Abstract
Manganese-enhanced magnetic resonance imaging (MRI) is an established neuroimaging method for signal enhancement, tract tracing, and functional studies in rodents. Along with the increasing availability of combined positron emission tomography (PET) and MRI scanners, the recent development of the positron-emitting isotope 52Mn has prompted interest in the use of Mn2+ as a dual-modality contrast agent. In this work, we characterized and compared the uptake of systemically delivered Mn2+ and radioactive 52Mn2+ in the rat brain for MRI and PET, respectively. Additionally, we examined the biodistribution of two formulations of 52Mn2+ in the rat. In MRI, maximum uptake was observed one day following delivery of the highest MnCl2 dose tested (60 mg/kg), with some brain regions showing delayed maximum enhancement 2–4 days following delivery. In PET, we observed low brain uptake after systemic delivery, with a maximum of approximately 0.2 %ID/g. We also studied the effect of final formulation vehicle (saline compared to MnCl2) on 52Mn2+ organ biodistribution and brain uptake. We observed that the addition of bulk Mn2+ carrier to 52Mn2+ in solution resulted in significantly reduced 52Mn2+ uptake in the majority of organs, including the brain. These results lay the groundwork for further development of 52Mn PET or dual Mn-enhanced PET/MR neuroimaging in rodents and indicate several interesting potential applications of 52Mn PET in other organs and systems.
Keywords: manganese-enhanced magnetic resonance imaging (MEMRI, MRI); positron emission tomography (PET); manganese; quantitative imaging; neuroimaging
1. Introduction
The T1-weighted signal-enhancing MRI contrast agent manganese (Mn2+) has been studied and applied in preclinical animal models and human subjects for imaging liver disease, cancer, cardiac viability, and brain function and structure (1–4). To avoid the toxic effects of this metal when using the large doses required for MR signal enhancement, only the chelated form Mn-DPDP (Magnafodipir, Teslascan®) and an oral MnCl2 agent (LumenHance®) have been approved for use in human patients (5). Because unchelated Mn2+ can be transported via Ca2+ channels, uptake and resulting T1 enhancement can indicate function and highlight structure in the heart and brain. Therefore, it has been applied in a variety of preclinical functional imaging studies, primarily in rodents (6–8). Mn2+ enhancement in the myocardium reflects viability, while uptake in the brain can reflect functional activation, connected neuronal tracts, or can be used for general tissue enhancement and cytoarchitecture visualization (7, 9). MnCl2 biodistribution studies in rats have also indicated high uptake of Mn2+ in a variety of other organs and glands including the liver, kidney, salivary glands, and pancreas (10).
Recently, the positron-emitting isotope 52Mn (t1/2 = 5.59 days) has been produced for PET imaging by several groups, including our own (11–16). Two factors have played a role in recent development of 52Mn as a PET contrast agent: first, the general interest in and investigation of longer-lived radiometals for antibody-based PET imaging (17, 18), and second, the development of simultaneous PET/MRI systems (19), with 52Mn2+ having potential as a dual-modality signal-enhancing contrast agent. Because PET imaging typically requires tracer doses, the availability of 52Mn could facilitate the clinical investigation and application of Mn2+-based imaging of function and viability that have been developed in preclinical models with manganese-enhanced MRI (MEMRI).
In the setting of neuroimaging, methods for MEMRI in the rodent have been developed for a variety of applications, including generalized cytoarchitecture enhancement, activation studies, and neuronal tract tracing (2, 9). Along with determining the best way to deliver Mn2+ for appropriate brain uptake and enhancement, several methods have already been proposed to minimize toxicity while delivering the maximum dose possible for sufficient imaging contrast (9, 20). For instance, intrathecal injection and osmotic pumps are intriguing alternative methods for Mn2+ administration (21, 22). Several other works also assert that slow systemic delivery via tail vein infusion can sufficiently reduce toxicity without the need for more specialized delivery techniques (23, 24). Specifically, one such study examined the effect of varying the time between contrast administration and imaging, and confirmed a correlation between R1 relaxation rate (R1=1/T1) and ex vivo elemental analysis of Mn uptake (24). This work showed maximum T1 change at one day following contrast administration, but did not address slightly later time points between 2–4 days, which are of interest to further reduce the potential combined effects of Mn2+ and isoflurane (for imaging anesthesia) on animal health. An additional motivation in our laboratory is related to the uptake of Mn2+ in specific brain regions, such as the striatum, for imaging grafted stem cells (12).
Compared to MEMRI, 52Mn PET has been much less thoroughly investigated in terms of contrast agent delivery, 52Mn biodistribution, PET image acquisition, and image reconstruction and analysis techniques. Few published studies address 52Mn PET, with the majority of these works focusing on production and separation of the isotope rather than in vivo imaging applications (11, 14, 15). As demonstrated by Topping and colleagues (11) and confirmed in our recent studies (12), relatively low brain uptake of 52Mn2+ (less than 0.5% ID/g) is observed in vivo. To improve brain uptake, 52Mn2+ supplementation with bulk MnCl2 may potentially increase circulation time and therefore allow more 52Mn2+ to be transported or diffuse into the brain, both of which are mechanisms of brain Mn2+ uptake (11, 25–28). The supplementation of 52Mn2+ with MnCl2 could also be of interest for simultaneous PET/MRI studies, further prompting more thorough investigation of this contrast agent preparation.
In this study, we characterize and directly compare the uptake of 52Mn2+ and non-radioactive Mn2+ in the rat brain using MRI and PET imaging, using similar contrast delivery and image time course protocols for both modalities. With these experiments, we aim to provide new knowledge regarding the contrast agent composition, dose, delivery method, and imaging time point to maximize contrast enhancement and 52Mn detectability in the rat brain. By testing the uptake and biodistribution characteristics of both no-carrier-added (NCA) and carrier-added (CA) 52Mn2+, we further hope to elucidate whether Mn2+ could be used as a dual-modality signal-enhancing contrast agent for PET/MR of the brain as well as other organs or systems of interest. For our further pursuit of manganese-based imaging of transplanted stem cells (12), the goal of these studies is to select the best contrast agent dose, formulation, delivery method, and imaging time point to facilitate sufficient Mn2+ supplementation for uptake and detection of cells over-expressing a Mn2+ transporter protein.
2. Results
2.1. Brain uptake and efflux measured by MEMRI
To observe the effect of MnCl2 dose and imaging time point on signal enhancement in the brain, we administered three doses of MnCl2 to rats (30, 45, and 60 mg/kg, N=3 per dose group) using slow i.v. infusion as described above. We then performed T1 mapping with MRI over the course of two weeks following MnCl2 administration. R1 relaxation rate (R1 = 1/T1) in the whole brain and in specific brain sub-regions was estimated from a set of variable flip angle spoiled gradient echo (SPGR) acquisitions at each time point.
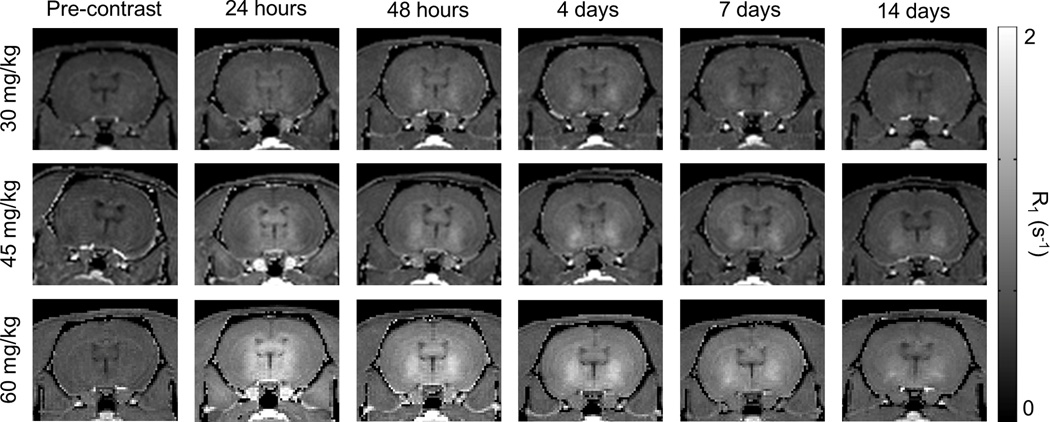
After T1 mapping and manual segmentation, whole-brain R1 enhancement was measured at all time points and doses. Representative R1 maps in a single subject for each dose group over the course of 2 weeks are shown in Fig. 1 and Supplementary Fig. 1. General T1 shortening was observed in the whole brain, with greatest enhancement near the ventricles and diffusing throughout neighboring regions over time. The greatest whole-brain R1 enhancement was observed one day following contrast administration for all doses tested (Fig. 2A). Due to a high level of inter-subject variability of uptake, significant differences were not observed between dose groups, although there was a clear trend toward increased signal enhancement in the rats from the higher dose groups compared to the 30 mg/kg dose group (Figs. 1 and 2A).
Figure 1.
Representative coronal R1 maps of three subjects delivered different doses of MnCl2 (30, 45, and 60 mg/kg). Imaging was performed and R1 maps were calculated prior to contrast administration and at five imaging time points over two weeks following administration. Higher contrast dose increases signal enhancement, particularly near the ventricles. Two weeks following contrast administration, signal enhancement remains, particularly in the vicinity of the striatum and globus pallidus.
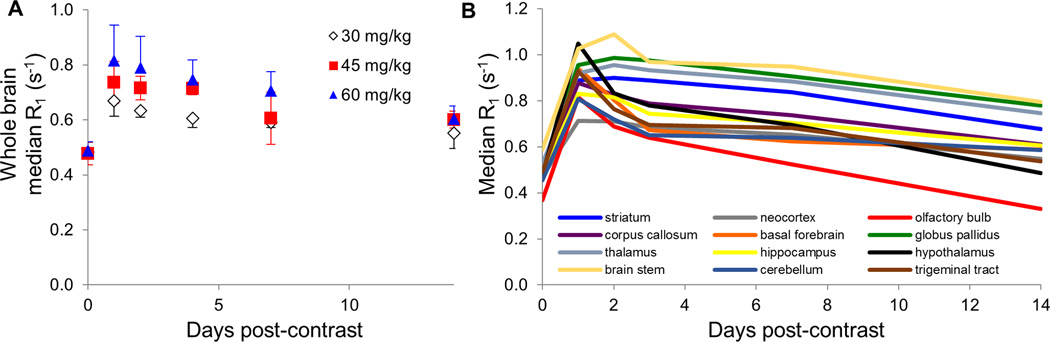
Figure 2.
Uptake of Mn in the whole brain and various brain regions measured with quantitative MRI. (A) Whole-brain average R1 relaxation rate reached a maximum 24 hours following contrast administration regardless of contrast dose ranging from 30–60 mg/kg MnCl2. Efflux from the brain was slow, with some enhancement remaining at the 14 day imaging time point. (B) Regional analysis of uptake of 60 mg/kg MnCl2 based on registration and segmentation of brain volumes to a rat brain atlas. Some brain regions (neocortex, corpus callosum, basal forebrain, hippocampus, hypothalamus, and cerebellum) reached maximum enhancement one day following contrast administration, while others (striatum, globus pallidus, thalamus, and brain stem) in some cases reached maximum at two or four days post-contrast.
Quantification of R1 relaxation rate in specific brain regions was performed via manual segmentation of the brain prior to registration to a Sprague Dawley rat brain atlas for regional delineation. We then determined R1 enhancement in the striatum, relevant for our stem cell imaging work, along with a variety of other brain regions of interest. Median R1 rates were determined in the striatum, neocortex, olfactory bulb, corpus callosum, basal forebrain, globus pallidus, thalamus, hippocampus, hypothalamus, brain stem, cerebellum, and trigeminal tract. The maximum R1 relaxation rate measured in each region for each dose level is shown in Table 1. Some regions, such as the neocortex, corpus callosum, basal forebrain, hippocampus, hypothalamus, and cerebellum, consistently reached maximum signal one day following contrast administration (Table 1 and Fig. 2). Other regions, in particular the striatum, globus pallidus, thalamus, and brain stem, reached their maximum signal level at either 1, 2, or 4 days following contrast administration, depending on dose delivered. Together, these results indicate that up to 60 mg/kg MnCl2 can be delivered for brain imaging between 1–4 days following contrast administration, depending on the enhancement level and brain structure(s) of interest.
Table 1.
Maximum R1 relaxation rate (s−1) reached by brain region and MnCl2 dose delivered, along with corresponding percent increase in R1 with respect to the pre-contrast measurement. Entries are color-coded by the imaging time point at which the maximum signal was reached; red = 24 hours, blue = 48 hours, green = 96 hours (4 days). No regions reached their maximum after the 96 hour imaging time point.
| MnCl2 Dose |
Striatum | Neocortex | Olfactory bulb | Corpus callosum | Basal forebrain | Globus pallidus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 mg/kg | 0.711 | 41% | 0.611 | 33% | 0.685 | 54% | 0.698 | 42% | 0.697 | 40% | 0.743 | 39% |
| 45 mg/kg | 0.825 | 70% | 0.656 | 43% | 0.736 | 76% | 0.766 | 58% | 0.851 | 65% | 0.904 | 72% |
| 60 mg/kg | 0.899 | 84% | 0.713 | 52% | 0.815 | 121% | 0.878 | 77% | 0.939 | 85% | 0.987 | 92% |
| Thalamus | Hippocampus | Hypothalamus | Brain stem | Cerebellum | Trigeminal tract |
|||||||
| 30 mg/kg | 0.747 | 37% | 0.702 | 49% | 0.814 | 48% | 0.8 | 41% | 0.635 | 37% | 0.659 | 48% |
| 45 mg/kg | 0.871 | 67% | 0.768 | 65% | 0.99 | 91% | 0.911 | 59% | 0.693 | 51% | 0.732 | 53% |
| 60 mg/kg | 0.956 | 82% | 0.83 | 74% | 1.049 | 120% | 1.088 | 85% | 0.809 | 78% | 0.929 | 90% |
2.2. Brain uptake of 52Mn2+ measured by gamma counting
To observe brain uptake of no-carrier-added (NCA, formulated in saline) and carrier-added (CA, formulated in 66.7 mM MnCl2 in saline) 52Mn2+, gamma counting was performed on resected rat brains at 24 and 48 hours following contrast agent administration (N=3 per group, Figure 3B). Significant increases in 52Mn2+ brain uptake were observed in subjects delivered NCA 52Mn2+ compared to those delivered CA 52Mn2+, by a factor of greater than 2.5 at both time points evaluated (Fig. 3A and B, p<0.01). Additionally, a trend toward approximately 30% increased uptake at 48 hours compared to 24 hours was observed for both contrast agent forms. The results of gamma counting indicate that significantly higher brain uptake of 52Mn2+ is observed when administered in no-carrier-added form.
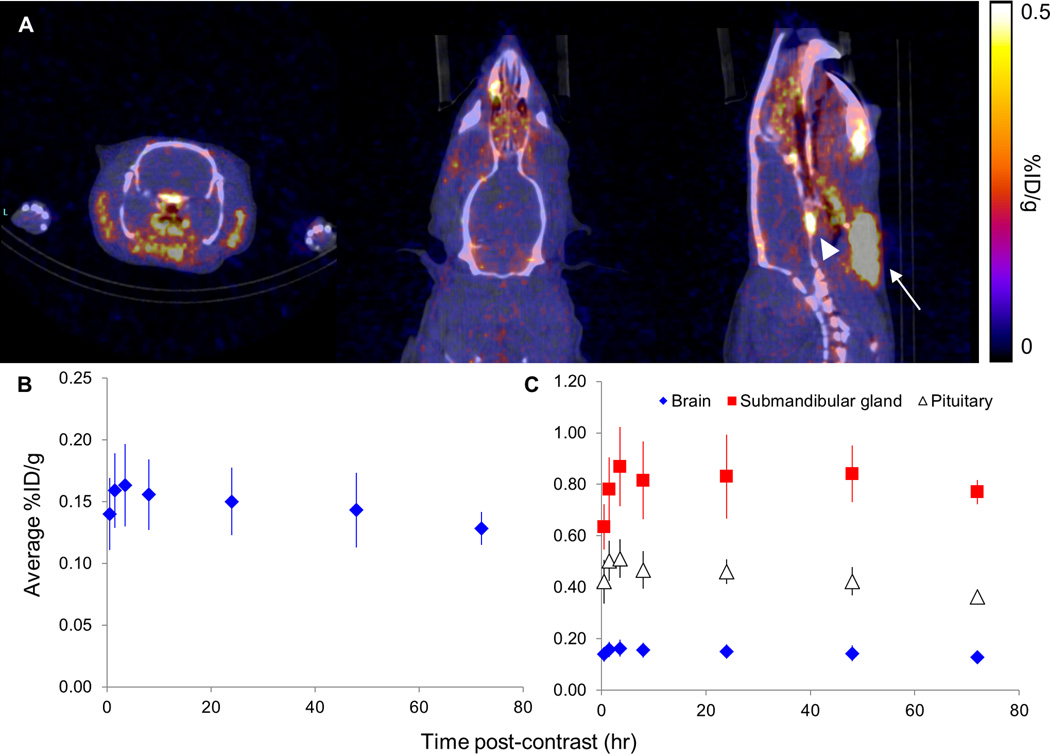
Figure 3.
Comparison of brain uptake of no-carrier-added (NCA) and carrier-added (CA) 52Mn with ex vivo gamma counting and in vivo PET/CT. We observed higher activity uptake of NCA 52Mn in both ex vivo gamma counting measurements of the excised brain at 24 and 48 hours (A, B) as well as in vivo with PET/CT for up to 7 days following contrast delivery (C). Ex vivo measurements are the average of 3 subjects per contrast agent formulation and time point, while in vivo PET/CT was performed on one subject per contrast agent formulation in this initial comparative imaging study.
2.3. In vivo brain uptake of 52Mn
In vivo full-body PET/CT was performed in six rats at various time points following administration of CA 52Mn2+ (N=1) and NCA 52Mn2+ (N=5). In order to examine brain uptake dynamics of the two formulations of 52Mn2+, ROI-based analysis on full-body PET images was used to compare brain uptake between two subjects delivered CA and NCA 52Mn2+, respectively, at multiple time points up to 7 days following administration. PET acquisition times ranged from 30–60 minutes due to the relatively long half-life of 52Mn (t1/2=5.59 days). In the subject delivered CA 52Mn2+, brain uptake between 0.08–0.10 %ID/g was observed, with maximum uptake measured at the earliest time point (24 hours) post injection (Fig. 3C). As expected, in the subject delivered NCA 52Mn2+, higher brain uptake of 0.10–0.16 %ID/g was observed. Surprisingly, the maximum brain uptake was measured at 2 hours following contrast administration, rather than at 48 hours, as gamma-counting results would suggest. Notably, we observed several regions of high 52Mn2+ uptake in the head and neck, including the pituitary and the submandibular glands (Fig. 4A).
Figure 4.
In vivo PET/CT of brain uptake of NCA 52Mn in the rat. (A) Coronal, axial, and sagittal views of the brain (left to right, respectively) from in vivo PET/CT of a rat four hours following administration of no-carrier-added (NCA) 52Mn. Submandibular gland = white arrow, pituitary = white arrowhead. (B) In four subjects, average brain uptake reached a maximum at four hours following contrast administration, after which a slow decrease in brain signal was observed. (C) High 52Mn uptake in the submandibular gland and pituitary, up to 0.869 and 0.512 %ID/g respectively, were measured at all imaging time points.
After observing maximum uptake of NCA 52Mn2+ at the 2 hour imaging time point, we further investigated the uptake dynamics of NCA 52Mn2+ in four additional subjects (Fig. 4). PET/CT images were acquired at multiple time points over the course of 8 hours and at 24, 48, and 72 hours (N=3 at 72 hour time point) following contrast administration. In these subjects, ROI-based time activity curves indicated uptake of 52Mn2+ in the first hour followed by a steady, slow decrease in retention over the course of 3 days (Fig. 4A and B). In order to understand the source of the discrepancy between brain uptake dynamics with gamma counting and PET/CT imaging, ROI-based analysis of submandibular glands and pituitary uptake was also performed on all subjects. Due to high uptake in these regions, signal spillover could affect quantification of adjacent brain uptake measurements (Fig. 4A). The submandibular glands exhibited uptake over the course of 4 hours, reaching maximum uptake of 0.869 %ID/g at 4 hours following contrast administration and followed by a slow decrease over the next 72 hours (Fig. 4C). Similarly, the pituitary showed maximum uptake at 4 hours, reaching 0.512 %ID/g. These signal levels correspond to 5.3 and 3.1 times the whole-brain average uptake at that time point, respectively. Together, these results indicate that although maximum brain uptake of 52Mn is measured in PET/CT 1–4 hours following administration, measurements may be skewed to higher than ground truth due to spillover effects from nearby high signal regions.
2.4. 52Mn full-body biodistribution
Due to the extensive application of MnCl2 as an MRI contrast agent in recent decades, the biodistribution of Mn2+ for rodent MRI has been previously investigated (10). However, the biodistribution and organ uptake of NCA and CA 52Mn has not yet been established in the literature. Therefore, in order to more thoroughly characterize this new PET agent prior to application for imaging tasks, ex vivo measurements of NCA and CA 52Mn2+ uptake in major organs were made at 4 and 48 hours following contrast delivery (Fig. 5A). At four hours following 52Mn delivery, the highest uptake of NCA 52Mn2+ was observed in the kidney (3.02 %ID/g), liver (2.75 %ID/g), pancreas (1.74 %ID/g), stomach (1.50 %ID/g), and submandibular gland (1.36 %ID/g). Interestingly, unlike in other organs, high pancreas and submandibular gland uptake was maintained at the 48-hour time point (2.49 and 1.24 %ID/g, respectively). Intestine uptake also remained relatively consistent from 4 to 48 hours following delivery (1.00 and 0.75 %ID/g, respectively). After CA 52Mn2+ delivery, the highest uptake was observed in the pancreas (1.94 %ID/g), which had approximately 2.5 times higher uptake than the next highest uptake observed in the submandibular gland (0.79 %ID/g). The uptake of CA 52Mn2+ was significantly reduced compared to the uptake of NCA 52Mn2+ at both time points in all organs tested with the exception of the bone, tail, blood, pancreas (significant difference at 4 hour time point only), and intestine (significant difference at 48 hour time point only) (p<0.05).
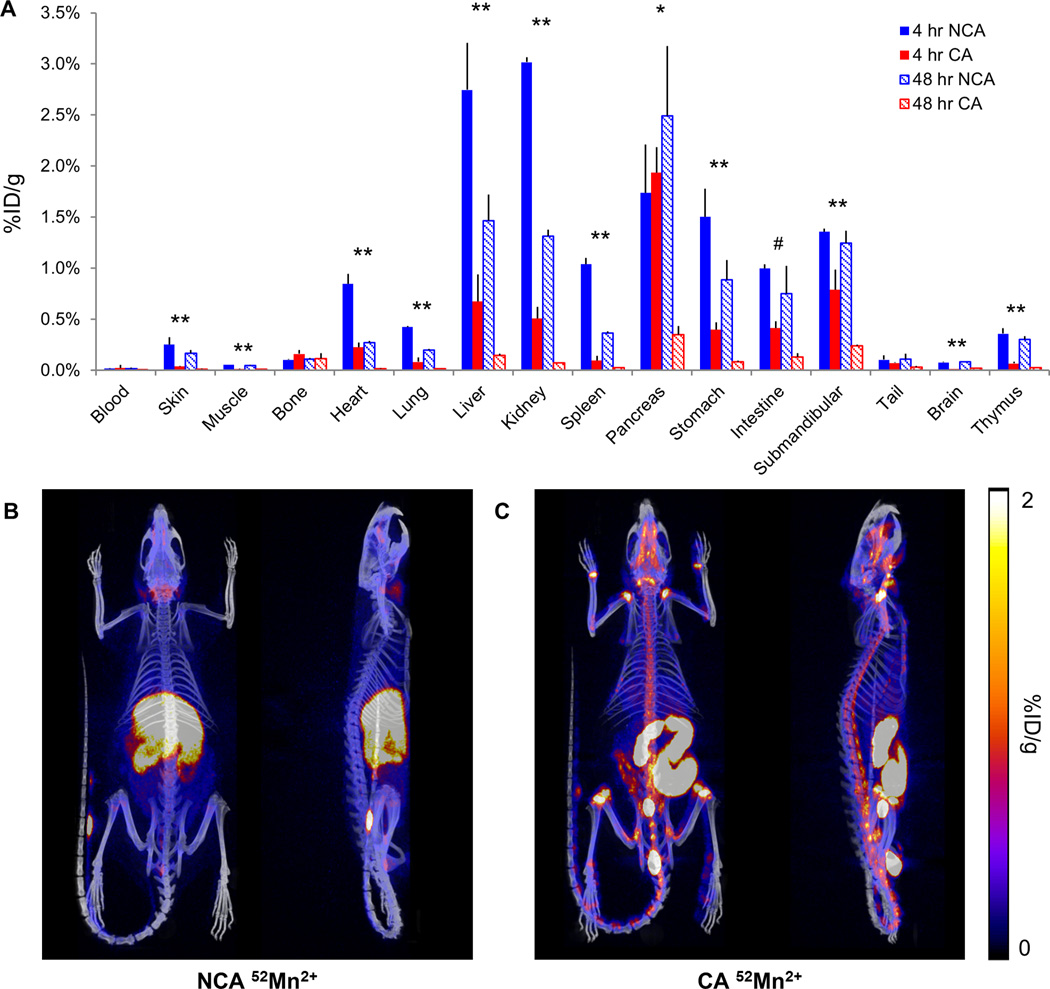
Figure 5.
Biodistribution of no-carrier-added (NCA) and carrier-added (CA) 52Mn in the rat. (A) Ex vivo biodistribution of NCA and CA 52Mn in the rat at 4 and 48 hours following administration; N=3 per contrast agent preparation and time point. A significant increase in uptake of NCA 52Mn compared to CA 52Mn was observed at both time points in the majority of organs tested (**p<0.05). In the pancreas and intestine, this increase was only significant at one time point (*p<0.05 at four hours, #p<0.05 at 48 hours). No significant differences were observed in the blood, bone, or tail. (B–C) Representative PET/CT overlay MIPs of full-body 52Mn uptake at 24 hours following contrast delivery from one subject per contrast agent formulation. (B) In the NCA subject, uptake is observed in the submandibular glands, nasal cavities, liver, kidneys, intestine, pancreas, and spleen. (C) In the CA subject, the majority of uptake is observed in the contents of the intestine and in the vicinity of the intervertebral and synovial joints.
Qualitative observations of CA and NCA 52Mn2+ biodistribution with in vivo PET/CT generally agreed with ex vivo biodistribution studies (Fig. 5B). At 24 hours following NCA 52Mn2+ delivery, high uptake was observed in the liver, kidneys, salivary glands, gastrointestinal tract, pancreas, and spleen. At the same time point following CA 52Mn2+ delivery, uptake was primarily observed in the contents of the intestinal tract and cecum as well as in the vicinity of intervertebral and synovial joints (Fig. 5C). Signal persisted in the gastrointestinal tract (NCA) and joints (CA) at later imaging time points (data not shown). Increased uptake of CA 52Mn2+ compared to NCA 52Mn2+ was observed in synovial and intervertebral joints at all in vivo imaging time points. In all subjects imaged with full-body PET/CT, we observed activity pooling in the tail at the injection site to varying degrees. This was possibly caused by movement of the injection needle during the slow infusion, which lasted between 15–45 minutes depending on bulk MnCl2 dose, and could be a contributor to the inter-subject variability of uptake measurements observed in vivo.
3. Discussion
In this work, we investigated the effects of contrast agent vehicle, dose, and acquisition time point on brain uptake and retention of Mn2+-based contrast agents for MRI and PET. The bulk doses of Mn2+ systemically delivered for MEMRI in the rodent brain are known to cause somnolence as well as side effects in the liver, heart, and brain (29, 30). By using a bulk MnCl2 delivery protocol that utilized slow infusion while maintaining body temperature and hydration, we observed minimal behavior and health side effects, even at the highest dose tested (60 mg/kg). Although we observed lethargy in rats immediately after delivery and recovery from anesthesia, all subjects recovered to normal demeanor and activity levels in less than 24 hours. Our observations support the methods used by other labs in rats, in which even higher total doses of 110–175 mg/kg MnCl2 were delivered (24, 31). In all measurements of Mn uptake in this work, inter-subject variability was observed. With whole-body PET/CT, we noted activity pooled in the tail, most likely caused by the needle moving slightly during the slow 15–45 minute infusion of contrast agent. This likely occurred in all subjects to some extent, not just those imaged with PET/CT, and therefore could be one source of inter-subject variability of brain uptake dynamics (along with normal biological and physiological variations). For gamma counting and PET/CT studies that require trace amounts of 52Mn2+, rather than large doses of bulk MnCl2, a faster infusion of NCA 52Mn2+ would likely be advisable. We hypothesize that this would minimize errors with needle placement, reducing the Mn2+ delivered to the tail rather than the blood pool and minimizing variability between subjects.
In MRI studies, we observed that greater MnCl2 dose resulted in increased R1 enhancement, in the whole brain and in a variety of individually analyzed brain regions. This maximum enhancement occurred at one day following contrast administration for the whole brain on average as well as for a majority of brain regions. The delayed maximum enhancement observed in some regions, most notably the striatum, thalamus, globus pallidus, and brain stem, may be a result of more prolonged uptake over greater than 24 hours and/or slower Mn2+ efflux from these brain regions. This indicates that neuroimaging studies focused on these regions may perform favorably at these later time points due to both increased enhancement and reduced combined negative effects of bulk MnCl2 administration and isoflurane anesthesia for imaging on study subjects.
The spatial pattern of R1 enhancement observed was characterized by a small amount of generalized enhancement with increased enhancement near the ventricles. This pattern agreed with other studies that show saturable active transport of Mn2+ to the brain parenchyma across the BBB with additional uptake via diffusion through the blood-CSF barrier, linearly correlated with the blood concentration of Mn2+ (25–29). Furthermore, we observed slow reduction of Mn2+ enhancement in the brain, consistent with previous studies establishing diffusion as the primary efflux mechanism (29, 32).
In gamma counting studies we observed that supplementing 52Mn2+ with bulk MnCl2 (CA 52Mn2+) resulted in reduced brain uptake by an average factor of greater than two. This is most likely due to competition between cold Mn2+ and 52Mn2+ for Mn2+ transporters into the brain (26, 29). At both 4 and 48 hours following contrast delivery, ex vivo biodistribution studies showed low plasma activity (<0.025 %ID/g) independent of contrast agent dose. These results indicate that, at the contrast doses and measurement times used for biodistribution studies in this work, adding bulk MnCl2 to 52Mn2+ prior to delivery does not significantly increase 52Mn2+ circulation time, which was previously hypothesized as a potential method to increase overall 52Mn brain uptake (11).
Regarding the timing window of maximum 52Mn2+ uptake in the brain, there was a disagreement between ex vivo gamma counting and in vivo PET imaging. Gamma counting experiments of the excised brain indicated a non-significant increase in brain activity from 24 to 48 hours, while ROI-based analysis of in vivo PET/CT indicated maximum brain uptake within the first eight hours. Based on imaging studies in six subjects, we observed maximum brain uptake 1–4 hours following NCA 52Mn2+ administration. However, the ROI-based brain uptake measurements may be artificially high due to spillover from nearby high-signal regions such as the submandibular glands and pituitary. An additional source of timing discrepancy may be the inter-subject variability of 52Mn delivery, which could affect the dynamics of 52Mn brain uptake through saturable transporters or the blood-CSF barrier. Nevertheless, this maximum uptake of approximately 0.2% ID/g may be too low for many 52Mn2+ neuroimaging applications, and alternate delivery methods such as blood-brain-barrier disruption or direct CSF delivery of 52Mn2+ may be worthy of future investigation. In studies in which an accumulation of 52Mn2+ is expected in a specific brain region of interest, such as for stem cell tracking, systemic delivery may prove sufficient when the appropriate formulation and imaging time point are utilized (12).
Systematic studies of 52Mn biodistribution in the rat, which have not previously been described in the literature, indicate patterns of retention and uptake that may be of interest for imaging applications outside the central nervous system. Although biodistribution studies were not the intended emphasis of this work, the interesting results warrant further discussion and investigation. We observed that the preparation of 52Mn2+ in MnCl2 solution caused reduced uptake in the majority of organs as well as kidney and liver excretion of much of the injected activity over the course of 4–48 hours following administration. Interestingly, full-body PET/CT after administration of CA 52Mn2+ showed uptake in intervertebral and synovial joints. Although this work does not focus on musculoskeletal applications of 52Mn PET, this uptake pattern may be of interest to other preclinical imaging researchers and warrants further investigation. Because Mn is an important osteotropic element, irregularity or change in 52Mn uptake in joints and bone may be indicative of underlying skeletal deficiencies or of response to treatment thereof, respectively (33).
In both CA and NCA preparations, 52Mn uptake greater than 0.5 %ID/g was observed in the liver, kidney, pancreas, and salivary glands. These results are in qualitative agreement with reports in the literature of Mn2+ biodistribution studies after non-radioactive MnCl2 administration (10). Interestingly, uptake in the pancreas and salivary glands was maintained until the later 48 hour time point after delivery of NCA 52Mn2+. We hypothesize that the long-term retention of 52Mn2+ in the pancreas and salivary glands, particularly following delivery of NCA 52Mn2+, may be attributable to the higher endogenous levels of manganese present in glandular organs such as these (10). Exchange between the endogenous and exogenous pools of Mn2+ following NCA 52Mn2+ administration would result in mitochondrial localization and sequestration (34). In the case of CA 52Mn2+, uptake may not be sustained for as long for several reasons. Because the secretory cells of the pancreas and salivary glands have mechanisms for Ca2+ efflux to maintain homeostasis, the higher levels of Mn2+ accumulating in the cell cytoplasm following CA 52Mn2+ delivery may exit the cells via this same pathway (35, 36). Furthermore, radiolabeled Mn2+ efflux may be stimulated by high extracellular Mn2+ concentrations, as has been observed in cultured rat astrocytes (37). This 52Mn2+ efflux would likely be far less dramatic following delivery of trace 52Mn2+ levels in the NCA formulation, resulting in greater retention of 52Mn2+ in the cell.
MR imaging in the majority of the organs in which high 52Mn2+ uptake was observed has previously been pursued experimentally and in clinical imaging settings (38–40). For diagnostic purposes, 52Mn2+ uptake may be reflective of acinar cell function in both the pancreas and salivary glands and β–cell function in the pancreas (38, 39). Particularly in NCA formulation, 52Mn uptake in the pancreas and salivary glands may increase the potential for PET-based diagnosis or disease monitoring, as much lower contrast doses would be required for PET than for MnCl2-enhanced MR studies. Furthermore, a shorter-lived isotope of manganese without confounding gamma emissions, 52Mn (t1/2 = 46 min, β+ = 97%) can also be produced on low-energy medical cyclotrons by the 50Cr(d,n) and 54Fe(p,α) reactions. The production of this isotope is currently underway at our institution, which may lead to greater clinical translatability of NCA Mn-based PET imaging.
4. Conclusions
MEMRI has been established as a useful preclinical neuroimaging method for observing brain tissue enhancement, neuron tract tracing, functional imaging, and stem cell detection. However, the doses required for T1-weighted signal enhancement are prohibitive for translation of these applications. Heightened interest in 52Mn PET imaging has resulted in its investigation as a novel imaging method with potential for reduced bulk Mn2+ dose, complementary imaging characteristics, and applications in simultaneous PET/MR. In these studies we have investigated several methods for preparation, delivery, and detection of Mn2+-based contrast agents with quantitative MEMRI and 52Mn PET. We observed that while the whole brain and many brain regions reach maximum R1 in MEMRI at 24 hours following MnCl2 delivery, several specific sub-regions show maximum enhancement at later time points. However, at the tracer doses of 52Mn2+ delivered for PET imaging, brain uptake was insufficient for practical application in most neuroimaging studies (in the case of an intact blood-brain barrier). With PET/CT and ex vivo gamma counting, we observed that the supplementation of tracer doses of 52Mn with MnCl2 reduced activity uptake in the brain and nearly all other organs. For this reason, along with the reduced bulk Mn2+ dose, NCA 52Mn2+ holds greater potential as the preferable contrast agent form for imaging applications. Despite a lack of promise in our 52Mn2+ neuroimaging results, these studies highlight several interesting areas of further study of 52Mn PET, including investigating specific 52Mn2+ uptake in the salivary glands, pancreas, and joints. In the future, additional development of methods to increase 52Mn2+ uptake in the brain could be of great interest.
5. Experimental
5.1. Animal experimentation
All animal experimentation was performed following NIH guidelines and in accordance with protocols approved by the University of Wisconsin Institutional Animal Care and Use Committee. Adult female Sprague Dawley rats were used for experiments. Rats were housed under controlled temperature and light conditions and had free access to food and water. Upon completion of experimentation, rats were euthanized by slowly introducing CO2 to the rat in an enclosed chamber.
5.2. Systemic delivery of Mn
In preparation for imaging, rats were delivered Mn2+ in the form of MnCl2 and/or 52Mn2+ in solution. Animals were anesthetized with light isoflurane (0.5–2% in oxygen) and placed on a warming pad to maintain body temperature. Contrast agent, prepared as described below, was delivered via tail vein with an infusion pump at a rate of 2 mL/hr. Following infusion, the infusion line was flushed with heparinized saline and animals were delivered 5 mL pre-warmed saline subcutaneously.
Prior to MRI, doses of 30, 45, and 60 mg/kg MnCl2 at a concentration of 66.7 mM in bicine-buffered saline were delivered to 9 animals (N=3 per group). 52Mn was prepared as described previously, with an average specific activity of 0.8 GBq/µmol (13). No-carrier-added (NCA) 52Mn2+ was prepared by diluting 52Mn2+ in saline to 1.5 mCi/mL (55.5 MBq/mL) then delivered at a dose of 2 mCi/kg (74 MBq/kg, N=6 for ex vivo gamma counting and N=4 for in vivo PET/CT). Alternatively, 52Mn2+ was diluted in 66.7 mM buffered MnCl2 solution at a concentration of 0.37 mCi/mL (13.7 MBq/mL). In this preparation, animals were delivered 2 mCi/kg (74 MBq/kg) 52Mn2+ in approximately 45 mg/kg MnCl2, which corresponds to the average dose used for MEMRI studies (N=6 for ex vivo gamma counting and N=1 for in vivo PET/CT). This contrast agent preparation is herein referred to as carrier-added (CA) 52Mn2+.
5.3. MEMRI
To observe brain Mn2+ uptake with MRI, in vivo R1 mapping was performed using a 4.7 T preclinical MRI scanner (Agilent Technologies, Santa Clara, CA, USA). At each imaging time point, a series of 3D SPGR images were acquired using the following pulse sequence parameters: TR = 5.96 ms; TE = 2.25 ms; flip angles (in degrees) = 3, 7, 12, 18, 25; matrix size = 64×128×128; field of view = 35×35×35 mm3; scan time = 4:54 min:sec per flip angle. Additionally, an actual flip-angle imaging (AFI) SPGR scan was acquired for flip angle mapping using the following parameters: TR1/TR2 = 5.9/29.5 ms; TE = 2.22 ms; flip angle = 55°; matrix size = 64×64×64; field of view = 35×35×35 mm3 (41). R1 times were estimated using weighted linear least squares estimation with flip angle correction (42). Images were acquired and R1 maps were calculated pre-contrast and at days 1, 2, 4, 7, and 14 following MnCl2 administration in order to analyze the in vivo dynamics of Mn2+ uptake and efflux.
To measure and quantify Mn2+ uptake in the whole brain and individual brain regions, brains were manually segmented from surrounding tissue at all imaging time points. Using FMRIB’s Linear Image Registration Tool, FLIRT, manually segmented images were registered to a Waxholm Space atlas of the Sprague Dawley rat brain (43–47). Following registration, the atlas was used to segment the following sub-regions for further quantification and analysis: striatum, neocortex, olfactory bulb, corpus callosum, basal forebrain, globus pallidus, thalamus, hippocampus, hypothalamus, brain stem, cerebellum, and trigeminal tract. T1 mapping, segmentation, registration, and image processing were performed in Matlab R2014b (The MathWorks, Natick, MA).
5.4. 52Mn gamma counting of brain uptake
To observe the whole brain uptake of 52Mn2+ following administration of NCA and CA 52Mn2+, subjects were sacrificed either 24 hours (N=3 per contrast agent group) or 48 hours (N=3 per contrast agent group) following intravenous infusion of 52Mn2+. The brain was immediately excised and rinsed once in distilled water, the olfactory bulb (and trigeminal nerves, if still intact) were removed, and the brain mass was measured. Brain-average 52Mn2+ uptake was measured by gamma counting using the average of three 2-minute counts with gating centered on the 744 keV gamma peak. Counting data was corrected for known gamma counter efficiency and decay-corrected to injection time to calculate the percent injected dose per gram (%ID/g) of brain tissue. A two-tailed Student’s t-test was used to compare the brain uptake of 52Mn2+ based on contrast agent form and measurement time point.
5.5. 52Mn PET
In vivo 52Mn PET/CT was performed in six subjects (N=5 with NCA 52Mn2+, N=1 with CA 52Mn2+) on a Siemens Inveon microPET/CT (Siemens Medical Solutions, Erlangen, Germany). PET acquisition time points varied between subjects, as an essential aspect of this experiment was to guide the design of a consistent 52Mn PET imaging protocol. In the first PET/CT experiment, two subjects were administered 2 mCi/kg 52Mn2+ (one CA, one NCA) and 30–60 minute full-body PET/CT scans were acquired 1, 2, 4, and 7 days later with a single static time frame. Additionally, the subject delivered NCA 52Mn2+ in this experiment was scanned 2 hours following delivery. This scan time point was not feasible for the other subject due to the negative combined effects of isoflurane and bulk MnCl2 immediately after delivery. In a second experiment, four subjects delivered 2 mCi/kg NCA 52Mn2+ were scanned for 30–60 minutes at 0.5, 1.5, 3.5, 7.5, 24, 48, and 72 hours following contrast delivery. Two of these subjects were additionally scanned at 7 days following delivery. For all full-body PET scans, continuous bed motion with 5 passes was used for PET acquisition due to the relative size of the rat compared to the PET scanner field of view.
For PET/CT reconstruction, attenuation maps were calculated using manual registration of full-body PET and CT images. Attenuation-corrected PET images were then reconstructed using a vendor-provided 2-dimensional ordered subset expectation maximization (OSEM) reconstruction algorithm. Scatter correction was not applied during reconstruction due to the tendency of this correction to reduce detectable signal in low-count brain imaging protocols, as has been observed in 15O[H2O] brain activation studies and was expected in this setting (48). For each subject and time point, the brain-average %ID/g was calculated by comparing the uptake in the manually delineated whole brain to the total delivered activity and normalizing by the mass of the brain, assuming tissue density equal to that of water.
5.6. 52Mn Biodistribution
Biodistribution of 52Mn2+ was measured with ex vivo organ gamma counting in order to examine the uptake of both NCA and CA 52Mn2+ in major organs of the rat. 52Mn2+ was prepared either at a concentration of 0.33 mCi/mL (12.2 MBq/mL) in saline (NCA) or at an activity concentration of 0.37 mCi/mL (13.7 MBq/mL) in 66.7 mM MnCl2. A target injection dose of 100 uCi was delivered to rats (N = 3 per time point and per contrast agent formulation) via tail vein infusion at a rate of 2 mL/hr for an average of 8–10 minutes. For CA 52Mn2+, the added carrier dose corresponded to approximately 4.5 mg/kg MnCl2. Animals were sacrificed and organs were harvested at 4 or 48 hours following initiation of contrast delivery. For each organ, the sample was weighed and activity was measured by gamma counting with a PerkinElmer Wizard 2480 (Waltham, MA, USA) to calculate %ID/g. For statistical analysis, a two-tailed Student’s t-test was used to compare the uptake of 52Mn2+ at 4 and 48 hours and for the two contrast agent formulations.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Justin Jeffery (University of Wisconsin-Madison) for his dedicated assistance with PET/CT acquisition and processing, along with Sam Hurley Ph.D. (FMRIB Centre, University of Oxford), and Cheng Guan Koay Ph.D. (Walter Reed National Military Medical Center, National Intrepid Center of Excellence), for contributing T1 mapping acquisition and processing tools. We greatly appreciate the assistance of Patrick J Lao and Tobey J Betthauser in reviewing the manuscript. This project was supported in part by funding from the University of Wisconsin Institute for Clinical and Translational Research, as a Clinical and Translational Science Award (CTSA) site of the National Center for Advancing Translational Sciences (NCATS) grant UL1TR000427. We would also like to acknowledge other funding sources, including the NIH (R01NS091540 to M.S., T32 GM008349 to R.H., T32 CA009206 to S.A.G.), the ALS Association (15-IIP-201 to M.S.), and the University of Wisconsin Foundation.
References
- 1.Regge D, Cirillo S, Macera A, Galatola G. Mangafodipir trisodium: review of its use as an injectable contrast medium for magnetic resonance imaging. Rep Med Imaging. 2009;2:55–68. [Google Scholar]
- 2.Massaad CA, Pautler RG. Manganese-enhanced magnetic resonance imaging (MEMRI) Methods Mol Biol. 2011;711:145–174. doi: 10.1007/978-1-61737-992-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suero-Abreu GA, Praveen Raju G, Aristizabal O, Volkova E, Wojcinski A, Houston EJ, Pham D, Szulc KU, Colon D, Joyner AL, Turnbull DH. In vivo Mn-enhanced MRI for early tumor detection and growth rate analysis in a mouse medulloblastoma model. Neoplasia. 2014;16(12):993–1006. doi: 10.1016/j.neo.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim PJ, Mahmoudi M, Ge X, Matsuura Y, Toma I, Metzler S, Kooreman NG, Ramunas J, Holbrook C, McConnell MV, Blau H, Harnish P, Rulifson E, Yang PC. Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium. Circ Res. 2015;116(7):e40–e50. doi: 10.1161/CIRCRESAHA.116.304668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Revisiting an old friend: manganese-based MRI contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(2):162–173. doi: 10.1002/wnan.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29(4):569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordhoy W, Anthonsen HW, Bruvold M, Jynge P, Krane J, Brurok H. Manganese ions as intracellular contrast agents: proton relaxation and calcium interactions in rat myocardium. NMR Biomed. 2003;16(2):82–95. doi: 10.1002/nbm.817. [DOI] [PubMed] [Google Scholar]
- 8.Bruvold M, Nordhoy W, Anthonsen HW, Brurok H, Jynge P. Manganese-calcium interactions with contrast media for cardiac magnetic resonance imaging: a study of manganese chloride supplemented with calcium gluconate in isolated Guinea pig hearts. Invest Radiol. 2005;40(3):117–125. doi: 10.1097/01.rli.0000153025.72638.63. [DOI] [PubMed] [Google Scholar]
- 9.Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17(8):532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- 10.Ni Y, Petre C, Bosmans H, Miao Y, Grant D, Baert AL, Marchal G. Comparison of manganese biodistribution and MR contrast enhancement in rats after intravenous injection of MnDPDP and MnCl2. Acta Radiol. 1997;38(4 Pt 2):700–707. doi: 10.1080/02841859709172402. [DOI] [PubMed] [Google Scholar]
- 11.Topping GJ, Schaffer P, Hoehr C, Ruth TJ, Sossi V. Manganese-52 positron emission tomography tracer characterization and initial results in phantoms and in vivo. Med Phys. 2013;40(4):042502. doi: 10.1118/1.4793756. [DOI] [PubMed] [Google Scholar]
- 12.Lewis CM, Graves SA, Hernandez R, Valdovinos HF, Barnhart TE, Cai W, Meyerand ME, Nickles RJ, Suzuki M. (52)Mn Production for PET/MRI Tracking Of Human Stem Cells Expressing Divalent Metal Transporter 1 (DMT1) Theranostics. 2015;5(3):227–239. doi: 10.7150/thno.10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graves SA, Hernandez R, Fonslet J, England CG, Valdovinos HF, Ellison PA, Barnhart TE, Elema DR, Theuer CP, Cai W, Nickles RJ, Severin GW. Novel Preparation Methods of (52)Mn for ImmunoPET Imaging. Bioconjug Chem. 2015;26(10):2118–2124. doi: 10.1021/acs.bioconjchem.5b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wooten AL, Lewis BC, Lapi SE. Cross-sections for (p,x) reactions on natural chromium for the production of (52,52m,54)Mn radioisotopes. Appl Radiat Isot. 2015;96:154–161. doi: 10.1016/j.apradiso.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daube ME, Nickles RJ. Development of myocardial perfusion tracers for positron emission tomography. Int J Nucl Med Biol. 1985;12(4):303–314. doi: 10.1016/0047-0740(85)90185-8. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz M, Spahn I, Coenen HH. Optimized separation procedure for production of no-carrier-added radiomanganese for positron emission tomography. Radiochimica Acta. 2015;103(12):893–899. [Google Scholar]
- 17.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET imaging with (8)(9)Zr: from radiochemistry to the clinic. Nucl Med Biol. 2013;40(1):3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shokeen M, Wadas TJ. The development of copper radiopharmaceuticals for imaging and therapy. Med Chem. 2011;7(5):413–429. doi: 10.2174/157340611796799177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torigian DA, Zaidi H, Kwee TC, Saboury B, Udupa JK, Cho ZH, Alavi A. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267(1):26–44. doi: 10.1148/radiol.13121038. [DOI] [PubMed] [Google Scholar]
- 20.Bade AN, Zhou B, McMillan J, Narayanasamy P, Veerubhotla R, Gendelman HE, Boska MD, Liu Y. Potential of N-acetylated-para-aminosalicylic acid to accelerate manganese enhancement decline for long-term MEMRI in rodent brain. J Neurosci Methods. 2015;251:92–98. doi: 10.1016/j.jneumeth.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CH, D'Arceuil HE, de Crespigny AJ. Direct CSF injection of MnCl(2) for dynamic manganese-enhanced MRI. Magn Reson Med. 2004;51(5):978–987. doi: 10.1002/mrm.20047. [DOI] [PubMed] [Google Scholar]
- 22.Canals S, Beyerlein M, Keller AL, Murayama Y, Logothetis NK. Magnetic resonance imaging of cortical connectivity in vivo. Neuroimage. 2008;40(2):458–472. doi: 10.1016/j.neuroimage.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med. 2005;53(3):640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- 24.Chuang KH, Koretsky AP, Sotak CH. Temporal changes in the T1 and T2 relaxation rates (DeltaR1 and DeltaR2) in the rat brain are consistent with the tissue-clearance rates of elemental manganese. Magn Reson Med. 2009;61(6):1528–1532. doi: 10.1002/mrm.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24(1):3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 26.Aschner M. The transport of manganese across the blood-brain barrier. Neurotoxicology. 2006;27(3):311–314. doi: 10.1016/j.neuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Yokel RA. Manganese flux across the blood-brain barrier. Neuromolecular Med. 2009;11(4):297–310. doi: 10.1007/s12017-009-8101-2. [DOI] [PubMed] [Google Scholar]
- 28.Murphy VA, Wadhwani KC, Smith QR, Rapoport SI. Saturable transport of manganese(II) across the rat blood-brain barrier. J Neurochem. 1991;57(3):948–954. doi: 10.1111/j.1471-4159.1991.tb08242.x. [DOI] [PubMed] [Google Scholar]
- 29.Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17(8):544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eschenko O, Canals S, Simanova I, Logothetis NK. Behavioral, electrophysiological and histopathological consequences of systemic manganese administration in MEMRI. Magn Reson Imaging. 2010;28(8):1165–1174. doi: 10.1016/j.mri.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Aoki I, Wu YJ, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage. 2004;22(3):1046–1059. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695(1):53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 33.Zofkova I, Nemcikova P, Matucha P. Trace elements and bone health. Clin Chem Lab Med. 2013;51(8):1555–1561. doi: 10.1515/cclm-2012-0868. [DOI] [PubMed] [Google Scholar]
- 34.Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol. 1992;115(1):1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- 35.Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742(1–3):103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11(1):4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 37.Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58(2):730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 38.Seshadri M, Hoy A. Manganese-enhanced MRI of salivary glands and head and neck tumors in living subjects. Magn Reson Med. 2010;64(3):902–906. doi: 10.1002/mrm.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaisse WJ, Maedler K. Imaging of the beta-cells of the islets of Langerhans. Diabetes Res Clin Pract. 2012;98(1):11–18. doi: 10.1016/j.diabres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Rief M, Huppertz A, Asbach P, Franiel T, Schwenke C, Hamm B, Taupitz M, Wagner M. Manganese-based oral contrast agent for liver magnetic resonance imaging: evaluation of the time course and dose response of liver signal intensity enhancement. Invest Radiol. 2010;45(9):565–571. doi: 10.1097/RLI.0b013e3181e9e120. [DOI] [PubMed] [Google Scholar]
- 41.Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007;57(1):192–200. doi: 10.1002/mrm.21120. [DOI] [PubMed] [Google Scholar]
- 42.Chang LC, Koay CG, Basser PJ, Pierpaoli C. Linear least-squares method for unbiased estimation of T1 from SPGR signals. Magn Reson Med. 2008;60(2):496–501. doi: 10.1002/mrm.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 45.Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG. Waxholm Space atlas of the Sprague Dawley rat brain. Neuroimage. 2014;97:374–386. doi: 10.1016/j.neuroimage.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kjonigsen LJ, Lillehaug S, Bjaalie JG, Witter MP, Leergaard TB. Waxholm Space atlas of the rat brain hippocampal region: three-dimensional delineations based on magnetic resonance and diffusion tensor imaging. Neuroimage. 2015;108:441–449. doi: 10.1016/j.neuroimage.2014.12.080. [DOI] [PubMed] [Google Scholar]
- 47.Sergejeva M, Papp EA, Bakker R, Gaudnek MA, Okamura-Oho Y, Boline J, Bjaalie JG, Hess A. Anatomical landmarks for registration of experimental image data to volumetric rodent brain atlasing templates. J Neurosci Methods. 2015;240:161–169. doi: 10.1016/j.jneumeth.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Zaidi H, Koral KF. Scatter modelling and compensation in emission tomography. Eur J Nucl Med Mol Imaging. 2004;31(5):761–782. doi: 10.1007/s00259-004-1495-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.