Abstract
Objective
Sleep disturbance has been identified as a risk factor for relapse in addiction to a range of substances. The relationship between sleep quality and treatment outcome has received relatively little attention in research on nonmedical use of prescription drugs (NMUPD). This study examined the within-person association between sleep quality and craving in medically detoxified patients in residence for the treatment of NMUPD.
Method
Participants (n= 68) provided daily reports of their sleep quality, negative affect (NA), positive affect (PA), and craving for an average of 9.36 (SD= 2.99) days. Within-person associations of sleep quality and craving were examined using multilevel modeling. Within-person mediation analyses were used to evaluate the mediating roles of NA and PA in the relationship between sleep quality and craving.
Results
Greater cravings were observed on days of lower than usual sleep quality (γ10 = −0.10, p = .003). Thirty-one percent of the overall association between sleep quality and craving was explained by PA, such that poorer sleep quality was associated with lower PA and, in turn, lower PA was associated with greater craving. No evidence emerged for an indirect association between sleep quality and craving through NA.
Conclusions
Daily fluctuations in sleep quality were associated with fluctuations in craving, an association partially explained by the association between sleep quality and daily PA. These data encourage further research on the relationship between sleep, affect, and craving in NMUPD patients, as well as in patients with other substance use disorders.
Keywords: opioids, affect, sleep, experience sampling, craving
1. INTRODUCTION
The annual quantity of painkillers prescribed in the United States has quadrupled since the year 2000 despite the absence of an increase in reported pain (SAMHSA, 2013). As a consequence, opioid analgesics are often diverted, resulting in nonmedical use of prescription drugs (NMUPD), substance use disorders and overdose deaths (Volkow & McLellan, 2016). Indeed, over the past two decades, the prevalence of prescription opioid dependence in the United States is increasingly recognized as a public health concern (Johnston et al., 2011; SAMHSA, 2013). Sleep disturbance has been identified as a risk factor for relapse in addiction to a range of substances (see Brower & Perron, 2010 for review), including alcohol (Brower, Aldrich, & Hall, 1998), nicotine (Boutou et al., 2008), and cocaine (Sofuoglu et al., 2005). Much less work has documented the role of sleep in relapse in the context of opioid dependence (see Dijkstra et al., 2008 for an exception) despite observations of sleep disruption during opiate withdrawal (Wang & Teichtahl, 2007). To gain further insight into the effects of sleep disruption in patients in treatment for NMUPD, this study examined the effects of sleep quality on craving – a proximal outcome associated with subsequent opioid use (Tsui et al., 2014) – through the indirect associations with positive affect (PA) and negative affect (NA) at the day-to-day, within-person level.
1.1. The Associations Between Sleep, Affect, and Craving
Sleep quality is negatively correlated with craving across a range of substances during drug withdrawal, including alcohol, tobacco, opiates, and cannabis (Serre et al., 2015; Lahti et al., 2011). The precise mechanism by which sleep impacts craving, however, remains unclear. Affective states, such as low PA or high NA, are likely to play a role in the relationship between disturbed sleep and craving.
1.1.1. Affect and Craving
Pervasive changes in affective experience persist following detoxification of patients dependent upon opioids. Persistent, drug dependence-associated changes in reward and memory brain circuitry are associated with sensitivity to drug-related cues and diminished sensitivity to non-drug rewards during drug abstinence (Koob & Mason, 2016; Volkow et al., 2004). In addition, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis with opioid dependence is associated with heightened sensitivity to stressors during drug abstinence (Koob & Kreek, 2007). These abstinence-associated effects on reward and stress response systems likely underlie the subjective experiences that patients report during protracted abstinence, and might contribute to the persistent desire for drugs even as patients are in residence and living apart from drug-related environmental triggers of craving (Ferguson & Shiffman, 2009; Kober & Mell, 2015).
Against this backdrop of "tonic" desire for drug, episodic craving might emerge, reflected in variability in levels of craving during abstinence in response to external or internal cues (Ferguson & Shiffman, 2009). When perceived, drug cues engender an incentive motivation for drugs (Robinson & Berridge, 2008), even after up to 30-days of abstinence (Childress et al., 1986). Cue-induced craving has a long history (Carter & Tiffany, 1999; Drummond, 2000) but internal states, including affective states (Baker et al., 2004; Otto et al., 2007), also constitute powerful cues or contexts for drug desire (Huhn et al., 2016).
The role for NA as a precipitant to drug craving has received considerable empirical support. Laboratory studies have demonstrated a positive association between NA and drug craving for a number substances, including opiates (Childress et al., 1994), alcohol, cocaine, and cigarettes (Conklin & Perkins, 2005; Litt et al., 1990; Sinha et al., 2000). Studies employing ecological momentary assessment (EMA) have also observed a positive association between NA and craving (Delfino et al., 2001; Epstein et al., 2009; Huhn et al., 2016).
The association between PA and craving, however, appears to be more complex than the association between NA and craving. Low PA has been associated with greater craving (Huhn et al., 2016; McHugh et al., 2013), and high levels of PA have been associated with both higher and lower levels of craving in the laboratory (Mason et al., 2008; Maude-Griffin & Tiffany, 1996; Schlauch et al., 2013; Tiffany & Drobes, 1990) and in the EMA (Bujarski et al., 2015; Epstein & Preston, 2010) literature. Emotional states that produce high PA might place individuals in a celebratory mood, encouraging drug use for hedonic reasons (Baker, Morse, & Sherman, 1987; Robinson & Berridge, 1993). Alternatively, high PA might serve to inhibit craving through the facilitation of self-regulation (Schlauch et al., 2013). Theoretically, among treatment-seeking patients, high relative to low PA might render participants more successful at managing cravings due to increased self-regulatory abilities and the capacity to experience reward from sources other than substances of abuse (Fredrickson, 2004). Consistent with this perspective, high PA has been shown to be associated with reduced cravings and lower urges among patients withdrawing from tobacco and/or alcohol (Schlauch et al., 2013; Zinser et al., 1992).
1.1.2. Sleep and Affect
Subjective sleep quality is associated with lower levels of subjective well-being (Lemola et al., 2013). Furthermore, sleep deprivation in the laboratory is related to greater NA and lower PA relative to normal sleep conditions (Franzen et al., 2008). Experience sampling studies, assessing natural variations in sleep, have observed a positive association between sleep quality and PA, as well as a negative association between sleep quality and NA (de Wild-Hartmann et al., 2013), although this effect has been more consistently observed for PA in EMA studies (e.g., Bower et al., 2010). Mechanisms underlying the association between sleep and affect might reflect an impaired ability to engage in affect regulation strategies following poor sleep quality (Mauss et al., 2012; Yoo et al., 2007).
1.2. The Present Study
To gain insight into the relationship between sleep quality and craving in NMUPD, the present study used EMA to examine the associations between sleep quality and craving at the daily level – both directly and through indirect associations between sleep, NA, and PA. Existing EMA studies have leveraged the method’s capacity to reduce retrospective bias (Schwarz, 2007) by asking participants to report emotions and experiences in the moment.
One strength of the intensive repeated measures design that has not yet been leveraged in the context of prescription opioid dependence and sleep, is the ability to disentangle within- and between-person variability. This allows for examination of the associations between day-to-day fluctuations in sleep (within-person) separately from the associations between person-to-person differences in sleep (between-person) and craving. This within-person approach is motivated by calls to increase our understanding of the dynamics involved in substance abuse recovery (McKay et al., 2006; Shiffman, 2009), at the micro, daily level (Zheng et al., 2015). The need for this approach is further underscored by findings of day-to-day variability in the constructs of craving, affect, and sleep (Bei et al., in press; Cleveland & Harris, 2010; Peacock et al., 2015).
In the current study, lower than usual sleep quality on a given night was hypothesized to be associated with higher than usual cravings during the following day, and this effect was predicted to be at least partially explained by an increase in NA and a decrease in PA following lower than usual sleep quality.
2. METHOD
2.1. Participants
Participants in the study were patients (n= 75; 30% female) recruited as part of a larger study at a residential drug and alcohol treatment facility in Wernersville, Pennsylvania. Participants ranged in age from 19 to 56 (M= 28.96, Range= 19–56) and had completed medically assisted withdrawal at the treatment center 10–14 days prior to the beginning of data collection. Patients were prescreened based on clinical charts at the residential treatment center; this information was used to initially identify participants that met study criteria who were then invited to take part in the study. Patient inclusion criteria were as follows: (1) capable and willing to comply with the research protocol; (2) met criteria for prescription opioid dependence as determined by clinical staff at the Caron Foundation using the Structured Clinical Interview for DSM-IV-TR (SCID; First et al., 2002), and Form-90D (Westerberg et al., 1998); (3) prescription opioids were the primary drug of choice; (4) over the age of 18; and (5) staying in residential treatment for at least 30 days. Exclusion criteria included (1) any history of serious mental illness (bipolar or schizophrenia) or psychosis, as diagnosed by the SCID; (2) intravenous drug use; (3) history of traumatic brain injury; and (4) because the other arms of the study included neuroimaging, current use of any opiate agonist (methadone or buprenorphine) or antagonist (Naltrexone). After self-selecting into the study, all participants provided written informed consent after a full explanation of the protocol as approved by the Pennsylvania State University College of Medicine Internal Review Board. For the present analyses, patients (n = 2) were excluded because they violated rules of the treatment center/study protocol after induction into the study, and participants with less than 2 days of sleep, affect, and craving data (n= 5) were also excluded, leaving 540 days nested within 68 persons. Participants provided data for a mean of 9.36 (SD= 2.99, Range= 2–14) days. In line with the increased rates of psychopathology in patients in treatment of NMUPD relative to normative samples (e.g., Cicero et al., 2008), participants in the sample were diagnosed with a range of mental health disorders, the most prevalent of which were generalized anxiety disorder (38.40%), attention deficit/hyperactivity disorder (29.41%), depressive disorder not otherwise specified (29.41%), and lifetime major depression disorder (23.53%). For further characterization of the sample, participant dependence on a range of substances as well as their use of medications during data collection are presented in Table 1. Notably, 44.12% of participants were prescribed medication to aid with their sleep during data collection.
Table 1.
Drug Dependence of Participants at Treatment Intake and Medication Use During the Study
| Participant Drug Dependence | ||
|---|---|---|
| N | % | |
| Poly-Substance Drug User | 31 | 45.59 |
| Alcohol Current Dependence | 10 | 14.71 |
| Tobacco Current Dependence | 28 | 41.18 |
| Cannabis Current Dependence | 24 | 35.29 |
| Tranquilizer Current Dependence | 0 | 0.00 |
| Sedative Current Dependence | 14 | 20.59 |
| Steroid Current Dependence | 0 | 0.00 |
| Stimulant Current Dependence | 8 | 11.76 |
| Cocaine Current Dependence | 6 | 8.82 |
| Hallucinogen Current Dependence | 0 | 0.00 |
| Inhalant Current Dependence | 1 | 1.47 |
| Opiate Current Dependence | 68 | 100.00 |
| Medications Administered During Data Collection | ||
| N | % | |
| Antidepressant | 34 | 50.00 |
| Muscle Relaxant | 2 | 2.94 |
| Opiate Antagonist | 12 | 17.65 |
| Narcotic Analgesic | 2 | 2.94 |
| Anticonvulsant | 8 | 11.76 |
| Antipsychotic | 6 | 8.82 |
| Alpha-2 Adrenergic Agonist | 21 | 30.88 |
| Melatonin | 10 | 14.71 |
| Norepinephrine Reuptake Inhibitor | 1 | 1.47 |
| Anxiolytic | 2 | 2.94 |
| Anticholinergic | 3 | 4.41 |
| Sleep Medication | 30 | 44.12 |
2.2. Procedure
Participants were equipped with smart phones programmed to administer a survey 4 times daily. On consecutive days, a preset alarm notified participants to complete surveys at morning, midday, mid-afternoon and evening times that did not conflict with their treatment program. Real time data were streamed to a secure server at the University campus to monitor compliance and data quality. The surveys took approximately 2–3 minutes each to complete. Research staff also used brief, in-person meetings to build rapport, answer participant questions, and manage any technical difficulties.
2.3. Measures
2.3.1. Craving
Craving was measured four times daily. Three items were employed with a five-point scale ranging from “strongly disagree” to “strongly agree”: 1) “Since last data entry, the idea of using drugs has intruded upon my thoughts”; 2) “Since last data entry, I have missed the feeling drugs can give me”; 3) “Since last data entry, I have thought about how satisfying drugs can be”. The stem for each item took the form of “Since waking” rather than “Since last data entry” for the morning assessments. An average craving score was created for each participant for each day of the study. A reliability analysis using a generalizability theory approach appropriate for intensive repeated measures data (see Bolger & Laurenceau, 2013) demonstrated that the reliable change score for the daily craving scale was .89, indicating that within-person change in craving could be assessed reliably with this scale.
2.3.2. Negative Affect
Participants rated their experience of NA 4 times per day with 8 items on a touchpoint scale ranging from 0 (Not at all) to 100 (Very): “angry,” “irritable,” “lonely,” “sad,” “guilty,” “ashamed,” “anxious,” “stressed”. An average daily negative affect score was created for each participant for each day of the study with at least one completed measurement occasion. The reliable change score for the daily negative affect variable was .89, indicating that within-person change in NA could be assessed reliably.
2.3.3. Positive Affect
Participants rated their experience of PA 4 times per day with 8 items on a touchpoint scale ranging from 0 (Not at all) to 100 (Very): “warmhearted,” “joyful,” “enthusiastic,” “happy,” “affectionate,” “loving,” “relaxed,” “calm”. An average daily PA score was created for each participant for each day of the study with at least one completed measurement occasion. The reliable change score for the daily negative affect variable was .91, indicating that within-person change in PA could be assessed reliably.
2.3.4. Sleep Quality
Sleep quality was measured during the morning assessment survey using an item adapted for daily reporting of sleep from conventional retrospective scales indexing subjective sleep quality (e.g., Ellis et al., 1981). Participants answered “How would you evaluate your sleep last night?” on a seven-point scale ranging from “Extremely light” to “Extremely deep.” Such daily, self-report sleep measures are more strongly correlated with objective sleep measures than retrospective, previous month reports (Lauderdale et al., 2008). They are also practical when frequent measurements are made to reduce participant burden, and have demonstrated favorable psychometric properties (e.g., Cappelleri et al., 2009). Daily sleep quality was significantly related to daily tiredness (β = −1.80) and drowsiness (β = −1.70) with days of better than usual sleep quality associated with lower than usual tiredness and drowsiness, suggesting that the item was a valid measure of sleep quality (further details on these supplementary analyses are available on request from the authors).
2.4. Data Analysis
The intensive repeated measures data were analyzed using multilevel models (Snijders & Bosker, 1999) to account for the nested nature of the data. A person-level sleep quality variable was calculated as the arithmetic mean across each individual’s repeated measures. A day-level sleep quality variable was calculated as deviations from these person-level means (Bolger & Laurenceau, 2013). Time, in the form of day in the study, was sample-mean centered.
A model for the association between sleep quality and craving was constructed as:
| (1) |
where cravingit is the craving variable for person i on day t; β0i indicates the expected craving in the middle of the study for an individual experiencing an average level of sleep quality for that person; β1i indicates within-person differences in craving associated with the day’s sleep quality; β2i indicates the effect of time in study on craving in order to account for time as a third variable (see Bolger & Laurenceau, 2013); and eit are day-specific residuals that were allowed to autocorrelate (AR1).
Person-specific intercepts and associations from the Level 1 model were specified at Level 2 as:
| (2) |
where the γs are sample-level parameters and the µs are residual between-person differences that may be correlated, but are uncorrelated with eit. Parameters γ11 through γ13 indicate how between-person differences in the within-person associations of day’s craving and sleep quality were moderated by usual sleep quality, age, and gender. The model was fit using the nlme package in R (Pinheiro et al., 2015) using maximum likelihood estimation, with incomplete data treated using missing at random assumptions. Statistical significance was evaluated at α = .05.
Other associations were tested but on finding no significant associations with craving, as well as unchanged results for the association between sleep quality and craving, the more parsimonious models were retained. These associations include a quadratic effect of time in addition to the linear effect of time; age at first use of opiates; and whether or not participants were prescribed medication to aid with their sleep during the study. Further, as mean scores across measurement occasions within days were used for the affect and craving variables, with days with at least one completed measurement occasion included, multilevel models predicting levels of positive affect, negative affect, and craving by within-person and between-person versions of the number of surveys completed per day, as well as a within- by between-person interaction, were used to test whether the number of surveys completed by participants was significantly associated with affect and craving scores. No significant differences emerged. As such, days with any data on affect and craving were included in the models.
Building from this model, two separate 1-1-1 multilevel mediation models (in which all three variables of interest are within-person, Level 1 variables) were employed to examine the role of sleep quality (X) on craving (Y) through the mediating pathway of affect (M; both PA and NA were tested in separate models). Mediation followed procedures described in Bolger and Laurenceau (2013). Person-mean deviated versions of sleep quality, NA, PA, and craving were created and the models were estimated in Mplus (Muthen & Muthen, 1998–2010). As per Bolger and Laurenceau (2013), possible linear time trends in the craving and affect outcomes during the study days were controlled for by regressing them on time. Quadratic effects of time on craving and affect were tested in addition to the linear effect of time but these effects were not significant. As such, the more parsimonious models were retained. The total effect was calculated using the formula from Kenny et al. (2003):
| (3) |
where c′ is the average direct effect of sleep quality on craving, ab is the product of the average X-to-M and M-to-Y effects, and σajbj is the covariance of between-person differences in the X-to-M and M-to-Y effects.
3. RESULTS
3.1. Descriptive statistics
On average, participants reported a mean sleep quality of 3.99 (SD= 1.36) and craving with a mean rating of 2.00 (SD= 1.75). Previous analyses have shown high inter-item reliability for the PA and NA factors (standardized Chronbach’s alpha for NA=0.93, and PA=0.95; Huhn et al., 2016). The Pearson’s r correlation between the PA and NA factors was r= −0.13. On average, participants reported a mean PA of 43.28 (SD= 18.57) and a mean NA of 33.13 (SD= 19.82).
3.2. Associations between Sleep Quality and Craving
Results from the multilevel model examining the effects of sleep quality on craving are shown in Table 2. In line with the hypothesis that the day’s sleep quality would affect craving, the within-person association between day’s sleep quality and craving was significant (γ10= −0.10, p= .003). That is, on days when participants’ sleep quality was higher than usual, their craving was lower than usual. Although a relatively small effect on average with an effect size of 0.13 (estimate for the within-person association divided by the within-person residual standard deviation, γ10/σe = 0.10/sqrt(0.60)), this within-person association ranged from −0.21 to 0.01 across persons. This within-person association between sleep quality and craving was not moderated by individuals’ usual sleep quality, age, or gender (γ11 to γ13, ps> .05).
Table 2.
Results of the Multilevel Model Examining the Association Between Sleep Quality and Craving
| Fixed Effects | Estimate | Standard Error |
|---|---|---|
| Intercept (γ00) | 2.08* | 0.11 |
| Day-Level Sleep Quality (γ10) | −0.10* | 0.03 |
| Time (γ20) | −0.03* | 0.01 |
| Person-Level Sleep Quality (γ01) | 0.04 | 0.12 |
| Age (γ02) | −0.02 | 0.01 |
| Gender (γ03) | −0.52 | 0.27 |
| Day-level Sleep Quality X Person-Level Sleep Quality (γ11) |
−0.03 | 0.04 |
| Day-Level Sleep Quality X Age (γ12) | 0.004 | 0.005 |
| Day-Level Sleep Quality X Gender (γ13) | 0.09 | 0.08 |
| Random Effects | Estimate | Confidence Interval |
| Intercept () | 0.83 | 0.56 – 1.21 |
| Day-Level Sleep Quality () | 0.01 | 0.001 – 0.12 |
| Correlation (ru0u1) | −0.11 | −0.71 – 0.58 |
| AR(1) | 0.13 | 0.02 – 0.24 |
| Residual () | 0.60 | 0.56 – 0.65 |
| Fit Indices | ||
| AIC | 1232.93 | |
| BIC | 1292.78 | |
Note: Nobservations =540 days nested within 68 persons; AIC = Akaike information criteria; BIC = Bayesian information criteria.
p < .05.
The between-person association between usual sleep quality and craving was not significant (γ01= 0.04, p= .77). The other between-person variables, age and gender, were also not significant predictors of craving (γ02 to γ03, ps> .05). Finally, there was a significant effect of time, such that craving decreased during the course of the study (γ20= −0.03, p< .001).
3.3. Associations between Sleep Quality, Negative Affect, and Craving
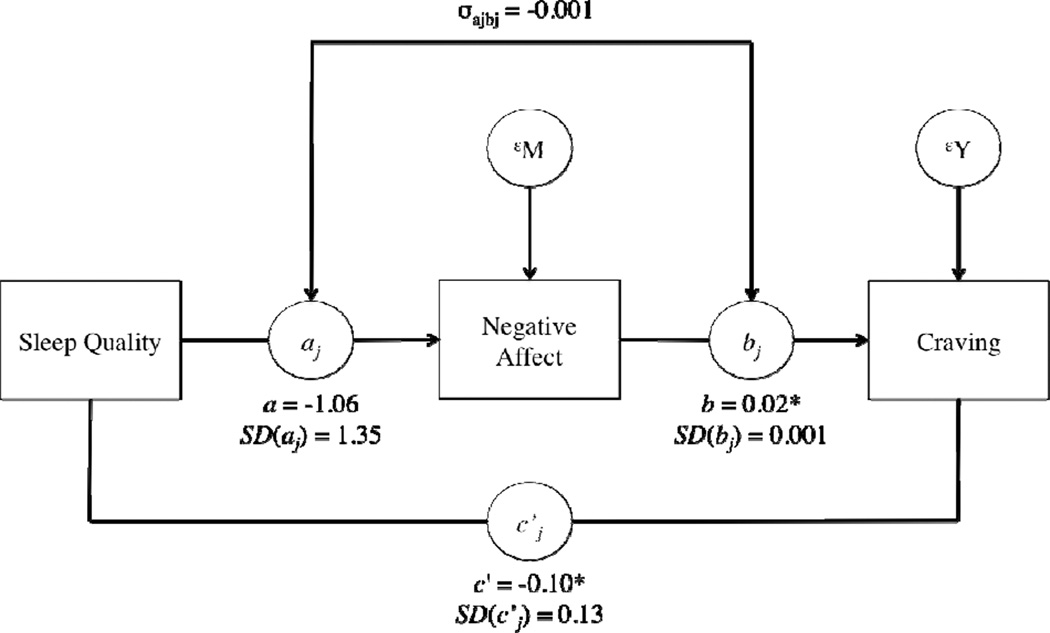
Results of the within-person mediation model are presented in figure 1. For the typical individual, sleep quality (X) did not significantly predict NA (M), B= −1.06, SE= 0.57, z= −1.86, p= .06. The NA (M) to craving (Y) slope for the average participant was 0.02, SE= 0.003, z= 5.23, p< .0001, indicating that on days when NA was higher than usual for that individual, craving was also higher than usual. There was also evidence of a direct association between sleep quality (X) and craving (Y) for the average participant, B= −0.10, SE= 0.03, z= −2.94, p <.01.
Figure 1.
Results for the within-person mediation for negative affect (see Bolger & Laurenceau, 2013). For ease of interpretability, we have omitted time as a predictor from the figure. Note: SD values indicate the between-person variability in the fixed effects. σajbj is the covariance of between-person differences in the X-to-M and M-to-Y effects *p < .05.
The total average effect of sleep quality on craving for the average participant was −0.12. Seventeen percent of the overall average relationship between sleep quality and craving was explained by NA but this effect had a standard error of 0.14 and was not statistically significant, z= 1.23, p= .22. Thus, NA did not mediate the association between sleep quality and craving.
3.4. Associations between Sleep Quality, Positive Affect, and Craving
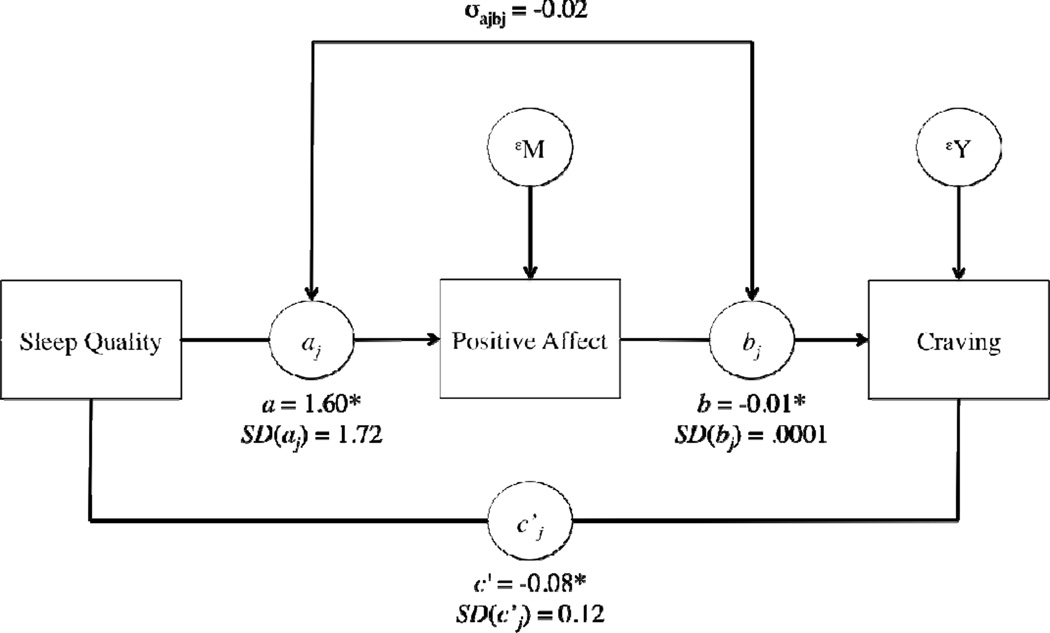
Results of the within-person mediation model are presented in figure 2. For the typical individual, sleep quality (X) significantly predicted PA (M), B= 1.60, SE= 0.63, z= 2.52, p= .01, indicating that days of higher than usual sleep quality were associated with higher than usual PA relative to days of lower than usual sleep quality. The PA (M) to craving (Y) slope for the average participant was −0.01, SE= 0.004, z= 2.15, p= .03, indicating that on days when PA was higher than usual for that individual, craving was lower than usual. There was also evidence of a direct association between sleep quality (X) and craving (Y) for the average participant, B= −0.08, SE= 0.04, z= −2.31, p=.02, such that, after adjusting for the association between PA and craving, better than usual sleep quality was associated with lower than usual craving.
Figure 2.
Results for the within-person mediation for positive affect (see Bolger & Laurenceau, 2013). For ease of interpretability, we have omitted time as a predictor from the figure. Note: SD values indicate the between-person variability in the fixed effects. σajbj is the covariance of between-person differences in the X-to-M and M-to-Y effects *p < .05.
The total average effect of sleep quality on craving for the average participant was −0.12. Thirty-one percent of the overall average relationship between sleep quality and craving was explained by PA. This effect had a standard error of 0.16 with a statistical significance of z= 2.00, p= .046. Thus, PA partially mediated the effect of sleep quality on craving.
4. DISCUSSION
4.1. Within-Person Association Between Sleep Quality and Craving
The present study found evidence for an association between sleep quality and craving among patients in residential treatment for prescription opioid dependence. A negative, within-person association between sleep quality and craving was observed when using EMA to assess these constructs in close temporal proximity to their occurrence. On days with better than usual sleep quality, participants reported less craving; this finding is consistent with research in other substance use disorders (e.g., Lahti et al., 2011) and extends previous research by considering NMUPD.
4.2. Sleep, Positive Affect, and Craving
Two separate mediation models were employed to gain insight into the relationship between sleep quality, affect, and craving. As hypothesized, day-level sleep quality was positively associated with daily PA, with PA partially mediating the association between sleep quality and craving. This finding is in line with previous research indicating an association between sleep quality and PA (Franzen et al., 2008) and PA and craving (Schlauch et al., 2013) and extends them to the NMUPD literature by explicitly testing the indirect path from sleep quality to craving through PA at the within-person level.
These findings are relevant to treatment of NMUPD in that clinicians might look for a combination of symptoms such as low PA and sleep disturbance as risk factors for high levels of drug craving, and thus, relapse. Opioid dependent patients in the early stages of recovery are likely to be at increased risk for craving on days when they experience sleep disturbance due partially to associated reductions in PA. From a clinical standpoint, this might call for a more personalized approach to patient care, e.g. medical treatments that target sleep disturbance to reduce the risk of relapse.
4.3. Sleep, Negative Affect, and Craving
Day-level NA was associated with craving such that craving was higher on days when NA was higher than usual. However, day-level sleep quality was not significantly associated with day-level NA. These findings were not in line with the hypothesis that sleep quality would be associated with NA. Due to the lack of EMA studies examining day to day variability in sleep and NA at the within-person level, our hypotheses were drawn from findings emerging primarily from laboratory studies and thus, the associations between less extreme, naturalistic variability in sleep and NA in situ might be of a different nature relative to the associations observed using sleep deprivation paradigms (e.g., Franzen et al., 2008). In line with this supposition, Bower and colleagues (2010) observed associations between sleep quality and PA, but not NA, in an EMA study. Such findings call for greater consideration of the associations between sleep and affect in naturalistic contexts.
The findings also point to the potential role of the circadian system in the regulation of affect and sleep. Circadian cycles are linked with PA more than NA (Clark et al., 1989; Murray et al., 2002) as noted by Bower et al. (2010) in interpreting the findings of associations between PA and sleep quality but not NA and sleep quality in their EMA study. Similar processes may also be at play in the current study. Indeed, disruptions in circadian rhythms have been observed across substance abusing samples (Birchler-Pedross et al., 2009; Ogeil et al., 2012; see Hasler et al., 2012, for review) with continued disruptions observed up to 30 days of abstinence in heroin-using samples for example (Li et al., 2009). Examining the contribution of the circadian system to affect and craving will require the use of specialized protocols capable of parsing out the effects of circadian timing from homeostatic sleep drive in future studies.
4.4. Limitations and Considerations for Future Research
Since the data in this study were collected from patients in a residential treatment facility, findings might not generalize beyond this context. Furthermore, the current study examined the association between sleep and craving, and not treatment outcome. Participants exhibited dependence for a range of substances and some were on medications (Table 1), including substances that exert influences on affect and sleep (Lydon et al., 2016; Oberndorfer et al., 2000; Weddington et al., 1990). While this polydrug and medication use does not allow conclusions to be drawn on associations between sleep, craving, affect, and NMUPD specifically, it is representative of NMUPD in the context of other drugs observed in nationally representative samples (McCabe et al., 2008; McCabe et al., 2007).
This study used a subjective measure of sleep quality. Although previous studies demonstrating effects of sleep on affect have used subjective sleep measures (e.g., Prather et al., 2013), a fuller understanding of the role of sleep on craving might be achieved by considering other measures of sleep dysregulation (for example, actigraphy-based measures which can provide information on sleep onset latency which is difficult to obtain through self-report) or alternative subjective measures of sleep that provide information on a number of facets of sleep (e.g., sleep duration and sleep disturbances; Buysse et al., 1989). Further, findings that rapid eye movement (REM) sleep is involved in emotional brain regulation (e.g., Gujar et al., 2011) are relevant to findings of associations between variations in sleep and affect and argue for the use of sleep measures capable of distinguishing different components of sleep physiology (e.g., polysomnography).
The focus on composite positive and negative affective states in the present study is in line with previous studies examining the association between affect and craving in NMUPD (e.g., Huhn et al., 2016), as well as studies that have examined the association between affect and drug craving more generally(e.g., McHugh et al., 2013; Schlauch et al., 2013). Future studies might gain further insight into the association between affect and craving by focusing on discrete affective states (e.g., Epstein et al., 2010).
Finally, drug craving decreased during the course of the study. This finding is in line with decreases in craving over the course of drug withdrawal observed in other studies across a range of substances (e.g., Shi et al., 2009; Wang et al., 2013; Weddington et al., 1990). This decrease in craving may reflect re-regulation of HPA axis and brain reward systems during prolonged drug abstinence, evidence for which has emerged in patients in treatment for heroin (Shi et al., 2009) and, more recently, prescription opioid-dependent patients (Bunce et al., 2015). Future research with intensive repeated measures will allow a characterization of the time course of drug craving through abstinence and to identify the relevance of changes in the functioning of the HPA axis and the brain reward systems in these changes.
4.5. Conclusion
This study demonstrated a within-person coupling between sleep quality and craving that was partially mediated by PA. The findings underscore the role of sleep disturbance as a risk factor for poor treatment outcomes in substance use (Brower & Perron, 2010), extending the findings to NMUPD, taking a within-person approach, and taking steps to identify the mechanisms through which sleep disturbances might affect craving. Future research is warranted to further elucidate the relationship between affect and sleep disturbance, particularly as it relates to the early stages of recovery from NMUPD.
Highlights.
Better than usual sleep quality was associated with lower than usual drug craving
Higher than usual positive affect was associated with lower than usual drug craving
The association between sleep quality and drug craving was partly mediated through positive affect
Acknowledgments
Role of Funding Sources
This study was supported by National Institute on Drug Abuse grant R01 DA035240. DML was supported by T32 DA017629 from the National Institute on Drug Abuse and an ISSBD-JJF Mentored Fellowship for Early Career Scholars. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors thank the Caron Treatment Center for hosting the research study, especially Cheryl Knepper, MA, Ken Thompson, MD, and Mike Early, for their continued support in our research efforts. The authors would also like to thank William Milchak for his ongoing collaborative involvement during the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
SCB, HHC, ED, and REM designed the study. ASH, JH, and DS oversaw data collection. DML performed the statistical analyses. All authors contributed to the writing of and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. In: Rivers PC, editor. The Nebraska symposium on motivation: alcohol Use and Abuse. Vol. 34. Lincolln, NE: University of Nebraska Press; 1986. pp. 257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews. doi: 10.1016/j.smrv.2015.06.003. (in press) [DOI] [PubMed] [Google Scholar]
- Birchler-Pedross A, Schröder CM, Münch M, Knoblauch V, Blatter K, Schnitzler-Sack C, Cajochen C. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. Journal of Biological Rhythms. 2009;24(3):232–242. doi: 10.1177/0748730409335546. [DOI] [PubMed] [Google Scholar]
- Bunce SC, Harris JD, Bixler EO, Taylor M, Muelly E, Deneke E, Meyer RE. Possible evidence for re-regulation of HPA axis and brain reward systems over time in treatment in prescription opioid-dependent patients. Journal of Addiction Medicine. 2015;9:53–60. doi: 10.1097/ADM.0000000000000087. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation & Emotion. 1989;13:205–234. [Google Scholar]
- Bolger N, Laurenceau J-P. Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Boutou AK, Tsiata EA, Pataka A, Kontou PK, Pitsiou GG, Argyropoulou P. Smoking cessation in clinical practice: predictors of six-month continuous abstinence in a sample of Greek smokers. Primary Care Respiratory Journal. 2008;17:32–38. doi: 10.3132/pcrj.2008.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower B, Bylsma LM, Morris BH, Rottenberg J. Poor reported sleep quality predicts low positive affect in daily life among health and mood-disordered persons. Journal of Sleep Research. 2010;19:323–332. doi: 10.1111/j.1365-2869.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective predictors of alcoholic relapse. Alcoholism: Clinical & Experimental Research. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Medical Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Roche DJO, Sheets ES, Krull JL, Guzman I, Ray LA. Modeling naturalistic craving, withdrawal, and affect during early nicotine abstinence: a pilot ecological momentary assessment study. Experimental and Clinical Psychopharmacology. 2015;23:81–89. doi: 10.1037/a0038861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Quality of Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O’Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal of Addiction. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;139:127–135. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Cleveland HH, Harris KS. The role of coping in moderating within-day associations between negative triggers and substance use cravings: a daily diary investigation. Addictive Behaviors. 2010;35:60–63. doi: 10.1016/j.addbeh.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- de Wild-Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, van Os J, Simons CJP. Day-to-day associations between subjective sleep and affect in regard to future depression in a female population-based sample. The British Journal of Psychiatry. 2013;202:407–412. doi: 10.1192/bjp.bp.112.123794. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Jamner LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine & Tobacco Research. 2001;3:235–248. doi: 10.1080/14622200110050466. [DOI] [PubMed] [Google Scholar]
- Dijkstra BAG, De Jong CAJ, Krabbe PFM, van der Staak CPF. Prediction of abstinence in opioid-dependent patients. Journal of Addiction Medicine. 2008;2:194–201. doi: 10.1097/ADM.0b013e31818a6596. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research. Addiction. 2000;95:129–144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary’s Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–97. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Daily life hour by hour, with and without cocaine: an ecological momentary assessment study. Psychopharmacology. 2010;211:223–232. doi: 10.1007/s00213-010-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J-L, Preston KL. Real-time electronic-diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- First MB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders: Patient Edition. Columbia University: Biometrics Research Department; 2005. [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationship between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The broaden-and-builf theory of positive emotions. Philisophical Transactions of the Royal Society B. 2004;359:1367–1377. doi: 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cerebral Cortex. 2011;21:115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Meyer RE, Harris JD, Ayaz H, Deneke E, Stankoski DM, Bunce SC. Evidence of anhedonia and differential reward processing in prefrontal cortex among post-withdrawal patients with prescription opiate dependence. Brain Research Bulletin. 2016 doi: 10.1016/j.brainresbull.2015.12.004. http://dx.doi.org/10.1016/j.brainresbull.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, Korchmaros JD, Bolger N. Lower level mediation in multilevel models. Psychological Methods. 2003;8:115–128. doi: 10.1037/1082-989x.8.2.115. [DOI] [PubMed] [Google Scholar]
- Kober H, Mell MM. Neural mechanisms underlying craving and the regulation of craving. In: Wilson SJ, editor. The Wiley-Blackwell Handbook on the Cognitive Neuroscience of Addiction. Chichester, UK: John Wiley & Sons; 2015. pp. 195–218. [Google Scholar]
- Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annual Review of Pharmacology & Toxicology. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American Journal of Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti T, Methuen T, Roine R, Seppa KL, Sinclair D, Partinen M, Alho H. The impacts of nitrous oxide gas on sleep quality during alcohol withdrawal. British Medical Journal. 2011;4:108–115. doi: 10.1186/1756-0500-4-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemola S, Lederman T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLOS One. 2013;8 doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Shi J, Epstein DH, Wang X, Zhang XL, Bao YP, Lu L. Circadian alteration in neurobiology during 30 days of abstinence in heroin users. Biological Psychiatry. 2009;65:905–912. doi: 10.1016/j.biopsych.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Kadden RM, Gaupp L. Reactivity to alcohol cues and induced moods in alcoholics. Addictive Behaviors. 1990;15:137–146. doi: 10.1016/0306-4603(90)90017-r. [DOI] [PubMed] [Google Scholar]
- Lydon DM, Ram N, Conroy DE, Pincus AL, Geier CF, Maggs JL. The within-person association between alcohol use and sleep duration and quality in situ: an experience sampling study. Addictive Behaviors. 2016;61:68–73. doi: 10.1016/j.addbeh.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Jasinksi DR. Physiological parameters of morphine dependence in main - tolerance, early abstinence, protracted withdrawal. Journal of Psychiatric Research. 1969;7:9–17. doi: 10.1016/0022-3956(69)90007-7. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology. 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. Production of smoking urges through imagery: the impact of affect and smoking abstinence. Experimental & Clinical Psychopharmacology. 1996;4:198–208. [Google Scholar]
- Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion. 2012;27:567–576. doi: 10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addictive Behaviors. 2008;33:1297–1305. doi: 10.1016/j.addbeh.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction. 2007;102:1920–1930. doi: 10.1111/j.1360-0443.2007.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Kaufman JS, Frost KH, Fitzmaurice GM, Weiss RD. Positive affect and stress reactivity in alcohol-dependent outpatients. Journal of Studies on Alcohol and Drugs. 2013;74:152–157. doi: 10.15288/jsad.2013.74.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clinical Psychology Review. 2006;26:109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiology International. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. Sixth. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- Ogeil RP, Rajaratnam SMW, Broadbear JH. Ecstasy and sleep disturbance: progress towards elucidating a role for the circadian system. Sleep & Biological Rhythms. 2012;10:3–13. [Google Scholar]
- Oberndorfer S, Saletu-Zyhlarz G, Saletu B. Effects of selective serotonin reuptake inhibitors on objective and subjective sleep quality. Neuropsychobiology. 2000;42(2):69–81. doi: 10.1159/000026676. [DOI] [PubMed] [Google Scholar]
- Otto MW, O’Cleirigh C, Pollack MH. Attending to emotional cues for drug abuse: bridging the gap between clinic and home behaviors. Science & Practice Perspectives. 2007;3(2):48–55. doi: 10.1151/spp073248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A, Cash C, Bruno R, Ferguson SG. Day-by-day variation in affect, arousal and alcohol consumption in young adults. Drug and Alcohol Review. 2015;34:588–594. doi: 10.1111/dar.12238. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package version 3.1-120. 2015 http://CRAN.R-project.org/package=nlme.
- Prather AA, Bogdan R, Hariri A. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosomatic Medicine. 2013;75:350–358. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schlauch RC, Gwynn-Shapiro D, Stasiewicz PR, Molnar DS, Lang AR. Affect and craving: positive and negative affect are differentially associated with approach and avoidance inclinations. Addictive Behaviors. 2013;38:1970–1979. doi: 10.1016/j.addbeh.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N. Retrospective and concurrent self-reports: the rationale for real-time data capture. In: Stone A, Shiffman S, Atienza A, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. New York, NY: Oxford University Press; 2007. pp. 11–26. [Google Scholar]
- Seere F, Fatseas M, Swendsen J, Auriacombe M. Are sleep disturbances associated with craving intensity? What is the influence of psychiatric comorbidity and type of substance on this relationship? A computerized ambulatory monitoring study in patients beginning treatment for addiction. Drug & Alcohol Depenendence. 2015;146:e94. [Google Scholar]
- Shi J, Li SX, Zhang XL, Wang X, Foll BL, Zhang XY, Lu L. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. The American Journal of Drug and Alcohol Abuse. 2009;35:267–272. doi: 10.1080/00952990902933878. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd. London, UK: Sage Publishers; 2012. [Google Scholar]
- Sofuoglu M, Dudish-Pulsen S, Poling J, Mooney M, Hatsukami DK. The effect of individual cocaine withdrawal symptoms on outcomes in cocaine users. Addictive Behaviors. 2005;30:1125–1134. doi: 10.1016/j.addbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the US. Center for behavioral Health Statistics and Quality Data Review (CBHSQ Data Review, Publication No. DR006) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addictive Behaviors. 1990;15:531–539. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, Stein MD. Craving predicts opioid use in opioid-dependent patients initiation buprenorphine treatment: a longitudinal study. The American Journal of Drug and Alcohol Abuse. 2014;40:163–169. doi: 10.3109/00952990.2013.848875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Medicine Reviews. 2007;11:35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled residential study. Archives of General Psychiatry. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Westerberg VS, Tonigan JS, Miller WR. Reliability of Form 90D: An instrument for quantifying drug use. Substance Abuse. 1998;19:179–189. doi: 10.1080/08897079809511386. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep - a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cleveland HH, Molenaar PCM, Harris KS. An alternative framework to investigating and understanding intraindividual processes in substance abuse recovery: an idiographic approach and demonstration. Evaluation Review. 2015;39:229–254. doi: 10.1177/0193841X14567313. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]