Abstract
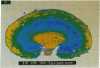
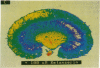
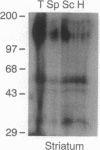
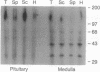
Dopamine (DA) produces a natriuretic/diuretic response in the kidney by mechanisms that are still not well understood. There is some indication that DA2 receptors may be involved in mediating the effects of DA, but little is known regarding the nature of this receptor in the kidney. Autoradiographic localization of [3H]spiperone, a DA2 antagonist, indicated that high-density binding was restricted to inner medullary collecting ducts (IMCDs). [3H]Spiperone binding was saturable, high affinity (Kd, 17.2 +/- 1.65 nM), and high density (Bmax, 935 +/- 83 fmol per mg of protein). The photosensitive spiperone analogue N-(p-azido-m-[125I]iodophenethyl)spiperone labeled similar sized proteins of Mr = 120,000 in membranes prepared from the kidney inner medulla, striatum, and pituitary. However, the rank-order competition profile for the [3H]spiperone binding in the kidney inner medulla differed from the DA2 receptor in striatum and pituitary and, furthermore, RNA (Northern) blot analyses of kidney inner medullary RNA with brain DA2 receptor oligonucleotide probes were negative. Functionally, DA stimulated prostaglandin E2 production by IMCD cells, an effect that could be blocked by the DA2 antagonist domperidone. These results indicate that the kidney inner medulla expresses a functional DA receptor that may represent a newly identified DA receptor subtype (here designated DA2K). Moreover, these results suggest that the kidney inner medulla may be a significant site at which DA, either directly or indirectly, influences water and electrolyte excretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amlaiky N., Caron M. G. Identification of the D2-dopamine receptor binding subunit in several mammalian tissues and species by photoaffinity labeling. J Neurochem. 1986 Jul;47(1):196–204. doi: 10.1111/j.1471-4159.1986.tb02850.x. [DOI] [PubMed] [Google Scholar]
- Andersen P. H., Gingrich J. A., Bates M. D., Dearry A., Falardeau P., Senogles S. E., Caron M. G. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990 Jun;11(6):231–236. doi: 10.1016/0165-6147(90)90249-8. [DOI] [PubMed] [Google Scholar]
- Baines A. D., Chan W. Production of urine free dopamine from DOPA; a micropuncture study. Life Sci. 1980 Jan 28;26(4):253–259. doi: 10.1016/0024-3205(80)90334-3. [DOI] [PubMed] [Google Scholar]
- Ball S. G., Lee M. R. The effect of carbidopa administration on urinary sodium excretion in man. Is dopamine an intrarenal natriuretic hormone? Br J Clin Pharmacol. 1977 Apr;4(2):115–119. doi: 10.1111/j.1365-2125.1977.tb00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chan Y. L. Cellular mechanisms of renal tubular transport of I-dopa and its derivatives in the rat: microperfusion studies. J Pharmacol Exp Ther. 1976 Oct;199(1):17–24. [PubMed] [Google Scholar]
- Chio C. L., Hess G. F., Graham R. S., Huff R. M. A second molecular form of D2 dopamine receptor in rat and bovine caudate nucleus. Nature. 1990 Jan 18;343(6255):266–269. doi: 10.1038/343266a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Felder C. C., McKelvey A. M., Gitler M. S., Eisner G. M., Jose P. A. Dopamine receptor subtypes in renal brush border and basolateral membranes. Kidney Int. 1989 Aug;36(2):183–193. doi: 10.1038/ki.1989.178. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Felder C. C., Eisner G. M., Jose P. A. The dopamine receptor in adult and maturing kidney. Am J Physiol. 1989 Sep;257(3 Pt 2):F315–F327. doi: 10.1152/ajprenal.1989.257.3.F315. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Nakamura K. T., Robillard J. E., Kanadjian M., Jose P. A. Dopamine receptors in the developing sheep kidney. Pediatr Nephrol. 1988 Jan;2(1):156–162. doi: 10.1007/BF00870397. [DOI] [PubMed] [Google Scholar]
- Frederickson E. D., Bradley T., Goldberg L. I. Blockade of renal effects of dopamine in the dog by the DA1 antagonist SCH 23390. Am J Physiol. 1985 Aug;249(2 Pt 2):F236–F240. doi: 10.1152/ajprenal.1985.249.2.F236. [DOI] [PubMed] [Google Scholar]
- Galbusera M., Garattini S., Remuzzi G., Mennini T. Catecholamine receptor binding in rat kidney: effect of aging. Kidney Int. 1988 Jun;33(6):1073–1077. doi: 10.1038/ki.1988.113. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972 Mar;24(1):1–29. [PubMed] [Google Scholar]
- Goldberg L. I., Kohli J. D. Peripheral pre- and post-synaptic dopamine receptors: are they different from dopamine receptors in the central nervous system? Commun Psychopharmacol. 1979;3(6):447–456. [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge S. S., Ricci A., Amenta F., Lokhandwala M. F. Evidence from functional and autoradiographic studies for the presence of tubular dopamine-1 receptors and their involvement in the renal effects of fenoldopam. J Pharmacol Exp Ther. 1989 Dec;251(3):1237–1245. [PubMed] [Google Scholar]
- Hegde S. S., Jadhav A. L., Lokhandwala M. F. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension. 1989 Jun;13(6 Pt 2):828–834. doi: 10.1161/01.hyp.13.6.828. [DOI] [PubMed] [Google Scholar]
- Hietala J., Syvälahti E., Röyttä M. Comparison of neuroleptic binding characteristics in rat striatum and renal cortex. J Recept Res. 1988;8(6):753–771. doi: 10.3109/10799898809049024. [DOI] [PubMed] [Google Scholar]
- Huo T., Healy D. P. Autoradiographic localization of dopamine DA1 receptors in rat kidney with [3H]Sch 23390. Am J Physiol. 1989 Sep;257(3 Pt 2):F414–F423. doi: 10.1152/ajprenal.1989.257.3.F414. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Lee M. R. Dopamine and the kidney. Clin Sci (Lond) 1982 May;62(5):439–448. doi: 10.1042/cs0620439. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Tollenaere J. P., Laduron P. M. Serotonergic component of neuroleptic receptors. Nature. 1978 Mar 9;272(5649):168–171. doi: 10.1038/272168a0. [DOI] [PubMed] [Google Scholar]
- Lokhandwala M. F., Barrett R. J. Cardiovascular dopamine receptors: physiological, pharmacological and therapeutic implications. J Auton Pharmacol. 1982 Sep;2(3):189–215. doi: 10.1111/j.1474-8673.1982.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Lorenz W., Lomasney J. W., Collins S., Regan J. W., Caron M. G., Lefkowitz R. J. Expression of three alpha 2-adrenergic receptor subtypes in rat tissues: implications for alpha 2 receptor classification. Mol Pharmacol. 1990 Nov;38(5):599–603. [PubMed] [Google Scholar]
- Manoogian C., Nadler J., Ehrlich L., Horton R. The renal vasodilating effect of dopamine is mediated by calcium flux and prostacyclin release in man. J Clin Endocrinol Metab. 1988 Apr;66(4):678–683. doi: 10.1210/jcem-66-4-678. [DOI] [PubMed] [Google Scholar]
- McGrath B., Bode K., Luxford A., Howden B., Jablonski P. Effects of dopamine on renal function in the rat isolated perfused kidney. Clin Exp Pharmacol Physiol. 1985 Jul-Aug;12(4):343–352. doi: 10.1111/j.1440-1681.1985.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Muto S., Tabei K., Asano Y., Imai M. Dopaminergic inhibition of the action of vasopressin on the cortical collecting tubule. Eur J Pharmacol. 1985 Aug 27;114(3):393–397. doi: 10.1016/0014-2999(85)90386-3. [DOI] [PubMed] [Google Scholar]
- Sato M., Dunn M. J. Interactions of vasopressin, prostaglandins, and cAMP in rat renal papillary collecting tubule cells in culture. Am J Physiol. 1984 Sep;247(3 Pt 2):F423–F433. doi: 10.1152/ajprenal.1984.247.3.F423. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Senogles S. E., Amlaiky N., Falardeau P., Caron M. G. Purification and characterization of the D2-dopamine receptor from bovine anterior pituitary. J Biol Chem. 1988 Dec 15;263(35):18996–19002. [PubMed] [Google Scholar]
- Seri I., Aperia A. Contribution of dopamine 2 receptors to dopamine-induced increase in glomerular filtration rate. Am J Physiol. 1988 Feb;254(2 Pt 2):F196–F201. doi: 10.1152/ajprenal.1988.254.2.F196. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stier C. T., Jr, Cowden E. A., Allison M. E. Effects of bromocriptine on single nephron and whole-kidney function in rats. J Pharmacol Exp Ther. 1982 Feb;220(2):366–370. [PubMed] [Google Scholar]
- Wassermann K., Huss R., Kullmann R. Dopamine-induced diuresis in the cat without changes in renal hemodynamics. Naunyn Schmiedebergs Arch Pharmacol. 1980 May;312(1):77–83. doi: 10.1007/BF00502578. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Takashina R., Takahasi H., Ijichi H. Role of renal nerves and dopamine on prostaglandin E release from the kidney of rats. Agents Actions Suppl. 1987;22:93–100. doi: 10.1007/978-3-0348-9299-5_10. [DOI] [PubMed] [Google Scholar]
- Zimlichman R., Levinson P. D., Kelly G., Stull R., Keiser H. R., Goldstein D. S. Derivation of urinary dopamine from plasma dopa. Clin Sci (Lond) 1988 Nov;75(5):515–520. doi: 10.1042/cs0750515. [DOI] [PubMed] [Google Scholar]