Abstract
Background
We performed a pilot evaluation of a new formulation of levonorgestrel butanoate (LB) designed to be a long-acting injectable (6 months) contraceptive to determine pharmacodynamic end-points in normal and obese BMI women.
Study design
Obese (BMI ≥ 30 kg/m2) and normal BMI, otherwise healthy, women received a single intramuscular injection of LB after ovulation was confirmed in a baseline cycle. The primary outcome was return of ovulation in days.
Results
A total of 14 women enrolled and completed the study [normal BMI n= 9, median BMI 22.7 kg/m2 (range 19.4 – 25.8); obese n= 5, median BMI 35.7 kg/m2 (30.1–39.2)]. The first 6 subjects (normal BMI = 4/9, obese BMI = 2/5) received 40 mg of LB, and the remaining 8 received 20 mg. All women except one returned to ovulation prior to 6 months. Return to ovulation occurred earlier in the obese group; 3/5 obese and 0/9 normal BMI subjects returned to ovulation within 90 days (p = 0.03). No serious adverse events were reported during the study.
Conclusion
Return to ovulation was earlier than 6 months in both BMI groups but more so in the obese BMI group.
Keywords: Contraceptive Injection, levonorgestrel butanoate, pharmacokinetics, obesity, body weight, contraception
Introduction
Depomedroxyprogesterone acetate (DMPA) is a progestin-only injectable method of hormonal contraception widely used in the US and throughout the world. Although the method is highly effective and acceptable when used correctly, the relatively short duration of activity (3 months) is widely accepted as a source of method discontinuation and failure.
Levonorgestrel (LNG) was first introduced to the U.S. market in 1973 as a progestin-only pill and a decade later in combination oral contraceptive (OCs). Since then, LNG has been one of the most popular and widely used progestins in the world. Levonorgestrel butanoate (LB) is an esterified analog of LNG. The butanoate ester prolongs the half-life of the drug. LB is formulated as an aqueous suspension that can be injected intramuscularly. The original batches were formulated and studied by the World Health Organization in the 1980s [1] with the goal of developing a method that would last for 2 months. The earlier studies compared three doses of LB (12.5, 25 and 50 mg) in subjects of low to normal BMI. These studies showed a clear dose response and the duration of action based on return to ovulation (12.5 mg = 105 days, 25mg = 231 days, 50mg = 265 days [2,3] with a great deal of individual variability (range) for the 25 mg dose. However, higher doses (50 mg) of the drug lasted for a much longer period (>6 months), leading to the prospect of developing a method that would last for a minimum of 4 months and possibly as long as 6 months. Unfortunately, earlier formulations of injectable LB were found to aggregate over time, resulting in loss of product stability and reproducibility of the clinical batches [2,3]. A new formulation of LB has been developed by CONRAD (Arlington, VA) and the National Institute of Child Health and Human Development (NICHD, Bethesda, MD) that has stabilizing agents to prevent aggregation. Studies with this new formulation indicated that the suspension maintains stable particle sizes over an extended period of time. The objective for developing this new LB preparation is to generate a product with reduced side effects and increased duration of action (>4 months) as compared to DMPA.
As an initial step in the development of this contraceptive method, we conducted this pilot study to characterize the new formulation and its duration of action. Based upon the results with earlier formulations of LB, a dose of 20 mg was expected to result in a stable PK profile and to yield a contraceptive effect for at least 16 weeks. Since the study product is progestin-only, ovulation suppression may not be the only mechanism of protection as cervical mucus may play a role [4,5] but for this pilot, we focused on duration of action as documented by return to ovulation. Since development of another long-acting estrogen-free hormonal contraceptive would particularly benefit obese women, as they have a higher risk of thromboembolic events [6], women with normal and obese BMI were included in the study.
Materials and Methods
We conducted a prospective open-label study at Oregon Health & Science University (OHSU) in Portland, Oregon from September 2011 to June 2012 The protocol was jointly developed by NICHD and CONRAD. The OHSU Institutional Review Board and OHSU Clinical & Translational Research Institute (OCTRI) approved the study protocol and all subjects underwent informed written consent.
The formulation, developed by CONRAD and NICHD, had been micronized to produce a particle size distribution consistent with the original WHO preparation (produced at Palmer Laboratories, UK). The final formulation consisted of a sterile suspension of the micronized LB (20 mg/mL) in an aqueous vehicle consisting of super refined polysorbate 80 (Tween® 80), spray dried sorbitan monopalmitate (Span® 40), sodium carboxymethylcellulose, sodium phosphate dibasic anhydrous, sodium phosphate monobasic dihydrate, and benzyl alcohol stored and dispensed in multi-use vials containing 2mL of volume or 40 mg of LB.
Otherwise healthy, obese (BMI ≥30 kg/m2) and normal BMI (<30 kg/m2) reproductive-aged (18–44 years old) women, were recruited. Subjects were required to be either heterosexually abstinent or, if heterosexually active, to use a non-hormonal, non-IUD method of contraception. Inclusion criteria included regular menstrual cycles, BMI ≤ 40 kg/m2, no recent use of hormonal contraception, no contraindications to hormonal contraception, no use of drugs known to interfere with the metabolism of sex steroids, and smoking <15 cigarettes/day.
A baseline observation cycle was required to confirm ovulatory status (a serum progesterone (P) of ≥ 3 ng/mL) and normal cervical mucus (modified Insler score of ≥7 at midcycle). After qualifying values were confirmed, subjects received a single intramuscular injection of LB during the first 5 days of the subsequent menses. Due to a dosing error, the first 6 subjects enrolled (normal BMI = 4, obese BMI = 2) received 40 mg of LB in a total injection volume of 2 mL. The remaining 8 subjects (normal BMI = 5, obese BMI = 3) received a dose of 20 mg in an injection volume of 1 mL. Compliance with drug treatment was not an issue as all women received the single intramuscular injection directly by a study staff member in the research clinic.
Outpatient visits were performed weekly until a dominant follicle (>12mm) was noted on transvaginal ultrasound (TVUS; GE LOGIQ 400 Proseries ultrasound, 7.5-MHZ; Fairfield, CT). At this point, twice weekly visits were performed until evidence of ovulation or resolution of the follicle. Ovulation was defined as an elevation of P > 3ng/mL during two consecutive samples within 10 days of the presence of a follicle >12mm. If a follicle resolved without evidence of ovulation, weekly visits resumed. If ovulation occurred, twice weekly visits were continued until the onset of menses. At this point, an exit visit was scheduled. The subject was also contacted by telephone 4– 6 weeks after the exit visit to record details of the next subsequent normal menses. At each treatment visit, serum was obtained to measure FSH, LH, estradiol (E2), and P. Sex hormone binding globulin (SHBG) levels were also assessed at the beginning and end of treatment.
Assay characteristics
Serum E2, P, LH, FSH and SHBG were analyzed by a Roche Cobas e411 chemiluminescence-based automatic clinical platform (Roche Diagnostics, Indianapolis, IN). The sensitivity of the E2, P, LH, FSH and SHBG assay for the Roche Cobas e411 is 5 pg/ml, 47 pg/ml, 0.1 mIU/ml, 0.1 mIU/ml, and 0.033 ug/ml, respectively. The intra- and inter-assay variation with the Roche Cobab e411 is consistently less than 10% for all assays. Quality control samples and validations were repeated prior to each assay run.
Statistical analysis
The primary outcome was time to ovulation in days as determined by growth of a dominant follicle > 12 mm on ultrasound observation accompanied by rising serum E2 followed by elevation of P ≥ 3 ng/mL. The planned pilot study enrollment was 15 subjects, including approximately 40% with a BMI ≥ 30kg/m2. All subjects who received any amount of study drug and provided any relevant data are included in the analyses.
Data analyses were performed using SAS version 9.2 (SAS inc, NC). Due to the small sample size, nonparametric method and descriptive analysis were used. Time from injection to ovulation in normal BMI and obese women were illustrated using Kaplan-Meier survival curve.
Results
Fourteen subjects signed informed consent (normal BMI n = 9; obese BMI, n= 5) and met eligibility criteria. With the exception of BMI (medians: normal 22.7 kg/m2, obese 35.7) and SHBG (medians: normal 6.6 ug/mL, obese 2.8 ug/mL), there were no notable differences in the baseline characteristics between the study groups (Table 1). There were no serious adverse events or pregnancies during the study period; the most common adverse events were pain at the injection site (20 mg group n = 2, 40 mg group n = 1; all reported within the first day of use) and headache (20 mg group n= 3, 40 mg group n=1; all reported after the first week of use or greater).
Table 1.
Demographics and baseline serum levels of gonadotropins and ovarian hormones and sex hormone binding globulin (SHBG)
| BMI < 30kg/m2 (n=9) | BMI ≥30kg/m2 (n=5) | |
|---|---|---|
| Age | 33 (24.0, 35.0) | 34 (27.0, 40.0) |
| BMI | 22.7 (21.5, 23.8) | 35.7 (34.3, 38.3) |
|
| ||
| LNG dose | ||
|
| ||
| LB 20 mg | 5 (56%) | 3 (60%) |
| LB 40 mg | 4 (44%) | 2 (40%) |
|
| ||
| Baseline serum levels | ||
|
| ||
| Estradiol-17b (pg/mL) | 38 (31, 65) | 47 (38, 49) |
| Progesterone (pg/mL) | 0.5 (0.3, 0.8) | 0.5 (0.5, 0.6) |
| LH (mIU/mL) | 4.3 (3.4, 5.5) | 4.5 (4.2, 5.8) |
| FSH (mIU/mL) | 6.3 (5.2, 6.6) | 5.7 (5.5, 6.8) |
| SHBG (ug/mL) | 6.6 (5.2, 7.4) | 2.8 (2.5, 2.9) |
Median and interquartile range for continuous variables, frequency and percentage for categorical variable
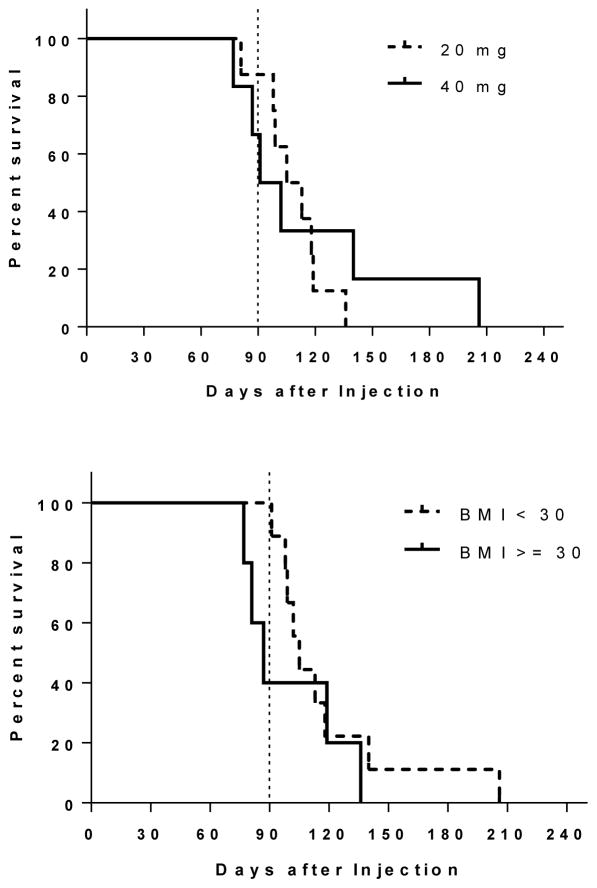
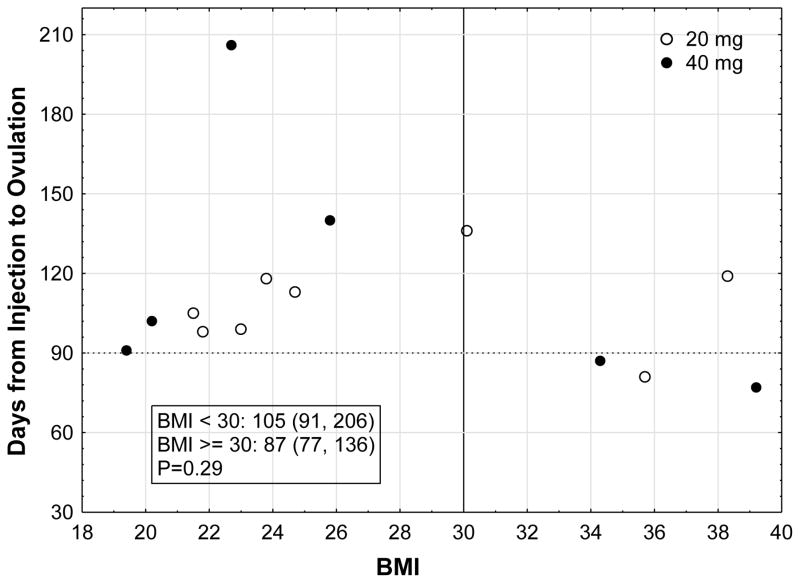
Both BMI groups experienced return to ovulation earlier than anticipated (Figure 1). All women except one returned to ovulation prior to 6 months. Figure 2 represents a scatter plot of all participants and their return to ovulation in days according to their BMI and LB dose. The earliest day of ovulation was day 77 in two women of obese BMI (39 and 36 mg/kg2) who received a 40mg and 20mg dose, respectively. The subject with the longest delay in return to ovulation at day 206 was a normal BMI woman who received a 40 mg dose. Mean LNG level at the time of ovulation for both BMI groups was at the lower limit of assay detection (~47 pg/mL) (see supplementary materials for LNG assay details). Although no subject in the normal BMI group had an ovulation prior to 88 days, 60% (3/5; Fisher p = 0.03) of the obese group did with two on day 77 and one on day 84. Two of these obese women received the 40 mg dose, and one received 20 mg.
Figure 1.
Kaplan-meier estimated ovulation-free survival curve (time to first ovulation following treatment; top graph 20mg vs. 40 mg LB doses, bottom graph BMI<30kg/m2 vs. BMI≥30kg/m2.
Figure 2.
Days to ovulation for each participant according to BMI and LB dose [text box includes median (range) of days to ovulation for each BMI group].
We did evaluate the pharmacokinetics of the LB preparation, but our small sample size did not permit proper statistical evaluation. These data are presented descriptively in the Supplemental Material available on line.
Discussion
A single intramuscular injection of LB did suppress ovulation in both obese and normal BMI women which likely translates to a high level of protection against pregnancy. Unfortunately, the duration of activity was shorter than anticipated in view of data from previous WHO studies with an earlier preparation [1–3]. Based on the earlier studies with LB (12.5, 25 and 50 mg), we anticipated a return to ovulation between 120–180 days. It is unclear why the duration of action of this new formulation was shorter than anticipated. Notably, and in contrast to the WHO studies, we did not see a dose response of the 40 versus 20 mg dose on duration of activity (97 versus 109 days respectively).
In developing this new formulation of LB, micronization was performed to produce a particle size distribution as close as possible to the original specifications reported for the batches used in the WHO studies. It is possible that differences in particle size distribution might have led to a difference in the observed duration of activity. Although the earlier studies looked promising for the potential of the drug to last for 4 months or longer, the original batches of LB experienced problems with aggregation that made the formulation unsuitable for clinical studies. This tendency toward aggregation may have resulted in a change to the particle size distribution at the time of injection compared with the size profile observed during manufacture. Our new formulation contained stabilizing components that inhibited this tendency toward particle aggregation. However, the current formulation did not achieve the goal of >16 weeks of activity and thus, did not reproduce the earlier WHO study results and did not provide an advantage in regard to duration of effect over the current commercially available DMPA injection. Enrollment was discontinued after 14 women participated because return to ovulation by <90 days in some individuals was clearly far short of the target goal of 4 months or longer. Although a shorter acting injectable may be desirable to women if it has a superior side effect profile and faster return to fertility than DMPA, this has not been identified as a priority area for product development.
We did experience a dosing error in this study; six subjects received double the planned dose. Although this represented a protocol violation, previous studies with earlier formulations of LB in humans conducted by WHO used doses of up to 50mg with no adverse effects. No serious adverse events or increase in side effects resulted from the higher dose. The data obtained serendipitously provided information on the PK and PD of the 40 mg dose which was to be studied at a future date.
Although the concept remains promising, the LB injection for female contraception needs to be reformulated if a longer duration of action is desired. Our results clarify and strengthen the conclusion that the pharmacodynamics (PD) of LNG is adversely affected by obesity [7–9]. These initial studies will aid in this work and our understanding of providing a contraceptive method that functions well in a real-world setting in which women are of varying sizes.
Supplementary Material
Implications.
Since return of ovulation was earlier than expected for this levonorgestrel butanoate injectable formulation, additional steps are needed to develop a preparation suitable as a longer lasting product.
Acknowledgments
Financial Support: Support for this research was from NICHD Contraception Clinical Trial Network HHSN275200403378I. The authors also acknowledge grant support from National Institutes of Health the OHSU Oregon Clinical & Translational Research Institute (NIH NCRR 1 UL1 RR024120), and the Endocrine Technologies Support Core at the Oregon National Primate Research Center (NIH P51 OD011092).
The authors would like to thank the Women’s Health Research Unit, the Department of Obstetrics & Gynecology, and the Oregon Clinical & Translational Research Institute at Oregon Health & Science University
Footnotes
Authors Disclosures: Dr. Edelman: consultant for World Health Organization, CDC, Gynuity Health Projects, Genzyme, and Agile Therapuetics. Nexplanon trainer for Merck. Author for UptoDate (Royalties received). Dr. Jensen has received payments for consulting from Bayer Healthcare, Merck, Agile Therapuetics, HRA Pharma, Teva, and the Population Council, and for giving talks for Bayer and Merck. His institution has also received research funding from Abbvie Pharmaceuticals, Bayer, the Population Council, the National Institute of Health, and the Bill & Melinda Gates Foundation. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crabbe P, Archer S, Benagiano G, et al. Long-acting contraceptive agents: design of the WHO Chemical Synthesis Programme. Steroids. 1983;41:243–53. doi: 10.1016/0039-128x(83)90095-8. [DOI] [PubMed] [Google Scholar]
- 2.Benagiano G, d’Arcangues C, Harris Requejo J, et al. The Special Programme of Research in Human Reproduction: Forty Years of Activities to Achieve Reproductive Health for All. Gynecol Obstet Invest. 2012;74:190–217. doi: 10.1159/000343067. [DOI] [PubMed] [Google Scholar]
- 3.Garza-Flores J, Hall PE, Perez-Palacios G. Long-Acting Hormonal Contraceptives for Women. J Steroid Biochem Molec Biol. 1991;40:697–704. doi: 10.1016/0960-0760(91)90293-e. [DOI] [PubMed] [Google Scholar]
- 4.Petta CA, Faundes A, Dunson TR, et al. Timing of onset of contraceptive effectiveness in Depo-Provera users. II. Effects on ovarian function. Fertil Steril. 1998;70:817–20. doi: 10.1016/s0015-0282(98)00309-4. [DOI] [PubMed] [Google Scholar]
- 5.Dunson TR, Blumenthal PD, Alvarez F, et al. Timing of onset of contraceptive effectiveness in Norplant implant users. Part I. Changes in cervical mucus. Fertil Steril. 1998;69:258–66. doi: 10.1016/s0015-0282(97)00476-7. [DOI] [PubMed] [Google Scholar]
- 6.Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139:289–96. doi: 10.1111/j.1365-2141.2007.06780.x. [DOI] [PubMed] [Google Scholar]
- 7.Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman AB, Cherala G, Munar MY, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception. 2012 doi: 10.1016/j.contraception.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314–23. doi: 10.1016/j.contraception.2010.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.