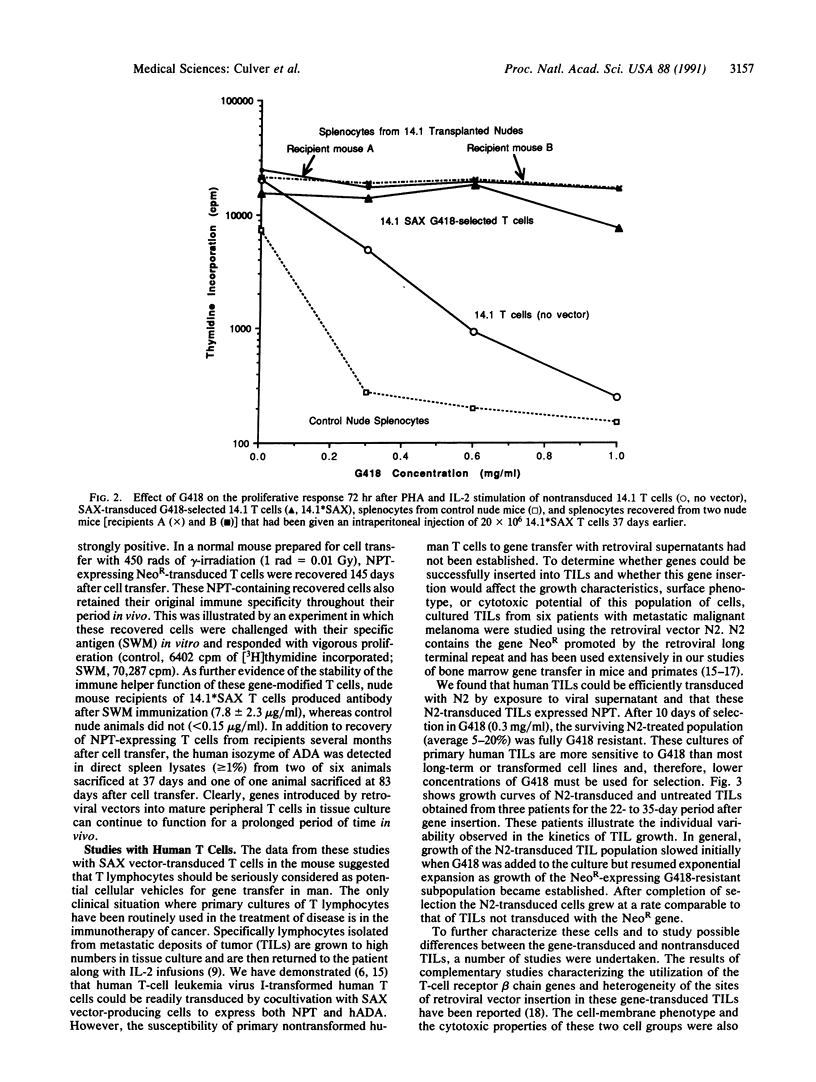
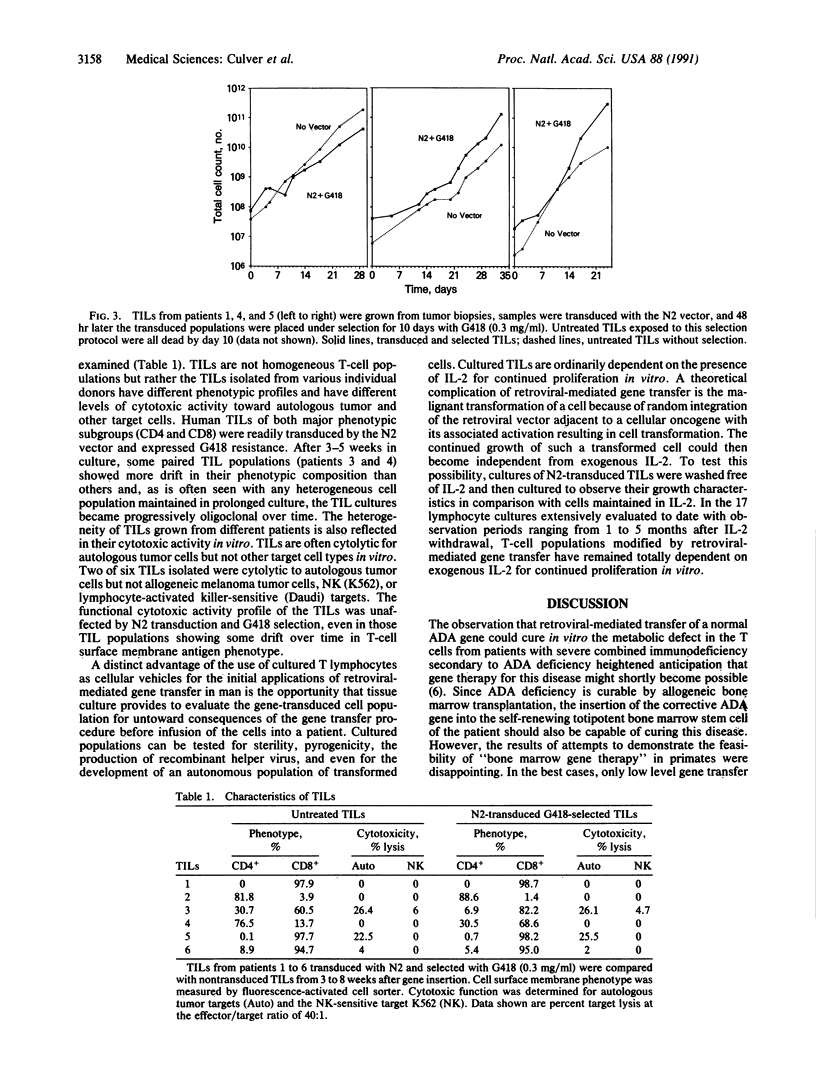
Abstract
The application of bone marrow gene therapy has been stalled by the inability to achieve stable high-level gene transfer and expression in the totipotent stem cells. We show that retroviral vectors can stably introduce genes into antigen-specific murine and human T lymphocytes in culture. Murine helper T cells were transduced with the retroviral vector SAX to express both neomycin-resistance and human adenosine deaminase genes. These cells were expanded in culture and selected for expression of neomycin resistance with G418. The gene insertion, selection, and culture expansion did not alter antigen specificity or growth characteristics of the T cells in vitro. To determine if cultured T cells might be used for gene therapy, their persistence and continued expression of the introduced genes was evaluated in nude mice transplanted with the SAX-transduced T cells. G418-resistant cells could be readily recovered from the spleens of recipients of transduced T cells for several months. In addition, recovered cells continued to produce human adenosine deaminase. Based on these observations, we studied cultured human tumor-infiltrating lymphocytes as a candidate cell for a trial of gene transfer in man. Exponential cultures of interleukin-2-stimulated tumor-infiltrating lymphocytes were efficiently transduced with the neomycin-resistance gene using the retroviral vector N2. Gene insertion and subsequent G418 selection did not substantially alter the growth characteristics, interleukin 2 dependence, membrane phenotype, or cytotoxicity profile of the transduced T cells. These studies provided a portion of the experimental evidence supporting the feasibility of the presently ongoing clinical trials of lymphocyte gene therapy in cancer as well as in patients with adenosine deaminase deficiency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I., Kawamura H., Matis L. A., Berzofsky J. A. T cell clones to two major T cell epitopes of myoglobin: effect of I-A/I-E restriction on epitope dominance. J Immunol. 1985 Oct;135(4):2628–2634. [PubMed] [Google Scholar]
- Cornetta K., Anderson W. F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J Virol Methods. 1989 Feb;23(2):187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Morgan R. A., Osborne W. R., Lee R. T., Lenschow D., Able C., Cornetta K., Anderson W. F., Blaese R. M. In vivo expression and survival of gene-modified T lymphocytes in rhesus monkeys. Hum Gene Ther. 1990 Winter;1(4):399–410. doi: 10.1089/hum.1990.1.4-399. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. Suppression of SV40-promoted gene expression by differentiation of preadipose cells. Genes Dev. 1988 Oct;2(10):1251–1257. doi: 10.1101/gad.2.10.1251. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Morecki S., Aebersold P., Cornetta K., Culver K., Freeman S., Director E., Lotze M. T., Blaese R. M., Anderson W. F. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci U S A. 1990 Jan;87(1):473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn D. B., Kantoff P. W., Eglitis M. A., McLachlin J. R., Moen R. C., Karson E., Zwiebel J. A., Nienhuis A., Karlsson S., O'Reilly R. Retroviral-mediated gene transfer into mammalian cells. Blood Cells. 1987;13(1-2):285–298. [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Cornetta K., Anderson W. F. Applications of the polymerase chain reaction in retroviral-mediated gene transfer and the analysis of gene-marked human TIL cells. Hum Gene Ther. 1990 Summer;1(2):135–149. doi: 10.1089/hum.1990.1.2-135. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Aebersold P., Cornetta K., Kasid A., Morgan R. A., Moen R., Karson E. M., Lotze M. T., Yang J. C., Topalian S. L. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990 Aug 30;323(9):570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Williams D. A. Expression of introduced genetic sequences in hematopoietic cells following retroviral-mediated gene transfer. Hum Gene Ther. 1990 Fall;1(3):229–239. doi: 10.1089/hum.1990.1.3-229. [DOI] [PubMed] [Google Scholar]




