Abstract
Most tumors display oncogene-driven reprogramming of several metabolic pathways, which are crucial to sustain their growth and proliferation. In recent years, both dietary and pharmacological approaches that target deregulated tumor metabolism are beginning to be considered for clinical applications. Dietary interventions exploit the ability of nutrient-restricted conditions to exert broad biological effects, protecting normal cells, organs and systems, while sensitizing a wide variety of cancer cells to cytotoxic therapies. On the other hand, drugs targeting enzymes or metabolites of crucial metabolic pathways can be highly specific and effective, but must be matched with a responsive tumor, which might rapidly adapt. In this Review, we illustrate how dietary and pharmacological therapies differ in their effect on tumor growth, proliferation and metabolism, and discuss the available preclinical and clinical evidence in favor or against each of them. We also indicate, when appropriate, how to optimize future investigations on metabolic therapies on the basis of tumor- and patient-related characteristics.
Keywords: cancer metabolism, fasting, fasting mimicking diet, pharmacologic metabolic therapies
Introduction
Several biological mechanisms underlying tumor initiation, progression and metastases have been elucidated in the last decades. The discovery of oncogenes and tumor suppressor genes (TSGs) has revealed the biochemical bases of some hallmarks of cancer, including unrestrained proliferation, independence from growth factor stimulation, and resistance to apoptosis (1). This has paved the way for targeting crucial biochemical pathways and improving prognosis of several malignancies, including breast, lung, renal and gastric tumors, as well as leukemias (2).
On the other hand, the impact of these discoveries has overshadowed the importance of metabolic aspects of tumor growth for a long time. Metabolism is the set of biochemical transformations that occur within cells, including catabolic processes leading to production of energy units (in the form of ATP or reducing equivalents) and anabolic processes leading to synthesis of complex biomolecules, such as proteins, lipids and DNA. Historically, the only metabolic pathway to be intensively studied and successfully targeted in cancer therapy is synthesis of nucleotides and deoxynucleotides (3, 4). Other key metabolic pathways have instead become the focus of intense research only recently, based on new discoveries and the efficacy plateau reached by standard anticancer treatments.
Similarly to highly proliferating healthy cells, cancer cells need a constant supply of ATP and anabolic precursors to sustain crucial biochemical processes, including DNA synthesis and repair, protein and lipid synthesis, post-translational modification of proteins, membrane and organelle formation and reassembly, vesicular transport of intracellular cargos and endocytosis. While mitochondrial oxidation of glucose-derived pyruvate is the preferred source of ATP for proliferating healthy cells, several cancer cells divert most of glycolysis-derived pyruvate from mitochondrial oxidation to the synthesis of lactate (5) (Figure 1). This phenomenon, known as “aerobic glycolysis”, is clinically exploited to detect increased uptake of the radiolabeled glucose analog 18F-fluoro-2-deoxy-D-glucose (or 18FDG) by tumors compared with normal tissues. 18FDG-based positron emission tomography (PET) is actually used for diagnostic and staging purposes, and also to monitor tumor response to therapies (6). Recent studies have shown that not only glycolysis, but many other metabolic pathways can be deregulated in tumor cells. Moreover, intimate connections between oncogenes/TSGs and metabolic reprogramming suggest that deregulated metabolism crucially contributes to unrestrained proliferation of cancers (5, 7). Given the large metabolic differences between healthy and neoplastic cells, there is a hope to selectively target tumor metabolism, while limiting toxicities to normal tissues.
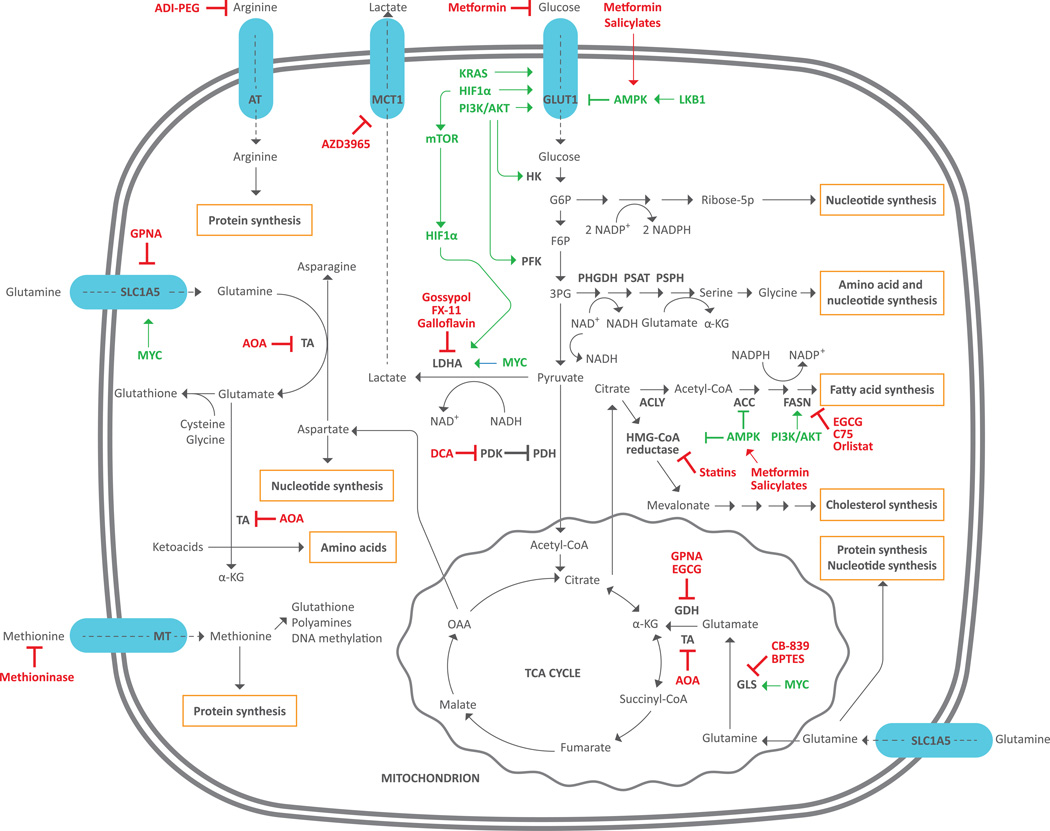
Figure 1. Main metabolic pathways deregulated in cancers and corresponding targeting drugs.
Cancer cells up-regulate both catabolic and anabolic pathways to optimize energy and macromolecule production. Glucose and glutamine are central biomolecules that provide cancer cells with most of energy and metabolites required for growth and proliferation. Glucose is uptaken by tumor cells through the GLUT1 transporter and enters glycolysis. The glycolytic intermediate G6P can be diverted to the pentose phosphate pathway to form ribose-5P (nucleotide synthesis) and reducing equivalents in the form of NADPH (anabolic processes). Another glycolytic intermediate, 3-PG, can be diverted to synthesis of serine and glycine, which can be incorporated into proteins or nucleotides, or used as precursors of other biomolecules. Finally, glucose-derived pyruvate can be converted to lactate by LDHA, oxidized in the mitochondrial TCA cycle or, finally, converted to citrate to fuel synthesis of FAs and cholesterol. Synthesis of FAs requires ACC and FASN enzymes while HMG-CoA reductase is the key enzyme for cholesterol synthesis. Glutamine enters tumor cells through the SLC1A5 transporter and is used for protein or nucleotide synthesis, or can be converted to glutamate and then α-KG. Finally, α-KG can be either oxidized in the mitochondrial TCA cycle, or undergo reductive metabolism to form citrate, thus contributing to FA and cholesterol synthesis. Cytoplasmic glutamine can be also transaminated to form amino acids from corresponding ketoacids. Arginine and methionine are uptaken from the external environment through specific transporters and then used for protein synthesis or other purposes.
ACC: acetyl-CoA carboxylase; ACLY: ATP citrate lyase; ADI-PEG: PEGylated arginine deiminase; AMPK: AMP-activated protein kinase; AOA: aminooxyacetate; AT: arginine transporter; DCA: dichloroacetate; FASN: fatty acid synthase; F6P: fructose 6 phosphate; GDH: glutamate dehydrogenase; GLS: glutaminase; GLUT1: glucose transporter 1; G6P: glucose-6 phosphate; HIF1-α: hypoxia-inducible factor 1; HK: hexokinase; LDHA: lactate dehydrogenase A; LKB1 (liver kinase B1); MT: methionine transporter; mTOR: mammalian target of rapamycin; NAD: nicotinamide adenine dinucleotide; NADP: nicotinamide adenine dinucleotide phosphate; OAA: oxaloacetate; PDH: pyruvate dehydrogenase; PDK: pyruvate dehydrogenase kinase; PFK: phosphofructokinase; PHGDH: phosphoglycerate dehydrogenase; PI3K: phosphatidylinositol 3-kinase; PSAT: phosphoserine aminotransferase; PSPH: phosphoserine phosphatase; Ribose 5-P: ribose 5-phosphate; TA: transaminase; TCA: tricarboxylic acid; α-KG: α-ketoglutarate; 3-PG: 3-phosphoglycerate.
Because cancer cell metabolism is complex and potentially heterogeneous within big tumor masses, recent technical advancements, including molecular magnetic resonance spectroscopy (MRS) and hyperpolarized magnetic resonance imaging (MRI), could offer a detailed picture of the utilization/production of several metabolites by in vivo human tumors in different disease localizations, thus accounting for metabolic heterogeneity. These techniques could also be useful to follow dynamical evolution of tumor metabolism during disease progression or in response to therapies (8, 9).
In the first part of this review we analyze the main metabolic cascades that are deregulated in tumors. A clear understanding of these pathways and their role in tumor cell proliferation and survival is essential to identify targets for effective therapies. In the second and third parts of the manuscript, we respectively review the dietary and pharmacological strategies that hold promise to successfully target tumor metabolism on the basis of available preclinical evidence.
Metabolic pathways that sustain cancer cell survival and proliferation
Glucose
Upregulated aerobic glycolysis provides several benefits to cancer cells (5). First, at physiological blood concentrations, glucose ensures sufficiently fast ATP production to fulfill energetic demands, while contemporarily fueling anabolic processes through biomass production; second, pyruvate-derived lactate, when excreted, creates an extracellular acid environment that recruits macrophages and other immune cells, thus favoring metastatization; third, pyruvate can be used to produce oxaloacetate (OAA) and the amino acids (AAs) alanine and aspartate, which take part in the synthesis of proteins or other biomolecules (5, 10). In summary, aerobic glycolysis can fulfill most of the energetic and metabolic needs of highly proliferating cancer cells, including AA biosynthesis when a proper source of nitrogen groups (usually deriving from glutamine) is also provided. In tumors, aerobic glycolysis is often stimulated by oncogenes, including PI3K and RAS, which induce expression of the glucose transporter gene GLUT1 and of the glycolytic enzymes hexokinase (HK) and phosphofructokinase (PFK), and contemporarily inhibit mitochondrial oxidation of pyruvate (Figure 1).
Recent in vivo studies on tumors showed that part of glucose-derived pyruvate can be diverted into the mitochondrial tricarboxylic acid (TCA) cycle to produce additional energy or intermediates for synthesis of fatty acids (FAs) or other nonessential AAs, such as glutamate and glutamine (11, 12). While reducing the dominance of aerobic glycolysis as the primary source of energy and anabolic precursors for rapidly proliferating tumor cells, these studies confirm glucose as the major metabolic substrate for in vivo malignancies.
Preclinical evidence suggests that targeting deregulated glucose metabolism is a potentially effective anticancer approach. Indeed, reducing extracellular glucose or inhibiting glycolysis through 2-deoxy-D-glucose (2-DG) induces proliferation arrest in several cancer cell lines and also synergizes with cytotoxic treatments to activate apoptosis (13, 14); these effects are especially strong in cells with compromised mitochondrial oxidative phosphorylation (15). Inhibiting lactate production by the lactate dehydrogenase A (LDHA) enzyme is another way to halt glycolysis progression by preventing NAD+ regeneration from NADH, which is toxic to highly glycolytic cancer cells (16). Moreover, dietary regimens that reduce glycemia also enhance the antitumor activity of chemotherapy and prolong survival of mice xenografted with human tumor cells (13). Finally, the hyperglycemic/diabetic state is associated with worse prognosis in glioblastoma multiforme (GBM), colorectal cancer and acute leukemia patients (17–20).
Two different approaches can be exploited to target aerobic glycolysis in cancer therapy: reducing blood glycemia (systemic approach), or inhibiting specific enzymes in the glycolytic cascade (cell-autonomous approach). The former strategy requires a careful selection of patient subgroups that, based on their glycemic state (hyperglycemic versus euglycemic), tumor avidity for glucose as detected through 18FDG-PET, or molecular tumor profile (e.g. RAS or PI3K activation), are more likely to benefit from it. In the cell-autonomous approach, the most suitable molecular targets need to be identified, and potent and selective inhibitors to be synthesized.
Amino acids (AAs)
Unrestrained tumor proliferation requires continuous replenishment of AAs to be used as building blocks for structural and enzymatic proteins, as precursors of essential biochemical components, including FAs, other AAs, nucleotides and the antioxidant glutathione, or, finally, as monocarbon unit donors. Similar to normal tissues, cancer cells are usually able to synthesize some AAs (referred to as “nonessential” ones), while they rely on blood supply of the remaining, “essential” ones. However, some tumors may loose the ability to synthesize one specific nonessential AA, thus becoming dependent from the external supply. This phenomenon is known as “auxotrophy”.
One potential strategy to target AA metabolism in tumors is to deplete circulating blood AAs through dietary or pharmacological interventions. However, because subtracting essential AAs might prove toxic to normal tissues, this strategy is reasonably exploitable only for auxotrophic tumors. One alternative strategy consists in targeting intracellular enzymes involved in the metabolism of specific AAs, without affecting their blood concentration.
Glutamine is a nonessential AA that can be obtained from dietary sources, protein degradation in muscle cells or de novo synthesis.
Several types of cancer cell lines, including some derived from breast and lung tumors, rely on glutamine supply to survive and proliferate (21, 22). Cancer cells can directly internalize glutamine from the extracellular environment (e.g. the blood) through the glutamine transporter SCL1A5 located on the plasmatic membrane (21). Alternatively, glutamine, as well as other AAs, can be derived from lysosomal degradation of extracellular proteins that are internalized through macropinocytosis (23). This latter mechanism has been described in RAS-mutated pancreatic and bladder cancers, which depend from extracellular glutamine, but become glutamine-independent if provided with sufficient amounts of extracellular albumin, as well as other proteins to be degraded in lysosomes (23).
In tumors, glutamine takes part in synthesis of proteins or nucleotides (24), stimulation of glucose uptake (25) and activation of the mammalian target of rapamycin (mTOR) (26, 27). However, most of intracellular glutamine is converted by the glutaminase (GLS) enzyme to glutamate, which is used as a precursor of glutathione or to produce alpha-ketoglutarate (α-KG) through reactions catalyzed either by the enzyme glutamate dehydrogenase (GDH), or by transaminases (TAs) (24, 27–30) (Figure 1). Finally, glutamine-derived α-KG can either undergo oxidative metabolism in mitochondrial TCA cycle (28), or reductive metabolism to form isocitrate and citrate in the “reverse TCA cycle”, thus contributing to FA and cholesterol synthesis (24, 29, 30) (Figure 1). The oncogenic protein MYC directly stimulates catabolic metabolism of glutamine by inducing expression of both glutamine transporter SLC1A5 and GLS genes (31); of note, GLS inhibition and glutamine deprivation halt proliferation of MYC-overexpressing tumor cells (30, 31). A recent paper has also shown increased oxidative metabolism of glutamine in PI3K-mutated, but not PI3K-WT, colorectal cancers (32). On the other hand, different studies suggest that glutamine uptake may be nonessential for in vivo GBM and lung tumors, in which glucose utilization is likely sufficient to satisfy both energetic and anaplerotic needs (11, 12, 33). Since glutamine intracellular utilization can significantly differ between in vitro-grown cancer cells and tumors that are embedded in their original microenvironment, tumor dependency should be assessed by measuring glutamine uptake and utilization in in vivo tumors, e.g. through NMR spectroscopy that tracks the fate of intravenously administered radiolabeled glutamine (12).
Because it is difficult to reproducibly modify glutamine blood concentration through dietary interventions, pharmacological strategies targeting glutamine transporters or glutamine-metabolizing enzymes seem to be the most promising approaches to target glutamine-addicted tumors.
Methionine is an essential AA taking part in protein synthesis, DNA and protein methylation, synthesis of glutathione and polyamines (34). Normal cells cannot synthesize methionine from other AAs, but they can produce it from homocysteine (Hcy). On the contrary, several tumor cell lines, including colon, breast and prostate ones, cannot proliferate and survive in the absence of methionine (35). Methionine contributes to activating oncogenic pathways in GBM, and methionine deprivation negatively impacts on tumor cell proliferation (36). Coherently with in vitro data, lowering dietary intake of methionine inhibits tumor growth in in vivo mouse and rat models, and also reduces tumor size synergistically with cytotoxic treatments (37). Dietary restriction of methionine is therefore a promising anticancer approach that deserves further investigation in future studies.
Arginine is a nonessential AA, used for protein synthesis, or as a precursor of nitric oxide (NO), polyamines, creatine, as well as glutamine and proline when these AAs are scarce (38). Moreover, arginine is involved in mTOR activation (26) and growth hormone (GH), insulin and insulin-like Growth Factor 1 (IGF-1) secretion, especially after strenuous physical exercise (39). Sources of circulating arginine include diet, protein degradation and de novo synthesis, which is initiated by the argininosuccinate synthetase 1 (ASS1) enzyme (40). Its dietary uptake becomes essential only in conditions of increased tissue growth (e.g. during childhood) or under specific stresses (such as inflammation) (41).
Several tumors, including some melanomas, hepatocellular carcinomas (HCCs) and mesotheliomas, epigenetically repress ASS1 expression through methylation of its promoter (42). These cancers depend on arginine uptake from the extracellular environment (the blood and/or nearby normal or tumor cells) to survive and proliferate (43). Despite causing tumor dependency from extracellular arginine, ASS1 inactivation confers specific metabolic advantages, including glutamine independence or aspartate-mediated enhancement of pyrimidine nucleotide production (44, 45). Reducing arginine availability in ASS1-repressing tumors holds promise as a potent and selective anticancer strategy.
Serine and glycine are nonessential AAs, which can be synthesized from the glycolytic intermediate 3-phosphoglycerate (3-PG) through a biochemical cascade initiated by the phosphoglycerate dehydrogenase (PHGDH) enzyme (Figure 1). They take part in redox balance and de novo synthesis of purines and glutathione, thus contributing to protein, DNA and lipid synthesis (46). Intracellular serine also stimulates cell proliferation through mTOR activation (47). Due to these multiple functions, highly proliferating cancer cells need continuous replenishment of both serine and glycine.
Some tumors uptake serine from the external environment (48), and could be sensitive to dietary restriction of serine-containing foods. Despite the lack of data in human subjects, experiments on mice demonstrate that limiting dietary intake of serine is safe, capable of lowering serine and glycine blood levels by about 50% and delaying tumor development (49). Moreover, serine restriction synergizes with metformin to inhibit growth of already established cancers (50). Other malignancies, especially triple-negative breast cancers (TNBCs) and melanomas, overexpress serine synthesis enzymes, especially PHGDH, through amplification of the PHGDH gene, thus becoming completely independent from external serine supply (51, 52). Suppressing PHGDH inhibits proliferation of PHGDH-overexpressing tumors and holds promise to target highly aggressive malignancies (48). The price paid by PGHDH-overexpressing cells for their serine-independency is the depletion of glycolytic intermediates. As a consequence, glucose deprivation or metformin-induced inhibition of mitochondrial oxidative phosphorylation could prove especially toxic to cancer cells relying on glucose-dependent synthesis of serine (Figure 1) (50).
Lipids
Lipids are essential components of cell membranes, contributing to their fluidity and to the activation of membrane-anchored signal transduction enzymes. Cancer cells depend on continuous replenishment of FAs to form new membranes and organelles. However, while most normal cells internalize dietary or fatty tissue-derived FAs that circulate in the bloodstream either as free or as part of lipoproteins, most cancer cells de novo synthetize their FAs independently from nutrient availability and hormone stimulation (53). FA synthesis begins with the conversion of citrate to acetyl-CoA and then acetoacetyl-CoA, which is finally elongated to form palmitate and other FAs. Crucial enzymes in this process are acetyl-CoA carboxylase (ACC), which catalyzes the limiting-step reaction of the cascade, and the multisubunit FA synthase (FASN) enzyme (Figure 1). Major sources of citrate to be used for FA synthesis are glucose (5) and glutamine-derived α-KG, especially under hypoxia or disruption of the mitochondrial oxidative machinery (24, 29, 30).
Crucially, FASN is overexpressed by most tumors, including breast, ovarian, lung, colon, endometrial, gastric and head and neck cancers (53, 54). Of note, MAPK/PI3K oncogenic pathways and FASN can activate each other through a positive feedback loop that couples cellular proliferation with metabolic processes (55). Moreover, FASN protein levels are associated with worse prognosis in human cancers (56, 57). Finally, pharmacological inhibition of FASN with cerulenin, C75, the anti-obesity drug orlistat, and green tea polyphenols, such as epigallocatechin-3-gallate (EGCG), results in significant in vitro and in vivo anticancer effects, which can be rescued by high FA extracellular concentrations (58, 59).
Targeting FA synthesis either indirectly (e.g. by inhibiting glycolysis/glutamine metabolism) or directly (by inhibiting ACC/FASN) holds promise to selectively affect FA metabolism in tumor cells. However, recent data suggest that some tumors can uptake extracellular lipids, especially lysophospholipids, that are either present in the bloodstream, or produced by nearby cells in the tumor microenvironment (60, 61). From a therapeutic perspective, this implies that inhibition of de novo FA synthesis should be combined with inhibition of extracellular lipid uptake to fully deplete tumor intracellular lipids.
Cholesterol
Cholesterol is another essential component of biological membranes and precursor of isoprenoids and steroid hormones. It is synthesized from acetyl-CoA through a series of biochemical reactions, whose first steps involve the condensation of 3 acetyl-CoA molecules to form hydroxymetylglutaryl-CoA (HMG-CoA). The rate-limiting step reaction of the cascade consists in the formation of mevalonate from HMG-CoA, which is catalyzed by the HMG-CoA reductase (HMGCR) enzyme. Interestingly, HMGCR is overexpressed in several tumors, leading to increased production of cholesterol and isoprenoids from glucose- or glutamine-derived acetyl-CoA (62). Moreover, HMGCR inhibition by statins (e.g. simvastatin or atorvastatin) halts proliferation or induces apoptosis in several tumor cell lines synergistically with chemotherapeutic agents (62–64). Targeting cholesterol metabolism is therefore a promising research topic in the field of tumor metabolism.
Ketone bodies
Under hypoglycemia or reduced glucose uptake, hepatocytes convert excess acetyl-CoA derived from FA β-oxidation to ketone bodies, especially acetoacetate and β-hydroxybutyrate, which are released into the bloodstream and used by peripheral cells to produce acetyl-CoA and fulfill energetic and biosynthetic requirements. FA-derived ketone bodies are therefore essential for normal tissues under conditions of glucose scarcity.
Different from healthy cells, most cancer cells cannot utilize ketone bodies as their primary energy source, mainly because they don’t usually express enzymes converting ketones to acetyl-CoA, and also because they are addicted to glucose for their energetic and biosynthetic needs (5, 65). Moreover, by forcing tumor cells to oxidize acetyl-CoA in mitochondria, ketone bodies may delay glycolysis progression and prove toxic to highly glycolytic tumors (66). Coherent with in vitro data, in vivo studies using mouse models have demonstrated reduced tumor growth and increased animal survival after increasing circulating ketone body concentration through specific dietary interventions (67, 68).
On the basis of preclinical evidences, increasing ketone bodies in the blood could synergize with glucose reduction or pharmacological glycolysis inhibition.
Insulin
Insulin is an essential pancreatic hormone that regulates carbohydrate and lipid metabolism by stimulating glucose uptake in peripheral cells and FA synthesis in the liver. It binds to insulin receptor (IR), which in turn activates the IR-stimulated (IRS)/RAS/RAF/MEK/MAPK and RAS/PI3K/AKT/mTOR signal transduction cascades (69) (Figure 2).
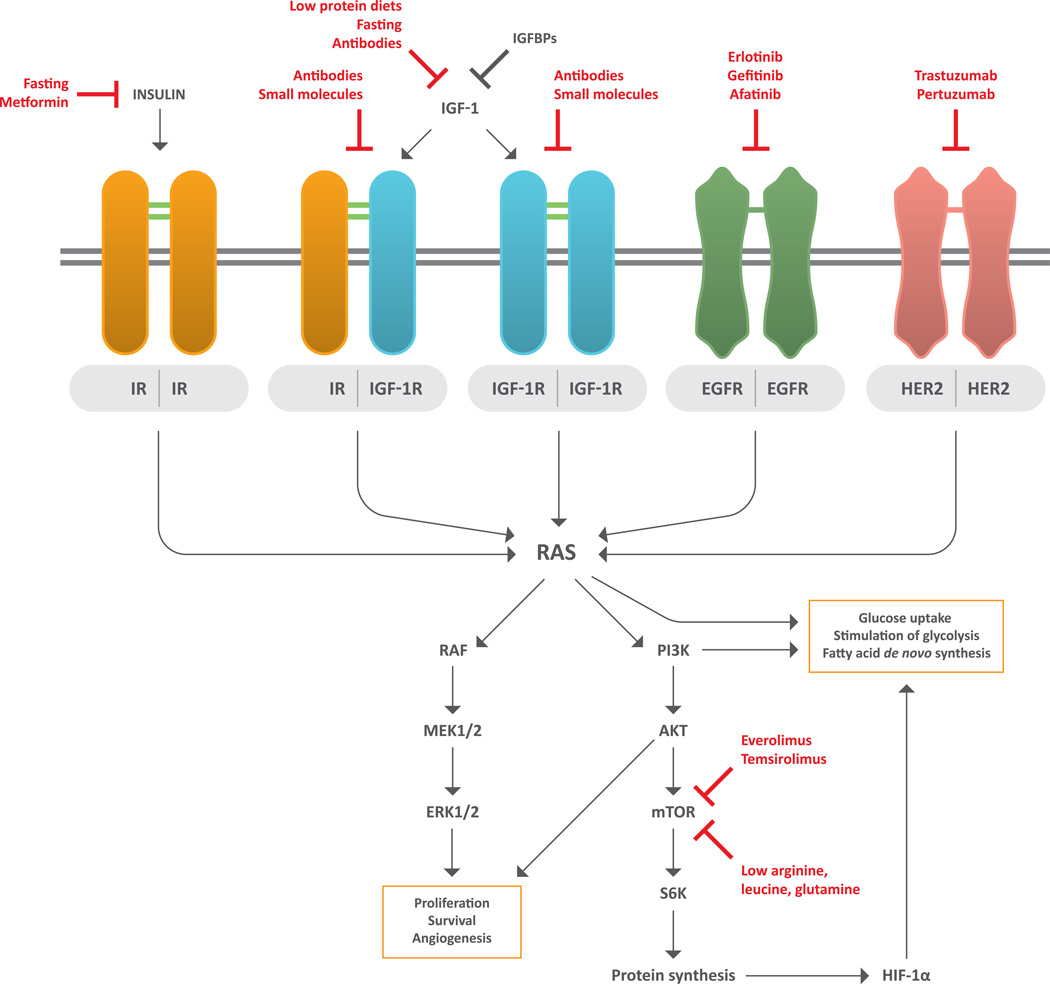
Figure 2. Connections between insulin/insulin-like growth factor 1 signaling and metabolic pathways in tumor cells.
Insulin receptor (IR) and IGF-1 receptor (IGF-1R) can either homo- or heterodimerize to activate their TK domains; this stimulates downstream RAS/RAF/MEK/ERK and RAS/PI3K/AKT/mTOR signal transduction pathways, which induce survival, proliferation, angiogenesis and ribosomal synthesis of several proteins, including hypoxia-induced factor 1α (HIF-1α). PI3K, RAS and HIF-1α promote crucial metabolic modifications in neoplastic cells, including glucose uptake and aerobic glycolysis, as well as de novo synthesis of fatty acids. Since RAS/RAF/MEK/ERK and RAS/PI3K/AKT/mTOR cascades can also be activated by other membrane receptors, including EGFR and HER2, combining inhibition of IGF-1/IGF-1R pathway with targeting of other TK receptors or their downstream mediators (e.g. mTOR) could synergistically inhibit cancer cell proliferation and survival.
Abbreviations. EGFR: epidermal growth factor receptor; HER2: human epidermal growth factor receptor 2; HIF1-α: hypoxia-induced factor 1-α; IGF-1: insulin-like growth factor 1; IGFBPs: IGF-1 binding proteins; IGF-1R: IGF-1 receptor; IR: insulin receptor; mTOR: mammalian target of rapamycin; PI3K: phosphoinositide 3-kinase; S6K: S6 kinase.
When added to cell growth media, physiological insulin concentrations stimulate cancer cell proliferation (70). Moreover, exogenous insulin and hyperinsulinemia accelerate tumor growth and metastases in animals, while IR inhibition reverses these effects (71, 72). Mechanisms responsible for insulin-mediated oncogenic activities include: 1) direct activation of IR and its downstream MAPK and PI3K/AKT signal transduction pathways (69); 2) insulin-stimulated IGF-1 production by hepatocytes; 3) direct activation of the IGF-1 receptor (IGF1R) by insulin. Retrospective studies suggest that hyperinsulinemic patients are more prone to develop aggressive tumors and to die of cancer (73, 74). However, it is presently unknown whether reducing blood insulin levels can affect already established tumors. Moreover, while blood insulin can be reduced through dietary (e.g. carbohydrate-restricted regimens) or pharmacological (e.g. metformin) approaches in hyperglycemic/hyperinsulinemic patients, euglycemic subjects would hardly benefit from this strategy because of the risk to cause insulin-dependent diabetes, thus increasing blood glucose and possibly overfeeding tumor cells.
IGF-1
The peptide hormone IGF-1 is produced by hepatocytes and other cell types when stimulated by GH, insulin (69) and protein-rich diets (75). IGF-1 plays its major physiological role during adolescence, when it promotes growth of several tissues, including bone cartilage (76). A significant fraction of circulating IGF-1 is bound to IGF-1 binding proteins (IGFBPs), especially IGFBP1, IGFBP2 and IGFBP3, which sequester IGF-1 and reduce its availability to target tissues (76).
Interestingly, chronically high blood IGF-1 levels are associated with increased tumor risk and worse cancer prognosis (73, 77, 78). IGF-1 binds to and activates IGF-1 TK receptors (namely IGF1R and IGF2R) on tumor cells, thus activating phosphorylation cascades that converge on RAS/RAF/MEK/MAPK or RAS/PI3K/AKT/mTOR pathway signaling, thus stimulating cell proliferation and inhibiting apoptosis (69). Targeting IGF-1 signaling could affect tumor growth, while contemporarily hampering IGF-1-induced immunodepression and stimulating an effective antitumor response (79, 80).
Disappointingly, studies conducted so far in metastatic breast, pancreatic and lung cancers patients failed to demonstrate the efficacy of IGF-1/IGF-1R axis inhibitors (81, 82). Better patient selection on the basis of circulating IGF-1 levels or activity of biochemical cascades downstream of IGF1R may improve effectiveness of IGF1R inhibition. Moreover, concomitant targeting of kinases that act in parallel (e.g. EGFR or HER2) or downstream (e.g. mTOR) of IGF1R to activate the same transduction pathways may synergize with IGF-1/IGF-1R inhibitors (83, 84) (Figure 2). Finally, dietary/pharmacological interventions that reduce hyperglycemia that can be induced by IGF-1 axis inhibition could significantly improve the efficacy of IGF-1R inhibitors (82).
Deregulation of TCA cycle enzymes in human cancers
Among emerging cancer-specific metabolic alterations, deregulation of different TCA cycle enzymes is interesting based on its potential therapeutic implications. Inactivation of the succinate dehydrogenase (SDH) in human paragangliomas, or fumarate hydratase (FH) in aggressive papillary kidney carcinomas, leads to accumulation of succinate and fumarate, respectively, which induce stabilization of hypoxia-inducible factor-1 (HIF-1) α and stimulate glycolytic metabolism (85, 86). Moreover, the TCA cycle isocitrate dehydrogenase (IDH) enzyme, which catalyzes the reversible conversion of isocitrate to α-KG, is frequently mutated in most low-grade astrocytomas and secondary glioblastomas, but also in acute myeloid leukemias, chondrosarcomas and cholangiocarcinomas (87). IDH1 mutations lead to a change of IDH1 enzymatic activity and to a significantly increased production of the α-KG metabolite 2-hydroxyglutarate (2-HG), which regulates DNA methylation and contributes to mTOR activation (88). Of note, two-dimensional (2D) correlation MRS has been used to detect in vivo 2-HG in glioma patients harboring IDH1 mutations; in the next future, this technique could be used as a noninvasive diagnostic tool and to study dynamical 2-HG changes during tumor progression or response to therapy (89).
Investigating how TCA cycle enzyme deregulation affects tumor growth will be crucial to design effective antitumor strategies, including IDH inhibitors or tyrosine kinase inhibitors (TKIs) (87, 90).
Dietary interventions
Dietary changes bear the potential to affect tumor growth by modifying the blood concentration of many biomolecules and metabolites that sustain cancer cell proliferation. However, given the variety of possible approaches and their complex and sometimes unpredictable impact on the multiplicity of blood metabolites, it is not a trivial aim to identify the ideal anticancer diet. Based on preclinical studies, this diet should target most deregulated metabolic pathways of tumor cells, without significantly affecting healthy tissues. In this overview, we will focus on those approaches that have a solid preclinical evidence of safety and antitumor activity, as summarized in Table 1. Following the observation that a moderate, chronic caloric restriction significantly reduces cancer incidence in non-human primates (91, 92) and other mammalian species (93), many currently investigated antitumor dietary approaches are based on the concept that restricting/modulating nutrient intake can reduce metabolite delivery to tumor cells.
Table 1.
Main characteristics of dietary interventions under clinical investigation
| Dietary intervention |
Metabolic effects | Potential clinical effects |
Risks and limitations |
Ongoing trials |
|---|---|---|---|---|
| Short-term fasting (STF) |
|
|
|
|
| Ketogenic diets |
|
|
|
|
| Fasting-mimicking diet (FMD) |
|
|
|
|
Fasting
Avoiding calorie intake for an average of 3–5 consecutive days, also referred to as “short term fasting” or STF, induces multiple systemic metabolic changes that could hamper cancer growth, including: a) reduction of blood glycemia; b) decreased insulinemia due to peripheral insulin sensitization; c) reduction of IGF-1 and increased IGFBP levels; d) increased blood ketone bodies (94, 95). While relative glycemia reduction depends on baseline glucose concentration and can be lacking in euglycemic subjects, IGF-1 and IGFBPs are more consistently reduced (by about 50%) and increased (by 5-fold), respectively, during STF (96, 97). Fasting can therefore affect both tumor metabolism and circulating growth factors. However, its effects on circulating AAs are still unclear. In healthy volunteers, fasting for 4–5 days induced a moderate increase in methionine, valine, leucine and isoleucine blood concentration, while poorly affecting or mildly reducing other AAs (98). However, due to significant differences in systemic AA metabolism between healthy and cancer patients, these results cannot be directly translated to the population of subjects with advanced tumors.
In in vitro experiments, STF is mimicked by short-term starvation (STS), consisting in the reduction of glucose and growth factors in cell growth media. STS sensitizes several cancer cell lines to different chemotherapeutic drugs, while relatively protecting normal cells (13, 79, 96). This differential stress response (DSR) may depend on the ability of nutrient-restricted healthy cells to halt proliferation and anabolic processes, and to activate catabolic processes (such as autophagy) and protective functions, such as DNA and protein repair, which preserve genome and proteome integrity during the resting state. On the contrary, most cancer cells are unable to halt proliferation and anabolism, even in conditions of nutrients scarcity; this exposes them to rapid ATP depletion and increased DNA damage by several chemotherapeutic agents, including alkylating agents and topoisomerase inhibitors (99). However, tumor cell sensitivity to nutrient and growth factor deprivation is not a general rule, because certain tumors with constitutively active PI3K/AKT pathway grow independently from IGF-1 and insulin stimulation, and are therefore insensitive to both in vitro starvation and in vivo caloric restriction (100). Understanding whether and how oncogenic pathways other than PI3K/AKT influence tumor response to starvation is of crucial importance to better select patients candidate to STF.
In in vivo mice models of several tumors, STF inhibits cancer growth similarly to chemotherapy (13). Moreover, fasting reduces CT-induced toxicities to several normal tissues and synergizes with CT or radiotherapy to kill tumor cells (13, 96). These effects translate into significant survival prolongation in animals fasting during chemotherapy. The DSR between normal and cancer tissues therefore creates a therapeutic window to specifically target cancer cells, while sparing normal tissues.
Recently, STS and STF have also shown synergistic in vitro and in vivo antitumor effects with a wide range of TKIs that inhibit growth of lung, breast and colorectal human cancers (101). The observed synergy can be explained through the ability of STS to potentiate TKI-induced inhibition of growth signaling cascades, including the MAPK and PI3K/AKT pathways, thus preventing rebound oncogene activation that occurs upon exposure to single TKI treatment (102). Despite preliminary, these evidences hold promise to extend the possible combinatorial applications of fasting to most clinical settings.
Ongoing trials are investigating the safety and efficacy of STF in advanced human cancers. Preliminary data indicate that STF is safe and potentially useful to reduce chemotherapy-induced side effects in humans with several types of tumors, without apparently compromising treatment efficacy (103). Patients loose as much as 10% of their weight during STF, but weight loss is usually reversible. In one recent study, stage II/III breast cancer patients undergoing STF during (neo-)adjuvant chemotherapy reported less hematological toxicities and less chemotherapy-induced DNA damage to circulating blood cells, including lymphocytes, compared to patients following a regular diet (104).
The potential impact of fasting on the balance between inhibitory and antitumor immune cell populations is another field of intensive research due to its possible dramatic consequences on the outcome of new immunotherapies in the treatment of several cancers. Promising in this respect is the preliminary finding that fasting reduces chemotherapy-induced lymphopenia in mice and humans (105), and that fasting-mediated antitumor effects depend on the presence T lymphocytes in mice (106).
Ketogenic diets
Commonly recommended diets ensure a daily caloric intake of 1500–2000 Kcal, with a net predominance of carbohydrates over proteins and fats (carbohydrates:proteints:fats ratio of around 60:20:20). On the other hand, diets chronically rich in fats and poor in simple and complex carbohydrates force the organism to switch from carbohydrate to FA metabolism, and are referred to as “ketogenic diets” (KDs), because increased FA oxidation raises blood levels of ketone bodies. Depending on the specific ratio of macronutrient composition (fats:proteins:carbohydrates), origin of fats (animal versus vegetal), and total calorie content, different KDs can produce quite different metabolic effects (107).
Based on the ability of normal, but not tumor brain cells to use ketone bodies as their primary energy source, KDs have been first proposed to treat brain malignancies (65, 108). The first reported study dates back to 1995, when two young children with grade III and IV astrocytomas recurring after extensive chemo- and radiotherapy treatment were given a specific KD, and both experienced reduced glucose uptake by the tumor and durable tumor control (109). Since then, several small studies have reported good tolerability and potential clinical activity of KDs in advanced tumors. In one study, 10 subjects with different metastatic cancers progressing on standard therapies and a positive 18FDG-PET scan were fed a KD with as few as 5% carbohydrate content for 28 days, while protein and fat ingestion was encouraged to maintain a stable total caloric intake. The dietary intervention was well tolerated and increased β-hydroxybutyrate blood concentration, while reducing glucose and insulin levels. Interestingly, some patients remained stable at one-month 18FDG-PET re-evaluation, and stability correlated with increased plasma ketones (110). In another study, 16 subjects with different end-stage cancers limited carbohydrate daily intake to less than 70 grams and were given meals rich in proteins and oils. This diet was globally quite well tolerated and five subjects with higher ketone body urinary excretion had stable disease after three months (111). Despite preliminary, these studies underscore the importance of blood and urinary ketones to monitor the metabolic effects of KDs. However, variability in total caloric content, fats:proteins:carbohydrates ratio, diet duration and combination with other therapies across several studies makes it difficult to drive conclusions about individual KDs. Prospective trials are currently testing the efficacy of different KDs in combination with CT and RT in GBM patients.
Looking for the most effective KD
Two crucial variables potentially affecting the impact of KDs on tumor metabolism are total calorie content and percent macronutrient composition. One study tested the role of moderate, chronic caloric restriction (40% daily reduction of total calories for 13 days) on blood glucose and IGF-1 in mice (112). Across different dietary regimens tested, the calorie-restricted KD with a fats:carbohydrates:proteins ratio of 60%:30%:10% reduced blood glucose and IGF-1 comparably to STF, and also protected mice from CT-induced side-effects. Although interesting, these results were obtained in animal models and should be confirmed in humans. Moreover, even if two dietary regimens produce similar systemic metabolic changes, their anticancer effects may significantly depend on kinetics of metabolite modifications. For example, fast reduction of glucose, IGF-1 and insulinemia as obtained with STF may be essential to induce metabolic crisis in rapidly proliferating cancers; on the contrary, slow reduction of the same metabolites may allow tumors to more easily adapt their metabolism and escape the insult. Since the goal of dietary interventions is to achieve prolonged tumor remissions and major survival effects against a variety of tumors, it is important to: 1) test specific dietary interventions in many different types of cancer models in vivo and in vitro; 2) combine the diet with different types of standard treatments matching the cancer type (e.g. chemotherapy or TKIs); 3) generate an intervention which is feasible, safe and can be easily prescribed.
Fasting-mimicking diet (FMD)
Attempting to design a tolerable diet that modifies systemic metabolism comparably to STF (therefore called “fasting mimicking diet” or FMD), a standardized, plant-based, low-calorie (500–1000 KCal/day), low-protein KD containing a fats:carbohydrates:proteins ratio of around 50%:40%:10% has been recently developed (113). When administered to human healthy volunteers for 5 consecutive days every one month, the FMD reversibly reduced body weight by 15%, blood glucose and IGF-1 by around 11% and 24%, respectively, while increasing ketone bodies and IGFBP-1 by around 3- and 1.5-fold. These changes were measured at least 5 days after the subjects had returned to their normal diet, suggesting the effects of the FMD are durable. The FMD is a safer and more acceptable alternative to STF, and produces metabolic and antitumor effects comparable to complete fasting, while potentially enhancing effective antitumor immunity (113, 114). As for STF, the impact of the FMD on circulating AAs is still unclear; however, since AAs are crucial for tumor growth, future studies on FMD should prioritize this aspect.
Prospective clinical trials are testing the FMD in combination with chemotherapy in patients with breast cancer.
Protein- and AA-restricted diets
Protein-restricted diets could potentially inhibit tumor growth by reducing AA supply to tumor cells and consequently affecting protein synthesis, AA-mediated mTOR activation and other metabolic processes. Preclinical experiments have shown that protein restriction inhibits in vivo tumor growth of melanoma but not breast cancer models, thus indicating its efficacy could be tumor-dependent (77). Other three major concerns can be raised against protein restriction in advanced tumors. First, clinical data of safety and efficacy are lacking. Second, nonessential AAs can be de novo synthesized by tumor and normal cells from precursors circulating in the blood, thus making general protein restriction unnecessary. Finally, prolonged protein deprivation can stimulate tumor-induced degradation of muscle cell proteins, thus precipitating sarcopenia, on the one side, and overfeeding the tumor with muscle cell-derived AAs, on the other side.
Dietary restriction of single AAs could be more safely used to exploit specific tumor auxotrophies. Based on preclinical data, methionine-restricted diets have been tested in populations of patients with advanced cancers and have shown a good tolerability profile (115–117). Overall, circulating blood methionine is reduced by about 60% upon methionine restriction, and some preliminary evidence of antineoplastic activity has also been reported. However, methionine represents an exception to other AAs, whose dietary deprivation is not consistently associated to fast and predictable reduction in blood concentration, and has also raised safety concerns (118). Moreover, normal cells in tumor microenvironment, including fibroblasts, endothelial and immune cells, can supply tumor cells with AAs deriving from autophagic degradation of their proteins, thus potentially limiting the impact of dietary restriction (119, 120).
Gut microbiota and diet
The gut microbiota is being increasingly recognized as a key regulator of the antitumor effect of cytotoxic chemotherapy and novel immune-directed therapies (121). Possible mechanisms to explain this interaction include bacteria-mediated regulation of immune cell activity and metabolic modulation. Indeed, gut-resident bacteria metabolize macromolecules introduced with diet, such as complex carbohydrates and proteins, producing and releasing short-chain fatty acids (SCFAs) and AAs into the bloodstream. In vivo experiments have shown that mice with intact gut microflora have lower levels of blood tryptophan and N-acetyltryptophan, but higher indoxyl sulfate and indole-3-propionic acid compared to bacteria-free animals (122). Another interesting study demonstrated that caloric restriction can induce microbiota changes that are associated with longer lifespan in mice (123). Future studies should focus on the effect of fasting/FMD on quantitative and qualitative composition of the gut microbiota, because bacterial metabolism could contribute to the metabolic changes occurring in fasting subjects. Moreover, the impact of antibiotics, which are frequently used by cancer patients to treat tumor-associated or chemotherapy-induced infections, should be further explored, and strategies to repopulate or reshape the gut microbiota, including probiotic supplementation or specific dietary interventions, should be investigated.
Pharmacological interventions
Widespread application of dietary interventions may be hindered by serious limitations. First, some subjects may not accept drastic dietary changes, especially fasting. Second, severely calorie-restricted regimens may damage patients with malnourishment or cachexia, a tumor- or chemotherapy-induced deadly syndrome that causes up to 20% of all cancer deaths. Cachexia is characterized by systemic inflammation, damage to normal tissues and weight loss, and is incompatible with significant caloric restriction, especially in its advanced stages (124). Third, diets can be difficult to standardize, and variability in diet composition or patient compliance can cause reproducibility problems in clinical trials (107). Finally, some tumors may be specifically addicted to one metabolic pathway and the pleiotropic effects induced by specific diets may be not strong or selective enough.
Metabolism-targeting pharmacological therapies are a valuable alternative to dietary approaches and aim to selectively target one specific metabolic pathway. Potential advantages of pharmacological approaches include the fact that they do not impose significant life-style changes to patients, and should not induce weight loss. Moreover, their dosages can be precisely titrated and their pharmacokinetic/pharmacodynamic effects reproducibly and precisely determined. The list of chemical compounds targeting tumor metabolism is long and in continuous expansion. In this paragraph we will focus on those molecules that appear as the most promising based on their biochemical mechanisms and preclinical/clinical data of tolerability and efficacy, so to justify ongoing clinical trials, as summarized in Table 2.
Table 2.
Main metabolic effects and clinical characteristics of metabolism-targeting drugs under clinical investigation*
| Drug | Metabolic effects | Potential clinical effects |
Risks and side effects |
Ongoing trials (tumor types) |
|---|---|---|---|---|
| Metformin |
|
|
|
lung: NCT02019979, NCT02285855, NCT02115464; prostate NCT02640534; breast NCT01310231; endometrial NCT02755844, NCT01797523; ovarian NCT02312661, NCT02122185; WDNETs NCT02294006; NCT02823691 |
| Aspirin |
|
|
|
prostate: NCT02420652; colorectal: NCT02607072, NCT00565708; breast NCT02602938, NCT02804815; lung NCT01707823; esophageal NCT02326779 |
| DCA |
|
|
|
H&N: NCT01386632 |
| Gossypol |
|
|
|
NSCLC: NCT01977209; B-CLL: NCT01003769 |
| AZD3965 |
|
|
|
several advanced tumors: NCT01791595 |
| CB-839 |
|
|
|
breast, lung, renal: NCT02771626; NCT02071862; leukemia: NCT02071927 |
| ADI-PEG |
|
|
|
liver, lung, uveal melanoma, glioma and mesothelioma: NCT02029690; prostate and NSCLC: NCT01497925; gastrointestinal: NCT02102022; mesothelioma: NCT02709512 |
|
EGCG/green tea extracts (GTEs) |
|
|
|
breast: NCT00949923; urothelial: NCT01993966; SCLC: NCT01317953 |
| Statins |
|
|
|
rectal: NCT02161822; NCT02569645; prostate NCT01992042; breast: NCT02483871; NCT02416427; bladder: NCT02360618 |
| Rapalogs |
|
|
|
breast, renal and pancreatic NCT02077933; endometrial and ovarian: NCT02188550; WDNETs: NCT01648465, NCT02294006 |
Only the most representative ongoing studies are reported, and for each of them the ClinicalTrials.gov Identifier is indicated
WDNETs: well-differentiated neuroendocrine tumors; H&N: Head and Neck; NSCLC: non-small cell lung cancer; B-CLL: B chronic lymphocytic leukemia; SCLC: small cell lung cancer
Metformin
Metformin, the reference drug for type II diabetes treatment, inhibits intestinal glucose uptake and liver neoglucogenesis, and also sensitizes peripheral tissues (mainly muscle and fat tissues) to insulin activity (125). In this way, metformin reduces blood glycemia and insulinemia in hyperglycemic/diabetic patients, but not in euglycemic ones. Of note, metformin use in diabetics has been consistently associated with reduced cancer risk (including breast, colon, pancreatic and liver malignancies) and better cancer prognosis compared with other anti-diabetic treatments (126–128).
Potential anticancer mechanisms of metformin include:
-
1)
Systemic metabolic activities: metformin significantly reduces blood glucose, insulinemia, cholesterol and triglycerides in hyperglycemic/diabetic subjects and in patients experiencing glucocorticoid-induced hyperglycemia (129). In one study on endometrial cancer patients, it also increased plasmatic ketone body concentration by about 5-fold (130), and has been proposed to reduce IGF-1 levels (131). Metformin can therefore potentially target different metabolic pathways that are associated with cancer progression and proliferation, mimicking fasting/FMD; however, different from fasting, most of metformin-induced effects are likely restricted to hyperglycemic/diabetic patients.
-
2)
Cell autonomous effects: metformin has in vitro anticancer activity that is likely mediated through inhibition of mitochondrial complex I, reduced NADH oxidation and increased AMP/ATP ratio. The consequent activation of AMP-kinase (AMPK) by its upstream LKB1 kinase results in: a) inhibition of mTOR signaling and protein translation; b) inhibition of ACC and FA synthesis, and upregulation of FA β-oxidation; c) inhibition of HMGCR and cholesterol synthesis (125). Metformin-induced shift from anabolic to catabolic processes, together with the energetic stress caused by reduced ATP levels, inhibits proliferation, and, in case of inability to do so, triggers apoptosis in cancer cell lines (132).
To date, only preliminary prospective data support metformin antitumor efficacy in human cancers (133–135). Although several prospective trials are now testing metformin in advanced tumors, lack of patient selection in most trials may limit their reliability. Disappointingly, three recent studies on patients with advanced pancreatic adenocarcinoma failed to demonstrate improved outcome with metformin in combination with different first- or second-line chemotherapeutic treatments (136–138). In future studies, it will be crucial to select patients who are more likely to benefit from metformin on the basis of systemic metabolic state (e.g. basal glycemia, ketone bodies, insulin, IGF-1 and IGFBPs levels), tumor genomic/metabolic signature (e.g. activation of the MAPK or IGF1R/PI3K/AKT/mTOR pathways, LKB1 activation state, glycolytic enzymes expression, addiction to specific AAs), and in vivo glucose avidity as measured through 18FDG-PET (139). It will be also important to design combinatorial therapies to exploit the potential synergism between metformin and other interventions, such chemotherapeutic or molecularly targeted drugs (Figure 1). Finally, the recently described effect of metformin on gut microbiota composition deserves further investigation in the light of the potentially significant contribution of intestinal bacteria to systemic metabolism and efficacy of anticancer therapies (140).
Aspirin
Aspirin, the most frequently used non-steroidal anti-inflammatory drug (NSAID), has shown interesting metabolic and antitumor properties. In one study on diabetic volunteers, high dose (6.7 grams/day) aspirin taken for two weeks significantly reduced fasting glycemia, C reactive protein, total cholesterol, triglycerides and FAs, with the most common side effects consisting of reversible hearing loss and tinnitus (141). Moreover, low-dose (75–160 mg) daily aspirin has been convincingly associated with reduced cancer incidence and mortality in prospective trials (142, 143). Finally, retrospective studies correlated aspirin use with reduced recurrence of PI3K-mutated, surgically resected colorectal cancers (144).
Putative antitumor mechanisms of aspirin include:
-
1)
Systemic activities: low-dose aspirin irreversibly inhibits cyclooxigenase 1 (COX1) enzyme and thromboxane A production in platelets, thus preventing platelet clot formation and, possibly, tumor cell migration and metastatization (145). At higher dosages, aspirin reduces systemic and local inflammation, and potentially impacts on systemic carbohydrate and lipid metabolism, especially in diabetic subjects.
-
2)
Cell autonomous effects: sodium salicylate, the main aspirin metabolite in human blood, activates AMPK and inhibits mTOR and FA synthesis in cancer cells lines (146). Another proposed anticancer mechanism of aspirin is inhibition of COX-2, which can drive carcinogenesis and is often overexpressed in human cancers, including colorectal cancer (147). Of note, in vitro antitumor effects of salicylates are achieved with drug concentrations obtainable after administration of high-dose aspirin.
While it is highly unlikely that high-dose aspirin can be safely administered to cancer patients for a prolonged time, brief exposures may be tolerated and possibly capable of strong synergistic anticancer effects with chemotherapy, metformin, or specific dietary interventions. The role of low-dose aspirin in established cancers is more uncertain, and ongoing prospective studies are investigating it as an adjuvant treatment after radical surgery.
Targeting aerobic glycolysis
The most direct way to target exaggerated aerobic glycolysis in tumors is to reduce glucose availability to cancer cells, which can be achieved through either dietary or pharmacological (e.g. metformin) interventions. However, while diabetic subjects could reasonably obtain a therapeutically relevant reduction of glycemia, euglycemic subjects would hardly benefit from such interventions, because lowering glycemia below 60–70 mg/dl for a prolonged period could irreversibly damage normal tissues, including the hearth and the brain. In euglycemic patients, pharmacological targeting of specific glycolytic enzymes could be the best-tolerated and most effective option. Several direct or indirect glycolysis inhibitors are under clinical investigation.
Dichloroacetate (DCA)
The enzyme pyruvate dehydrogenase kinase (PDK) is overexpressed in several tumors, where it inhibits pyruvate dehydrogenase (PDH) and diverts pyruvate to lactate, thus contributing to their glycolytic phenotype (148). By inhibiting PDK, DCA stimulates the conversion of pyruvate to acetyl-CoA and mitochondrial oxidation of acetyl groups (Figure 1) (149). Moreover, it induces mitochondrial depolarization and increases production of reactive oxygen species (150). Interestingly, DCA inhibits in vitro and in vivo (mouse models) tumor growth, and could synergize with metformin to co-target aerobic glycolysis and mitochondrial oxidative phosphorylation (151, 152). Preliminary clinical studies reported on the antitumor efficacy of DCA in human cancers, with some prolonged tumor remissions in highly pre-treated GBM patients (153). Of note, DCA has been used for decades in oral formulation for treating children with inborn mitochondrial defects, and is considered safe at standard dosage (154). Prospective trials are currently testing the efficacy of this promising drug in several tumors.
LDHA inhibitors
LDHA inhibition prevents regeneration of NAD+, which is crucially required for glycolysis progression (Figure 1) (16). Clinical trials investigating the LDHA inhibitor gossypol in different solid cancers are ongoing, but serious concerns exist about its poor tolerability and antitumor in vivo efficacy (155). Another way to target lactate metabolism is to inhibit its export from cancer cells into the extracellular environment. Indeed, accumulating intracellular lactate forces the reversible reaction catalyzed by LDHA in the direction of pyruvate production, again preventing NAD+ regeneration. AZD3965 is an inhibitor of the lactate transporter MCT1, which is expressed at high levels on the cell membrane of several tumors, including breast, colorectal cancers and gliomas; moreover, high MCT1 levels predict poor patient outcomes (156, 157). Clinical studies are testing the tolerability and antitumor effectiveness of AZD3965 in solid tumors.
Targeting glutamine metabolism
Conversion of glutamine to α-KG is a two-step process involving glutamate as an intermediate metabolite (Figure 1). Inhibitors of GLS, including BPTES and CB-839, GDH, such as GPNA or EGCG, or transaminases (TAs), such as aminooxyacetate (AOA), could all target the first steps of intracellular glutamine metabolism (32, 158). In a recent study, CB-839 reduced production of glutamine-derived glutamate, glutathione and TCA intermediates, and displayed significant antitumor activity against TNBC cell lines (159). CB-839 is under clinical investigation in several solid and hematological malignancies. Another recent paper reported GPT2 transaminase overexpression in glutamine-addicted breast cancer cell lines (160). In this study, the general TA inhibitor AOA reduced tumor cell viability, which was rescued by adding aspartate to the growth medium; this confirms glutamine contribution to neo-synthesis of other AAs (10). Interestingly, AOA has been clinically tested to treat tinnitus, and its toxicity profile is well known (161). Identifying glutamine-dependent tumors on the basis of biological/immunohistochemistry (such as MYC overexpression) characteristics or functional imaging (such as in vivo uptake of glutamine analogs) will prove crucial to select patients who are more likely to respond to inhibitors of glutamine metabolism (162).
Methioninase
Although methionine-restricted diets reduce plasmatic methionine concentration by more than 50% after only one day (117), longer restriction is not associated with further decrease, likely because muscle protein degradation can compensate for methionine reduction and consequently limit the effectiveness of dietary deprivation (124). Pharmacological approaches to optimize methionine depletion include the bacterial methionine-degrading enzyme methioninase, which inhibits growth of mouse models of colon, lung and brain tumors, especially in combination with cytotoxic treatments (163–165). Of note, methioninase reduces blood methionine independently from patient diet, which can be difficult to control. Moreover, metioninase could counteract homeostatic, rebound increases of methionine absorption or systemic production. Finally, methioninase would deplete not only systemic, but also intratumoral methionine that could be produced by normal cells in tumor microenvironment or by sub-clones of adapted cancer cells. Low half-life and high immunogenicity of methioninase currently limit its long-lasting efficacy (166), but investigating alternative strategies to optimize methionine depletion in advanced human cancers should be encouraged.
ADI-PEG
Depleting circulating arginine is predicted to be lethal for ASS1-repressing malignancies. However, limiting dietary arginine intake is unlikely to be effective, because the liver and the kidneys are able to synthesize and release it in the bloodstream. ADI-PEG, a pegylated (polyethylene glycol conjugated) form of the Mycoplasma-isolated arginine deiminase (ADI) enzyme that catabolizes arginine to citrulline and ammonia, is a promising arginine-depleting therapy (167). Repeated ADI-PEG administrations are well tolerated and induce rapid and durable plasmatic arginine reduction. Of note, clinically significant disease stabilizations have been observed in advanced melanomas and HCC patients treated with ADI-PEG monotherapy, and efficacy correlated with prolonged arginine depletion (168). Resistance mechanisms to ADI-PEG include the production of ADI-PEG-inactivating antibodies by B-lymphocytes and derepression of ASS1 in tumor cells, which become independent from external supply (168–170). Ongoing phase II/III trials are testing ADI-PEG in advanced HCCs, mesotheliomas and NSCLCs; based on the experimentally observed synergy between arginine depletion and interference with DNA synthesis/replication in ASS1-repressing tumors, some of these studies are testing ADI-PEG in combination with cisplatin and pemetrexed (171).
Targeting FA metabolism
Since most tumors de novo synthesize FAs from intracellular intermediates (Figure 1), reducing plasmatic triglycerides and cholesterol levels with dietary or pharmacological interventions is unlikely to effectively target lipid metabolism in tumors. Instead, pharmacological inhibition of FA synthesis is a potentially more effective strategy. Cerulenin and C75 are well-studied inhibitors of FASN; unfortunately, they cause severe side effects, including anorexia and loss of adipose mass due to massive lipolysis, which preclude their administration to cancer patients (172). On the other hand, EGCG, some of its synthetic derivatives, and some flavonoids (such as quercetin and luteonin), seem to be safer FASN inhibitors; developing tolerated and more effective EGCG derivatives is a promising field of current research (173). ACC is another possible pharmacological target to inhibit FA synthesis (174). Both metformin and salicylates induce phosphorylation and inhibition of ACC in tumor cell lines, and their potential in vivo antitumor activity may be in part related to inhibition of FA synthesis (125, 146). Finally, combined inhibition of aerobic glycolysis and glutamine metabolism with dietary or pharmacological strategies is another way to deplete intracellular FA precursors.
Statins
Based on in vitro and preclinical in vivo activity, the cholesterol lowering drugs statins have been evaluated as anticancer agents (175). Prospective studies have shown some antitumor activity, especially with lipophilic statins, in patients with advanced malignancies (176, 177). In particular, one study suggested that HMGCR-overexpressing human breast cancers may be especially sensitive to atorvastatin (177). Statins have also shown preliminary synergistic antitumor activity with gefitinib in patients with NSCLC (178). However, clinical benefit in unselected cancer populations has been globally modest so far. Identifying pathological/biological tumor characteristics that predict benefit from statins (e.g. activation of RAS/PI3K/AKT pathway or expression levels of HMGCR) and investigating effective concomitant cytotoxic/metabolic therapies will be crucial steps to optimize antitumor therapy with these drugs.
Rapalogs
mTOR inhibition induces antitumor effects by inhibiting protein synthesis and cell proliferation. mTOR inhibitors are globally referred to as “rapalogs”, and include the ancestor drug, rapamycin, and its derivatives everolimus and temsirolimus. Everolimus is currently approved for the treatment of advanced renal, well-differentiated neuroendocrine and hormone receptor-positive breast tumors (179). Interestingly, mTOR is also involved in glucose metabolism by stimulating transcription of HIF-1α and consequently up-regulating GLUT1. For this reason, solid rationale exists for contemporarily inhibiting mTOR and aerobic glycolysis to synergistically kill tumor cells (26). In particular, combining rapalogs with metformin could produce the following synergistic effects: 1) enhanced mTOR inhibition via metformin-induced AMPK activation; 2) contemporary targeting of protein translation (via mTOR inhibition) and de novo synthesis of FAs and cholesterol (via metformin-induced inhibition of ACC and HMGCR); 3) reduction of rapalog-induced hyperglycemia, which could partially hamper the antitumor activity of mTOR inhibitors. Several phase I/II trials are now testing the combination of rapalogs and metformin in advanced solid tumors.
Conclusions and perspectives
The rationale behind selectively targeting cancer metabolism while sparing normal tissues relies on the different ways that normal and cancer cells produce and utilize energy and metabolites, and their consequently different response to starvation conditions.
In this respect, both dietary and pharmacological interventions are potentially highly effective strategies that could synergize with classical cytotoxic treatments causing oxidative and metabolic stress, or molecularly targeted therapies that inhibit crucial signal transduction pathways. Which, if any, of the two approaches (dietary or pharmacological) is more effective as an adjuvant to standard treatments is actually unclear, and will be the focus of intensive future research. Both strategies hold significant advantages and disadvantages (Table 3). Dietary interventions produce pleiotropic effects on different metabolic pathways, with a likely broader antineoplastic activity. Moreover, they are cheap and already available. The major limitation of extreme calorie-restricted regimens is their poor acceptability by some subjects or their inapplicability to cachectic patients. Because cachexia usually arises in the latest tumor stages and most metastatic patients are not cachectic at diagnosis, drastic dietary interventions are more likely to be tolerated and effective when used in the first-line treatment setting.
Table 3.
Major advantages and disadvantages of dietary and pharmacological interventions targeting tumor metabolism
| Metabolic intervention | Potential advantages | Potential disadvantages |
|---|---|---|
|
Dietary (fasting ketogenic diets, FMD) |
|
|
| Pharmacological |
|
|
On the other hand, pharmacological therapies target tumor-specific metabolic alterations and could be better accepted by cancer patients, but require synthesis and investigation of highly selective compounds, with significant costs and longer waiting times. Moreover, tumor cell adaptation to inhibition of single metabolic pathways could lead to fast selection of resistant sub-clones.
Due to recognized intertumor and intratumor heterogeneity, improving metabolic therapies at best will probably require the identification of crucial metabolic alterations in single tumors. For example, in vivo glucose avidity as detected by 18FDG-PET, or overexpression of glycolytic enzymes at immunohistochemistry may help identify tumors that are especially sensitive to reduction of blood glycemia and/or metformin treatment. Similarly, glutamine-addicted cancers, as detected by in vivo tumor catabolism of 18F-(2S,4R)4-fluoroglutamine, may respond to inhibitors of glutamine metabolism (162). Finally, low tumor ASS1 levels as detected by immunohistochemistry may predict response to ADI-PEG or other arginine-depleting strategies, and high methionine tumor uptake could be associated with efficacy of methioninase or dietary methionine deprivation (42).
Combining pleiotropic effects of dietary interventions with pharmacological targeting of the metabolic pathway(s) to which an individual tumor is addicted may enhance antitumor efficacy of either strategy, while contemporarily preventing tumor adaptation (Figure 3).
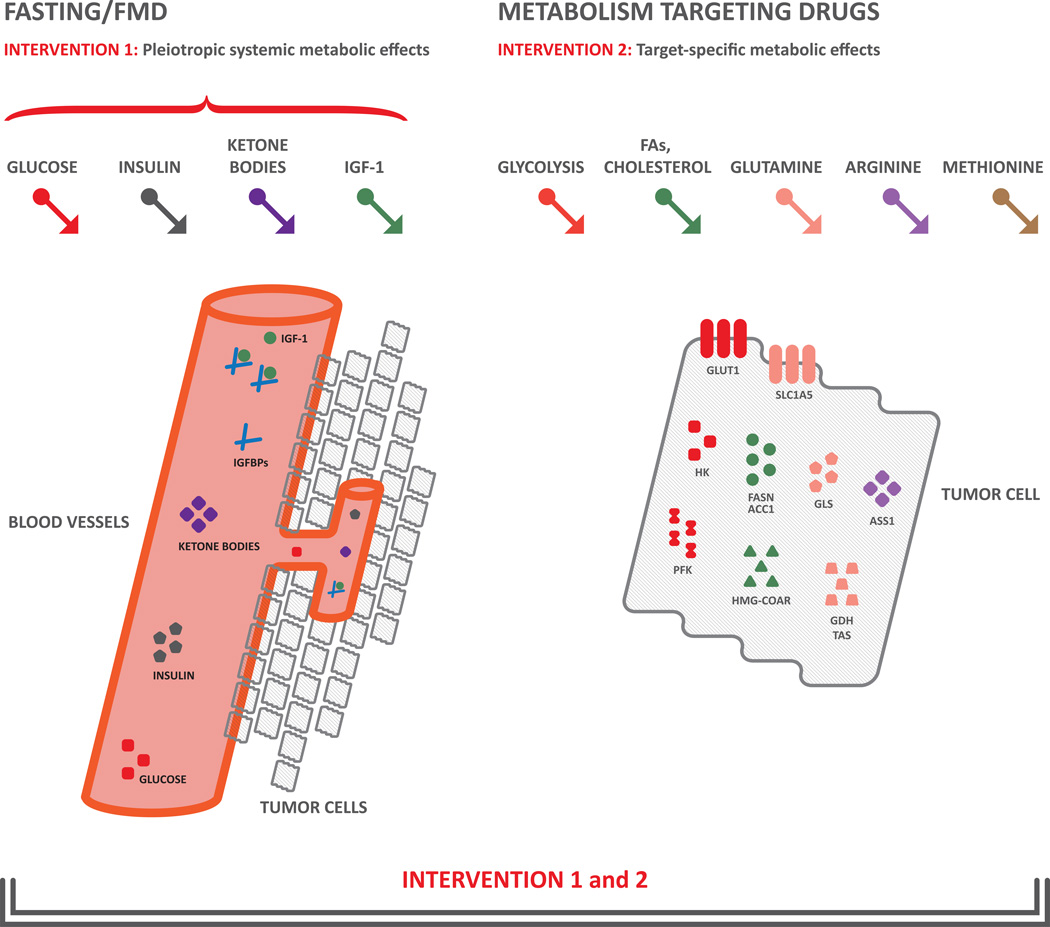
Figure 3. Rationale for combining dietary interventions and drugs targeting specific metabolic pathways in cancers.
Fasting and FMD (left part of the figure) impact on systemic metabolism through induction of pleiotropic metabolic effects, including reduction of glycemia, insulin and IGF-1 levels, and increase of ketone bodies and IGFBPs. On the other hand, pharmacologic approaches (right part of the figure) have the potential to selectively inhibit the specific metabolic pathway(s), such as glycolysis, glutamine, arginine, methionine, FAs and cholesterol metabolism, to which a single tumor may be addicted. Combining the two strategies could produce synergistic and selective anticancer effects .
Abbreviations. IGF-1: insulin-like growth factor 1; FAs: fatty acids; GLUT1: glucose transporter 1; HK: hexokinase; PFK: phosphofructokinase; FASN: fatty acid synthase; ACC1: acetyl-CoA carboxylase; HMGCR: hydroxymethylglutaryl-CoA reductase; GLS: glutaminase; GDH: glutamate dehydrogenase; TAs: transaminases; ASS1: argininosuccinate synthase 1
Statement of Significance.
To our knowledge, this is the first review article that comprehensively analyses the preclinical and preliminary clinical experimental foundations of both dietary and pharmacological metabolic interventions in cancer therapy. Among several promising therapies, we propose treatment personalization on the basis of tumor genetics, tumor metabolism and patient systemic metabolism.
Acknowledgments
The authors thank Dr. Fabio Picchini for carefully reading the manuscript, helpful scientific discussions and significant graphical improvements of figures.
Footnotes
Conflicts of interest disclosure: Valter Longo has equity interest in L-Nutra, a company that develops and sells medical food. The other authors declare no conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Huang M, Shen A, Ding J, Geng M. Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci. 2014;35:41–50. doi: 10.1016/j.tips.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11:282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 4.Mathews CK. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat Rev Cancer. 2015;15:528–539. doi: 10.1038/nrc3981. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, et al. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin Cancer Res. 2007;13:3460–3469. doi: 10.1158/1078-0432.CCR-07-0074. [DOI] [PubMed] [Google Scholar]
- 7.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues TB, Serrao EM, Kennedy BW, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C–labeled glucose. Nat Med. 2014;20:93–97. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, et al. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. 2010;29:1641–1652. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- 15.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. The Journal of clinical investigation. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 18.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27:1082–1086. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. The Journal of pediatrics. 2009;155:73–78. doi: 10.1016/j.jpeds.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 20.Ali NA, O’Brien JM, Jr, Blum W, Byrd JC, Klisovic RB, Marcucci G, et al. Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer. 2007;110:96–102. doi: 10.1002/cncr.22777. [DOI] [PubMed] [Google Scholar]
- 21.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A. 2009;106:14878–14883. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Zou Y, Mao D, Sun D, Gao G, Shi J, et al. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J Cell Biol. 2014;206:173–182. doi: 10.1083/jcb.201403009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. The Journal of biological chemistry. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 29.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao Y, Samuels Y, Li Q, Krokowski D, Guan BJ, Wang C, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun. 2016;7:11971. doi: 10.1038/ncomms11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein JD. Methionine metabolism in mammals. The Journal of nutritional biochemistry. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 35.Cavuoto P, Fenech MF. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat Rev. 2012;38:726–736. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Palanichamy K, Thirumoorthy K, Kanji S, Gordon N, Singh R, Jacob JR, et al. Methionine and Kynurenine Activate Oncogenic Kinases in Glioblastoma, and Methionine Deprivation Compromises Proliferation. Clin Cancer Res. 2016;22:3513–3523. doi: 10.1158/1078-0432.CCR-15-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poirson-Bichat F, Goncalves RA, Miccoli L, Dutrillaux B, Poupon MF. Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res. 2000;6:643–653. [PubMed] [Google Scholar]
- 38.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. The Journal of nutrition. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 39.Zajac A, Poprzecki S, Zebrowska A, Chalimoniuk M, Langfort J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. Journal of strength and conditioning research / National Strength & Conditioning Association. 2010;24:1082–1090. doi: 10.1519/JSC.0b013e3181d321ff. [DOI] [PubMed] [Google Scholar]
- 40.Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. European journal of biochemistry / FEBS. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- 41.Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56:427–438. doi: 10.1016/s0753-3322(02)00273-1. [DOI] [PubMed] [Google Scholar]
- 42.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- 43.Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. International journal of cancer Journal international du cancer. 2010;126:2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- 44.Long Y, Tsai WB, Wangpaichitr M, Tsukamoto T, Savaraj N, Feun LG, et al. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther. 2013;12:2581–2590. doi: 10.1158/1535-7163.MCT-13-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinovich S, Adler L, Yizhak K, Sarver A, Silberman A, Agron S, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527:379–383. doi: 10.1038/nature15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeBerardinis RJ. Serine metabolism: some tumors take the road less traveled. Cell Metab. 2011;14:285–286. doi: 10.1016/j.cmet.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gravel SP, Hulea L, Toban N, Birman E, Blouin MJ, Zakikhani M, et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014;74:7521–7533. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- 51.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature genetics. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 54.Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Experimental cell research. 2002;279:80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- 55.Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci U S A. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visca P, Sebastiani V, Botti C, Diodoro MG, Lasagni RP, Romagnoli F, et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer research. 2004;24:4169–4173. [PubMed] [Google Scholar]
- 57.Kapur P, Rakheja D, Roy LC, Hoang MP. Fatty acid synthase expression in cutaneous melanocytic neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18:1107–1112. doi: 10.1038/modpathol.3800395. [DOI] [PubMed] [Google Scholar]
- 58.Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611–4615. [PubMed] [Google Scholar]
- 59.Pizer ES, Wood FD, Pasternack GR, Kuhajda FP. Fatty acid synthase (FAS): a target for cytotoxic antimetabolites in HL60 promyelocytic leukemia cells. Cancer Res. 1996;56:745–751. [PubMed] [Google Scholar]
- 60.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato S, Smalley S, Sadarangani A, Chen-Lin K, Oliva B, Branes J, et al. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. Journal of cellular and molecular medicine. 2010;14:1180–1193. doi: 10.1111/j.1582-4934.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bocci G, Fioravanti A, Orlandi P, Bernardini N, Collecchi P, Del Tacca M, et al. Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br J Cancer. 2005;93:319–330. doi: 10.1038/sj.bjc.6602720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feleszko W, Jakobisiak M. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 2000;6:1198–1199. [PubMed] [Google Scholar]
- 65.Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fine EJ, Miller A, Quadros EV, Sequeira JM, Feinman RD. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer cell international. 2009;9:14. doi: 10.1186/1475-2867-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. International journal of cancer Journal international du cancer. 2014;135:1711–1720. doi: 10.1002/ijc.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 70.Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21:310–320. doi: 10.1097/00006676-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson RD, Gallagher EJ, Cohen D, Tobin-Hess A, Alikhani N, Novosyadlyy R, et al. Hyperinsulinemia promotes metastasis to the lung in a mouse model of Her2-mediated breast cancer. Endocrine-related cancer. 2013;20:391–401. doi: 10.1530/ERC-12-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Archives of physiology and biochemistry. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 75.Isley WL, Underwood LE, Clemmons DR. Dietary components that regulate serum somatomedin-C concentrations in humans. The Journal of clinical investigation. 1983;71:175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine reviews. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 77.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. The Lancet Oncology. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang CT, Chang MC, Chen YL, Chen TC, Chen CA, Cheng WF. Insulin-like growth factors inhibit dendritic cell-mediated anti-tumor immunity through regulating ERK1/2 phosphorylation and p38 dephosphorylation. Cancer letters. 2015;359:117–126. doi: 10.1016/j.canlet.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2014;32:2059–2066. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King H, Aleksic T, Haluska P, Macaulay VM. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev. 2014;40:1096–1105. doi: 10.1016/j.ctrv.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–8332. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 84.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 86.Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27:599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- 88.Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, et al. 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab. 2015;22:508–515. doi: 10.1016/j.cmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4:116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saha SK, Gordan JD, Kleinstiver BP, Vu P, Najem MS, Yeo JC, et al. Isocitrate Dehydrogenase Mutations Confer Dasatinib Hypersensitivity and SRC Dependence in Intrahepatic Cholangiocarcinoma. Cancer Discov. 2016;6:727–739. doi: 10.1158/2159-8290.CD-15-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norrelund H, Frystyk J, Jorgensen JO, Moller N, Christiansen JS, Orskov H, et al. The effect of growth hormone on the insulin-like growth factor system during fasting. The Journal of clinical endocrinology and metabolism. 2003;88:3292–3298. doi: 10.1210/jc.2002-021983. [DOI] [PubMed] [Google Scholar]
- 95.Cotterill AM, Holly JM, Wass JA. The regulation of insulin-like growth factor binding protein (IGFBP)-1 during prolonged fasting. Clinical endocrinology. 1993;39:357–362. doi: 10.1111/j.1365-2265.1993.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 96.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. West J Med. 1982;137:379–399. [PMC free article] [PubMed] [Google Scholar]
- 98.Felig P, Owen OE, Wahren J, Cahill GF., Jr Amino acid metabolism during prolonged starvation. The Journal of clinical investigation. 1969;48:584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Izyumov DS, Avetisyan AV, Pletjushkina OY, Sakharov DV, Wirtz KW, Chernyak BV, et al. “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochimica et biophysica acta. 2004;1658:141–147. doi: 10.1016/j.bbabio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caffa I, D’Agostino V, Damonte P, Soncini D, Cea M, Monacelli F, et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget. 2015;6:11820–11832. doi: 10.18632/oncotarget.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging. 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. doi: 10.1186/s12885-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhamija R, Eckert S, Wirrell E. Ketogenic diet. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2013;40:158–167. doi: 10.1017/s0317167100013676. [DOI] [PubMed] [Google Scholar]
- 108.Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutrition & metabolism. 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. Journal of the American College of Nutrition. 1995;14:202–208. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 110.Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–1035. doi: 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutrition & metabolism. 2011;8:54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Experimental gerontology. 2013;48:1120–1128. doi: 10.1016/j.exger.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell. 2016;30:136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thivat E, Farges MC, Bacin F, D’Incan M, Mouret-Reynier MA, Cellarier E, et al. Phase II trial of the association of a methionine-free diet with cystemustine therapy in melanoma and glioma. Anticancer research. 2009;29:5235–5240. [PubMed] [Google Scholar]
- 116.Goseki N, Yamazaki S, Shimojyu K, Kando F, Maruyama M, Endo M, et al. Synergistic effect of methionine-depleting total parenteral nutrition with 5-fluorouracil on human gastric cancer: a randomized, prospective clinical trial. Japanese journal of cancer research : Gann. 1995;86:484–489. doi: 10.1111/j.1349-7006.1995.tb03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durando X, Farges MC, Buc E, Abrial C, Petorin-Lesens C, Gillet B, et al. Dietary methionine restriction with FOLFOX regimen as first line therapy of metastatic colorectal cancer: a feasibility study. Oncology. 2010;78:205–209. doi: 10.1159/000313700. [DOI] [PubMed] [Google Scholar]
- 118.Sugimura T, Birnbaum SM, Winitz M, Greenstein JP. Quantitative nutritional studies with water-soluble, chemically defined diets. IX. Further studies on d-glucosaminecontaining diets. Archives of biochemistry and biophysics. 1959;83:521–527. doi: 10.1016/0003-9861(59)90060-8. [DOI] [PubMed] [Google Scholar]
- 119.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016 doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. doi: 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 125.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. American journal of epidemiology. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 127.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. The oncologist. 2013;18:1248–1255. doi: 10.1634/theoncologist.2013-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vu K, Busaidy N, Cabanillas ME, Konopleva M, Faderl S, Thomas DA, et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clinical lymphoma, myeloma & leukemia. 2012;12:355–362. doi: 10.1016/j.clml.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer medicine. 2015;4:161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soliman PT, Broaddus R, Westin SN, Iglesias DA, Burzawa JK, Zhang Q, et al. Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer: A window of opportunity study. Journal of Clinical Oncology. 2014:32. doi: 10.1016/j.ygyno.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast cancer research and treatment. 2014;148:81–90. doi: 10.1007/s10549-014-3141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bartschi D, et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09) European urology. 2014;66:468–474. doi: 10.1016/j.eururo.2013.12.057. [DOI] [PubMed] [Google Scholar]
- 135.Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer. 2016;114:281–289. doi: 10.1038/bjc.2015.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 137.Braghiroli MI, de Celis Ferrari AC, Pfiffer TE, Alex AK, Nebuloni D, Carneiro AS, et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience. 2015;9:563. doi: 10.3332/ecancer.2015.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, et al. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22:1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 139.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 140.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. The Journal of clinical investigation. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 143.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 144.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31:4297–4305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 145.Paez Espinosa EV, Murad JP, Khasawneh FT. Aspirin: pharmacology and clinical applications. Thrombosis. 2012;2012:173124. doi: 10.1155/2012/173124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferrandez A, Prescott S, Burt RW. COX-2 and colorectal cancer. Current pharmaceutical design. 2003;9:2229–2251. doi: 10.2174/1381612033454036. [DOI] [PubMed] [Google Scholar]
- 148.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 149.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism: clinical and experimental. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 150.Glushakova LG, Judge S, Cruz A, Pourang D, Mathews CE, Stacpoole PW. Increased superoxide accumulation in pyruvate dehydrogenase complex deficient fibroblasts. Molecular genetics and metabolism. 2011;104:255–260. doi: 10.1016/j.ymgme.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 152.Voltan R, Rimondi E, Melloni E, Gilli P, Bertolasi V, Casciano F, et al. Metformin combined with sodium dichloroacetate promotes B leukemic cell death by suppressing anti-apoptotic protein Mcl-1. Oncotarget. 2016;7:18965–18977. doi: 10.18632/oncotarget.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra4. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 154.Berendzen K, Theriaque DW, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 155.Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, et al. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:781–785. doi: 10.1097/JTO.0b013e31820a0ea6. [DOI] [PubMed] [Google Scholar]
- 156.Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 157.Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Archiv : an international journal of pathology. 2008;452:139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 158.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. The Journal of biological chemistry. 2006;281:10214–10221. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 159.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 160.Korangath P, Teo WW, Sadik H, Han L, Mori N, Huijts CM, et al. Targeting Glutamine Metabolism in Breast Cancer with Aminooxyacetate. Clin Cancer Res. 2015;21:3263–3273. doi: 10.1158/1078-0432.CCR-14-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guth PS, Risey J, Briner W, Blair P, Reed HT, Bryant G, et al. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. The Annals of otology, rhinology, and laryngology. 1990;99:74–79. doi: 10.1177/000348949009900113. [DOI] [PubMed] [Google Scholar]
- 162.Lieberman BP, Ploessl K, Wang L, Qu W, Zha Z, Wise DR, et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011;52:1947–1955. doi: 10.2967/jnumed.111.093815. [DOI] [PubMed] [Google Scholar]
- 163.Tan Y, Sun X, Xu M, Tan X, Sasson A, Rashidi B, et al. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin Cancer Res. 1999;5:2157–2163. [PubMed] [Google Scholar]
- 164.Yoshioka T, Wada T, Uchida N, Maki H, Yoshida H, Ide N, et al. Anticancer efficacy in vivo and in vitro, synergy with 5-fluorouracil, and safety of recombinant methioninase. Cancer Res. 1998;58:2583–2587. [PubMed] [Google Scholar]
- 165.Hu J, Cheung NK. Methionine depletion with recombinant methioninase: in vitro and in vivo efficacy against neuroblastoma and its synergism with chemotherapeutic drugs. International journal of cancer Journal international du cancer. 2009;124:1700–1706. doi: 10.1002/ijc.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc Natl Acad Sci U S A. 2011;108:1272–1277. doi: 10.1073/pnas.1014739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wheatley DN. Controlling cancer by restricting arginine availability--arginine-catabolizing enzymes as anticancer agents. Anti-cancer drugs. 2004;15:825–833. doi: 10.1097/00001813-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 168.Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010;28:2220–2226. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- 169.Ott PA, Carvajal RD, Pandit-Taskar N, Jungbluth AA, Hoffman EW, Wu BW, et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Investigational new drugs. 2013;31:425–434. doi: 10.1007/s10637-012-9862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8:3223–3233. doi: 10.1158/1535-7163.MCT-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nicholson LJ, Smith PR, Hiller L, Szlosarek PW, Kimberley C, Sehouli J, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. International journal of cancer Journal international du cancer. 2009;125:1454–1463. doi: 10.1002/ijc.24546. [DOI] [PubMed] [Google Scholar]
- 172.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Puig T, Turrado C, Benhamu B, Aguilar H, Relat J, Ortega-Gutierrez S, et al. Novel Inhibitors of Fatty Acid Synthase with Anticancer Activity. Clin Cancer Res. 2009;15:7608–7615. doi: 10.1158/1078-0432.CCR-09-0856. [DOI] [PubMed] [Google Scholar]
- 174.Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cellular and molecular life sciences : CMLS. 2005;62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pisanti S, Picardi P, Ciaglia E, D’Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological research. 2014;88:84–98. doi: 10.1016/j.phrs.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 176.Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2:483–491. [PubMed] [Google Scholar]
- 177.Bjarnadottir O, Romero Q, Bendahl PO, Jirstrom K, Ryden L, Loman N, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast cancer research and treatment. 2013;138:499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 178.Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17:1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 179.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]