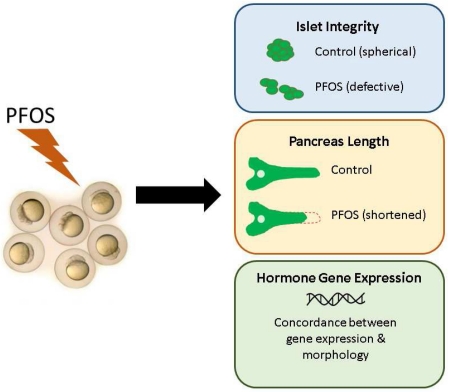
Abstract
Perfluorooctanesulfonic acid (PFOS) is a ubiquitous environmental contaminant, previously utilized as a non-stick application for consumer products and firefighting foam. It can cross the placenta, and has been repeatedly associated with increased risk for diabetes in epidemiological studies. Here, we sought to establish the hazard posed by embryonic PFOS exposures on the developing pancreas in a model vertebrate embryo, and develop criteria for an adverse outcome pathway (AOP) framework to study the developmental origins of metabolic dysfunction. Zebrafish (Danio rerio) embryos were exposed to 16, 32, or 64 μM PFOS beginning at the mid-blastula transition. We assessed embryo health, size, and islet morphology in Tg(insulin-GFP) embryos at 48, 96 and 168 hpf, and pancreas length in Tg(ptf1a-GFP) embryos at 96 and 168 hpf. QPCR was used to measure gene expression of endocrine and exocrine hormones, digestive peptides, and transcription factors to determine whether these could be used as a predictive measure in an AOP. Embryos exposed to PFOS showed anomalous islet morphology and decreased islet size and pancreas length in a U-shaped dose-response curve, which resemble congenital defects associated with increased risk for diabetes in humans. Expression of genes encoding islet hormones and exocrine digestive peptides followed a similar pattern, as did total larval growth. Our results demonstrate that embryonic PFOS exposures can disrupt pancreatic organogenesis in ways that mimic human congenital defects known to predispose individuals to diabetes; however, future study of the association between these defects and metabolic dysfunction are needed to establish an improved AOP framework.
Keywords: pancreas development, insulin, islets, β cells, embryo, exocrine pancreas
Graphical abstract
INTRODUCTION
The global prevalence of diabetes has been rapidly increasing in recent decades (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 2014). Both Type 1 and Type 2 diabetes manifest as hyperglycemia related to reduced beta cell mass, either due to autoimmune destruction of the insulin-producing beta cells in Type 1, or insulin resistance with loss of beta cell mass in Type 2 diabetes. Recent studies have demonstrated that chemical exposures are capable of reducing beta cell mass. However, the consequences of developmental exposures on the rapidly growing and maturing islets require identification.
While genetic sources of metabolic dysregulation are known to contribute to diabetic etiology in the adult, there is a growing body of evidence supporting the link between developmental environmental exposures and occurrence of diabetes later in life (Inadera, 2013; Simmons, 2006; Simmons, 2007). Numerous studies have investigated how physiological and pharmacological conditions influence beta cell health in adolescents and adults; however, very little is known about how these conditions may impact the sensitive developing pancreas, and whether these developmental consequences may manifest as metabolic dysfunction in adulthood. These gaps in our knowledge warrant investigation to produce a robust, predictive adverse outcome pathway (AOP) to study the developmental origins of diabetes and metabolic disease.
Islet architecture plays an important role in the governance of islet physiology and endocrinology, and variant morphologies can be observed concurrent with diabetic phenotypes and hyperglycemia (Bosco et al., 2010; Cabrera et al., 2006; Kilimnik et al., 2011; Kim et al., 2009). An increased risk of metabolic disease and pancreatitis has been associated with four congenital pancreatic malformations found in the human population: pancreas divisum, ectopic pancreatic tissue, dorsal pancreatic agenesis, and annular pancreas. Pancreas divisum and ectopic pancreatic tissue are predicted to occur in approximately 10% of the population (Prasad et al., 2001; Varshney and Johnson, 1999; Vaughn et al., 1998), while the other two anomalies are considered rare. Unlike most congenital defects which manifest as life-threatening or debilitating conditions, these pancreatic defects result in largely mild phenotypic outcomes and thus often go undetected, although they have been associated with increased risk for diabetes and pancreatitis in adulthood (Balakrishnan et al., 2006; Concepcion et al., 2014; Gentile and Fiorente, 1999; Gilinsky et al., 1987; Lindstrom et al., 1990; Mitchell et al., 2004; Shoji et al., 2013). The causes of these malformations are largely unknown, but do not appear to be genetic in nature. This suggests that these congenital pancreatic defects occur in response to environmental stimuli.
Pancreas development is difficult to observe during embryonic development in mammalian models, as it requires highly invasive procedures. Building upon an understanding of highly conserved vertebrate developmental processes, the zebrafish embryo is a well-established model for studying pancreas development (reviewed in (Kinkel and Prince, 2009; Tiso et al., 2009)). Because zebrafish embryos are transparent and fertilized externally, this allows for direct visualization of developing pancreas structures throughout the developmental timecourse.
Both the endocrine and exocrine pancreas can be easily visualized during organogenesis using transgenic zebrafish models (Tiso et al., 2009). The pancreas is formed from two anlages that emerge from the endoderm and fuse together and extend dorsally during organogenesis. The endocrine pancreas houses the islets of Langerhans, which largely consist of the insulin-producing beta cells, but also include other cell types that secrete hormones that regulate nutrient metabolism and comprise the glucose homeostasis feedback system. These include alpha cells that produce glucagon, delta cells that produce somatostatin, epsilon cells that produce ghrelin, and gamma cells (also called pancreatic polypeptide cells) that produce pancreatic polypeptide. The islets are embedded in the exocrine pancreas tissue, which functions to produce digestive enzymes that drain into ducts feeding into the duodenum. Transgenic zebrafish, such as those engineered to express fluorescent proteins in beta cells (Tg(ins:GFP)) and in the exocrine pancreas tissue (Tg(ptf1a:GFP)) (diIorio et al., 2002; Lin et al., 2004), present a unique opportunity to study the effects of toxicant exposures on this sensitive target tissue in a live vertebrate embryo in real time, and determine the relationship between toxicant exposures and pancreatic defects.
One anthropogenic contaminant that might contribute to pancreatic malformations is perfluorooctanesulfonic acid (PFOS), which has been repeatedly associated with metabolic dysfunction. PFOS is a surfactant previously found in non-stick application products, such as Teflon and Scotchgard, until it was phased out of production in the United States in 2002. It is highly persistent in the environment and in the body, with a half-life of approximately 5 years in human serum (Olsen et al., 2007), though estimated to be roughly 12 days in the blood of rainbow trout (Martin et al., 2003). Humans are almost ubiquitously exposed to PFOS, which has been detected in >98% of human serum samples (Calafat et al., 2007) and also found in human pancreas tissue (Maestri et al., 2006). Detection in both cord blood and amniotic fluid samples demonstrates that PFOS can also cross the placental barrier, indicating an exposure risk to the developing fetus (Inoue et al., 2004; Toft et al., 2016). Numerous studies have associated PFOS exposures with markers for metabolic syndrome and diabetes, such as elevated insulin and cholesterol, insulin resistance, and altered beta cell function (Lin et al., 2009; Lv et al., 2013; Nelson et al., 2010; Wan et al., 2014). However, the pathological consequences of PFOS exposure for the fetal pancreas, as well as the underlying mechanism of PFOS-induced metabolic dysfunction, remain unknown.
Our study objective was to identify whether embryonic exposure to PFOS may alter the structure and function of the developing pancreas. We hypothesized that embryonic PFOS exposures would reduce β cell mass and disrupt the glucoregulatory axis. Here, we utilize the zebrafish embryo model to visualize malformations of the developing pancreas, and develop criteria for use in an AOP to interrogate the developmental origins of metabolic dysfunction.
MATERIALS & METHODS
CHEMICALS
Heptadecafluorooctanesulfonic acid (PFOS) was purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific (Pittsburgh, PA). Stock solutions [160-640 mM] for embryo exposures were prepared by dissolving PFOS into DMSO, and stored at room temperature in glass bottles inside of light-prohibitive containers until use. All experimental procedures involving PFOS were performed using appropriate safety precautions.
ANIMALS AND HUSBANDRY
Transgenic zebrafish of the Tg(ins:GFP) (diIorio et al., 2002) and Tg(ptf1a:GFP) strains were each obtained as a heterozygous population from Dr. Philip diIorio at the University of Massachusetts Medical School (Worchester, MA) and bred in house to homozygosity. The Tg(ins-GFP) strain expresses green fluorescence in the insulin-producing beta cells, allowing for visualization of pancreatic islets. The Tg(ptf1a:GFP) strain expresses green fluorescence in the exocrine pancreas tissues, and also in the retina and parts of the brain (Godinho et al., 2005; Lin et al., 2004).
Adult fish were housed in an Aquaneering zebrafish system maintained at 28.5°C in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and with approval from the University of Massachusetts Amherst Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3551-01). Fish were maintained on a 14 h light:10 h dark daily cycle, and provided the recommended amount of GEMMA Micro 300 (Skretting; Westbrook, ME) once daily. Breeding populations were housed in tanks containing roughly 15 males and 30 females.
Embryos were collected from breeding tanks 0-1 hour post fertilization (hpf), washed, and housed with no more than 25 other embryos in glass 100 mm petri dishes containing 0.3× Danieau’s medium (17 mM NaCl, 2 mM KCl, 0.12mM MgSO4, 1.8mM Ca(NO3)2, 1.5mM HEPES, pH 7.6) throughout the experiments.
EXPOSURES
At 3 hours post fertilization (hpf), embryos staged at the mid-blastula transition were exposed to PFOS solutions with a total of 0.01% DMSO v/v in a total of 20 ml of 0.3× Danieau’s medium. Final concentrations of PFOS were 0 (DMSO control), 16, 32, or 64 μM, and were refreshed daily to mimic subchronic developmental exposures. These concentrations were chosen based upon exposures used in other zebrafish studies (Chen et al., 2014; Wang et al., 2011; Zheng et al., 2011), and to optimize islet effects while minimizing effects on gross anatomy and embryo survival. All embryos were manually dechorionated using watchmaker’s forceps at 24 hpf and debris removed from dishes prior to refreshing exposures. Experiments were replicated 3-4 times on groups of 8-12 embryos per concentration.
MICROSCOPY
Tg(ins-GFP) embryos and larvae were imaged at 48, 96, and 168 hpf to observe morphogenesis of the primary islet, and later formation of the secondary islets. Tg(ptf1a-GFP) larvae were imaged at 96 and 168 hpf to observe the extension of the exocrine pancreas, indicative of total pancreas length. All imaging was performed using an inverted fluorescence microscope (EVOS FL Auto, Life Technologies, Pittsburgh, PA) equipped with a GFP filter. Embryos and larvae were washed thoroughly and briefly anaesthetized in 2% v/v MS-222 solution (prepared as 4 mg/ml tricaine powder in water, pH buffered, and stored at −20°C until use). Embryos and larvae were mounted in drops of 3% methylcellulose for imaging, and oriented for optimal pancreas visualization. Images were acquired using 10× and 20× objectives for magnification of islets, and 4× magnification for exocrine pancreas visualization. Because images were obtained on an inverted microscope, images presented in figures have been mirror-flipped to reflect the actual orientation.
RNA ISOLATION AND REVERSE TRANSCRIPTION
RNA was collected from embryos at 48 hpf and 96 hpf for targeted examination of pancreas-relevant gene expression. Embryos were collected into RNAlater (Fisher Scientific) and stored at −80°F until RNA isolation. At 48 hpf, 8-10 embryos were pooled per sample for a total of 6-9 samples per exposure group; at 96 hpf, 5-7 eleutheroembryos were pooled per sample for a total of 4-5 samples per exposure group. Eleutheroembryos are those that have hatched, but are not yet independently feeding and are still dependent on their yolk for nutrition.
Samples were processed with the GeneJET RNA Purification Kit (Fisher Scientific; Waltham, MA) according to manufacturer instructions. RNA concentrations were determined using a BioDrop μLITE spectrophotometer (BioDrop; Cambridge, UK). For 48 hpf samples, 500 ng RNA underwent reverse transcription for cDNA conversion using the iScript cDNA Synthesis Kit (Bio-Rad). For 96 hpf samples, 1 μg of RNA was reverse transcribed into cDNA. Upon completion, cDNA was stored at −20°C until use.
QUANTITATIVE PCR
Prior to qPCR, cDNA was diluted to a working stock of 0.25 ng/μl for use in reactions. Quantitative PCR was performed using a Bio-Rad CFX Connect Real-Time PCR Detection System in a 20 μl reaction mixture containing 10 μl 2× iQ SYBR Green Supermix (Bio-Rad), 5 pM of each primer, 5 μl water, and 1 ng (4 μl) of cDNA template. Primers used in this study have been previously published (Timme-Laragy et al., 2015). Previously designed and optimized primers for β-actin (actb), beta-2-macroglobulin (b2m), and preproinsulin a (insa) are provided in Supplementary Table 1. Endocrine pancreas gene expression was investigated at 48 and 96 hpf using commercially available PrimePCR primers (Bio-Rad) for pancreatic and duodenal homeobox 1 (pdx1), somatostatin 2 (sst2), glucagon a (gcga), and ghrelin (ghrl). Exocrine pancreas gene expression was examined at 96 hpf using PrimePCR primers for pancreas specific transcription factor 1a (ptf1a), trypsin (try), chymotrypsinogen B1 (ctrb1), and pancreatic amylase 2a (amy2a). Data was visualized and analyzed using the Bio-Rad CFX Manager software, and fold-changes were calculated using the ΔΔCT method (Livak and Schmittgen, 2001). Treatment did not significantly affect the expression the housekeeping genes, actb or b2m, and all fold changes were standardized relative to actb expression.
STATISTICAL ANALYSIS
All data is presented as the mean ± SEM. Independent t-tests and ANOVAs were used to test for statistical significance using IBM SPSS software. Fisher’s Exact Test was used to test for significant differences in the prevalence of secondary islets and islet morphological variants. A confidence level of 95% (α=0.05) was used.
RESULTS
ISLET SIZE
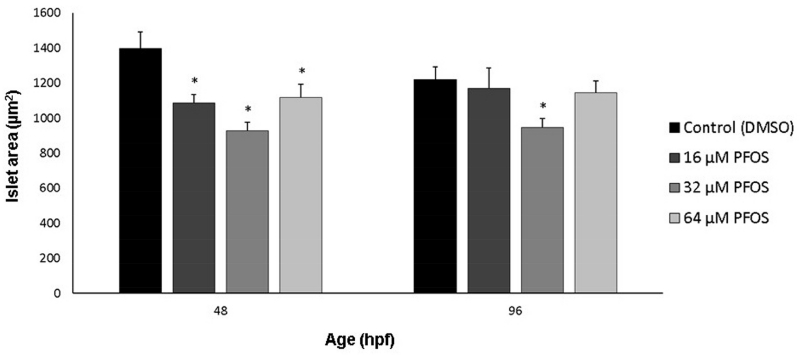
Diabetic phenotypes are often characterized by decreased beta cell mass [reviewed in (Akirav et al., 2008; Karaca et al., 2009; Matveyenko and Butler, 2008)]. To assess whether PFOS exposures could reduce beta cell mass during development, we measured area of the beta cells labeled with GFP driven by the insulin promoter. Primary islet size was quantified for embryos at 48 hpf and eleutheroembryos at 96 hpf, a time point previously shown to be sensitive to toxicological perturbation of pancreatic organogenesis (Timme-Laragy et al., 2015). At 48 hpf, islet area decreased following a non-monotonic response (Fig. 1). A decrease of islet area was observed for 16 and 32 μM PFOS exposures compared to controls, though there was a moderate attenuation of this effect at the highest concentration of 64 μM (p<0.01, p=0.03, and p=0.04, respectively). For islet size in 96 hpf eleutheroembryos, a similar non-monotonic, U-shaped response was observed. As at the earlier developmental stage, the most severe decrease of islet area was observed in the eleutheroembryos exposed to 32 μM PFOS (p<0.01).
Fig 1.
PFOS decreases islet area at 48 and 96 hpf. Islet area was measured in Tg(insulin-GFP) embryos using EVOS software. Islet area was decreased along a U-shaped curve. Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=30-45 embryos at 48 hpf; n=20-25 eleutheroembryos at 96 hpf
ISLET MORPHOLOGY
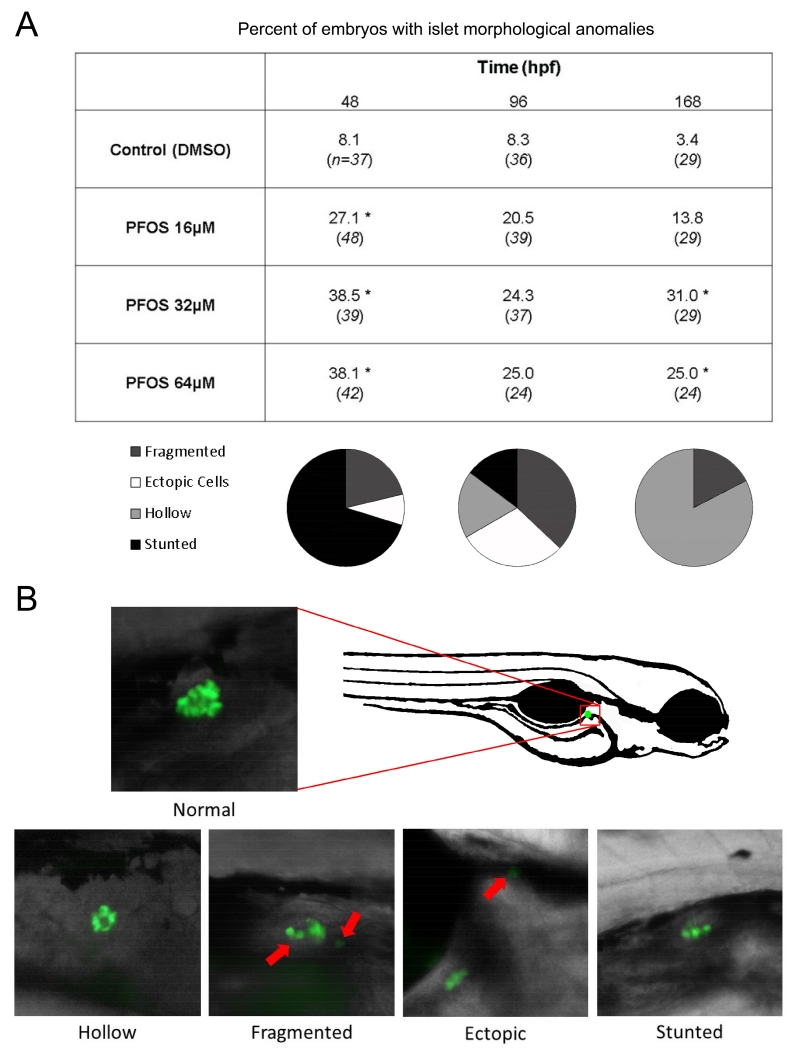
To observe whether PFOS exposures could produce anomalous islet morphology in addition to decreased islet areas, embryos and larvae were examined for morphological variants of the primary islet at 48, 96, and 168 hpf. Normally, islets are spherical, compact, and located near the 4th somite after 24 hpf. We previously identified several examples of anomalous islet morphology from toxicological perturbation with PCB-126, including islet fragmentation, ectopic beta cells, and hypomorphic islets (Timme-Laragy et al., 2015). Here, we also observed these morphologies as well as several newly identified variants due to PFOS exposures. The prevalence of total islet malformations was elevated in embryos and larvae at all time points for all PFOS exposure concentrations respective to controls (Fig. 2A). The distribution of morphologies was also time sensitive (Fig. 2B). At 48 hpf, 16, 32, and 64 μM PFOS significantly increased the incidence of anomalous morphologies (p=0.05, p<0.01, and p<0.01, respectively), primarily due to an increased number of stunted islets, which appear as a thin row of beta cells rather than a spherical mass. At 96 hpf, the frequency of total islet variants more than doubled for all PFOS exposures due to increased incidence of islets that appeared hollow/ring-shaped or fragmented, though these changes were not statistically significant (p>0.05). At 168 hpf, PFOS increased islet variant frequency for 16 (p>0.05), and especially 32 and 64 μM exposures (p=0.01 and p=0.04), and hollow islet morphology was the most commonly observed variant.
Fig 2.
PFOS exposure increases the frequency of anomalous pancreas morphologies during development. (A) Islet morphology was examined in Tg(insulin-GFP) transgenic fish at 48, 96, and 168 hpf after subchronic PFOS exposure beginning at 3 hpf. Islets were screened for fragmentation, hollowness, and severely stunted growth (shown in B at 20× magnification). Numbers presented are the percent of embryos/larvae with variant islets. Italicized numbers are the number of embryos/larvae sampled, cumulative across several study replicates. Fewer than 5% of embryos and larvae were severely deformed at the time of sampling, and were excluded from pancreas imaging. The distribution of islet morphologies are shown in pie charts under each respective time point, indicating a difference in the types of variants observed throughout development. No significant temporal differences were observed. The position of the islet within the zebrafish is shown (B, left). Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=30-45 embryos at 48 hpf; n=20-25 eleutheroembryos at 96 hpf; n=24-29 larvae at 168 hpf
SECONDARY ISLETS
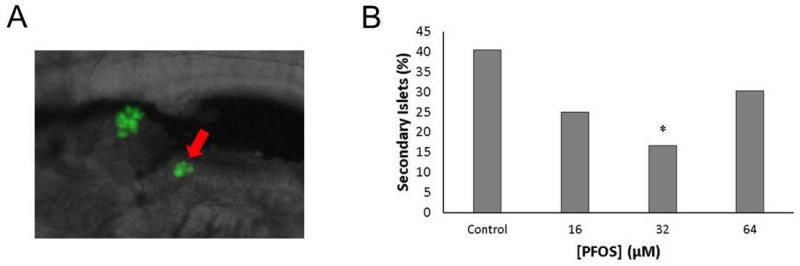
The mature pancreas has many secondary islets throughout the length of its entire tissue, which begin to appear in zebrafish between 5-7 dpf. The number of secondary islets in the pancreas and timing of their development can be sensitive to pharmacological stimuli (Wang et al., 2015). To assess whether PFOS alters the timing of secondary islet formation, the number of larvae with secondary islets was quantified for all PFOS exposures (Fig. 3). Approximately 40% of control larvae developed at least one secondary islet at 168 hpf. Compared to controls, the number of PFOS-exposed larvae with secondary islets decreased following the same U-shaped, non-monotonic response as observed with islet area. Larvae exposed to 32 μM were more than 59% less likely to have begun developing secondary islets than controls (p=0.04), though there were no significant differences between controls and 16 or 64 μM PFOS exposed larvae.
Fig 3.
PFOS exposure delays formation of secondary islets. (A) Secondary islets are characterized by one or more beta cells developing after the primary islet (arrow), typically after 120 hpf. (B) The number of secondary islets at 7 dpf was quantified in Tg(insulin-GFP) larvae. Incidence of islet defects was 19/47 (40%) in controls, 9/36 (25%) in the 16 μM group, 6/36 (17%) in the 32 μM group, and 13/43 (30%) in the 64 μM group. Bars represent the percent of larvae with secondary islets. Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=36-47 larvae per group.
FISH GROWTH
Endocrine disruption is often coupled with perturbations in developmental growth and metabolic programming. Therefore, fish length at 168 hpf was measured to determine whether the concentrations of PFOS exposure used in this study altered the overall growth of the embryos and larvae (Fig. S1). Total fish length was unchanged in larvae exposed to 16 μM PFOS. Larvae exposed to 32 μM (p=0.07) and 64 μM PFOS were 2% (p<0.01) and 1.5% (p=0.07) smaller, respectively. We observed no mortality. Because several other studies examined embryotoxicity of PFOS in zebrafish and observed increased incidence of delayed swim bladder inflation and spinal lordosis, we assessed these outcomes at 96 and 168 hpf respectively (Supplemental Table 2). There was a slight decrease in the percent of eleutheroembryos with inflated swim bladders at 96 hpf and an increase in the number of eleutheroembryos with lordosis at 168 hpf, though none of these differences were statistically significant.
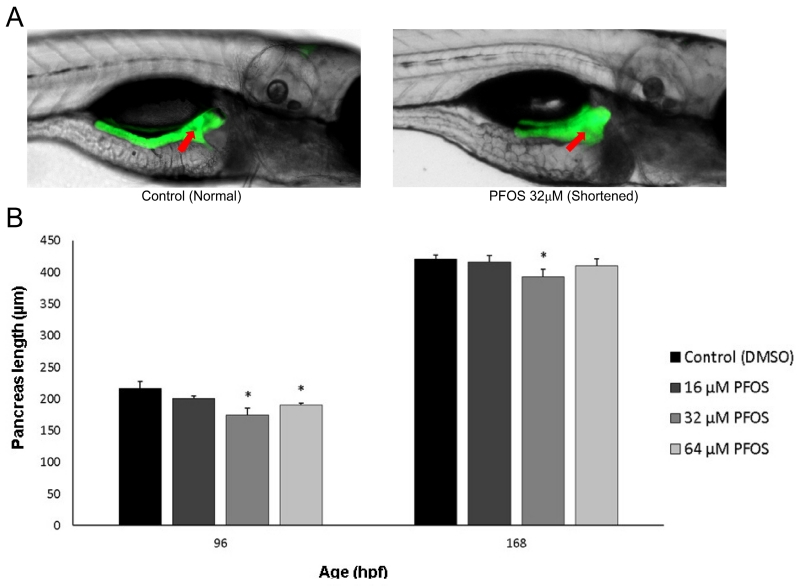
PANCREAS LENGTH
The pancreas is predominantly composed of exocrine tissue, which lengthens in the posterior direction between 48-96 hpf during zebrafish development. Though primary islets form in the region proximal to the gut (pancreas head), greater concentrations of secondary islets develop throughout the distal body and tail regions of the pancreas once exocrine extension has completed (Elayat et al., 1995; Wang et al., 2013; Wittingen and Frey, 1974). Pancreas length has been inversely associated with incidence of diabetes in adulthood (Agabi and Akhigbe, 2016), likely due to the shortening of the islet-dense regions. Here, we wanted to observe whether these shortened pancreases could be observed during development and whether toxicological perturbation may contribute to this phenotype. Pancreas extension was observed at 96 and 168 hpf, and the length from the center of the primary islet to the posterior tip of the exocrine pancreas was measured in ptf1a transgenic fish (Fig. 4A). Pancreas length was decreased by 7-20% at 96 hpf and by 1-7% at 168 hpf, both following the characteristic U-shaped non-monotonic dose-response curve observed in our other measures (Fig. 4B). Pancreas length was significantly decreased in 96 hpf eleutheroembryos exposed to 32 and 64 μM PFOS, with the greatest decrease occurring with exposure to 32 μM (p=0.01 and p=0.04, respectively). At 168 hpf, only larvae exposed to 32 μM PFOS showed significant reduction of pancreas length compared to controls compared to controls (p=0.04). The relative pancreas length was calculated for each fish (pancreas length/fish length) to identify any associations between pancreas and total body growth (Fig. S2). There were no significant changes in relative pancreas length due to PFOS exposures, though there was a subtle, linear, dose-dependent decrease.
Fig 4.
PFOS exposure decreases exocrine pancreas length at 96 and 168 hpf. (A) Pancreas length was measured in Tg(ptf1a-GFP) transgenic fish, shown at 168 hpf. Pancreas length was measured by quantifying the distance from the center of the islet (arrow) to the posterior tail of the pancreas. A control pancreas of normal length is shown at left, and a PFOS-exposed and shortened pancreas is shown at right. (B) Pancreas length is significantly decreased in fish exposed to 32 and 64 μM PFOS at 96 hpf, and to 32 μM PFOS at 168 hpf. Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=22-28 larvae
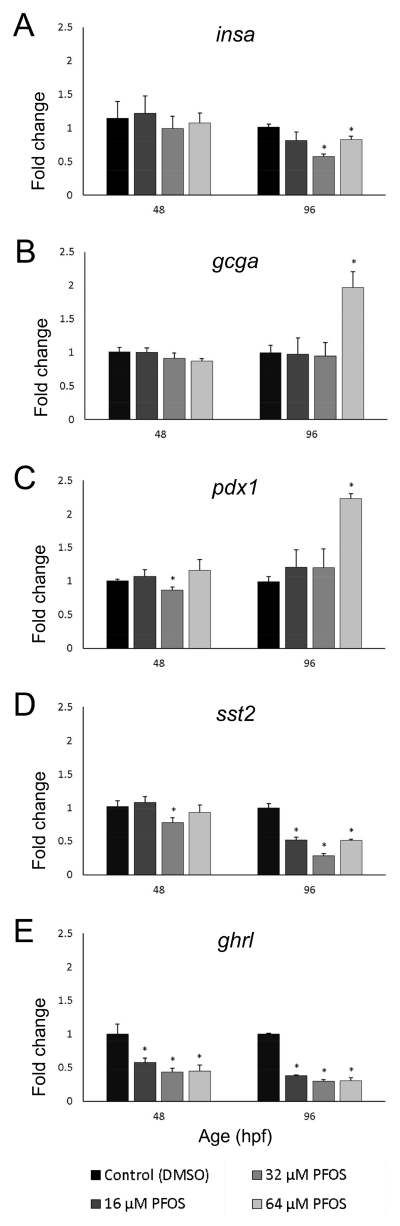
ENDOCRINE GENE EXPRESSION
Because PFOS produced structural changes of the developing pancreas in our embryos and larvae, gene expression of endocrine pancreatic hormones and transcription factors was quantified to observe whether any functional changes were produced by PFOS exposures. Here, we quantified gene expression of several major hormones and endocrine transcription factors. Insulin (Insa) is secreted by beta cells and stimulates the uptake of glucose from the blood into tissues. Glucagon a (Gcga), the hormone stimulating breakdown of glucose stores into free glucose, is secreted from islet alpha cells and often has an inverse relationship with insulin. Somatostatin 2 (Sst2) belongs to a family of genes with a myriad of endocrinology roles, including inhibition of insa expression, and is secreted from delta cells. Ghrelin (Ghrl), also an inhibitor of insa expression, is produced in hunger conditions by the islet epsilon cells and functions to counteract the action of the anorexic hormone leptin (produced by fat cells) in the brain. Pancreatic and duodenal homeobox 1 (Pdx1) is an endocrine pancreas-specific transcription factor that governs the expression of glucoregulatory genes, including insa. Together, these hormones and factors help govern endocrine function and glucose homeostasis for the entire organism.
Exposure to PFOS disrupted expression of genes which govern the glucoregulatory hormone axis in islet cells. At 48 hpf, islet sizes and insa expression were not concordant, since gene expression was unchanged by treatment. However, at 96 hpf, eleutheroembryos exposed to 32 and 64 μM PFOS had reduced insa expression compared to controls at 96 hpf (p<0.01 and p=0.05, respectively), which was concordant with islet size data (Fig. 5A). Expression of gcga was relatively stable at both 48 and 96 hpf (Fig. 5B), but exposure to 64 μM PFOS nearly doubled expression (p=0.01) at 96 hpf. Expression of transcription factor pdx1 also nearly doubled following 64 μM PFOS exposure in 96 hpf eleutheroembryos (p<0.01) compared to controls (Fig. 5C), and was also significantly decreased by 32 μM PFOS exposure in 48 hpf embryos (p=0.04).
Fig 5.
Embryonic PFOS exposure alters pancreas endocrine gene expression. RNA was isolated from embryos collected at 48 and 96 hpf, following subchronic PFOS exposure since 3 hpf. Expression of insa (A), gcga (B), pdx1 (C), sst2 (D), and ghrl (E) was analyzed using qPCR and the ΔΔCT method. Bars represent the average fold change (relative to beta actin; shown on y-axis) and the control group, and stars represent a PFOS-associated statistically significant change of expression from the control group. Age of the embryos and eleutheroembryos is shown on the x-axis in hpf. Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=7-9 samples of 9 pooled embryos at 48 hpf; n=4-5 samples of 5 pooled eleutheroembryos at 96 hpf
Expression of sst2 was decreased by more than 20% in 48 hpf embryos exposed to 32 μM PFOS (p=0.05; Fig. 5D). All PFOS exposures significantly decreased sst2 expression in 96 hpf eleutheroembryos compared to controls (p<0.01 for all concentrations), and followed a non-monotonic U-shaped response curve. Ghrelin expression was sensitive to PFOS exposures at both 48 and 96 hpf (Fig. 5E). Exposures of 16, 32, or 64 μM PFOS significantly decreased ghrl expression in embryos by nearly 50% (p=0.03, p<0.01 and p<0.01, respectively). This same response was observed at 96 hpf, with the 16 and 64 μM exposures halving ghrl expression (p<0.01) and the 32 μM exposure decreasing expression by over 70% (p<0.01).
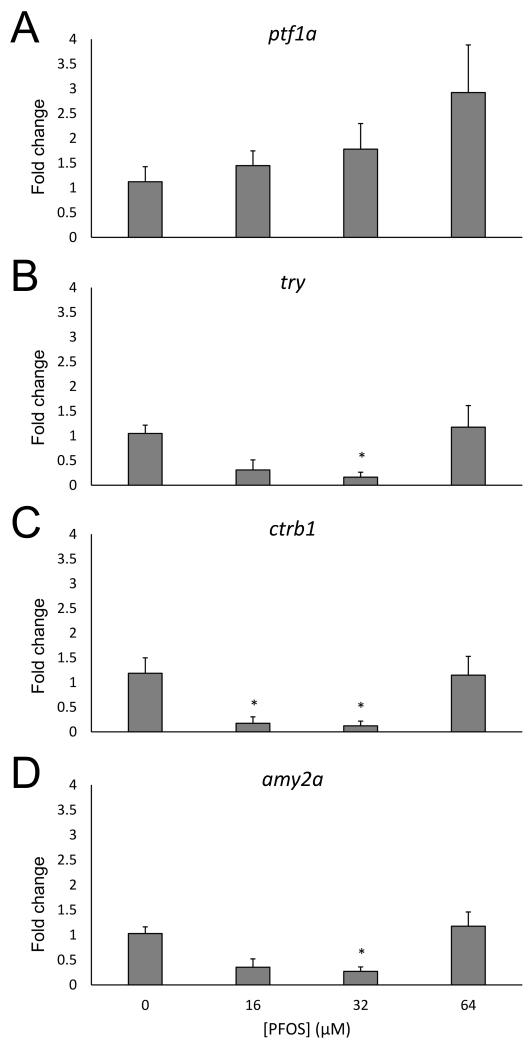
EXOCRINE GENE EXPRESSION
Both the endocrine and exocrine pancreas play important roles in glucose homeostasis, either through the secretion of glucoregulatory hormones into the vasculature or of digestive peptides. Since pancreas length was shortened, as quantified by measuring the length of the exocrine pancreas, we wanted to assess whether these structural changes co-occurred with gene expression alterations that may be crucial for exocrine pancreas development and function. Likewise, this would allow us to examine whether the effects of PFOS are specific to only the endocrine pancreas. Expression of these genes was only characterized at 96 hpf, due to the lack of exocrine architecture in the embryonic pancreas at 48 hpf. First, expression of transcription factor ptf1a was assessed to determine whether the altered exocrine pancreas structure, as visualized by using the Tg(ptf1a-GFP) transgenic line, is correlated with ptf1a gene expression (Fig. 6A). While an increasing trend was observed, there was no significant change in ptf1a expression.
Fig 6.
Embryonic PFOS exposure alters pancreas exocrine gene expression. RNA was isolated from 96 hpf following subchronic PFOS exposure since 3 hpf. Expression of ptf1a (A), try (B), ctrb1 (C), and amy2a (D) was analyzed using qPCR and the ΔΔCT method. Bars represent the average fold change (relative to beta actin; shown on y-axis) and the control group, and stars represent a PFOS-associated statistically significant change of expression from the control group. Asterisks (*) indicate a difference between designated treatment group and the controls (p<0.05); n=4-5 samples of 5 pooled eleutheroembryos at 96 hpf
We also measured expression of several digestive enzymes synthesized in the exocrine pancreas. The dose-dependent expression profiles of the proteases trypsin (try, Fig. 6B) and chymotrypsinogen B1 (ctrb1, Fig. 6C) were similar. Expression of try decreased at the 16 μM (p=0.06) and 32 μM concentrations, though was only statistically significant for the 32 μM concentration (p<0.01). Expression of ctrb1 was significantly decreased at both the 16 and 32 μM PFOS concentrations (p=0.02 and p=0.01, respectively). For both proteases, the effect was attenuated for the 64 μM treated eleutheroembryos. We also examined the expression of the carbohydrate digestive enzyme amy2a, the form of amylase produced by the exocrine pancreas (Fig. 6D). Expression of amy2a was also decreased by both the 16 μM (p=0.06) and 32 μM PFOS exposures, though only significantly for the 32 μM concentration (p<0.01). Also similar to the other proteases, this effect was attenuated in the embryos exposed to 64 μM.
DISCUSSION
Incidence of diabetes and metabolic syndrome, especially among children, has been rapidly increasing in the United States, presenting an emerging public health and economic crisis (D’Adamo and Caprio, 2011; Dabelea et al., 2014; Li et al., 2009; Silverstein et al., 2005). Though genetics and lifestyle are well known to increase risk for these disorders, the contribution of the chemical environment is not well understood. To better understand the contributions of environmental toxicant exposures to the developmental origins of diabetes, we investigated the health consequences of PFOS on organogenesis of the pancreas, an organ central to digestive function and glucoregulation. We also propose new developmental criteria to contribute to an AOP framework for the developmental origins of metabolic dysfunction using the zebrafish model. Pancreatic organogenesis is a highly conserved process across vertebrates; zebrafish are an ideal model for these studies due to the rapid development of transparent embryos and availability of transgenic models which enable in vivo observation of the developing pancreas in real time. Thus, we are able to quantify the effect of contaminants directly in the pancreas of living vertebrate embryos. In this study, we investigated whether embryonic exposure to the ubiquitous contaminant PFOS may disrupt pancreas development.
We observed increased incidence of hypomorphic and defective islets in PFOS-exposed embryos and larvae compared to controls (Figs. 1 and 2). Since islet size and architecture have been associated with both Type 1 and Type 2 diabetes, this data suggests that developmental PFOS could increase risk for diabetes later in life. These observed morphologies, coupled with a matching dose-response for hormone gene expression, suggest that the acute effects of PFOS exposures during early development are likely to result in insulin deficiency. In this study, Tg(ins:gfp) zebrafish were used to visualize islet architecture, specifically beta cells. We observed altered size and morphology, but we cannot attribute this decrease to fewer beta cells. In future work we will quantify whether the perturbations observed in this study translate to decreased numbers of beta cells and/or impact the architecture, or influence other islet cell types such as alpha cells.
With respect to the endocrine pancreas, we observed a similar dose response for many of our morphological and gene expression endpoints. The high degree of concordance between these endpoints suggests that pancreas morphologies and hormones might be predictive of each other during embryonic development. If so, these measures could be utilized in an AOP framework for understanding embryonic contributions to diabetes. Further, exocrine pancreas endpoints such as pancreas length and digestive peptide expression also followed a U-shaped dose-response when exposed to the same PFOS concentrations. This also suggests that developmentally susceptible windows of the endocrine and exocrine pancreas tissues may be similar.
Expression of insa was intially lowered by PFOS exposures, but attenuated by the highest concentration (Fig. 5). This attenuation was complemented by nearly doubled expression of pdx1 and gcga at 96 hpf. These data suggest that this increased pdx1 expression might directly increase the expression of insa and gcga since pdx1 serves as a transcription factor for the two hormones. It is possible that this increase of pdx1 expression causes the attenuation of islet effects at the highest PFOS concentration for all of the islet morphology and gene expression data, and future study of causality is necessary. The mechanism by which pdx1 is induced by PFOS exposure warrants further research, though it has been shown to be sensitive to oxidative stress (Harmon et al., 2005; Hoarau et al., 2014; Kaneto et al., 1999). PFOS has been repeatedly demonstrated to induce oxidative stress across a variety of tissues and model organisms, including the zebrafish embryo (Chen et al., 2012; Hu et al., 2005; Liu et al., 2007; Shi and Zhou, 2010). More work is required to explore the mechanisms by which oxidative stress may influence these signaling pathways.
PFOS exposures increased the incidence of variant islets throughout development. Exposures produced the greatest percentage of islet variants at 48 hpf, and these percentages decreased until 7 dpf (Fig. 2). The decreasing percentage of islet variants suggests that either these morphologies are not completely persistent or that compensation may occur. In particular, the zebrafish has a greater regenerative capacity compared to humans. In this study, we utilized a repeated daily PFOS exposure in order to minimize regenerative time and more closely mimic a constant exposure produced by the human in utero environment. The incidence of variants observed in the same population decreased between 2-7 dpf by 50-80%. Juvenile and adult zebrafish could be used to study the resilience and sensitivity of beta cells during specific windows of the lifecourse.
We have identified specific morphological islet variants during development. Because pancreatic malformations have been associated with increased risk for diabetes, understanding the causes and consequences of these anomalies could help us to improve and expand an AOP for developmental contributions to diabetes and other pancreatic diseases (Balakrishnan et al., 2006; Concepcion et al., 2014; Gentile and Fiorente, 1999; Gilinsky et al., 1987; Lindstrom et al., 1990; Mitchell et al., 2004; Shoji et al., 2013). The prevalence of these morphological variants within the control group suggests that there is some innate variability in these developmental processes regardless of exposures, as we have recently shown (Sant et al., 2016). The background prevalence of these variants in our controls of 3-8% falls within the estimated background rate for humans based upon clinical data (Prasad et al., 2001; Varshney and Johnson, 1999; Vaughn et al., 1998). The variants observed in this study appear to be morphologically congruent to developmental anomalies observed in humans, suggesting that the zebrafish may be an appropriate model organism for studying and understanding human congenital pancreatic defects.
We have shown that PFOS exposures during organogenesis may alter the length of the pancreas (Fig. 4), a measure associated with diabetic phenotypes in humans. To our knowledge, this is the first study to causally link embryonic exposures with congenitally shortened pancreas. Because the majority of islets are eventually concentrated in the distal body and tail regions of the pancreas, it is possible that a shortened pancreas could reduce the number of total islets due to loss of habitable area. Dorsal pancreatic agenesis, the partial or complete lack of a pancreatic tail, is uncommon, but rarely diagnosed due to the mild phenotypic consequences. The zebrafish provides an excellent model to test for the relationship between pancreas length and metabolic dysfunction, as the pancreas can be easily imaged in the living organism. Because of the novelty of this finding, more work is necessary to understand the types of compounds that could affect pancreas length, and the mechanisms by which they may act.
In this study, pancreas length was observed using ptf1a transgenic zebrafish, where green fluorescence is present throughout their exocrine pancreas. To validate these findings, we also analyzed ptf1a gene expression. Unexpectedly, ptf1a expression was not significantly changed and did not follow the same U-shaped response observed for pancreas length. Instead, there is an increasing trend for ptf1a gene expression (Fig. 6). Though this data did not confirm the exocrine pancreas length dose-response, ptf1a is not a pancreas-restricted transcription factor, and isan important transcription factor in the central nervous system (Aldinger and Elsen, 2008; Kani et al.; Pashos et al., 2013; Sellick et al., 2004). The hindbrain expression of ptf1a was visible in our embryo model (Fig. 4A). Because gene expression was quantified using whole embryos instead of pancreas tissue alone, the contribution of these other tissues may be confounding this data and therefore, ptf1a may not be a good candidate for an AOP framework.
Though the pancreas length decreased, we also characterized gene expression of several digestive enzymes to better understand whether the observed exocrine pancreas structure was associated with altered exocrine function. Expression of proteases try and ctrb1 as well as of the glycolytic enzyme amy2a was decreased by 16 and 32 μM PFOS exposures; however, this effect was attenuated by 64 μM PFOS exposure (Fig. 6). Though this dose-response does follow along with pancreas length, it was interesting that the high-dose PFOS exposure was unable to produce the structural and expression changes observed at lower concentrations. Further work should investigate whether PFOS alters the uptake, distribution, and utilization of nutrients during organogenesis, since these factors have been implicated in the developmental origins of diseases such as diabetes.
This work provides several developmental outcomes of PFOS exposure in the pancreas. However, the contributions of these pancreatic variants to overall developmental progress and growth remain unknown. Here, a modest decrease of total larval length was measured due to PFOS exposure. Though a 2% decrease of fish length is a mild phenotypic change, it is not insignificant. In the United States, there is only a modest 4-6% difference in fetal length between the median infant length and fetuses small for gestational age, defined as the lowest 10th percentile for fetal growth (Fenton and Kim, 2013). A longitudinal study should be performed to observe whether these pancreas morphologies and physiological consequences persist beyond early developmental stages, or whether juvenile or adult fish are able to “catch up” and correct for previous deficiencies. It is important to identify whether these developmental consequences will ultimately manifest as metabolic dysfunction throughout the lifecourse in order to better define prenatal parameters for interrogation in a developmental origins of diabetes AOP.
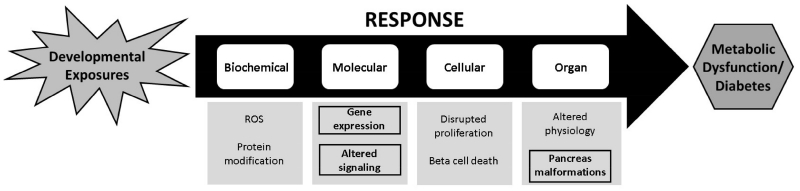
There are many gaps in our understanding of an AOP for the development of diabetes produced by early life exposures. Numerous studies have examined epidemiological associations between developmental exposures and metabolic dysfunction, or the pathological consequences of toxicant exposures on adult beta cells. However, pancreas teratogenesis has rarely been studied, but may provide a link between the embryonic biochemical and molecular changes and the pathological outcomes later in life. In this study, we have addressed several of these gaps by elucidating changes in gene expression and signaling, as well as structural anomalies in the embryonic pancreas (Fig. 7, shown in bold). Future studies will further investigate the mechanistic basis of these structural changes, and how they manifest as metabolic dysfunction throughout the lifecourse.
Fig 7.
This study helps to expand an AOP framework for the developmental origins of metabolic dysfunction and diabetes. Findings of this study (highlighted in black boxes) provide new criteria for use in an AOP framework for the association between developmental exposures and metabolic dysfunction. This framework (flowing from left to right) has guided the identification of several key biochemical, molecular, cellular, and organ changes that lead to these disorders; however, the effects of exposures such as PFOS on pancreas structure had not been studied. In the future, we seek to elucidate a mechanism by which these exposures may cause dysmorphogenesis of the endocrine and exocrine pancreas, and further how these structural anomalies are associated with the development of metabolic dysfunction later in the lifecourse.
CONCLUSION
In conclusion, we have identified specific morphological and likely functional consequences of PFOS-induced perturbation of pancreatic organogenesis in both endocrine and exocrine tissues for the purpose of expanding and improving an AOP. This work establishes a foundation for future toxicology studies of the developing pancreas. We seek to establish a predictive AOP framework for understanding the embryonic contributions to diabetes risk through studying the mechanisms by which these morphological consequences may increase risk for diabetes later in the lifecourse. In the future, we will continue to pursue the coordinated characterization of the pancreatic biochemical, molecular, and morphological consequences of toxicological perturbations during these newly identified key windows of developmental susceptibility.
Supplementary Material
Highlights.
Developmental PFOS exposures decreased the size of beta cell mass in the primary Islet of Langerhans in the zebrafish embryo.
PFOS exposures increased the incidence of islet malformations and shortened pancreas length, which recapitulate congenital defects known to increase risk for diabetes in humans.
Abnormal pancreas development is a previously unidentified hazard of developmental PFOS exposures.
ACKNOWLEDGEMENTS
We are grateful to the late Dr. Philip J. diIorio for helpful conversations and guidance, as well as providing the fish used in this study. We thank the following students for exceptional fish care: Aviraj Basnet, Sarah Brown, Shana Fleischman, Sadia Islam, Yankel Karasik, Derek Luthi, Karen Melendez, Michelle Rousseau, Paul Sinno, Christopher Sparages, Olivia Venezia, Felicia Wang, Kaylee Williams, and Jiali Xu. The authors declare they have no actual or potential competing financial interests.
FUNDING
This work was supported in part by the National Institutes of Health (R01 ES025748 to ART-L), and a University of Massachusetts Amherst Commonwealth Honors College Research grant (to KAB.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agabi JO, Akhigbe AO. Comparative sonographic evaluation of the anteroposterior dimensions of the pancreas in diabetics and nondiabetics. Niger J Clin Pract. 2016;19:175–181. doi: 10.4103/1119-3077.175969. [DOI] [PubMed] [Google Scholar]
- Akirav E, Kushner JA, Herold KC. β-Cell Mass and Type 1 Diabetes: Going, Going, Gone? Diabetes. 2008;57:2883–2888. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Elsen GE. Ptf1a Is a Molecular Determinant for Both Glutamatergic and GABAergic Neurons in the Hindbrain. The Journal of neuroscience. 2008;28:338–339. doi: 10.1523/JNEUROSCI.5139-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Narayanan VA, Siyad I, Radhakrishnan L, Nair P. Agenesis of the dorsal pancreas with chronic calcific pancreatitis. case report, review of the literature and genetic basis. JOP. 2006;7:651–659. [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique Arrangement of α- and β-Cells in Human Islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Tal TL, Bai C, Tilton SC, Jin D, Yang D, Huang C, Dong Q. Early life perfluorooctanesulphonic acid (PFOS) exposure impairs zebrafish organogenesis. Aquat Toxicol. 2014;150:124–132. doi: 10.1016/j.aquatox.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhang L, Yue J.-q., Lv Z.-q., Xia W, Wan Y.-j., Li Y.-y., Xu S.-q. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reproductive Toxicology. 2012;33:538–545. doi: 10.1016/j.reprotox.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Concepcion JP, Reh CS, Daniels M, Liu X, Paz VP, Ye H, Highland HM, Hanis CL, Greeley SAW. Neonatal Diabetes, Gallbladder Agenesis, Duodenal Atresia, and Intestinal Malrotation Caused by a Novel Homozygous Mutation in RFX6. Pediatric diabetes. 2014;15:67–72. doi: 10.1111/pedi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo E, Caprio S. Type 2 Diabetes in Youth: Epidemiology and Pathophysiology. Diabetes Care. 2011;34:S161–S165. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog Is Required Early in Pancreatic Islet Development. Developmental Biology. 2002;244:75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. Journal of Anatomy. 1995;186:629–637. [PMC free article] [PubMed] [Google Scholar]
- Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: Structure, function, evolution, and AHR-dependent regulation in vivo. Archives of Biochemistry and Biophysics. 2005;441:151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13:1–13. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile M, Fiorente P. Esophageal, duodenal, rectoanal and biliary atresia, intestinal malrotation, malformed/hypoplastic pancreas, and hypospadias: further evidence of a new distinct syndrome. Am J Med Genet. 1999;87:82–83. [PubMed] [Google Scholar]
- Gilinsky NH, Lewis JW, Flueck JA, Fried AM. Annular pancreas associated with diffuse chronic pancreatitis. Am J Gastroenterol. 1987;82:681–684. [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong ROL. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Harmon JS, Stein R, Robertson RP. Oxidative Stress-mediated, Post-translational Loss of MafA Protein as a Contributing Mechanism to Loss of Insulin Gene Expression in Glucotoxic Beta Cells. Journal of biological chemistry. 2005;280:11107–11113. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- Hoarau E, Chandra V, Rustin P, Scharfmann R, Duvillie B. Pro-oxidant/antioxidant balance controls pancreatic [beta]-cell differentiation through the ERK1/2 pathway. Cell Death Dis. 2014;5:e1487. doi: 10.1038/cddis.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jones PD, Celius T, Giesy JP. Identification of genes responsive to PFOS using gene expression profiling. Environmental Toxicology and Pharmacology. 2005;19:57–70. doi: 10.1016/j.etap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Inadera H. Developmental origins of obesity and type 2 diabetes: molecular aspects and role of chemicals. Environ Health Prev Med. 2013;18:185–197. doi: 10.1007/s12199-013-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane Sulfonate (PFOS) and Related Perfluorinated Compounds in Human Maternal and Cord Blood Samples: Assessment of PFOS Exposure in a Susceptible Population during Pregnancy. Environ Health Perspect. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- Kani S, Bae Y-K, Shimizu T, Tanabe K, Satou C, Parsons MJ, Scott E, Higashijima S.-i., Hibi M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Developmental Biology. 343:1–17. doi: 10.1016/j.ydbio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Karaca M, Magnan C, Kargar C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 2009;35:77–84. doi: 10.1016/j.diabet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, Hara M. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients with Type 2 Diabetes. PLoS ONE. 2011;6:e27445. doi: 10.1371/journal.pone.0027445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: A comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: Zebrafish as a model for pancreas development and function. BioEssays: news and reviews in molecular, cellular and developmental biology. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of Pre-Diabetes and Its Association With Clustering of Cardiometabolic Risk Factors and Hyperinsulinemia Among U.S. Adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32:342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32:702–707. doi: 10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Developmental Biology. 2004;274:491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lindstrom E, von Schenck H, Ihse I. Pancreatic exocrine and endocrine function in patients with pancreas divisum and abdominal pain. Int J Pancreatol. 1990;6:17–24. doi: 10.1007/BF02924340. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu K, Shi X, Wang J, Lam PKS, Wu RSS, Zhou B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus) Aquatic Toxicology. 2007;82:135–143. doi: 10.1016/j.aquatox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, Wei J, Lin Y, Jiang Y, Wang Y, Shu B, Xu B, Xu S. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28:532–542. doi: 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- Maestri L, Negri S, Ferrari M, Ghittori S, Fabris F, Danesino P, Imbriani M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry. 2006;20:2728–2734. doi: 10.1002/rcm.2661. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DCG. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss) Environmental Toxicology and Chemistry. 2003;22:196–204. [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC. Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10:23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Punthakee Z, Lo B, Bernard C, Chong K, Newman C, Cartier L, Desilets V, Cutz E, Hansen IL, Riley P, Polychronakos C. Neonatal diabetes, with hypoplastic pancreas, intestinal atresia and gall bladder hypoplasia: search for the aetiology of a new autosomal recessive syndrome. Diabetologia. 2004;47:2160–2167. doi: 10.1007/s00125-004-1576-3. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Diabetes Information Clearinghouse (NDIC); 2014. [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population. Environmental health perspectives. 2010;118:197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashos E, Park JT, Leach S, Fisher S. Distinct enhancers of ptf1a mediate specification and expansion of ventral pancreas in zebrafish. Developmental Biology. 2013;381:471–481. doi: 10.1016/j.ydbio.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TR, Gupta SD, Bhatnagar V. Ectopic pancreas associated with a choledochal cyst and extrahepatic biliary atresia. Pediatric Surgery International. 2001;17:552–554. doi: 10.1007/s003830100607. [DOI] [PubMed] [Google Scholar]
- Sant K, Jacobs H, Xu J, Borofski K, Moss L, Moss J, Timme-Laragy A. Assessment of Toxicological Perturbations and Variants of Pancreatic Islet Development in the Zebrafish Model. Toxics. 2016;4:20. doi: 10.3390/toxics4030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, J Coleman R, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, Goodwin G, Houlston RS. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhou B. The Role of Nrf2 and MAPK Pathways in PFOS-Induced Oxidative Stress in Zebrafish Embryos. Toxicological Sciences. 2010;115:391–400. doi: 10.1093/toxsci/kfq066. [DOI] [PubMed] [Google Scholar]
- Shoji F, Takeo S, Shikada Y, Katsura M. Anterior mediastinal gastroenteric cyst containing pancreatic tissue influenced the diabetes mellitus status. Interactive Cardiovascular and Thoracic Surgery. 2013;16:413–415. doi: 10.1093/icvts/ivs496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N. Care of Children and Adolescents With Type 1 Diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of diabetes: The role of oxidative stress. Free Radical Biology and Medicine. 2006;40:917–922. doi: 10.1016/j.freeradbiomed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of diabetes: the role of epigenetic mechanisms. Curr Opin Endocrinol Diabetes Obes. 2007;14:13–16. doi: 10.1097/MED.0b013e328013da5b. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy A, Sant K, Rousseau M, diIorio P. Deviant development of pancreatic beta cells from embryonic exposure to PCB-126 in zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. 2015;178:25–32. doi: 10.1016/j.cbpc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Tiso N, Moro E, Argenton F. Zebrafish pancreas development. Mol Cell Endocrinol. 2009;312:24–30. doi: 10.1016/j.mce.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Toft G, Jönsson BAG, Bonde JP, Nørgaard-Pedersen B, Hougaard DM, Cohen A, Lindh CH, Ivell R, Anand-Ivell R, Lindhard MS. Perfluorooctane Sulfonate Concentrations in Amniotic Fluid, Biomarkers of Fetal Leydig Cell Function, and Cryptorchidism and Hypospadias in Danish Boys (1980–1996) Environmental health perspectives. 2016;124:151–156. doi: 10.1289/ehp.1409288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney S, Johnson CD. Pancreas divisum. Int J Pancreatol. 1999;25:135–141. doi: 10.1385/IJGC:25:2:135. [DOI] [PubMed] [Google Scholar]
- Vaughn DD, Jabra AA, Fishman EK. Pancreatic disease in children and young adults: evaluation with CT. Radiographics. 1998;18:1171–1187. doi: 10.1148/radiographics.18.5.9747614. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Leung PY, Wong CKC. Perinatal Exposure to Perfluorooctane Sulfonate Affects Glucose Metabolism in Adult Offspring. PLoS ONE. 2014;9:e87137. doi: 10.1371/journal.pone.0087137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Rajpurohit SK, Delaspre F, Walker SL, White DT, Ceasrine A, Kuruvilla R, Li RJ, Shim JS, Liu JO, Parsons MJ, Mumm JS. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. Elife. 2015:4. doi: 10.7554/eLife.08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen J, Lin K, Chen Y, Hu W, Tanguay RL, Huang C, Dong Q. CHRONIC ZEBRAFISH PFOS EXPOSURE ALTERS SEX RATIO AND MATERNAL RELATED EFFECTS IN F1 OFFSPRING. Environmental Toxicology and Chemistry / Setac. 2011;30:2073–2080. doi: 10.1002/etc.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, Millis JM, Witkowski P, Hara M. Regional Differences in Islet Distribution in the Human Pancreas - Preferential Beta-Cell Loss in the Head Region in Patients with Type 2 Diabetes. PLoS ONE. 2013;8:e67454. doi: 10.1371/journal.pone.0067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfinger A, Arkhipova V, Meyer D. Cell type and tissue specific function of islet genes in zebrafish pancreas development. Developmental Biology. 2013;378:25–37. doi: 10.1016/j.ydbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg. 1974;179:412–414. doi: 10.1097/00000658-197404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Liu HL, Shi W, Wei S, Giesy JP, Yu HX. Effects of perfluorinated compounds on development of zebrafish embryos. Environ Sci Pollut Res Int. 2011;19:2498–2505. doi: 10.1007/s11356-012-0977-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.