Abstract
Objective(s)
To estimate the incidence of lipid and glucose abnormalities and assess their association with exposure to antiretroviral (ARV) regimens among perinatally HIV-infected Latin American children.
Design
Longitudinal cohort study.
Methods
Data were analyzed from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) Pediatric Latin American Countries Epidemiologic Study (PLACES). The incidence of dyslipidemia (total cholesterol>200mg/dL, HDL<35mg/dL, LDL≥130mg/dL, triglycerides>110mg/dL [age<10 years] or >150mg/dL [≥10 years]) and fasting glucose abnormalities (homeostasis model assessment of insulin resistance >2.5 [Tanner Stage 1] or >4.0 [Tanner Stage>1]; impaired glucose: 110 to <126mg/dL; diabetes: ≥126 mg/dL) was estimated. Proportional hazards regression was used to evaluate the risk of abnormalities associated with ARV regimen, adjusted for covariates.
Results
There were 385 children eligible for analysis (mean age 6.6 years). Incident cholesterol abnormalities were reported in 18.1% of participants (95% confidence interval [CI] 14.1–22.8%), HDL and LDL cholesterol abnormalities in 19.6% (15.1–24.7%) and 15.0% (11.3–19.5%), respectively, and triglyceride abnormalities in 44.2% (37.7–50.8%). In multivariable analysis, ARV regimen was only associated with triglyceride abnormalities; participants receiving a protease inhibitor-containing (PI) regimen were 3.6 times as likely to experience a triglyceride abnormality as those receiving no ARVs (95% CI: 1.3–10.5; p=0.0167). The cumulative incidence of insulin resistance was 3.8% (1.8–7.1%); there were no incident cases of diabetes and only two of impaired fasting glucose.
Conclusions
Children receiving PI-containing regimens were at increased risk of developing triglyceride abnormalities. Continued monitoring of lipid levels in children receiving PI-containing regimens appears warranted.
Keywords: children, HIV infection, protease inhibitors, dyslipidemia, glucose abnormalities
INTRODUCTION
Combination antiretroviral therapy (cART) has demonstrated unquestionable value in reducing morbidity and mortality among HIV-infected children [1, 2]. Despite these benefits, studies have found an association between cART and metabolic abnormalities including dyslipidemia and glucose abnormalities, such as insulin resistance (IR) and type II diabetes [3, 4, 5]. The persistence of these associations over time may increase metabolic risk [6] and is of concern as HIV-infected children are expected to receive antiretroviral therapy (ART) for life [7]. In a previous study from an older version of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) pediatric protocol we showed that Latin American HIV-infected children using protease inhibitors (PI)-containing cART regimens were approximately three times more likely to develop hypercholesterolemia and hypertriglyceridemia, compared to children on non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing cART [3]. However, this study did not require fasting, so did not analyze cholesterol subsets nor glucose abnormalities. Studies from cohorts in the United States have shown similar results. Children receiving PI-containing cART regimens present higher rates of hyperlipidemia in comparison to those not receiving PIs: 29% vs. 10%, respectively [8]. The Pediatric AIDS Clinical Trials Group (PACTG) 219C cohort study demonstrated that PI use was associated with increased risk of developing hypercholesterolemia, with 4.8 cases per 100 person-years of use of cART regimens containing PIs, compared to 0.7 cases per 100 person-years of use of cART combinations without PIs [9].
Disorders of glucose metabolism, ranging from reduction in insulin sensitivity to impaired glucose tolerance and diabetes mellitus, have also recently been recognized in HIV-infected adults [10, 11]. However, these disorders have not been sufficiently studied in HIV-infected children and adolescents receiving cART [10]. Although HIV-1 infection alone may be independently linked to decreased insulin sensitivity [12], it has been fairly well established that PIs are associated with the emergence of IR in HIV-infected adults. These same processes occur in children, but are less apparent clinically [10]. A cohort study found an IR prevalence of 15.2% in pre-pubertal and pubertal children with HIV infection [5]. However, initial cross-sectional analyses of the NISDI Pediatric Latin American Countries Epidemiologic Study (PLACES) data did not confirm these results; we previously found a 6.8% prevalence of IR among Latin American children, but no association of IR with ARV use [4]. The objective of the current study was to expand on the prior analyses of the NISDI PLACES cohort using longitudinal methods to estimate the incidence of lipid and glucose metabolic abnormalities and examine their association with type of antiretroviral regimen received.
METHODS
NISDI PLACES Cohort
NISDI PLACES was a prospective cohort study that enrolled perinatally HIV-infected children younger than 6 years of age, from 2008 to 2011, at 14 clinical sites in Latin America (12 sites in Brazil, one in Peru and one in Mexico). PLACES also enrolled participants that were younger than 6 years of age at the time of enrollment to the earlier version of the NISDI pediatric protocol. A description of this cohort has been published elsewhere [4, 13]. The protocol was approved by the ethical review boards of each clinical center, the sponsoring institution (NICHD), the data management and statistical center (Westat, Rockville, MD), and the Brazilian National Ethics Committee (CONEP). Informed consent was obtained from the parents or guardians of participants.
Study Population
Per protocol, children were asked to come in a fasting state for testing, once a year after the age of five [3]. Fasting lipid and glucose testing results obtained for children five years of age or older served as the basis for analyzing the prevalence and incidence of the study outcomes. Accordingly, children enrolled in the NISDI PLACES protocol who were five years old or older at enrollment were eligible to participate, as were younger children beginning at the point during study follow-up when they reached age five. Children with a diagnosis of type I diabetes, nephrotic or nephritic syndrome, uremia, or hypothyroidism at baseline were excluded from the study, as these conditions could result in abnormal levels of the study outcomes.
Study Definitions
The following cutoffs were used to define an abnormal result for the different lipid measures: total cholesterol >200 mg/dL; HDL cholesterol <35 mg/dL; LDL cholesterol ≥130 mg/dL and TG>110 mg/dL for age <10 years or TG>150 mg/dL for age ≥10 years. In accordance with World Health Organization (WHO) definitions, diabetes was defined as a fasting glucose ≥126 mg/dL; a fasting glucose result of ≥110 to <126 mg/dL was considered as impaired fasting glucose [14]. The Centers for Disease Control and Prevention (CDC) definition of impaired fasting glucose (fasting glucose result of ≥100 to 125 mg/dL) was also evaluated [15]. The homeostatic model assessment of insulin resistance (HOMA-IR) was defined on the basis of the standard formulation of fasting insulin times fasting glucose, divided by 405 [16]. Insulin resistance was defined as HOMA-IR >2.5 in those with Tanner stage equal to 1 or HOMA-IR >4.0 in those with Tanner stage >1. Tanner Stage for puberty rating was determined by the attending physician based on physical examination at study appointments. Since Tanner Stage is not assessed for males less than 9 years of age or females less than 7 years of age, these children were assigned as Stage I for the analyses.
Adherence to ART was assessed through patient or caregiver self-report, using a structured questionnaire developed for use by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) as part of standard practice in PACTG studies [17]. ART adherence was based on the total number of doses missed relative to the total number of expected doses included in the participant’s treatment regimen during the three-day period just before follow-up visits, which were conducted every six months.
Statistical Analysis
Characteristics of the study population at eligibility were examined using simple descriptive statistics; categorical-scaled variables were described using frequencies and percentages, while continuous variables were examined in terms of means, standard deviations (SD) and medians. Prevalence rates (and 95% confidence intervals [CIs]) were calculated separately for each study outcome measure based on the first available fasting test result (‘baseline’) reported for each outcome. Cumulative incidence rates (and 95% CIs) were derived based on the occurrence of abnormal glucose or lipid measures during study follow-up among children who had an initial normal fasting measure (non-prevalent cases).
The risk of metabolic abnormalities associated with ART use was modeled on the basis of the time-to-event using Cox proportional hazards regression modeling. The association of a number of patient-level characteristics with abnormal lipid levels was explored in preliminary univariate proportional hazard regression analyses to screen for candidate covariates for inclusion in multivariate modeling using an alpha ≤0.1 for covariate screening. ART regimen, age, HIV RNA and CD4 percent were modeled as possible time-varying predictors of the study outcomes; the time-varying predictor variables were updated in the analyses to reflect any changes that occurred during study follow-up prior to the outcome events. In order to avoid assigning risk to recently initiated ART regimens and those that ceased being taken long before the timing of the outcome events, a window of 14 to 183 days was used in capturing the ART regimen; if the ART regimen changed less than 14 days before the outcome assessment, the patient’s previous ART combination was used in the multivariable analysis for the relevant study visit. This same window was used in capturing the other time-varying covariate values. The fixed covariates of gender and country of origin were included in the final multivariable models in order to adjust for potential residual confounding due to differences among study participants.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
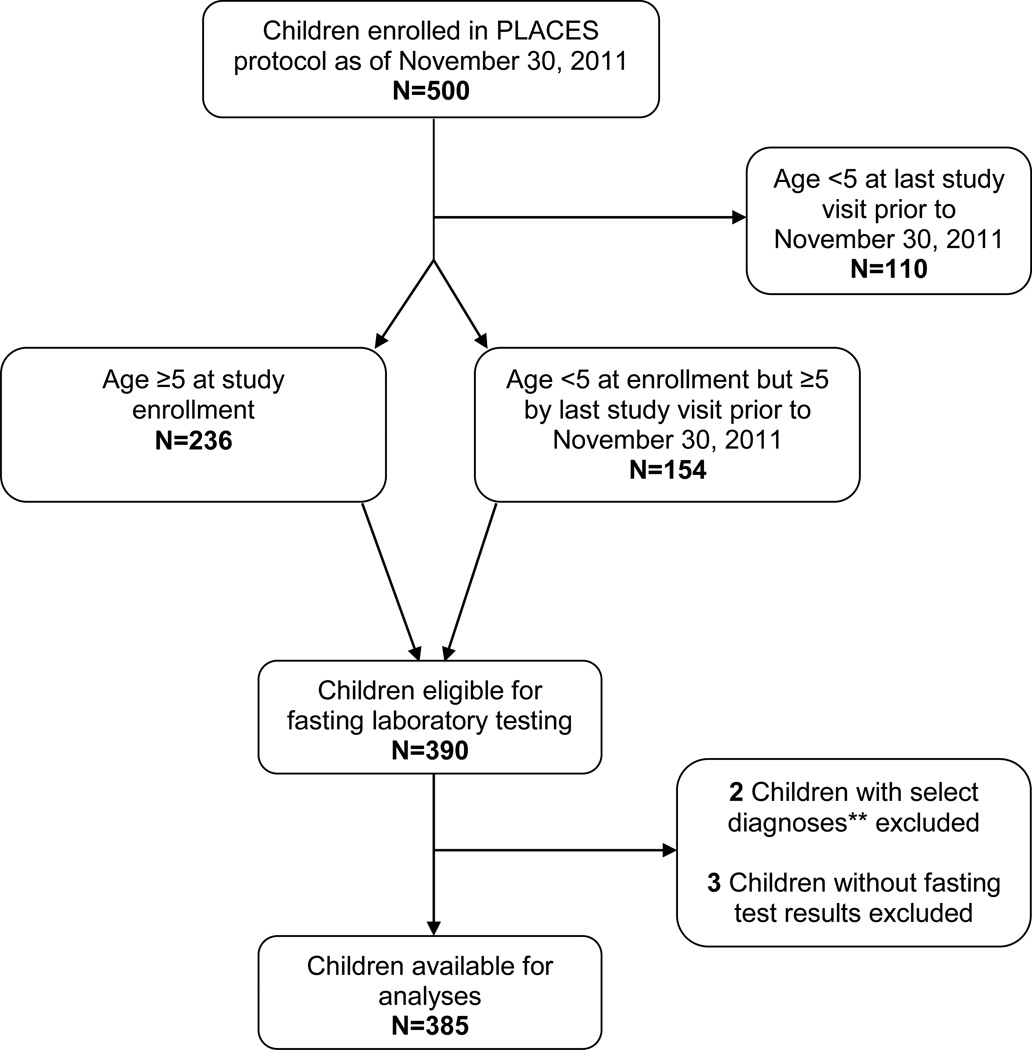
Among the 500 children enrolled on the NISDI PLACES protocol, 115 were excluded from the present analysis for the following reasons: 110 patients were younger than 5 years of age at their last study visit and therefore not subject to fasting metabolic laboratory testing, two were diagnosed with diseases associated with abnormalities of lipid or glucose metabolism identified for exclusion (both had hypothyroidism), and three did not have fasting laboratory test results available for analysis (Figure 1). The final analysis population consisted of 385 children that met the eligibility criteria for the lipid profile analysis; 342 of these children were eligible for insulin quantification and 345 for glucose profile analysis.
Figure 1. Study Population Derivation.
** Any child with a diagnosis of type I diabetes, nephrotic or nephritic syndrome, uremia, or hypothyroidism was excluded.
Table 1 summarizes the characteristics of the study population at eligibility (N=385). The average age (±SD) of study participants eligible for this analysis was 6.6 (±2.0) years and the majority of them (51.2%) were female. Most subjects (67.9%) were below the gender-specific age cut-off for assessment of Tanner Stage and set to Stage 1 for purposes of the analysis. Most of the participants were classified as having normal body mass index (BMI) (83.5%), height (84.7%) and weight (87.2%) for age according to the WHO standards [18]. Table 2 presents the HIV data at baseline, the mean (±SD) log10 viral load was 3.1 (±1.3), with 34.2% having undetectable (<400 copies/mL) HIV RNA levels; most had high CD4 measures (71.6% with CD4 percent >25%, 88.3% with CD4 absolute count ≥500 cells/mm3). WHO immunological staging was classified as ‘none or not significant’ for 61.3%. The majority of participants (89.4%) had received ART prior to becoming eligible for the present analysis, with a mean (±SD) duration of use of 4.6 (±2.8) years; 55.6% were currently receiving a PI-containing regimen at study eligibility. The most common regimens at eligibility included 3TC/ZDV/LPV/r (n=83), 3TC/ZDV/EFV (n=36), 3TC/d4T/LPV/r (n=32), ddI/ZDV/LPV/r (n=32) and 3TC/ZDV/NVP (n=20). Self-reported adherence to ART was high among study participants; 89.4% indicated that they had taken all prescribed ART doses during the 3 days prior to the eligibility visit.
Table 1.
Sociodemographic and anthropometric characteristics of the study population at eligibility (N=385)
| Characteristic1 | |
|---|---|
| Age (years): | |
| Mean (SD) | 6.6 (2.0) |
| Median | 5.0 |
| Gender: n (%) | |
| Female | 197 (51.2) |
| Male | 188 (48.8) |
| Race: n (%) | |
| White | 155 (40.3) |
| Black | 84 (21.8) |
| Mestizo | 144 (37.4) |
| Mulato | 2 (0.5) |
| Country: n (%) | |
| Brazil | 301 (78.2) |
| Mexico | 58 (15.1) |
| Peru | 26 (6.8) |
| Tanner Stage: n (%)2 | |
| 1 | 353 (92.2) |
| 2 | 22 (5.7) |
| 3 | 6 (1.6) |
| 4 | 2 (0.5) |
| 5 | 0 |
| Missing | 2 |
| WHO BMI for age z-score: n (%) | |
| <−2 SD (underweight) | 11 (3.4) |
| −2 SD to +1 SD (normal) | 268 (83.5) |
| >+1 SD (overweight) | 30 (9.3) |
| >+2 SD (obesity) | 12 (3.7) |
| Missing | 64 |
| WHO height for age z-score: n (%) | |
| <−2 SD | 48 (15.0) |
| Normal (−2 SD to +2 SD) | 272 (84.7) |
| >+2 SD | 1 (0.3) |
| Missing | 64 |
| WHO weight for age z-score: n (%) | |
| <−2 SD | 28 (10.3) |
| Normal (−2 SD to +2 SD) | 238 (87.2) |
| >+2 SD | 7 (2.6) |
| Missing | 112 |
Where not available at time of eligibility, the most recent result prior to eligibility was selected. Missing data were not included in percentage calculations.
Since Tanner Stage is not assessed for males less than 9 years of age or females less than 7 years of age, these children were all set to Stage 1 for the analyses.
Table 2.
HIV data Results (N=385)
| Characteristic1 | |
|---|---|
| Log10 viral load (copies/mL): | |
| Mean (SD) | 3.1 (1.3) |
| Median | 2.6 |
| Viral load: n (%) | |
| Undetectable (<400 copies/mL) | 130 (34.2) |
| Detectable (≥ 400 copies/mL) | 250 (65.8) |
| Missing | 5 |
| CD4 count percent: n (%) | |
| <15% | 19 (5.0) |
| 15 to 25% | 89 (23.4) |
| >25% | 272 (71.6) |
| Missing | 5 |
| CD4 count (cells/mm3): n (%) | |
| <200 | 5 (1.3) |
| 200–499 | 40 (10.4) |
| ≥500 | 340 (88.3) |
| CDC Classification: n (%) | |
| N | 28 (7.3) |
| A | 105 (27.3) |
| B | 116 (30.2) |
| C | 135 (35.2) |
| Missing | 1 |
| WHO immunological staging: n (%) | |
| None or not significant | 233 (61.3) |
| Mild | 43 (11.3) |
| Advanced | 33 (8.7) |
| Severe | 71 (18.7) |
| Missing | 5 |
| Any ART prior to eligibility: n (%) | |
| Yes | 344 (89.4) |
| No | 41 (10.6) |
| Duration of exposure to ART prior to eligibility (years): | |
| Mean (SD) | 4.6 (2.8) |
| Median | 4.5 |
| Current ART regimen type: n (%) | |
| No ART | 68 (17.7) |
| PI-containing regimen | 214 (55.6) |
| Non-PI-containing regimen | 103 (26.8) |
| Self-reported ART adherence among those receiving ART2
: n (%) |
|
| 100% adherence | 279 (89.4) |
| <100% adherence | 33 (10.6) |
| Missing | 73 |
Where not available at time of eligibility, the most recent result prior to eligibility was selected. Missing data were not included in percentage calculations.
Percent adherence was determined in terms of numbers of ART doses missed relative to doses expected to be taken during the 3 day period prior to the study visit.
There were no participants using statins prior to study eligibility. Only 21 (5.5%) subjects received glucocorticoids (all but one received systemically), which included hydrocortisone, dexamethasone, budesonide, prednisone or prednisolone (data not shown). Also, there were few reports at or prior to eligibility of diagnoses of obesity (2.1%) or lipodystrophy (fat accumulation, 1%; fat loss, 2.9%), and there were no reports of type II diabetes.
The mean (±SD) fasting glucose and insulin levels at baseline were 78.4 (±8.9) mg/dL and 4.2 (±4.3) mU/I, respectively. Mean (±SD) HOMA-IR at baseline was 0.84 (±1.04). The mean (±SD) total, HDL and LDL cholesterol levels and TG levels at baseline were 162.0 (±35.2), 44.4 (±13.9), 96.1 (±30.2), and 106.9 (±55.3) mg/dL, respectively.
Table 3 summarizes the prevalence and the cumulative incidence of lipid, fasting glucose and HOMA-IR abnormalities during study follow-up. A total of 58 (18.1%; 95% CI: 14.1–22.8%) of 320 children developed hypercholesterolemia after baseline. The cumulative incidence of abnormal HDL and LDL was 19.6% (95% CI: 15.1–24.7) and 15.0% (95% CI: 11.3–19.5), respectively. A total of 103 children developed hypertriglyceridemia after baseline, corresponding to a cumulative incidence of 44.2% (95% CI: 37.7–50.8). The cumulative incidence of insulin resistance was 3.8% (95% CI: 1.8–7.1). Although there were two subjects with diabetes at baseline (glucose ≥126 mg/dL), there were no incident cases; per WHO definition (glucose 110–125 mg/dL), there were two incident cases of impaired fasting glucose, although there were none at baseline. Applying the CDC definition for impaired glucose instead (glucose 100–125 mg/dL), there were two cases at baseline and six incident cases. Among incident cases with a subsequent laboratory measure available, the persistence of dyslipidemia at a subsequent laboratory measure was as follows: 61.1% for hypercholesterolemia, 46.7% for abnormal HDL, 52.4% for abnormal LDL, and 57.5% for hypertriglyceridemia (data not shown).
Table 3.
Prevalence and cumulative incidence of lipid, HOMA-IR, and fasting glucose abnormalities.
| Prevalence1 | Cumulative Incidence on Study | |||||
|---|---|---|---|---|---|---|
| Abnormal Laboratory Measure | Total N | N | Percent (95% CI2) | Total N3 | N | Percent (95% CI2) |
| Total cholesterol (>200 mg/dL) | 385 | 45 | 11.7 (8.7 – 15.3) | 320 | 58 | 18.1 (14.1 – 22.8) |
| HDL cholesterol (<35 mg/dL) | 385 | 87 | 22.6 (18.5 – 27.1) | 281 | 55 | 19.6 (15.1 – 24.7) |
| LDL cholesterol (≥130 mg/dL) | 385 | 46 | 12.0 (8.9 – 15.6) | 319 | 48 | 15.0 (11.3 – 19.5) |
| Triglycerides (age-specific)4 | 385 | 138 | 35.8 (31.1 – 40.9) | 233 | 103 | 44.2 (37.7 – 50.8) |
| Glucose: | ||||||
| Diabetes (≥126 mg/dL) | 345 | 2 | 0.6 (0.1 – 2.1) | 265 | 0 | 0.0 (0.0 – 1.4) |
| Impaired (110 to <126 mg/dL) | 345 | 0 | 0.0 (0.0 – 1.1) | 265 | 2 | 0.8 (0.1 – 2.7) |
| HOMA-IR (Tanner stage-specific)5 | 342 | 18 | 5.3 (3.2 – 8.2) | 236 | 9 | 3.8 (1.8 – 7.1) |
Prevalence was defined based on the first available fasting test result for each measure.
CI = Confidence Interval; Exact Clopper-Pearson 95% CIs are shown.
The number missing results for determining the cumulative incidence of study outcomes (among those with a normal baseline result) was as follows: total cholesterol=12; HDL=9; LDL=12; triglycerides=8; Glucose=77; HOMA-IR=85. Missing are not included in ‘Total N’ or in percent calculations.
Age < 10 years: abnormal triglycerides >110 mg/dL; Age ≥ 10 years: abnormal triglycerides >150mg/dL.
Tanner Stage =1: abnormal HOMA-IR>2.5; Tanner stage >1: abnormal HOMA-IR>4.0.
Table 4 shows the results of the Cox proportional hazards regression models examining ART use, adjusted for age, gender, country, and HIV RNA. The model for abnormal HDL is also adjusted for CD4 percent.
Table 4.
Proportional hazards regression modeling of risk of developing metabolic abnormalities associated with type of ART regimen received
| Total cholesterol | HDL cholesterol | LDL cholesterol | Triglycerides | |||||
|---|---|---|---|---|---|---|---|---|
| Covariate | HR (95% CI)1 | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Type of ART regimen: | ||||||||
| PI-containing | 1.6 (0.5–5.7) | 0.46 | 0.6 (0.2–1.5) | 0.27 | 1.5 (0.4–5.4) | 0.56 | 3.6 (1.3–10.5) | 0.0167 |
| Non-PI-containing | 0.6 (0.1–2.8) | 0.52 | 0.4 (0.1–1.3) | 0.12 | 0.6 (0.1–2.8) | 0.52 | 1.5 (0.5–4.5) | 0.51 |
| No ART | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Age (1-year increase in age) | 1.0 (0.9–1.2) | 0.82 | 1.1 (0.9–1.3) | 0.26 | 0.9 (0.8–1.1) | 0.32 | 1.1 (1.0–1.2) | 0.22 |
| Gender: | ||||||||
| Male | 1.4 (0.7–2.9) | 0.30 | 0.7 (0.3–1.3) | 0.25 | 0.7 (0.4–1.6) | 0.44 | 0.5 (0.3–0.8) | 0.0029 |
| Female | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Country: | ||||||||
| Brazil | 0.5 (0.3–1.1) | 0.0745 | 0.7 (0.3–1.6) | 0.46 | 1.2 (0.5–2.9) | 0.70 | 0.3 (0.2–0.5) | <0.0001 |
| Mexico/Peru | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| HIV RNA: | ||||||||
| ≥400 copies/mL | 0.4 (0.2–0.9) | 0.0178 | 1.2 (0.6–2.6) | 0.57 | 0.4 (0.2–1.0) | 0.0376 | 0.7 (0.5–1.2) | 0.21 |
| <400 copies/mL | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| CD4%: | ||||||||
| ≤25% | - | - | 2.5 (1.2–5.0) | 0.0128 | - | - | - | - |
| >25% | 1.0 | |||||||
HRs (hazard ratios) and 95% confidence intervals (95% CI) obtained from proportional hazards regression modeling.
Hypercholesterolemia
ART regimen was not associated with the development of hypercholesterolemia; the p-values for the comparisons of PI-containing and non-PI-containing regimens to no ART were 0.46 and 0.52, respectively. The risk of developing an abnormal total cholesterol was significantly lower among those with HIV RNA ≥400 copies/mL than those with RNA below this cutpoint (hazard ratio [HR]=0.4, 95% CI: 0.2–0.9; p=0.0178). The risk of experiencing an abnormal total cholesterol level was not associated with subject’s age, gender, or country of origin (p>0.07).
Abnormal HDL cholesterol
The risk of developing abnormal HDL cholesterol was not associated with type of ART regimen, age, gender, country or HIV RNA (P>0.1). However, the risk of developing an abnormal HDL cholesterol was significantly higher among those with CD4 percent ≤25% compared to those with CD4 percent above 25% (HR=2.5, 95% CI: 1.2–5.0; p=0.0128).
Abnormal LDL cholesterol
Type of ART regimen received was not associated with risk of abnormal LDL cholesterol level (p>0.5). Subjects with HIV RNA ≥400 copies/mL had a reduced risk of experiencing an abnormal LDL cholesterol compared to those with HIV RNA below this cutpoint (HR=0.4, 95% CI: 0.2–1.0; p=0.0376). There was no association between the risk of developing abnormal LDL cholesterol and subject’s age, gender or country (p>0.3).
Hypertriglyceridemia
Children on a PI-containing regimen were nearly four times as likely to experience an abnormal TG measure as those receiving no ART (HR=3.6, 95% CI: 1.3–10.5; p=0.0167). Those receiving a non-PI-containing regimen were at increased risk of experiencing an abnormal triglyceride measure compared to those receiving no ART, but this difference was not significant (HR=1.5, 95% CI: 0.5–4.5; p=0.51). The risk of hypertriglyceridemia was significantly lower among males than females (HR= 0.5, 95% CI: 0.3–0.8; p=0.0029) and also among those enrolled in Brazil compared to Mexico or Peru (HR=0.3, 95% CI: 0.2–0.5; p<0.0001). Risk of hypertriglyceridemia was not associated with age (p=0.22) or HIV RNA (p=0.21).
DISCUSSION
In the present study, the cumulative incidence of lipid abnormalities ranged from a low of 15.0% for abnormal LDL cholesterol to a high of 44.2% for triglyceride abnormalities. The incidence of glucose and HOMA-IR abnormalities was quite small in the study population (<4%). The type of ART regimen received was associated only with the risk of developing a triglyceride abnormality in multivariable modeling. The results indicate that the risk of developing a triglyceride abnormality among those receiving a PI-containing regimen was over three times that of those receiving no ART. Subjects with detectable viral load were at lower risk of experiencing an abnormal total or LDL cholesterol, possibly due to less exposure to antiretroviral treatment.
Children in our study were generally in good health, with most having normal BMI, height and weight, and an immunological profile where the majority presented with high CD4 levels, in accordance with high levels of self-reported ART adherence. With a mean duration of ART exposure of 4.6 years and approximately 56% receiving a PI-containing regimen, our study found a prevalence of dyslipidemia at baseline similar to what has been published and consistent with the preliminary report on baseline data from the NISDI cohort [4]. The cumulative incidence results support the need for further investigation into the cause of these rather high rates of lipid abnormalities. In a clinical trial in South Africa, Strehlau et al, found that initiation of PI-based regimens resulted in significant increases in total cholesterol, LDL and HDL cholesterol and decreases in the total cholesterol: HDL ratio and TG [19]. To our knowledge, the largest longitudinal study that examined the association of PIs and other ART with lipid levels in HIV-infected children was the study of Tassiopoulos et al. In contrast to our findings, this study, which focused only on hypercholesterolemia because fasting was not required, concluded that PI-based (boosted and nonboosted) regimens and use of NNRTIs were significant risk factors for hypercholesterolemia. [9].
Our results indicate that, although complications of glucose metabolism are uncommon, they do exist in HIV-infected children as shown in other studies [1, 20]. In addition to the 5.3% of the children we observed with abnormal HOMA-IR at baseline, 3.8% developed abnormal HOMA-IR during the course of study follow-up. This suggests that the proportion of children with insulin resistance is likely to continue to increase, as ART can interact with the physiologic insulin resistance induced by puberty itself [20]. The glucose outcomes did not include sufficient numbers of events (abnormalities) to support proportional hazards regression modeling.
A potential limitation of our analysis is that we did not evaluate factors such as maternal dyslipidemia or family history of heart disease, but this is not likely to have had a major influence on our findings, as some authors indicate these factors are unlikely to be associated with ART use [9, 19]. We recognize that our study is a secondary analysis of infrequent measures, with imprecise determination of the onset of abnormalities. Nevertheless, the cohort study was conducted using standardized data collection instruments and fasting measurements, with a relatively large sample size. Another limitation is the self-reported adherence measure used for this study, as another analysis of NISDI study data showed that it “demonstrated some association with viral load, but may not be adequate for reliably identifying non-adherence.” [21] This measure is likely over-estimating adherence, which may explain why only 34.2% of the subjects had undetectable viral load despite high rates of adherence. Additionally, although it would have been of interest to investigate the influence of receipt of individual ARVs on the study outcomes, the numbers were not sufficient to support such analyses.
Secondary to HIV infection itself, the explanation for the association between PIs and abnormal lipid levels remains unclear, with some hypotheses considering their potential to increase lipid synthesis by liver enzymes or by inhibition of proteins involved in lipid metabolism and adipocyte differentiation [9]. The mechanism involved in glucose homeostasis alteration, especially insulin resistance, is also still under investigation, but seems to be related to the direct inhibition of the GLUT4 (insulin-responsive facilitative glucose transporter isoform 4) attributed to the use of HAART including PIs and nucleoside reverse transcriptase inhibitors (NRTIs) [5, 22].
While we found some evidence of an association of ART including PIs with dyslipidemia, we agree with other authors that, despite the potential adverse consequences of treatment of HIV-infected children with PIs, the benefits outweigh the toxicities, and that there is the need for ongoing metabolic monitoring of treated children [3, 7, 9, 19]. According to the UNAIDS 2014 Global Report, there were 783,000 children in June 2014 receiving antiretroviral treatment, an increase of three percent in comparison to 2013 [23]. Based on current WHO guidelines that all HIV-infected children should be treated with cART, the number of children starting presumably lifelong cART at young ages will increase worldwide [24]. Those children will possibly experience drug-related adverse events and toxicities that are increasingly recognized and represent one of the most common reasons for treatment discontinuation or switch [25]. Metabolic complications of long term ART exposure remain an on-going problem for perinatally HIV-infected children, potentially affecting their overall quality of life and influencing treatment adherence [1]. Further complicating the issue, it is difficult to separate the impact of HIV infection from ART on serum lipid concentrations. Finally, because clinical events are not expected until adulthood, longitudinal analyses, or at least tracking of perinatally HIV-infected youth into adulthood, will be important.
Additional studies are necessary to determine whether these metabolic abnormalities are associated with an increased risk of cardiovascular disease and how this scenario can be managed in children and adolescents [9]. Associations between ART use in HIV-infected children and adolescents and glucose metabolism disturbances should continue to be investigated in future studies.
Acknowledgments
Principal investigators, co-principal investigators, study coordinators, data management center representatives, and NICHD staff include: Brazil: Belo Horizonte: Jorge A. Pinto, Flávia F. Faleiro, Marcelle M. Maia (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/ Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Luis Felipe Moreira, Ivete Gomes (Hospital Geral Nova de Iguacu – HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Rosa Dea Sperhacke, Marcelo Goldani, Carmem Lúcia Oliveira da Silva, Margery Bohrer Zanetello (Universidade de Caxias do Sul /Hospital de Clínicas de Porto Alegre); Regis Kreitchmann, Marcelo Comerlato Scotta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Maria Célia Cervi, Márcia L. Isaac, Fernanda Tomé Sturzbecher, Bento V. Moura Negrini (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Maria Leticia Cruz, Leon Claude Sidi, Maria Isabel Gouvêa, Mariza Curto Saavedra, Clarisse Bressan, Fernanda Cavalcanti A. Jundi (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Daisy Maria Machado (Escola Paulista de Medicina-Universidade Federal de São Paulo); Marinella Della Negra, Wladimir Queiroz, Yu Ching Lian (Instituto de Infectologia Emilio Ribas); Mexico: Mexico City: Noris Pavía-Ruz, Dulce Morales-Pérez, Karla Ojeda-Diezbarroso (Hospital Infantil de México Federico Gómez); Peru: Lima: Jorge O. Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”-Sección de Epidemiologia, UNMSM), María Castillo Díaz (Instituto Nacional de Salud del Niño), Mary Felissa Reyes Vega (Instituto de Medicina Tropical “Daniel Alcides Carrión” - Sección de Epidemiologia, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, Margot Krauss, Sue Li, Karen Megazzini, Orlando Ortega, James Korelitz, Sharon Sothern de Sanchez, Sonia K. Stoszek, Qilu Yu (Westat, Rockville, MD, USA) NICHD: Rohan Hazra, Lynne M. Mofenson, George K. Siberry (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
Source of Funding: Supported by NICHD Contracts N01-HD-3-3345 (2002–2007), HHSN267200800001C (2007–2012), and HHSN275201300003C (2012–2017).
Footnotes
Oral presentation of the abstract at INFECTO 2015, Gramado, RS – Brazil in August 29th, 2015.
Conflicts of Interest:
The authors have no other funding or conflicts of interest to declare.
Contributor Information
MP Paganella, Laboratório de Pesquisa em HIV/AIDS, Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil; Postgraduate Program in Epidemiology, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil.
RA Cohen, Westat, Rockville, MD, USA.
DR Harris, Westat, Rockville, MD, USA.
RS Kuchenbecker, Postgraduate Program in Epidemiology, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil.
RD Sperhacke, Laboratório de Pesquisa em HIV/AIDS, Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil.
SK Kato, Laboratório de Pesquisa em HIV/AIDS, Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil; Departamento de Saúde Coletiva, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, RS, Brazil; Faculdade de Matemática; Departamento de Estatística; Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brazil.
CLO Silva, Hospital de Clinicas de Porto Alegre, Porto Alegre, RS, Brazil.
FT Sturzbecher, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo.
RHS Oliveira, Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), Universidade Federal do Rio de Janeiro (UFRJ).
N Pavía Ruz, Hospital Infantil de México Federico Gómez.
R Hazra, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Bethesda, MD, USA.
REFERENCES
- 1.Barlow-Mosha L, Eckard AR, McComsey GA, et al. Metabolic complications and treatment of perinatally HIV-Infected children and adolescents. JIAS. 2013;16:18600. doi: 10.7448/IAS.16.1.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidari S, Mofenson LM, Hobbs CV, et al. Unresolved antiretroviral treatment management issues in HIV-infected children. J Acquir Immune Defic Syndr. 2012;59(2):161–169. doi: 10.1097/QAI.0b013e3182427029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewinski M, Megazzini K, Hance LF, et al. Dyslipidemia in a cohort of HIV-infected Latin American children receiving highly active antiretroviral therapy. J Tropic Ped. 2010;57(5):324–332. doi: 10.1093/tropej/fmq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazra R, Hance LF, Monteiro JP, et al. Insulin resistance and glucose and lipid concentrations in a cohort of perinatally HIV-infected Latin American children. Pediatr Infect Dis J. 2013;32(7):757–759. doi: 10.1097/INF.0b013e318286c774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geffner ME, Patel K, Miller TL, et al. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Pædiatr. 2011;76(6):386–391. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimock D, Thomas V, Cushing A, et al. Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. 2011;60(6):874–880. doi: 10.1016/j.metabol.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhoads MP, Lanigan J, Smith CJ, et al. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr. 2011;57(5):404–412. doi: 10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- 8.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23(6):661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tassiopoulos K, Williams PL, Seage GR, et al. Association of Hypercholesterolemia Incidence With Antiretroviral Treatment, Including Protease Inhibitors, Among Perinatally HIV-Infected Children. J Acquir Immune Defic Syndr. 2008;47(5):607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosso R, Parodi A, d’Annunzio G, et al. Evaluation of insulin resistance in a cohort of HIV-infected youth. Eur J Endocrinol. 2007;157(5):655–659. doi: 10.1530/EJE-07-0414. [DOI] [PubMed] [Google Scholar]
- 11.Hadigan C. Diabetes, insulin resistance, and HIV. Curr Infect Dis Rep. 2006;8(1):69–75. doi: 10.1007/s11908-006-0037-1. [DOI] [PubMed] [Google Scholar]
- 12.Palios J, Kadoglou NPE, Lampropoulos S. The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: the emerging role of adipokines. Exp Diabetes Res. 2011;2012:103063. doi: 10.1155/2012/103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazra R, Stoszek SK, Freimanis Hance L, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38(5):1207–1214. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO/IDF. Geneva, Switzerland: 2006. [Accessed 05,13,2013]. WHO Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; p. 46. Available at: http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf. [Google Scholar]
- 15.CDC. CDC Statements on Diabetes Issues. [Accessed 05,13,2013];2011 Available at: http://www.cdc.gov/diabetes/news/class.htm.
- 16.Mathews DR, Hosker JP, Rudenski AS, et al. Homesostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Van Dyke RB, Lee S, Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 18.WHO. The WHO Child Growth Standards. [Accessed 05,19,2015]; Available at: http://www.who.int/childgrowth/standards/en/
- 19.Strehlau R, Coovadia A, Abrams EJ, et al. Lipid profiles in young HIV-infected children initiating and changing antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60(4):369–376. doi: 10.1097/QAI.0b013e318243760b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beregszaszi M, Dollfus C, Levine M, et al. Longitudinal Evaluation and Risk Factors of Lipodystrophy and Associated Metabolic Changes in HIV-Infected Children. J Acquir Immune Defic Syndr. 2005;40(2):161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 21.Duarte HA, Harris DR, Tassiopoulos K, Leister E, Negrini SFBM, Ferreira FF, Cruz MLS, Pinto J, Allison S, Hazra R for the NISDI PLACES Study Group. Relationship between viral load and behavioral measures of adherence to antiretroviral therapy in children living with HIV in Latin America. Braz J Infect Dis. 2015;19(3):263–271. doi: 10.1016/j.bjid.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez AD, Balasubramanyam A. Dysregulation of glucose metabolism in HIV patients: epidemiology, mechanisms, and management. Endocrine. 2012;41:1–10. doi: 10.1007/s12020-011-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS. Geneva (Switzerland): UNAIDS; 2014. [Accessed 12,04,2014]. UNAIDS World AIDS Day Report 2014; pp. 1–40. Available at: http://www.unaids.org/en/resources/campaigns/World-AIDS-Day-Report-2014. [Google Scholar]
- 24.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. [Accessed 12, 01, 2015]; Available at: http://apps.who.int/iris/bitstream/10665/198064/1/9789241509893_eng.pdf?ua=1.
- 25.Prosperi MC, Fabbiani M, Fanti I, et al. Predictors of first-line antiretroviral therapy discontinuation due to drug-related adverse events in HIV-infected patients: a retrospective cohort study. BMC Infect Dis. 2012;12:296. doi: 10.1186/1471-2334-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]