Abstract
Nonmedical benzodiazepine use is common among adults with opioid use disorder; however, little is known about this co-occurrence. Anxiety sensitivity--the fear of anxiety symptoms and sensations--motivates behaviors to escape and avoid distressing states, and accordingly is associated with coping motives for substance use. This might be particularly relevant among women, who report using substances to cope with negative emotions more often than men. The aim of the current study was to examine whether nonmedical benzodiazepine use was associated with higher anxiety sensitivity among treatment-seeking adults diagnosed with opioid use disorder, and to investigate whether gender moderated this association. A sample of adults (ranging in age from 18–81 years) receiving inpatient treatment for opioid use disorder (N=257) completed measures of anxiety, anxiety sensitivity, and benzodiazepine use frequency. Results of an analysis of variance indicated that frequency of past-month nonmedical benzodiazepine use was associated with significantly higher anxiety sensitivity. This effect remained when controlling for the effect of anxiety symptoms (F[1, 251] = 3.91, p = .049, ηp2=.02). Gender moderated this association, and post-hoc analyses found a strong association between nonmedical benzodiazepine use and anxiety sensitivity in women, and not men. Anxiety sensitivity, which can be reduced with treatment, might be a candidate therapeutic target in this population, particularly in women.
Keywords: opioid use disorder, benzodiazepines, anxiety sensitivity, gender differences
1. Introduction
Nonmedical benzodiazepine use is common among those with opioid use disorder (K. W. Chen et al., 2011; Substance Abuse and Mental Health Services Administration, 2016), and is associated with worse outcomes in opioid use disorder treatment (Peles, Schreiber, & Adelson, 2010). The use of benzodiazepines among those with opioid use disorder is particularly concerning because of the risk for overdose when opioids and benzodiazepines are combined (Dasgupta et al., 2015; Park, Saitz, Ganoczy, Ilgen, & Bohnert, 2015). Benzodiazepines were involved in more than 30% of opioid overdoses in the United States in 2011, reflecting a more than doubling of the proportion of opioid overdoses involving benzodiazepines during the previous decade (L. H. Chen, Hedegaard, & Warner, 2014). Thus, reducing nonmedical benzodiazepine use among patients with opioid use disorder is an urgent clinical need. For the purpose of this paper, we define nonmedical benzodiazepine use as: use without a prescription, or at a frequency or quantity higher than prescribed (Compton & Volkow, 2006).
Anxiety is associated with nonmedical benzodiazepine use among those with opioid use disorder, consistent with the anxiolytic effects of benzodiazepines (K. W. Chen et al., 2011; Lavie, Fatseas, Denis, & Auriacombe, 2009). The desire to relieve anxiety is among the most commonly reported reasons for initiating and maintaining nonmedical benzodiazepine use among those with opioid use disorder (Fatseas, Lavie, Denis, & Auriacombe, 2009; Vogel et al., 2013). Those with a high sensitivity to anxiety might be particularly vulnerability to nonmedical benzodiazepine use. Anxiety sensitivity, a risk factor for anxiety disorder development (Calkins et al., 2009; Schmidt et al., 2010), is defined as the fear of anxiety symptoms and sensations (Peterson & Reiss, 1992; Reiss, Peterson, Gursky, & McNally, 1986). Anxiety sensitivity is hypothesized to amplify anxiety symptoms and thus to motivate attempts to avoid or escape these sensations. Individuals with higher anxiety sensitivity report using substances to cope with negative emotional states more frequently than those with low anxiety sensitivity (Bonn-Miller, Zvolensky, & Bernstein, 2007; Johnson, Mullin, Marshall, Bonn-Miller, & Zvolensky, 2010; Novak, Burgess, Clark, Zvolensky, & Brown, 2003). Moreover, anxiety sensitivity might also motivate reduction of other distressing states, such as pain (Ocanez, McHugh, & Otto, 2010), and withdrawal symptoms (Langdon et al., 2013; Zvolensky, Farris, Guillot, & Leventhal, 2014). Although anxiety itself fluctuates, anxiety sensitivity is a trait-like characteristic that reflects a tendency to respond fearfully to anxiety symptoms and sensations. Importantly, anxiety sensitivity is a key therapeutic target in the treatment of anxiety disorders, and has been shown to mediate anxiety symptom change (Smits, Powers, Cho, & Telch, 2004).
One previously published study has evaluated the association between anxiety sensitivity and nonmedical benzodiazepine use. Sixty-eight participants receiving methadone maintenance therapy for opioid dependence found that those with higher anxiety sensitivity were more likely to report lifetime nonmedical benzodiazepine use (Hearon et al., 2011). This effect was moderated by gender, with a stronger link between anxiety sensitivity and nonmedical benzodiazepine use in women relative to men (Hearon et al., 2011). However, this previous study did not control for the effect of anxiety, which is also elevated among those who use benzodiazepines nonmedically, and is correlated with anxiety sensitivity. Controlling for anxiety is important to rule out the confound of current fluctuations in anxiety (e.g., elevation related to drug withdrawal).
The aim of the present study was to evaluate the association between nonmedical benzodiazepine use and anxiety sensitivity among adults with opioid use disorder. Specifically, we hypothesized that (1) nonmedical benzodiazepine use in the past month would be associated with higher anxiety sensitivity, and (2) gender would moderate this relationship, such that the association between nonmedical benzodiazepine use and anxiety sensitivity would be stronger in women compared to men.
2. Methods
2.1 Participants
Adults seeking treatment for opioid use disorder (N=257) were recruited from the inpatient detoxification unit of a private, academically-affiliated psychiatric hospital as part of a larger study of individuals in treatment for substance use disorders. Inclusion criteria required that participants were at least 18 years old, met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (American Psychiatric Association, 1994) criteria for opioid dependence, and were receiving treatment for a substance use disorder. Only those with an acute psychiatric or medical condition that would interfere with the ability to complete study procedures were excluded from participation. Otherwise, those with co-occurring substance use disorders, as well as other non-acute psychiatric disorders, were eligible to participate.
In the parent study, 68% of participants who were offered study participation agreed and were enrolled. Of the remaining people approached, 15% declined participation; the primary reasons for refusal were: not interested, pending discharge from the inpatient unit, concerns about privacy/confidentiality, and feeling ill. The remaining individuals were unable to be enrolled because of delays (e.g., participant asked the staff to come back later, needed to meet with clinical staff, had a visitor, etc.).
A total of 266 of the 702 (37.9%) individuals enrolled in the parent study were diagnosed with a primary opioid use disorder. Of these, 9 participants did not fully complete the anxiety sensitivity measure, or did not identify as either male or female; therefore, 257 individuals were included in the present analysis.
2.2 Procedure
Study procedures were reviewed and approved by the local Institutional Review Board. Following a presentation by a member of the research staff, interested patients were given a complete study description and provided written, informed consent. Participants completed a battery of self-report measures during one session lasting approximately 30 minutes. The average length of stay on the treatment unit is approximately 4 days, and participants generally completed the study on day 2 or 3 of their stay (i.e., not on the day of admission or discharge).
2.3 Measures
Participants self-reported demographic information, including age, gender, race, and employment status. Psychiatric and substance use disorder diagnoses were extracted from patients’ medical records.
The Overall Anxiety Severity and Impairment Scale (OASIS; Norman, Cissell, Means-Christensen, & Stein, 2006) was used to assess anxiety symptoms. The OASIS is a brief (5-item) measure of anxiety that includes questions about frequency, severity, and interference of anxiety symptoms in the previous week. Responses for each item range from 0 to 4, with a higher score indicating more anxiety symptoms and greater severity of these symptoms (Campbell-Sills et al., 2009).
Nonmedical benzodiazepine use in the month prior to hospitalization was assessed using the Brief Addiction Monitor (BAM; Cacciola et al., 2013), a 17-item self-report measure of substance use severity. Participants selected ranges for the number of days of substance use in the previous 30 days (e.g., 0, 1–3, 4–8, 9–15, or 16–30 days). The BAM has demonstrated good test-retest reliability, sensitivity to change, and predictive validity (Cacciola et al., 2013). For the purpose of this study, the BAM item assessing use of “sedatives/tranquilizers” was split into two separate items, one assessing use of “benzodiazepines,” and the other assessing use of “other sedatives/tranquilizers.” Instructions specified that benzodiazepine use referred to use at a dose or frequency higher than prescribed, or without a prescription (i.e., nonmedical use).
The Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007) is a self-report questionnaire used to measure fear of arousal-related sensations. Participants rate their agreement with 18 statements from 0, “very little,” to 4, “very much,” for a possible range of total scores from 0–72, with higher scores indicating greater anxiety sensitivity. This measure has shown excellent internal consistency across a variety of populations (Taylor et al., 2007). The ASI-3 has demonstrated small to moderate correlations with anxiety symptoms, suggesting that anxiety sensitivity is similar to, yet distinct from, anxiety symptoms (Wheaton, Deacon, McGrath, Berman, & Abramowitz, 2012).
2.4. Data Analysis
All variables were evaluated for skewness to determine appropriate statistical tests. We then conducted χ2 and t-tests to examine differences in sociodemographic and clinical characteristics between those with and without past-month nonmedical benzodiazepine use. Clinical variables of interest included the following markers of psychiatric and substance use severity: diagnosis of an anxiety disorder, other psychiatric disorder, or co-occurring substance use disorder (not including benzodiazepines or opioids), and presence of heroin use. Variables with significant group differences were included as covariates in the main analyses. Age, gender, and anxiety symptoms (OASIS total score) were planned covariates.
In an unadjusted analysis, we examined the association between past-month nonmedical benzodiazepine use frequency (i.e., days of use) and anxiety sensitivity using one-way analysis of variance (ANOVA). To address the main study aims, we conducted an analysis of covariance (ANCOVA) with Anxiety Sensitivity Index-3 total score as the dependent variable, controlling for covariates. This model included the gender by nonmedical benzodiazepine use frequency interaction term to test whether gender moderated the association between use and anxiety sensitivity. All analyses were conducted in SPSS Version 20.
3. Results
Sociodemographic and clinical characteristics of the sample are displayed in Table 1. More than half of the sample (55.3%) reported nonmedical use of benzodiazepines in the 30 days prior to admission, consistent with the literature suggesting very high rates (i.e., 50% and higher) of benzodiazepine use among those in treatment for opioid use disorder (see Jones, Mogali, & Comer, 2012). There were no significant differences between those with and without past-month nonmedical benzodiazepine use on age, gender, race, or employment status, or any clinical variables, with the exception of anxiety symptoms (OASIS total score).
Table 1.
Sample Characteristics by Past-Month Benzodiazepine Use
| Variable | Total Sample (N=257) | No Use (n=115) | Any Use (n=142) | χ2/t | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 28.4 (10) | 29.7 (11.5) | 27.4 (8.4) | 1.84 | 0.07 |
| Caucasian, % | 94.9% | 92.2% | 94.4% | 0.16 | 0.69 |
| Female, % | 27.2% | 28.7% | 26.1% | 0.22 | 0.64 |
| Employed, % | 39.8% | 36.6% | 42.3% | 0.83 | 0.36 |
| Substance use and clinical characteristics | |||||
| Any of past-month heroin use, % | 76.7% | 72.2% | 80.3% | 2.33 | 0.13 |
| * Other co-occurring substance use disorder, % | 46.3% | 37.4% | 57.0% | 1.74 | 0.19 |
| Co-occurring psychiatric disorder, % | 59.9% | 54.8% | 64.1% | 2.29 | 0.13 |
| ** Co-occurring anxiety disorder, % | 23.7% | 23.5% | 23.9% | 0.01 | 0.93 |
| OASIS total score, mean (SD) | 11.4 (4.6) | 10.5 (4.9) | 12.1 (4.3) | −2.73 | 0.01 |
Note: OASIS = Overall Anxiety Severity and Impairment Scale.
Other co-occurring substance use disorder does not include benzodiazepine use disorder.
Anxiety disorder diagnosis is based on DSM-IV criteria and therefore includes posttraumatic stress disorder.
The mean ASI-3 score of the sample was 23.6 (SD = 15.8), indicating a high level of anxiety sensitivity (Allan et al., 2014). There were no differences between men and women in age (t[255] = −0.81, p = .42), anxiety sensitivity (t[255] = 1.40, p = .16), or prevalence of past-month nonmedical benzodiazepine use (χ2[1] = .22, p = .64).
Results of the ANOVA indicated that higher frequency of nonmedical benzodiazepine use was associated with higher anxiety sensitivity (F(1, 255) = 7.51, p = .007, ηp2=.03). Results of the ANCOVA model found that nonmedical benzodiazepine use was significantly associated with anxiety sensitivity (F[1, 251] = 3.91, p = .049, ηp2=.02), when controlling for the effects of age, gender, anxiety symptoms, and the interaction of gender and nonmedical benzodiazepine use. The interaction between gender and nonmedical benzodiazepine use was also significant (F[1, 251] = 9.37, p = .002, ηp2=.04). There were also main effects of gender (F[1, 251] = 8.55 p = .004, ηp2=.03) and anxiety symptoms (F[1, 251] = 10.45, p <.001, ηp2=.30). The overall model predicted 34% of the variance in ASI-3 total score (F[5, 251] = 26.36, p < .001, ηp2 = .34).
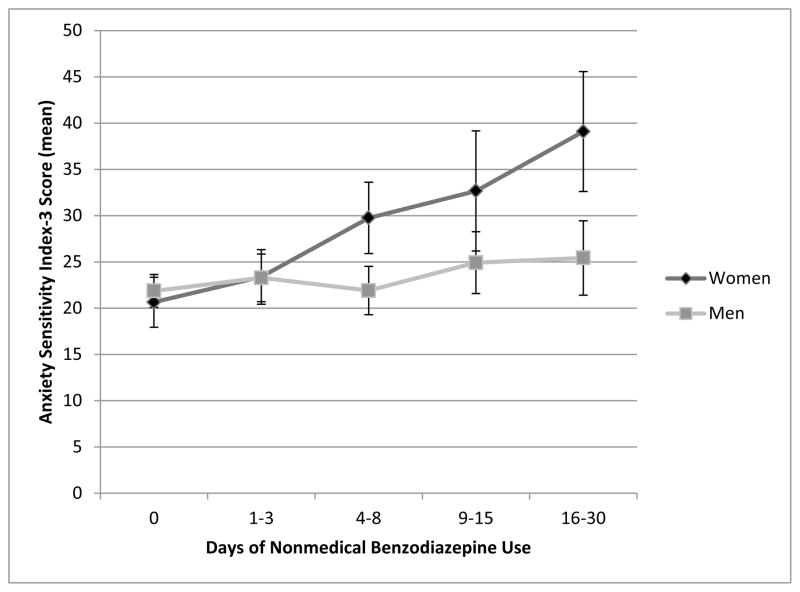
We examined the nature of the interaction effect by testing the association between nonmedical benzodiazepine use frequency and anxiety sensitivity for men and women, separately. The ANCOVA model adjusting for age and anxiety symptoms (OASIS total score) was calculated separately for men and women. Among women, there was a statistically significant effect of nonmedical benzodiazepine use on anxiety sensitivity (F[1, 66] = 8.23, p = .006, ηp2=.11); however, this effect was not present among men (F[1, 183] = 0.70, p = .40, ηp2=.004). Figure 1 presents ASI-3 total scores by frequency of nonmedical benzodiazepine use separately for women and men.
Figure 1.
Association between Anxiety Sensitivity and Days of Nonmedical Benzodiazepine Use by Gender.
4. Discussion
This study examined the association between anxiety sensitivity and nonmedical benzodiazepine use in a sample of adults receiving treatment for opioid use disorder. Results indicated that greater frequency of nonmedical benzodiazepine use was associated with higher anxiety sensitivity. However, this effect was qualified by a significant moderational effect of gender. Specifically, this effect was largely driven by female participants, as indicated by a strong association between nonmedical benzodiazepine use and anxiety sensitivity in women, but not men.
The literature on the nonmedical use of benzodiazepines among those with opioid use disorder suggests two potential explanations for the co-occurring nonmedical use of benzodiazepines and opioids. First, in some cases, nonmedical use of these medications might be an attempt to mitigate symptoms of anxiety, sleep disruption, or acute or protracted withdrawal (Fatseas et al., 2009; Vogel et al., 2013). Consistent with this perspective, our findings suggest that anxiety sensitivity, a well-established motivator of escape and avoidance of anxiety and other distressing states, is higher among those with opioid use disorder who more frequently engage in nonmedical benzodiazepine use. Thus, untreated or undertreated symptoms of anxiety and other distressing affective and somatic symptoms (e.g., opioid withdrawal) might be a risk factor for the nonmedical use of benzodiazepines in this population, particularly among those who are highly sensitive to these states.
Nonetheless, the nonmedical use of benzodiazepines for its rewarding properties (i.e., to get high) is also common (Fatseas et al., 2009; Vogel et al., 2013), and might reflect an alternative pathway to nonmedical use, particularly among those with opioid use disorder. Both animal and human laboratory studies have found that benzodiazepines enhance the reinforcing effects of opioids (Lintzeris, Mitchell, Bond, Nestor, & Strang, 2007; Walker & Ettenberg, 2001, 2003), consistent with self-report by patients that they often use benzodiazepines to enhance the “high” of opioids (Stitzer, Griffiths, McLellan, Grabowski, & Hawthorne, 1981). It is likely that each of these pathways, in part, contribute to the elevated prevalence of nonmedical benzodiazepine use among those with opioid use disorder. Further studies assessing such motives for use (e.g., the possibility that benzodiazepines were used prior to treatment entry to mitigate symptoms of withdrawal) will help to clarify this association.
Our findings further suggest that gender might be an important variable moderating these pathways. Specifically, the association between nonmedical benzodiazepine use and anxiety sensitivity was driven largely by women, consistent with a prior study (Hearon et al., 2011). Women have higher rates of anxiety disorders than men in both the general population (Kessler, Chiu, Demler, Merikangas, & Walters, 2005), and among those with substance use disorders (Conway, Compton, Stinson, & Grant, 2006). Moreover, studies on motives for substance use suggest that men and women might, on average, differ in their perceived motives for use. Specifically, women report use of substances to cope with negative affective states more often than men (Boyd, Austic, Epstein-Ngo, Veliz, & McCabe, 2015; McHugh et al., 2013; Terry-McElrath, O’Malley, & Johnston, 2009), and some studies suggest that men are more likely to report enhancement motives (i.e., to get high or experiment; Ham, Zamboanga, Bacon, & Garcia, 2009; Kuntsche, Knibbe, Gmel, & Engels, 2006; Leigh & Neighbors, 2009; McCabe, Cranford, Boyd, & Teter, 2007; Terry-McElrath et al., 2009). Thus, women might be particularly susceptible to the use of benzodiazepines to manage anxiety and other negative affective states. Future research designed to test these gender differences are needed to better understand these trends in the literature. This study further highlights the importance of considering not only main effects of gender (reflecting average difference between men and women), but also interaction effects, which may indicate different mechanisms based on gender.
Understanding the role of anxiety sensitivity in nonmedical benzodiazepine use has several clinical implications. Anxiety sensitivity might be a pertinent clinical variable for the discontinuation of nonmedical benzodiazepine use. Studies on the discontinuation of benzodiazepine treatment among those with anxiety disorders suggest that anxiety sensitivity plays an important role in taper success. Older adults with high anxiety sensitivity are less likely to consider tapering off of their medication, even controlling for benzodiazepine dose (Cook, Biyanova, Thompson, & Coyne, 2007). Cognitive-behavioral therapy targeting anxiety sensitivity increases the likelihood of successful benzodiazepine taper among those with anxiety disorders (Otto et al., 2010; Otto et al., 1993). Those with greater reductions in anxiety sensitivity in such treatments are more likely to successfully discontinue benzodiazepines (Bruce, Spiegel, Gregg, & Nuzzarello, 1995; Bruce, Spiegel, & Hegel, 1999), suggesting that anxiety sensitivity reductions might serve as a mechanism of this treatment effect. Thus, elevated anxiety sensitivity might be a therapeutic target for reduction of nonmedical benzodiazepine use among those with opioid use disorder. Because causality cannot be determined in the current study, it is uncertain whether higher anxiety sensitivity is a risk factor for the initiation of nonmedical benzodiazepine use, a result of use, or reflective of a bi-directional relationship. Studies examining the temporal associations among these variables will help to determine whether anxiety sensitivity might also be a useful target for prevention.
These findings also highlight the importance of adequate attention to anxiety in those with opioid use disorder. Approximately one-quarter of the individuals in our sample were diagnosed with an anxiety disorder, and the average level of anxiety sensitivity was comparable to populations with an anxiety disorder (Allan et al., 2014; Taylor et al., 2007). It is possible that nonmedical benzodiazepine use in this population is related to undertreated or unrecognized clinical anxiety, particularly among women. Prior research suggests that coping with distress is a significant motivating factor for nonmedical benzodiazepine use among the majority of those with opioid use disorder who use benzodiazepines nonmedically (Fatseas et al., 2009). Adequately addressing symptoms of anxiety (and other negative affective states) among individuals presenting to treatment for an opioid use disorder might be necessary to successfully reduce nonmedical benzodiazepine use in this population.
There are several limitations to the current study. As noted above, this study was cross-sectional and thus causality cannot be inferred, and the temporal associations between these variables are unknown. Second, this sample consisted of adults receiving treatment, and thus it is unknown whether these results would generalize to non-treatment seeking samples. Moreover, our sample was primarily Caucasian and data were not collected on income or education; replication in more heterogeneous samples is needed. The average age of this sample was 28 years, with only 5% of the sample age 50 years or older. Given that concomitant prescribing of opioids and benzodiazepines is higher among older adults than any other age group (Hwang et al., 2016), and that nonmedical use of these medications is increasing in this age group (Schepis & McCabe, 2016), future research should address treatment targets for combination benzodiazepine and opioid use among older adults.
We only assessed nonmedical benzodiazepine use and do not have data on whether any participants were prescribed a benzodiazepine that they were taking as prescribed. It is possible that the association between anxiety sensitivity and benzodiazepine use was mitigated by the failure to account for prescribed benzodiazepines. More fine-grained examination of benzodiazepine use in this population (including variables such as benzodiazepine dose, history, and medical vs. nonmedical use) will clarify this association, particularly considering the challenges of distinguishing medical from nonmedical use of any prescription drug (McHugh, Nielsen, & Weiss, 2015). Our measure of substance use consisted of the previous 30 days, and thus it is unknown whether participants had a history of nonmedical benzodiazepine use outside of the month prior to entering treatment. Participants were receiving treatment at the time of this study, and were undergoing medical detoxification, and thus anxiety symptoms were likely impacted by fluctuations associated with early sobriety; however, anxiety sensitivity--a trait-level construct--is not anticipated to substantively change with such fluctuations.
In light of these limitations, there are a number of promising future directions for this research area. First, prospective studies investigating whether high anxiety sensitivity precedes nonmedical benzodiazepine use in those with opioid use disorder will help to determine whether this is a relevant target for preventive efforts. Second, studies assessing motives for nonmedical benzodiazepine use (i.e., use to address un- or under-treated anxiety vs. use for rewarding properties) will help clarify: (1) whether observed gender differences are attributable to differences in motives for using benzodiazepines, and (2) potential interventions for reducing nonmedical benzodiazepine use and sufficiently addressing anxiety. Third, anxiety sensitivity might be a pertinent risk factor for nonmedical benzodiazepine use even among those without opioid use disorder; indeed, this effect may be stronger among those who do not also use opioids because this population also uses to enhance the opioid high. Future studies are needed to establish the generalizability of this finding.
Nonmedical benzodiazepine use is highly prevalent among those with opioid use disorder, and is associated with significant risks, including increased overdose risk when benzodiazepines and opioids are combined. Yet, surprisingly little is known about nonmedical benzodiazepine use among those with opioid use disorders. The results from this study indicated that past-month nonmedical benzodiazepine use was associated with higher anxiety sensitivity among inpatients with opioid use disorder. This finding was driven by a strong association between these variables among women. Although studies are needed to better understand the nature of this association, anxiety sensitivity might be a promising treatment target among those with opioid use disorder who use benzodiazepines nonmedically, especially women.
Highlights.
Benzodiazepine abuse is common among those with opioid use disorder.
Our sample consisted of adults receiving treatment for opioid use disorder.
Frequent past-month benzodiazepine abuse was associated with high anxiety sensitivity.
The link between benzodiazepine abuse and anxiety sensitivity was unique to women.
Women may be particularly susceptible to abusing benzodiazepines to manage anxiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan NP, Raines AM, Capron DW, Norr AM, Zvolensky MJ, Schmidt NB. Identification of anxiety sensitivity classes and clinical cut-scores in a sample of adult smokers: results from a factor mixture model. Journal of Anxiety Disorders. 2014;28:696–703. doi: 10.1016/j.janxdis.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ, Bernstein A. Marijuana use motives: concurrent relations to frequency of past 30-day use and anxiety sensitivity among young adult marijuana smokers. Addictive Behaviors. 2007;32:49–62. doi: 10.1016/j.addbeh.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Boyd CJ, Austic E, Epstein-Ngo Q, Veliz PT, McCabe SE. A prospective study of adolescents’ nonmedical use of anxiolytic and sleep medication. Psychology of Addictive Behaviors. 2015;29:184–191. doi: 10.1037/adb0000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TJ, Spiegel DA, Gregg SF, Nuzzarello A. Predictors of alprazolam discontinuation with and without cognitive behavior therapy in panic disorder. American Journal of Psychiatry. 1995;152:1156–1160. doi: 10.1176/ajp.152.8.1156. [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Spiegel DA, Hegel MT. Cognitive-behavioral therapy helps prevent relapse and recurrence of panic disorder following alprazolam discontinuation: a long-term follow-up of the Peoria and Dartmouth studies. Journal of Consulting and Clinical Psychology. 1999;67:151–156. doi: 10.1037//0022-006x.67.1.151. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Otto MW, Cohen LS, Soares CN, Vitonis AF, Hearon BA, Harlow BL. Psychosocial predictors of the onset of anxiety disorders in women: results from a prospective 3-year longitudinal study. Journal of Anxiety Disorders. 2009;23:1165–1169. doi: 10.1016/j.janxdis.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, … Stein MB. Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS) Journal of Affective Disorders. 2009;112:92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Berger CC, Forde DP, D’Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011;11:90. doi: 10.1186/1471-244x-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Hedegaard H, Warner M. Drug-poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. NCHS Data Brief. 2014;(166):1–8. [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug and Alcohol Dependence. 2006;83(Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Cook JM, Biyanova T, Thompson R, Coyne JC. Older primary care patients’ willingness to consider discontinuation of chronic benzodiazepines. General Hospital Psychiatry. 2007;29:396–401. doi: 10.1016/j.genhosppsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Medicine. 2015;17:85–98. doi: 10.1111/pme.12907. [DOI] [PubMed] [Google Scholar]
- Fatseas M, Lavie E, Denis C, Auriacombe M. Self-perceived motivation for benzodiazepine use and behavior related to benzodiazepine use among opiate-dependent patients. Journal of Substance Abuse Treatment. 2009;37:407–411. doi: 10.1016/j.jsat.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Ham LS, Zamboanga BL, Bacon AK, Garcia TA. Drinking motives as mediators of social anxiety and hazardous drinking among college students. Cognitive Behavioral Therapy. 2009;38:133–145. doi: 10.1080/16506070802610889. [DOI] [PubMed] [Google Scholar]
- Hearon BA, Calkins AW, Halperin DM, McHugh RK, Murray HW, Otto MW. Anxiety sensitivity and illicit sedative use among opiate-dependent women and men. American Journal of Drug and Alcohol Abuse. 2011;37:43–47. doi: 10.3109/00952990.2010.535581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. American Journal of Preventive Medicine. 2016;51:151–160. doi: 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson K, Mullin JL, Marshall EC, Bonn-Miller MO, Zvolensky M. Exploring the mediational role of coping motives for marijuana use in terms of the relation between anxiety sensitivity and marijuana dependence. American Journal on Addiction. 2010;19:277–282. doi: 10.1111/j.1521-0391.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug and Alcohol Dependence. 2012;125:8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addictive Behaviors. 2006;31:1844–1857. doi: 10.1016/j.addbeh.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of Studies on Alcohol and Drugs. 2013;74:469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J, Neighbors C. Enhancement Motives Mediate the Positive Association Between Mind/Body Awareness and College Student Drinking. Journal of Social and Clinical Psychology. 2009;28:650–669. doi: 10.1521/jscp.2009.28.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Mitchell TB, Bond AJ, Nestor L, Strang J. Pharmacodynamics of diazepam co-administered with methadone or buprenorphine under high dose conditions in opioid dependent patients. Drug and Alcohol Dependence. 2007;91:187–194. doi: 10.1016/j.drugalcdep.2007.05.019. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ, Teter CJ. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addictive Behaviors. 2007;32:562–575. doi: 10.1016/j.addbeh.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Devito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, … Weiss RD. Gender differences in a clinical trial for prescription opioid dependence. Journal of Substance Abuse Treatment. 2013;45:38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. Journal of Substance Abuse Treatment. 2015;48:1–7. doi: 10.1016/j.jsat.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, Stein MB. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS) Depression and Anxiety. 2006;23:245–249. doi: 10.1002/da.20182. [DOI] [PubMed] [Google Scholar]
- Novak A, Burgess ES, Clark M, Zvolensky MJ, Brown RA. Anxiety sensitivity, self-reported motives for alcohol and nicotine use, and level of consumption. Journal of Anxiety Disorders. 2003;17:165–180. doi: 10.1016/s0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Ocanez KL, McHugh RK, Otto MW. A meta-analytic review of the association between anxiety sensitivity and pain. Depression and Anxiety. 2010;27:760–767. doi: 10.1002/da.20681. [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, Pollack MH. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behaviour Research and Therapy. 2010;48:720–727. doi: 10.1016/j.brat.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Sachs GS, Reiter SR, Meltzer-Brody S, Rosenbaum JF. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. American Journal of Psychiatry. 1993;150:1485–1490. doi: 10.1176/ajp.150.10.1485. [DOI] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. 15-Year survival and retention of patients in a general hospital-affiliated methadone maintenance treatment (MMT) center in Israel. Drug and Alcohol Dependence. 2010;107:141–148. doi: 10.1016/j.drugalcdep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index revised manual. Worthington, OH: International Diagnostic Systems Publishing Corportation; 1992. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Schepis TS, McCabe SE. Trends in older adult nonmedical prescription drug use prevalence: Results from the 2002–2003 and 2012–2013 National Survey on Drug Use and Health. Addictive Behaviors. 2016;60:219–222. doi: 10.1016/j.addbeh.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB, Keough ME, Mitchell MA, Reynolds EK, Macpherson L, Zvolensky MJ, Lejuez CW. Anxiety sensitivity: prospective prediction of anxiety among early adolescents. Journal of Anxiety Disorders. 2010;24:503–508. doi: 10.1016/j.janxdis.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Powers MB, Cho Y, Telch MJ. Mechanism of change in cognitive-behavioral treatment of panic disorder: evidence for the fear of fear mediational hypothesis. Journal of Consulting and Clinical Psychology. 2004;72:646–652. doi: 10.1037/0022-006x.72.4.646. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Griffiths RR, McLellan AT, Grabowski J, Hawthorne JW. Diazepam use among methadone maintenance patients: patterns and dosages. Drug and Alcohol Dependence. 1981;8:189–199. doi: 10.1016/0376-8716(81)90061-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health, 2014: Inter-university Consortium for Political and Social Research (ICPSR) [distributor] 2016. [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, … Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychoogicall Assessment. 2007;19:176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD. Reasons for drug use among American youth by consumption level, gender, and race/ethnicity: 1976–2005. Journal of Drug Issues. 2009;39:677–714. doi: 10.1177/002204260903900310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M, Knopfli B, Schmid O, Prica M, Strasser J, Prieto L, … Dursteler-Macfarland KM. Treatment or “high”: benzodiazepine use in patients on injectable heroin or oral opioids. Addictive Behaviors. 2013;38:2477–2484. doi: 10.1016/j.addbeh.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Benzodiazepine modulation of opiate reward. Experimental and Clinical Psychopharmacology. 2001;9:191–197. doi: 10.1037//1064-1297.9.2.191. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. The effects of alprazolam on conditioned place preferences produced by intravenous heroin. Pharmacology, Biochemistry and Behavior. 2003;75:75–80. doi: 10.1016/s0091-3057(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Wheaton MG, Deacon BJ, McGrath PB, Berman NC, Abramowitz JS. Dimensions of anxiety sensitivity in the anxiety disorders: Evaluation of the ASI-3. Journal of Anxiety Disorders. 2012;26:401–408. doi: 10.1016/j.janxdis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, Leventhal AM. Anxiety sensitivity as an amplifier of subjective and behavioral tobacco abstinence effects. Drug and Alcohol Dependence. 2014;142:224–230. doi: 10.1016/j.drugalcdep.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]