyrase (Fig. 2; not shown for U937). These data show that IL-8 is responsible for most of the neutrophil migration toward supernatants from Pam3CSK4-stimulated monocytes and that apyrase diminished neutrophil migration specifically by inhibiting IL-8 secretion. As controls, apyrase itself, when added only at the beginning of the transmigration assay, did not affect neutrophil migration toward the supernatants of monocytes stimulated with LPS or toward IL-8 added to the bottom chamber (data not shown). The remaining ~40% of neutrophil migration may be due to another chemokine and/or other functional molecule(s) for which the secretion is independent of the presence of nucleotides. As our work focused on nucleotide-dependent signalling, this unidentified molecule was not studied. Note that Pam3CSK4 did not trigger neutrophil migration per se in this model (Fig. 2) and that it does not seem to be necessary to remove it from the supernatants before the migration assay. It is also noteworthy that this neutrophil migration was not due to Pam3CSK4-activated IL-8 secretion by HUVEC or neutrophils, which, in these cells, was relatively low and nucleotide-independent (data not shown).
TLR2-activated IL-8 release in monocytic cell lines requires concomitant P2 receptor activation
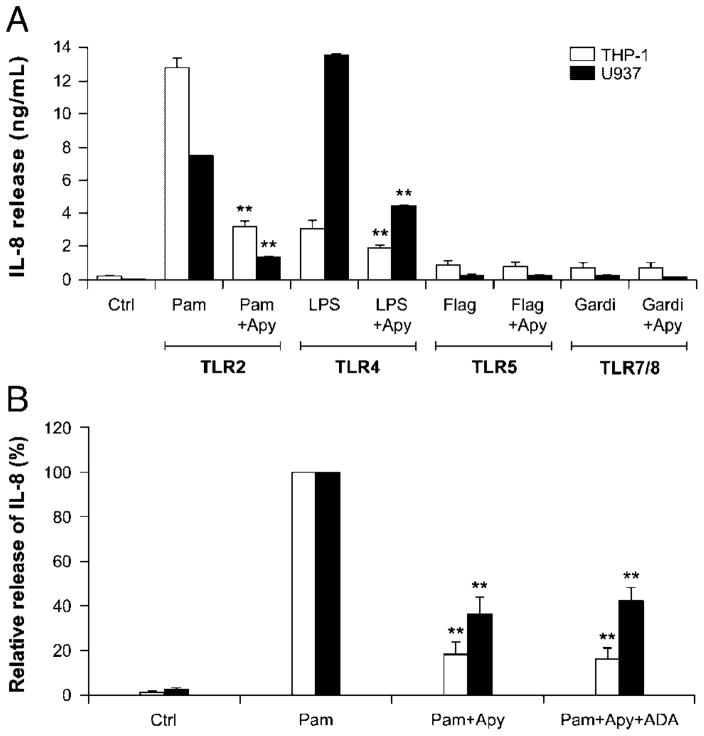
The above neutrophil transmigration assays suggest that extra-cellular nucleotides regulate neutrophil migration by controlling IL-8 release from TLR2-induced human monocytic cell lines. To test this possibility, we determined IL-8 release in the supernatants of cells stimulated in different conditions. We also analyzed the supernatants of THP-1 and U937 cells stimulated with LPS as a positive control [14]. In both monocytic cell lines, the stimulation was carried out for 2 and 18 h as IL-8 secretion accumulates over time [20, 21]. As the stimulation of monocytic cells with Pam3CSK4 yielded ten-fold more IL-8 secretion at 18 h than at 2 h, the former stimulation time was used in subsequent experiments. Apyrase specifically inhibited IL-8 release induced by Pam3CSK4 (80% and ~75% decrease for 2 and 18 h, respectively; data not shown for 2 h and Fig. 3A for 18 h). The fact that the secretion was affected at these two time points implies that both release and synthesis are involved. The inhibition of IL-8 secretion (Fig. 3) and neutrophil migration (Fig. 2) by apyrase suggest both the involvement of extracellular nucleotides in these processes and that the intrinsic ectonucleotidase activity at the surface of monocytes is insufficient to prevent P2 receptor activation caused by Pam3CSK4 treatment, at least not completely.
Figure 3.
Among monocytic expressed TLR, TLR2 and TLR4 induce IL-8 release that is dependent on the presence of extracellular nucleotides. (A) THP-1 (empty bars) and U937 (filled bars) cells (106/mL) were stimulated for 18 h with the indicated PAMP (Pam3CSK4, Pam, 1 μg/mL; lipopolysaccharide, LPS, 100 ng/mL; flagellin, Flag, 100 ng/mL; gardiquimod, Gardi, 1 μg/mL) or vehicle (control, Ctrl) in the presence or absence of a nucleotide scavenger (recombinant apyrase, Apy, 2 U/mL) as well as in the additional presence or absence of adenosine deaminase (ADA, 2 U/mL), where indicated. Apyrase and/or ADA were added to the cells 15 min before the addition of the indicated PAMP. Secreted IL-8 was measured by ELISA. (B) Relative release of IL-8. Release of IL-8 induced by Pam3CSK4 where 100% represents 12.9 ng/mL for THP-1 and 7.5 ng/mL for U937. Data show mean+SEM of three or four assays performed in duplicate or triplicate. **p<0.01.
Adenosine is a potent anti-inflammatory molecule that might have partially inhibited IL-8 synthesis/release in the above experiments. Indeed, adenosine might, in theory, have been generated from the hydrolysis of endogenously secreted ATP by endogenous ectonucleotidases and/or exogenous apyrase and endogenous ecto-5′-nucleotidase, and/or alkaline phosphatases. To exclude a potential effect of adenosine in these assays, monocytes were stimulated, together with Pam3CSK4 and apyrase, in the presence of adenosine deaminase (ADA) to convert adenosine to inosine, as we did previously [14]. The addition of exogenous ADA may be necessary as the endogenously expressed enzyme might have been insufficient to hydrolyze the formed adenosine sufficiently rapid, as for intrinsic ectonucleotidases degrading tri- and diphosphonucleosides that did not prevent P2-dependent IL-8 secretion from Pam3CSK4-stimulated monocytes. ADA did not affect IL-8 release in these samples (Fig. 3B). These data excluded the involvement of adenosine from TLR2-induced IL-8 release leaving nucleotide receptors as the only candidate(s) responsible for this effect.
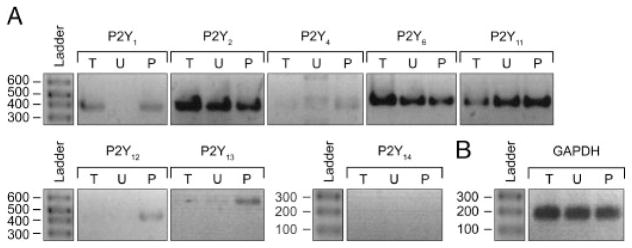
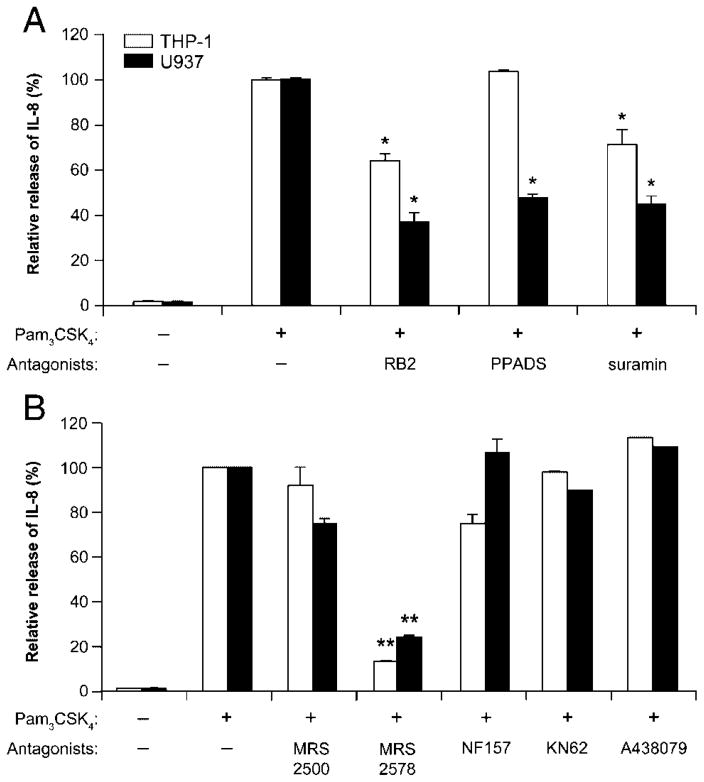
We next aimed to determine the P2 receptor(s) subtype responsible for this IL-8 release. RT-PCR assays showed that both monocytic cell lines tested expressed P2X1, P2X7 (data not shown and [15]), and several P2Y receptors (P2Y1, 2, 6, 11; Fig. 4). To define which of these receptors activated IL-8 release, THP-1 and U937 cells were stimulated with Pam3CSK4 in the presence of the following P2 receptor antagonists: reactive blue 2 (RB2), pyridoxal-phosphate-6-azophenyl-2, 4-disulfonate (PPADS) and suramin (general P2 receptor antagonists), MRS2500 (selective for P2Y1), MRS2578 (P2Y6), NF157 (P2Y11 and P2X1), and KN62 and A438079 (P2X7). In both cell lines, IL-8 release was strongly inhibited by MRS2578 (~80% inhibition), RB2, and suramin (Fig. 5). In U937 cells, IL-8 release was, in addition to the former molecules, partially inhibited by PPADS (Fig. 5A). As NF157, KN62, A438079, and MRS2500 were ineffective, the involvement of P2X receptors, P2Y1 and P2Y11 could be excluded. These data suggested that Pam3CSK4-induced IL-8 release from monocytic cell lines was mediated by the P2Y6 nucleotide receptor. However, since there is no specific antagonist for P2Y2, which is also suramin-sensitive and PPADS-insensitive [22], we could not exclude that this receptor was also involved in monocyte Pam3CSK4-induced IL-8 release. Indeed, further experiments showed that the addition of exogenous agonists of this receptor, 100 μM ATP or UTP, with a suboptimal concentration of Pam3CSK4 (0.1 ng/mL for monocytic cells and 0.3 ng/mL for human primary monocytes) also resulted in IL-8 release (Table 1). Note that the stimulation of monocytes with UDP in the presence of suboptimal concentration of Pam3CSK4 had, as expected, a synergistic effect on IL-8 release from these cells (Table 1). In contrast with a previous work by Warny et al. [15] where their THP-1 cell line released IL-8 exclusively due to UDP, we have observed here that ATP and UTP alone also induced IL-8 secretion in our THP-1 cell line. This discrepancy could be due to the distinct parental monocytic cells used in these two works. Note that it was not surprising that the THP-1 cells investigated here responded to the latter nucleotides as the RT-PCR data for these cells showed P2Y receptors expression responding to these nucleotides (Fig. 4). Note also that lower concentrations of these nucleotides alone were ineffective (data not shown).
Figure 4.
RT-PCR analysis of P2 receptor expression in monocytic THP-1 and U937 cells. RNA extracts were subjected to RT-PCR with P2Y receptor-specific primers (A) and primers for the housekeeping gene GAPDH (B). For each primer set, lanes 1, 2 and 3 correspond to amplifications from THP-1 (T), U937 (U), as well as freshly isolated PBMC (P) used as a positive control, respectively. The molecular size markers (ladder) are shown in base pairs on the left side of each group. The data presented are representative of two independent experiments with two different sets of primers.
Figure 5.
Effect of P2 receptor antagonists on Pam3CSK4-induced IL-8 secretion by monocytic cells. THP-1 and U937 cells (106/mL) were preincubated for 30 min with (A) the non-selective P2 receptor antagonists RB2, PPADS and suramin (100 μM) or (B) selective antagonists of P2Y1 (MRS2500, 1 μM), P2Y6 (MRS2578, 10 μM), P2Y11/P2X1 (NF157, 1 μM) and P2X7 antagonists (KN62, 3 μM or A438079, 10 μM) and subsequently stimulated with Pam3CSK4 (1 μg/mL) for 18 h. Secreted IL-8 was measured by ELISA. Data show mean+SEM of at least three experiments performed with cells at different passages. One hundred percent was set for the release of IL-8 induced by Pam3CSK4, which was consistently in the order of 11 ng/mL for THP-1 and 7 ng/mL for U937. *p<0.05; **p<0.01.
Table 1.
Comparative level of IL-8 secreted by monocytic cells and human primary monocytes (Mo) activated by a suboptimal concentration of Pam3CSK4 in the presence or absence of P2Y2 or P2Y6 agonistsa)
| IL-8 release (pg/mL)
|
|||
|---|---|---|---|
| THP-1 | U937 | Mo | |
| Basal release (vehicle) | 50±1 | 35±2 | 419±9 |
| Pam3CSK4 | 62±2.5§ | 37±5 | 696±9§ |
| ATP | 520±2§§ | 45±0.5 | 690±69NS |
| UTP | 483±1§§ | 40±0.5 | 534±67NS |
| UDP | 484±1§§ | 39±0.5 | 750±26§§ |
| Pam3CSK4+ATP | 1015±5*, †† | 471±5**, †† | 864±12† |
| Pam3CSK4+UTP | 965±5*, †† | 442±5**, †† | 684±11NS |
| Pam3CSK4+UDP | 970±3*, †† | 442±3**, †† | 1747±60*, † |
Cells (1 × 106/mL) were stimulated with suboptimal concentration of
Pam3CSK4 (0.1 ng/mL for THP-1 and U937 cells, or 0.3 ng/mL for Mo)
for 18 and 5 h, respectively, in the presence or absence of ATP, UTP or UDP (100 μM). The freshly isolated monocytes were plated on 24 wells and the next day, the medium was replaced with a fresh one before stimulation. The values presented are the mean±SEM of at least three experiments carried out in duplicate or triplicate. p values were calculated to compare the followings. Each value for nucleotide alone or Pam3CSK4 alone was compared with the one for vehicle control (basal release).
p<0.05;
p<0.01.
Pam3CSK4+either ATP, UTP or UDP was compared with the respective nucleotide alone (*p<0.05; **p<0.01), or with Pam3CSK4 alone (†p<0.05; ††p<0.01). NS, not significant.
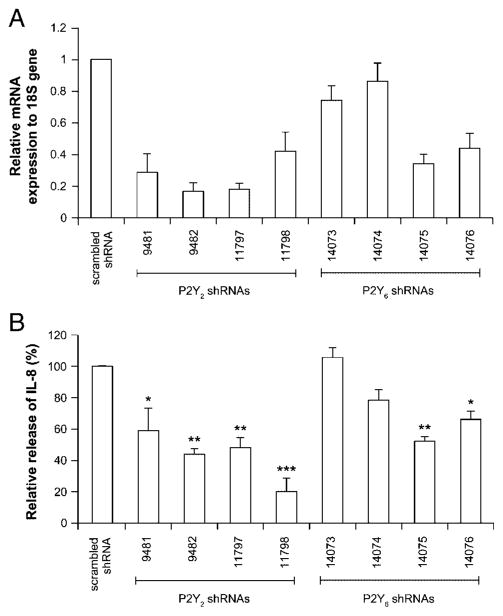
As a more direct test of the role of P2Y2 receptor, and also of P2Y6, in IL-8 release from THP-1 cells, we knockdown these receptors with predesigned shRNA. As shown in Fig. 6A, the relative expression of mRNA for P2Y2 and P2Y6 genes were decreased for most of them by 50–80% when compared with the control cells expressing scrambled shRNA. Due to the lack of reliable Ab against P2Y2 and P2Y6, we were unable to confirm the knockdown of these receptors at the protein level. Interestingly, the incubation of the knockdown cells with Pam3CSK4 resulted in a reduced secretion of IL-8 compared with the scrambled shRNA cells (control; Fig. 6B), suggesting that both P2Y2 and P2Y6 receptors were involved in IL-8 release from THP-1 cells. Note that the two shRNA that did not succeed to reduce the mRNA level of P2Y6, namely 14073 and 14074, were alsoinefficient blocking IL-8 release, further confirming the specificity of our assay.
Figure 6.
Downregulation of either P2Y2 or P2Y6 receptor impairs IL-8 release in THP-1 cells. THP-1 cells (3 × 106/5 mL) were infected with lentivirus carrying scrambled shRNA or shRNA targeting human P2Y2 or P2Y6 mRNA as described in the Materials and methods. (A) Knockdown quantification of P2Y2 and P2Y6 mRNA levels by qRT-PCR. (B) The shRNA treated cells were stimulated with Pam3CSK4 as before and IL-8 secretion measured. Data show mean+SEM of four different experiments, each performed in duplicate or triplicate. One hundred percent was set with the media of Pam3CSK4-treated THP-1 and represents 18±4 ng/mL secreted IL-8. *p<0.05; **p<0.01; ***p<0.001.
Extracellular nucleotides/P2 receptors are involved in IL-8 secretion by Pam3CSK4-stimulated human primary monocytes
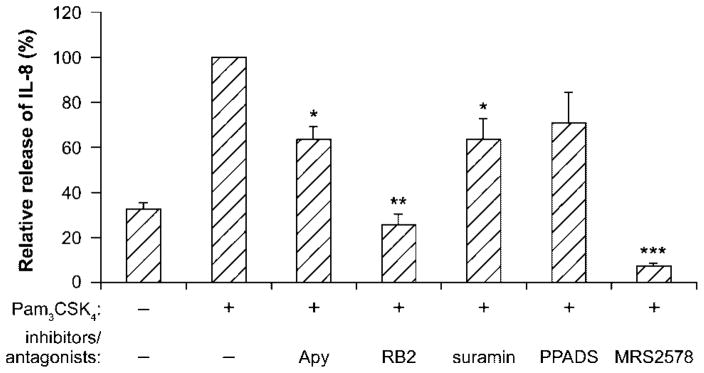
The above experiments showed that IL-8 release activated by TLR2 stimulation with Pam3CSK4 of both monocytic cell lines was mediated by extracellular nucleotides. To confirm this finding in human primary monocytes, these cells, freshly isolated from human peripheral blood, were stimulated with Pam3CSK4 in the presence or absence of either apyrase or P2 antagonists. In agreement with the results obtained for monocytic cells, IL-8 release from primary monocytes was strongly inhibited by all inhibitors used except PPADS (Fig. 7). These data suggest that, as in monocytic cell lines, extracellular nucleotides also mediate IL-8 secretion from Pam3CSK4-stimulated human primary monocytes. Note that the concentration of Pam3CSK4 used for priming monocytes was one hundredth of the one used for the monocytic cell lines, which was determined by dose-response curves (data not shown).
Figure 7.
Extracellular nucleotides/P2 receptors are involved in IL-8 secretion by Pam3CSK4-stimulated human primary monocytes. Huma n primary monocytes (5 × 105/mL) were stimulated with Pam3CSK4 (10 ng/mL) for 5 h in the presence or absence of semi-purified apyrase (2 U/mL) and/or P2 receptor antagonists (100 μM RB2, suramin, PPADS, or 10 μM MRS2578, respectively). Secreted IL-8 was quantified by ELISA. Data show mean+SEM of six experiments, each performed in duplicate or triplicate using cells from different blood donors. One hundred percent was set with the media of Pam3CSK4-treated monocytes and represents 18±7 ng/mL secreted IL-8. *p<0.05; **p<0.01; ***p<0.001.
Extracellular nucleotides are not involved in TLR5- and TLR8-induced IL-8 release by monocytic cells
As THP-1 and U937 also express TLR activated by PAMP other than LPS and lipopeptides (TLR5, 7/8) [23, 24], we next examined whether or not the stimulation of these receptors induce IL-8 release, and if so, if it was in a nucleotide-dependent manner, as for LPS and Pam3CSK4. TLR5 was stimulated with flagellin and TLR7/8 with gardiquimod. The involvement of extracellular nucleotides was again tested with apyrase. In both monocytic cell lines, apyrase did not affect IL-8 release induced by flagellin or gardiquimod, which were however far less potent to induce IL-8 release than LPS and Pam3CSK4 (Fig. 3A).
Discussion
This study shows that extracellular nucleotides are essential for TLR2 to induce neutrophil migration in vivo and in vitro. Indeed, the co-administration of Pam3CSK4 (an agonist of TLR1/2) with apyrase to deplete extracellular nucleotides significantly diminished neutrophil migration in the mouse air pouch compared to neutrophil migration in the air pouches administered with Pam3CSK4 alone (Fig. 1). This suggests that the administration of Pam3CSK4 in the air pouch induces the release of nucleotide(s) that serve as danger signal molecules triggering neutrophil recruitment via P2 receptor activation. In agreement with these data, apyrase also inhibited Pam3CSK4-induced migration of human neutrophils through the layer of endothelial cells in a Boyden chamber assay. Specifically, the media of monocytes stimulated with Pam3CSK4 in the presence of apyrase induced markedly lesser neutrophil migration than the media of monocytes stimulated in the absence of this enzyme, which correlated with IL-8 content of these supernatants. We further confirmed that IL-8 secretion from Pam3CSK4-treated monocytic THP-1 and U937 cells, as well as human primary monocytes, is nucleotide-dependent and defined two receptors involved in this process. Indeed, IL-8 secretion was significantly reduced by apyrase, general P2 receptor antagonists, a specific P2Y6 antagonist, as well as by shRNA targeting P2Y2 and P2Y6. This nucleotide-dependent IL-8 secretion from Pam3CSK4-stimulated monocyte may be of primary importance in Gram-positive bacteria infection as to our knowledge and experience, other cells like endothelial cells (HUVEC) and human primary neutrophils produce only little amounts of IL-8 in response to TLR2 stimulation (data not shown).
Obviously, neutrophil migration in the air pouch did not involve IL-8 as mice do not produce this chemokine. Studies suggest that the chemokine MIP-2 is a mouse counterpart of IL-8. Interestingly, Bar et al. [25] showed that UDP via P2Y6 activation potentiates LPS-induced release of MIP-2 from WT mouse peritoneal macrophages and that this effect is absent in P2Y6 deficient macrophages. The authors however did not test whether LPS or other PAMP such Pam3CSK4-induced MIP-2 release was nucleotide-dependent and whether UDP would also synergize with these molecules. In our assays, apyrase did not affect the production of MIP-2 in Pam3CSK4-treated air pouches (data not shown). It is possible that Pam3CSK4-induced neutrophil migration in the air pouch may be mediated by chemokines other than MIP-2. For example, CXCL12 (SDF1-α) enhances human CD34+ hematopoietic stem cells migration induced by UTP [26]. Alternatively, this migration could involve chemokine-independent mechanisms such as the activation of the neutrophil P2Y2 receptor that is instrumental for the migration of these cells caused by the Gram-positive bacteria Staphylococcus aureus in vivo [27] or expression of adhesion molecules involved in neutrophil migration, e.g. integrins [28].
The results presented in this study show that a concomitant activation of TLR2 and nucleotide receptors is required for neutrophil migration both in vivo and in vitro. It is likely that nucleotides involved in this effect may be liberated from monocytes as a result of TLR2 activation but it is not excluded that this synergistic effect involves a spontaneous (basal) release of nucleotides by monocytes such as the one observed in lymphocytes. Interestingly, these cells are able to maintain micromolar pericellular ATP concentrations called ATP halo that are sufficient for purinergic receptor activation [29]. However, this ATP halo is somehow resistant to hydrolysis by apyrase and therefore unlikely to be involved in Pam3CSK4-induced IL-8 secretion from monocytes, which was inhibited by this enzyme (Fig. 3). In line with the synergism between TLR2 and nucleotides, the addition of exogenous nucleotides increased IL-8 secretion due to a suboptimal concentration of Pam3CSK4 (Table 1).
We have shown here that the monocyte P2Y6 stimulation was involved in TLR2-activated IL-8 release from human monocytes. This nucleotide receptor is also required for IL-8 secretion due to TLR4 responding to LPS of Gram-negative bacteria in both THP-1 and human primary monocytes [14, 15, 30]. The P2Y6 receptor has also been shown to activate the release of other cytokines and chemokines, such as TNF-α, MCP-1 and IP-10 from U937 cells, and CCL20 (MIP-3α) from human monocytes and monocyte-derived dendritic cells [30, 31]. It is noteworthy that in the latter cells, CCL20 release could also be induced by ATP-γS, suggesting that again two or more P2 receptors can be involved [31]. The results presented in Fig. 6, which are in agreement with the one depicted in Fig. 5, suggest also a novel role of the P2Y2 receptor in IL-8 release. We have to note that our previous works did not exclude a function of P2Y2 in LPS-induced IL-8 secretion [14, 15]. Other previous studies showed that the activation of this receptor was coupled to MCP-1 release from rat alveolar and peritoneal macrophages [22] and IL-6 release from human airway epithelia [32].
Altogether, the results reported in this paper suggest that the P2Y2 and P2Y6 receptors may play an important role in the innate immune response resulting from TLR2 activation. As IL-8 secreted by a variety of inflammatory cells including monocytes has been implicated in a number of inflammatory/infectious diseases, such as rheumatoid arthritis [33], inflammatory bowel disease [34], psoriasis [35, 36], palmoplantar pustulosis [37, 38], asthma and chronic obstructive pulmonary disease [39], targeting P2Y2 and/or P2Y6 receptors may reveal as novel therapeutic approaches to fight these diseases. These data are also in agreement with a new concept proposing that extracellular nucleotides serve as endogenous danger-signaling molecules called alarmins that are rapidly released in stress conditions and resolve the innate immune response [40].
Materials and methods
Materials
LPS from Escherichia coli O111:B4, nucleotides (ATP, ADP, UTP and UDP), PPADS and suramin were purchased from Sigma Chemical (St. Louis, MO, USA). Pam3CSK4, flagellin from Salmonella typhimurium and gardiquimod were purchased from Invivogen (San Diego, CA, USA). ADA was provided by Roche Diagnostics (Indianapolis, IN, USA). MRS2500, MRS2578, NF157, KN62 and A438079 were purchased from Tocris Bioscience (Bristol, UK) and RB2 from ICN Biochemicals, (Aurora, OH, USA). Recombinant and semi-purified potato apyrase grade VII were bought from New England BioLabs (Ontario, Canada) and Sigma, respectively. Human recombinant IL-8 (hrIL-8) was purchased from Medicorp (Montreal, Canada), IL-8 neutralizing Ab MAB208 from R&D Systems (Minneapolis, MN, USA) and a matching isotype mouse IgG1 Ab (used as a control in Boyden chamber transmigration assays) from Sigma. RPMI-1640 medium, FBS (heat-inactivated by a 30 min incubation at 56°C), HEPES, sodium pyruvate and glutamate were obtained from Wisent (St-Bruno, Canada) and glucose from Fisher (NJ, USA).
TLR agonists (Pam3CSK4, LPS, flagellin and gardiquimod), nucleotides and P2 receptor antagonists (RB2, PPADS, suramin, MRS2500, NF157 and A438079) were reconstituted in endotoxin-free water (Sigma). Prior to cell stimulation, LPS was sonicated for 10 min. Before use, ADA and recombinant apyrase were dialyzed against sterile 0.9% NaCl and 10 mM HEPES, pH 7.4, in a Slide-A-Lyser cassette (MWCO 3500, Pierce, Rockford, IL, USA). Dialysis was performed to remove ammonium sulfate present in the commercial preparation of ADA that induces calcium mobilization in cells and glycerol present in the preparation of recombinant apyrase that induced non-specific IL-8 release in human primary monocytes. For P2 receptor inhibition assays, MRS2578 and KN62 were dissolved in sterile DMSO (Sigma) at the concentration of 100 and 1.5 mM, respectively, and further diluted with RPMI-1640 plus 10% heat-inactivated FBS to obtain 10 and 3 μM, respectively. Appropriate controls containing 0.01 and 0.2% DMSO were performed.
Neutrophil migration in vivo (mouse air pouch)
The animal protection committee of Université Laval (Québec, QC, Canada) approved the experimental protocol. Air pouches were formed on the dorsum of 10–12 wk old female CD-1® mice (Charles River, St.-Colomban, Canada) by subcutaneous (sc) injection of 4 mL of sterile air on day 0 and 3 mL on day 4. On day 7, samples of 1 mL containing Pam3CSK4 (10 ng/mL), with or without apyrase (2 U), or the diluent (PBS) alone were injected into the air pouches. The mice were sacrificed 4 h later by CO2 asphyxiation. The cells that migrated in the pouches were collected by a 2 mL wash with 5 mM EDTA in sterile PBS, and counted with a hemocytometer. Leukocyte subpopulations were distinguished by flow cytometry, which showed that neutrophils accounted for 80–95% of accumulated cells while other cell populations (e.g. monocyte/macrophage) were negligible.
Neutrophil migration in vitro
Neutrophil transmigration assay was carried out in a Boyden chamber system [14]. Briefly, cell culture inserts (3 μM pore size) were used to form dual compartments in a Falcon™ 24 well culture plate (Becton Dickinson, Franklin Lakes, NJ, USA). The polyethylene membrane filters (6.4 mm diameter) of the inserts were coated successively with 1% w/v gelatine (overnight; Sigma Chemical), 0.006% v/v stabilized human fibronectin (2 h; Biomedical Technologies, Stoughton, MA, USA) and 15 × 104 HUVEC (2 days to become confluent). For neutrophil migration, the supernatants of Pam3CSK4-stimulated cells (0.7 mL) were loaded to the lower compartment of the Boyden chamber and freshly isolated human neutrophils (106 cells in 0.2 mL RPMI-1640 with 5% heat-inactivated FBS) to the upper chamber on the HUVEC monolayer. In each assay, positive control for neutrophil migration was performed using hrIL-8 (2 ng/mL) in culture medium of THP-1 and U937 and also a negative control using culture medium alone was done. Neutrophils were allowed to migrate for 3 h at 37°C and 5% CO2. The transmigrated neutrophils in the bottom chamber were collected and counted with a hemocytometer. Depending on the assay, from 0.3 to 0.63 × 106 of the neutrophils loaded to the upper chamber for migration were recruited to the bottom chamber by media of Pam3CSK4-stimulated monocytes. These numbers are expressed as 100% in Fig. 6.
Cell lines
Human acute monocytic leukemia THP-1 cells (a kind gift from Dr. S. Bourgoin, CRRI, CRCHUL, Québec) were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 10 mM HEPES and 1 mM sodium pyruvate in a humid atmosphere containing 5% CO2. Human leukemic monocytic lymphoma cells U937 (a kind gift from Dr. M. J. Tremblay, CRI, CRCHUL, Québec) were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 10 mM HEPES, 1 mM sodium pyruvate, 1 mM glutamate and 4.5 g/mL glucose in a humid atmosphere containing 5% CO2. Both THP-1 and U937 cells were cultured for up to 1 month at a density of 5–8 × 105 cells/mL, which was maintained by passages performed every 2–3 days. HUVEC (Cambrex Bio Science, Walkersville, MD, USA) were cultured in a complete EGM Bulletkit medium (Cambrex Bio Science). Cells were used for experiments between passage four and five.
Isolation of human primary neutrophils and monocytes
Human primary monocytes and neutrophils were isolated as described before [14]. Briefly, venous blood of healthy volunteers was collected on isocitrate anticoagulant solution and centrifuged (250 g, 10 min, 24°C), and the resulting platelet-rich plasma was discarded. Leukocytes were obtained following erythrocyte sedimentation in 2% Dextran T-500 and centrifuged (525 g, 20 min, 24°C) through a 10 mL Ficoll-Paque cushion (Wisent). Neutrophils were recovered from the pellet and PBMC from the interface. Neutrophils were subjected to a 15 s hypotonic lysis to remove contaminating erythrocytes, whereas monocytes were purified further from PBMC by depleting contaminating lymphocytes. To this end, PBMC were mixed with an equal volume of HBSS containing Ca2+, seeded on a 5 cm petri dish and allowed to adhere for 30 min at 37°C. Nonadherent lymphocytes were discarded while the attached monocytes were washed four times with PBS without Ca2+ and subsequently detached by 15 min incubation with accutase (Innovative cell technologies, San Diego, CA, USA) at 37°C. Purified monocytes and neutrophils were centrifuged (1000 g, 5 min, 24°C), resuspended in RPMI-1640 medium with 5% heat-inactivated FBS and used for the experiments described in the following subsections Monocytes stimulation and conditioned media and Neutrophil migration in vitro.
Monocyte stimulation and conditioned media
Monocytic cells (THP-1 or U937; 106 cells/mL) or human primary monocytes (5 × 105 cells/mL) were placed in a 5 mL sterile tube and stimulated with the following PAMP: Pam3CSK4 (1 μg/mL for the cell lines and 10 ng/mL for primary monocytes), LPS (100 ng/mL), flagellin (100 ng/mL) or gardiquimod (1 μg/mL), in the presence or absence of recombinant apyrase (2 U/mL) or P2 receptor antagonists (added to the cells 30 min before the addition of the stimulus). The stimulation of THP-1 and U937 cells were carried out for 18 h and human primary monocytes for 5 h at 37°C in a humid atmosphere containing 5% CO2. The resulted conditioned media were centrifuged (1000 g, 10 min, 24°C), the supernatants collected and frozen at −80°C until used for IL-8 quantification by ELISA and/or neutrophil transmigration assays.
IL-8 ELISA
IL-8 secreted by monocytic cell lines and human primary monocytes was quantified by Flexia ELISA kit (Medicorp), following the manufacturer’s instructions. The recombinant protein hrIL-8 was used as a standard.
RT-PCR
Total RNA was isolated from the cells using Trizol as recommended by the manufacturer (Invitrogen) and quantified spectro-photometrically at 260 nm. One microgram of RNA was reverse transcribed to cDNA in 20 μL of the reaction mixture containing 50 μM oligo(dt)20, 10 mM dNTPs, 40 U/mL RNase Out, 200 U/μL Superscript III reverse transcriptase dissolved in the supplied buffer (all from Invitrogen) and 0.1 mM dithiothreitol (Roche, Ontario, Canada). The reactions were performed for 60 min at 50°C and stopped by heating at 70°C for 15 min followed by the addition of 2 U E. coli RNase H (Invitrogen). The PCR reactions were performed in 25 μL of the reaction mixture containing 1 μL cDNA, 10 pmol of the primer, 10 mM dNTP, 5 U/μL of Taq DNA polymerase (New England BioLabs). After initial denaturation for 4 min at 94°C, the amplifications were carried out for 35 cycles of denaturation at 95°C for 45 s, annealing at primer specific temperature for 45 s and extension at 74°C for 45 s. The PCR was ended by 7 min incubation at 74°C. The same program was used for the amplification of the gene of reference, which was GAPDH. Sequences of primers, expected PCR fragment sizes and human sequence GenBank accession numbers are listed in Table 2. As a control for contaminations of the RNA preparation with genomic DNA, the crude product of the RNA extraction procedure without any reverse transcription reaction was used as template for a PCR reaction. No signal was detected in these samples without cDNA synthesis indicating that they were free of genomic DNA. RT-PCR products were separated on 1.8% agarose gels containing ethidium bromide and photographed under UV illumination.
Table 2.
Primer pairs for PCR and products size
| Gene | Forward primer | Reverse primer | Genbank | Length (bp) |
|---|---|---|---|---|
| RT-PCR | ||||
| P2RY1 | CCCTGGGCCGGCCTCAAAAAGAAGAATG | CAAGCCGGGCCCTCAAGTTCATCGTTTTC | NM_002563 | 389 |
| P2RY2 | GCTACAGG GCCGCTTCAACGAGGACTTC | GGCAGGCCAGCACCAACACCCACAC | NM_176072 | 429 |
| P2RY4 | CCACCTGGCATTGTCAGACACC | GAGTGACCAGGCAGGGCACGC | NM_176072 | 425 |
| P2RY6 | CCCTGCTGGCCTGCTACTGTCTCCTG | CTAATTCTCCGCATGGTTTGGGGTTGG | NM_002565 | 456 |
| P2RY11 | CCCCCGCTGGCCGCCTACCTCTATCC | CGCAGCCCAACCCCGCCAGCACCAG | NM_176797 | 397 |
| P2RY12 | CTAAGATTCTCTCTGTTGTCATCTG | ACAGAGTGCTCTCTTTCACATAG | NM_002566 | 432 |
| P2RY13 | TGTGTCGTTTTTCTTCGGTG | TGCTGCCAAAAAGAGAGTTG | NM_022788 | 578 |
| P2RY14 | CGCAACATATTCAGCATCGTGT | GCTGTAATGAGCTTCGGTCTGAC | BC041116 | 102 |
| P2RX1 | GCTACGTGGTGCAAGAGTCA | GTAGTTGGTCCCGTTCTCCA | NM_014879 | 215 |
| P2RX7 | AAGCTGTACCAGCGGAAAGA | GCTCTTGGCCTTCTGTTTTG | NM_002562 | 202 |
| GAPDH | CGACCACTTTGTCAAGCTCA | AGGGGTCTACA GGCAACTG | NM_002046 | 228 |
| qPCR | ||||
| P2RY2 | Z-tail-GCATCCTGACCTGGAGAGCAa) | CAGGTCTGCTGCCATCGC | NM_002564 | 51 |
| P2RY6 | GGCCTCCCTGAACATAGGAAA | Z-tail-CATTGTCCCATTCCATGGC | NM_004154 | 52 |
| 18S | Z-tail-CGGTACAGTGAAACTGCGAATG | CCAAAGGAACCATAACTGATTTAATGA | M10098 | 51 |
The Z-tail for qPCR is ACTGAACCTGACCGTACA. It was excluded from the qPCR product lengths presented in base pairs.
P2Y2 and P2Y6 knockdown
A partial P2Y2 and P2Y6 depletion was achieved by lentiviral infection of THP-1 cells according to the protocol adapted from the Invitrogen’s ViraPower™ Lentiviral Expression System by the Gene Expression Lab. Lentiviral vectors containing scrambled shRNA (control) or shRNAs targeting human P2Y2 (clones 9481, 9482, 11797 and 11798; Sigma) and P2Y6 (clones 14073, 14074, 14075 and 14076; Sigma) were produced in 293FT cells. On the day of infection (day 1), 3 × 106 THP-1 cells (in a T-25 cell culture flask containing 5 mL of the RPMI with 6 μg/mL Polybrene) were incubated with the indicated viral vector overnight at 37°C in a humid atmosphere containing 5% CO2. On day 2, the medium was replaced with fresh THP-1 culture medium. On day 3, fresh RPMI containing puromycine (0.75 μg/mL) was added to select for stably infected cells and the medium was then replaced every 3 days for 15 days with a fresh one. Depletion of P2Y2 and P2Y6 mRNA in positively selected THP-1 cells was confirmed by quantitative qRT-PCR (“Centre de Génomique de Qué,” CHUQ, Canada; Table 2). The P2Y2 and P2Y6 depleted cells and control THP-1 cells infected with lentivirus expressing scrambled shRNA were used for IL-8 secretion assays.
Statistical analysis
Student’s t-test was performed using Excel software (Microsoft® Office OneNote™ 2003).
Acknowledgments
We thank Mr. S. Simard from the Gene Quantification core laboratory of the Centre de Génomique de Québec for qPCR analysis. This work was supported by grants to J. S. from the Canadian Institutes of Health Research (CIHR; MOP-68957 and MOP-93683) and from The Arthritis Society of Canada (TAS; RG07/127). J. S. was a recipient of a new investigator award from the CIHR, F. B. Y. of a scholarship from Fonds de la Recherche sur l’Arthrite et les Maladies Rhumatismales (FRAMR) and F. K. of a fellowship from the CIHR/Wyeth Pharmaceuticals.
Abbreviations
- ADA
adenosine deaminase
- HUVEC
human umbilical vein endothelial cells
- PPADS
pyridoxal-phosphate-6-azophenyl-2, 4-disulfonate
- RB2
reactive blue 2
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 3.Ishii KJ, Uematsu S, Akira S. “Toll” gates for future immunotherapy. Curr Pharm Des. 2006;12:4135–4142. doi: 10.2174/138161206778743484. [DOI] [PubMed] [Google Scholar]
- 4.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans. 2003;31:637–642. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- 7.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Kim HY, Lee JY, Kim KH, Jang MK, Lee JH, Yoo JY, et al. Macrolide-affected Toll-like receptor 4 expression from Helicobacter pylori-infected monocytes does not modify interleukin-8 production. FEMS Immunol Med Microbiol. 2005;44:171–176. doi: 10.1016/j.femsim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 12.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Kukulski F, Ben Yebdri F, Lefebvre J, Warny M, Tessier PA, Sévigny J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81:1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warny M, Aboudola S, Robson SC, Sévigny J, Communi D, Soltoff SP, Kelly CP. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 17.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 18.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martin-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 20.Palfreyman RW, Watson ML, Eden C, Smith AW. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect Immun. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messer RL, Lewis JB, Wataha JC, Adams Y, Tseng WY. Cytokine secretion from monocytes persists differentially after activator removal-One mechanism of long-term biological response to implants. J Biomed Mater Res B Appl Biomater. 2007;83:58–63. doi: 10.1002/jbm.b.30766. [DOI] [PubMed] [Google Scholar]
- 22.Stokes L, Surprenant A. Purinergic P2Y2 receptors induce increased MCP-1/CCL2 synthesis and release from rat alveolar and peritoneal macrophages. J Immunol. 2007;179:6016–6023. doi: 10.4049/jimmunol.179.9.6016. [DOI] [PubMed] [Google Scholar]
- 23.Omueti KO, Beyer JM, Johnson CM, Lyle EA, Tapping RI. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem. 2005;280:36616–36625. doi: 10.1074/jbc.M504320200. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- 26.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 28.Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- 29.Yegutkin GG, Mikhailov A, Samburski SS, Jalkanen S. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ectoadenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17:3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox MA, Gomes B, Palmer K, Du K, Wiekowski M, Wilburn B, Petro M, et al. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467–473. doi: 10.1016/j.bbrc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Marcet B, Horckmans M, Libert F, Hassid S, Boeynaems JM, Communi D. Extracellular nucleotides regulate CCL20 release from human primary airway epithelial cells, monocytes and monocyte-derived dendritic cells. J Cell Physiol. 2007;211:716–727. doi: 10.1002/jcp.20979. [DOI] [PubMed] [Google Scholar]
- 32.Douillet CD, Robinson WP, III, Milano PM, Boucher RC, Rich PB. Nucleotides induce IL-6 release from human airway epithelia via P2Y2 and p38 MAPK-dependent pathways. Am J Physiol Lung Cell Mol Physiol. 2006;291:L734–746. doi: 10.1152/ajplung.00389.2005. [DOI] [PubMed] [Google Scholar]
- 33.Seitz M, Dewald B, Gerber N, Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991;87:463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm MC, Elsbury SK, Pavli P, Doe WF. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–98. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder JM, Gregory H, Young J, Christophers E. Neutrophil-activating proteins in psoriasis. J Invest Dermatol. 1992;98:241–247. doi: 10.1111/1523-1747.ep12556058. [DOI] [PubMed] [Google Scholar]
- 36.Wiedow O, Luademann J, Utecht B. Elafin is a potent inhibitor of proteinase 3. Biochem Biophys Res Commun. 1991;174:6–10. doi: 10.1016/0006-291x(91)90476-n. [DOI] [PubMed] [Google Scholar]
- 37.Anttila HS, Reitamo S, Erkko P, Ceska M, Moser B, Baggiolini M. Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J Invest Dermatol. 1992;98:96–101. doi: 10.1111/1523-1747.ep12495817. [DOI] [PubMed] [Google Scholar]
- 38.Ozawa M, Terui T, Tagami H. Localization of IL-8 and complement components in lesional skin of psoriasis vulgaris and pustulosis palmaris et plantaris. Dermatology. 2005;211:249–255. doi: 10.1159/000087019. [DOI] [PubMed] [Google Scholar]
- 39.Stemmler S, Arinir U, Klein W, Rohde G, Hoffjan S, Wirkus N, Reinitz-Rademacher K, et al. Association of interleukin-8 receptor alpha polymorphisms with chronic obstructive pulmonary disease and asthma. Genes Immun. 2005;6:225–230. doi: 10.1038/sj.gene.6364181. [DOI] [PubMed] [Google Scholar]
- 40.Grbic DM, Degagne E, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180:2659–2668. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]