Abstract
People with Parkinson’s disease (PD) typically demonstrate impaired anticipatory postural adjustments (APAs) that shift the body center of mass forward (imbalance) and over the stance leg (unloading) prior to gait initiation. APAs are known to be smallest when people with PD are in their OFF-medication state compared to ON-medication or healthy controls. The aim of this pilot study is to validate a previously developed method for the assessment of gait initiation on PD patients in OFF state with body-worn, inertial sensors. Ten subjects with mild-to-moderate idiopathic PD and twelve healthy controls of similar age performed three gait initiation trials. The spatio-temporal parameters of APAs were extracted from three wearable sensors, placed on the shins and on the lower back, and validated with two force plates. Temporal parameters extracted from sensors and force plates, as well as the trunk medio-lateral acceleration and the correspondent displacement of the center of pressure, were significantly correlated. Subjects with PD showed hypometric adjustments in the medio-lateral direction (p-value < 0.003) and increased duration of the unloading phase (p-value = 0.04). The unloading phase was significantly longer than the imbalance (p-value = 0.003) only in subjects with PD. The validity of the method of quantifying APAs from inertial sensors was confirmed in PD subjects by comparison with force plates. Sensitivity in discriminating PD patients from healthy controls was proven by both spatial and temporal parameters. Objective measures of gait initiation deficits with wearable technology provides valuable instrument for the assessment of gait initiation in clinical environments.
Keywords: Inertial sensors, Force plate, Accelerometry, Spatio-temporal parameters, APA phases, Levodopa
1. Introduction
Gait initiation is a complex transitional task, typically impaired in Parkinson’s disease (PD). PD symptoms include akinesia, bradykinesia, tremor, postural and gait instability. Furthermore, anticipatory postural adjustments (APAs), the transient phase between quiet standing and a voluntary movement [1], are reduced, affecting balance and fall risk [2].
Motor deficits are mainly due to a progressive deterioration of the dopaminergic neurons in the basal ganglia [3]. Hence, symptoms are typically improved by replacing dopamine with L-dopa or dopaminergic agonists [4–7], therefore, measuring APAs in the ON state might not be representative of motor impairments.
We recently presented an instrumented method for evaluating APAs preceding gait initiation and stair climbing in subjects with PD in their ON-medication state by using inertial sensors [8]. Our method differs from those adopted in previous studies [9–11] because of the ability to characterize both the imbalance (from the APA onset to the heel-off of the stepping limb) and unloading (from the heel-off to the toe-off of the same limb) phases preceding the step execution. Unlike force plates and EMGs, a wireless, body-worn sensor approach to measuring APAs enables measurement of postural preparation for movement in clinical settings, even though optimal placements and outcomes have still to be identified [12,13].
The aims of this study are i) to evaluate the validity of our recently developed algorithm on PD patients in the OFF-medication state and ii) to assess the accuracy and sensitivity of the method. Due to our previous results [8], we expect the algorithm to accurately detect the APAs’ phases and deficits in PD subjects in the OFF-medication state.
2. Methods
2.1 Participants
Ten subjects with mild-to-moderate idiopathic PD (age, mean ± SD: 67.2 ± 5 yrs, UPDRS III: 27.5 ± 9, H&Y stage: 2.5 ± 0.5, 2 female) and 12 healthy controls (age 68 ± 5 yrs, 3 female) participated to the study, after giving informed consent according to the Oregon Health & Science University Institutional Review Board. No significant difference in age and BMI was found between groups. Subjects were excluded when presenting any neurological disorder other than PD or conditions that could affect balance. Subjects with PD were tested in their practical OFF-medication state, after at least 12 hours washout from antiparkinson medications.
2.2 Procedures
Participants stood with feet externally rotated on separate, side-by-side force plates at heel-to-heel distance of 10 cm [9]. They performed 3 gait initiation trials starting with their most affected leg at comfortable pace. Initial foot position was made consistent by tracing feet outlines on the plates.
Data were collected from 3 IMUs (APDM Inc., USA), fixed with elastic bands on the trunk (L5), and on both shins. Signals were acquired at 128 Hz and resampled at 50 Hz to match our previous study [8]. Ground reaction forces and center of pressure (CoP) displacement were measured via the force plates, considered as gold standard, at 480 Hz.
CoP displacement was filtered with a fourth-order, zero-lag, low-pass Butterworth filter with a 10 Hz cut-off frequency [10]. APAs quantification, consisting of the imbalance and unloading phases, were calculated from 3 automatically-detected time points: 1) APA onset, 2) heel-off, and 3) toe-off [8,14]. Trunk accelerations, transformed to horizontal-vertical coordinate system, and angular velocities from the shins were filtered using a fourth-order, zero-lag, low-pass Butterworth filter with a 3.5 Hz cut-off frequency [10]. The time points were extracted with the previously reported procedure [8]: i) APA onset as the instant in which the ML trunk acceleration exceeded a threshold set as twice the SD of the signal during the initial quiet standing, ii) heel-off as the instant in which the ML angular velocity of the stepping limb became higher than 7% of the signal’s first peak value, and iii) toe-off as the subsequent instant when the signal became lower than 25% of the peak value. Mean absolute errors (MAEs) between instants recognized from force plates and IMUs were averaged among all subjects. As previously proposed [8], the amplitude of the ML trunk acceleration and of the CoP displacement were extracted and the durations of the phases and of the entire APAs were measured from force plates and wearable sensors.
2.3 Statistical analyses
For each subject, variables were averaged over the trials. The relationship between parameters from the force plates and from IMUs was investigated through linear correlation and Bland-Altman analyses. The method sensitivity was assessed by comparison of the correlated spatio-temporal parameters through Student’s t-test. Comparison of the durations of the two phases was assessed by using a paired t-test.
All the analyses were performed with R (R Foundation for Statistical Computing, Vienna, Austria), with level of significance set at 0.05.
3. Results
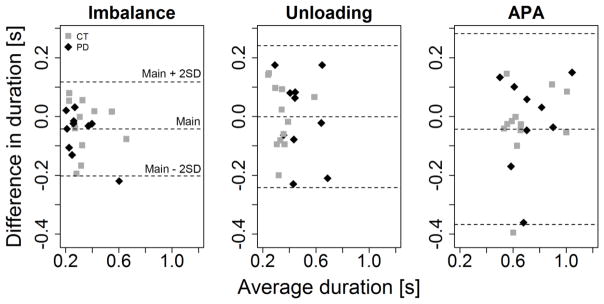
Significant correlations between IMU and force plate measures were detected (p-value < 0.05). Considering spatial parameters, only the ML measures were significantly correlated (Table 1). Bland-Altman analysis showed no obvious relation between the difference and the mean duration of the phases measured with the different systems (figure 1).
Table 1.
Linear correlation between inertial sensors and force plates measures
| Phase | AP | ML | Δt |
|---|---|---|---|
| Imbalance | 0.29 (0.210) | 0.60 (0.004 *) | 0.82 (< 0.001 *) |
| Unloading | 0.05 (0.830) | 0.60 (0.003 *) | 0.63 (0.002 *) |
| APA (Imbalance + Unloading) | 0.23 (0.300) | 0.42 (0.050) | 0.72 (< 0.001 *) |
Correlation between CoP displacement and trunk acceleration in the antero-posterior (AP) and medio-lateral (ML) directions, and between phase durations (Δt) measured by force plates and wearable sensors. Pearson’s correlation coefficients (r) and the correspondent p-values are reported. Significant correlations (p-value < 0.05) are marked with *.
Figure 1. Bland-Altman plot APA phases duration.
Bland-Altman plot comparing duration of the imbalance (left) and unloading (center) phases and of the entire APA (left) calculated from force plates and wearable sensors. The central dotted lines represent the mean difference between the devices, while the upper and lower lines represent the limits of agreement. CT: control subjects (in black); PD: subjects with PD (in red).
No significant differences were found between PD and control subjects considering MAEs for APA onset (CT, mean ± SD: 0.06 ± 0.02 s; PD, mean ± SD: 0.06 ± 0.03 s; p-value = 0.33), heel-off (CT: 0.08 ± 0.03 s; PD: 0.08 ± 0.03 s; p-value = 0.27), and toe-off (CT: 0.07 ± 0.04 s; PD: 0.07 ± 0.04 s; p-value = 0.68) instants.
As reported in Table 2 (Trunk results), PD subjects showed smaller ML amplitudes in all the phases. The unloading phase resulted to be significantly longer for PD than control subjects and significantly longer than the imbalance phase for PD subjects (p-value = 0.003), but not for controls (p-value = 0.72).
Table 2.
Comparison of parameters extracted in subjects with PD and healthy controls (HC) from force plate and wearable sensors respectively.
| CoP
|
Trunk
|
||||||
|---|---|---|---|---|---|---|---|
| HC | PD | p-value | HC | PD | p-value | ||
| Imbalance | ML | 40.04 ± 11.91 | 22.72 ± 8.44 | < 0.001 * | 0.38 ± 0.14 | 0.15 ± 0.02 | < 0.001 * |
| Δt | 0.33 ± 0.14 | 0.28 ± 0.10 | 0.31 | 0.33 ± 0.10 | 0.29 ± 0.07 | 0.28 | |
| Unloading | ML | 149.82 ± 23.28 | 115.96 ± 21.48 | 0.003 * | 1.06 ± 0.32 | 0.67 ± 0.20 | 0.003 * |
| Δt | 0.32 ± 0.05 | 0.48 ± 0.14 | 0.005 * | 0.34 ± 0.11 | 0.48 ± 0.17 | 0.04 * | |
| APA | ML | 109.57 ± 16.78 | 93.57 ± 21.13 | 0.07 | 0.68 ± 0.26 | 0.53 ± 0.23 | 0.18 |
| Δt | 0.69 ± 0.20 | 0.76 ± 0.22 | 0.46 | 0.70 ± 0.17 | 0.73 ± 0.17 | 0.72 | |
CoP displacement (ML; mean ± SD [mm]), trunk acceleration (ML; mean ± SD [m/s2]) and durations (Δt; mean ± SD [s]) of the different phases and the entire test. Significant differences (p-value < 0.05) are marked with *.
Force plate analysis confirmed all the results (Table 2, CoP results).
4. Discussion
In this study, we investigated the validity, accuracy and sensitivity of our previously developed, IMU-based method for gait initiation assessment on subjects with PD in their OFF-medication state.
Our findings confirmed the validity of the algorithm for the considered population. The significant correlation between parameters from IMUs and force plates supported the possibility to adopt IMUs to assess APAs outside a laboratory setting.
Considering the accuracy, the MAE values between the instants detected from IMUs and from force plates are consistent with results of our former work. In our opinion, this result further supports the method robustness.
Concerning the sensitivity, it was possible to discriminate PD and controls on the basis of APAs alterations. In fact, subjects with PD showed a significantly smaller medio-lateral trunk acceleration in all the phases and took significantly more time to perform the unloading phase compared to healthy controls. A significant difference in the duration of the phases was limited to the PD group. Even though similar results have been observed in previous laboratory studies [7,14,15], the reduced amplitude of spatial parameters and the prolonged durations of the unloading phase were novelties when considering IMU-based analyses.
The study’s main limitation is represented by the small number of participants, thus these preliminary results must be confirmed on a larger population. Future investigations are desirable to evaluate the repeatability of the method and its sensitivity in detecting ON-OFF differences, possibly leading to the adoption of the method for a fine tuning of medications and Deep Brain Stimulation (DBS) parameters.
Highlights.
Our new method is intended for Gait Initiation (GI) examination in OFF state;
PD subjects in OFF state showed reduced spatio-temporal parameters during GI;
PD subjects took more time than controls to perform the unloading phase;
A difference between the durations of the GI phases was limited to the PD group.
Acknowledgments
This publication was mainly funded by the Italian Ministry of Health [Ricerca Corrente and Ricerca Finalizzata: grant n. GR-2009-1604984] with the support from NIH [grant RC1 NS068678 and R42 HD071760-03] for data collection.
Footnotes
Conflict of interests
OHSU and Dr. Horak have a significant financial interest in, and is employ of APDM, a company that has a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Degani AM, Danna-Dos-Santos A, Latash ML. Postural preparation to making a step: is there a “motor program” for postural preparation? [accessed August 30, 2016];J Appl Biomech. 2007 23:261–74. doi: 10.1123/jab.23.4.261. http://www.ncbi.nlm.nih.gov/pubmed/18089924. [DOI] [PubMed] [Google Scholar]
- 2.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35:ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 3.LeWitt PA. New levodopa therapeutic strategies. Parkinsonism Relat Disord. 2016;22(Suppl 1):S37–40. doi: 10.1016/j.parkreldis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Hoskovcová M, Dušek P, Sieger T, Brožová H, Zárubová K, Bezdíček O, Šprdlík O, Jech R, Štochl J, Roth J, Růžička E. Predicting Falls in Parkinson Disease: What Is the Value of Instrumented Testing in OFF Medication State? PLoS One. 2015;10:e0139849. doi: 10.1371/journal.pone.0139849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancini M, Rocchi L, Horak FB, Chiari L. Effects of Parkinson’s disease and levodopa on functional limits of stability. 2008;23:450–458. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov Disord. 2015;30:1361–70. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson’s disease: Influence of initial stance conditions. Neurosci Lett. 2006;406:128–32. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Bonora G, Carpinella I, Cattaneo D, Chiari L, Ferrarin M. A new instrumented method for the evaluation of gait initiation and step climbing based on inertial sensors: a pilot application in Parkinson’s disease. J Neuroeng Rehabil. 2015;12:45. doi: 10.1186/s12984-015-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancini M, Chiari L, Holmstrom L, Salarian A, Horak FB. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture. 2016;43:125–131. doi: 10.1016/j.gaitpost.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancini M, Zampieri C, Carlson-Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: An accelerometer-based approach. Eur J Neurol. 2009;16:1028–1034. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Mendez R, Sekine M, Tamura T. Detection of anticipatory postural adjustments prior to gait initiation using inertial wearable sensors. J Neuroeng Rehabil. 2011;8:17. doi: 10.1186/1743-0003-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubble RP, Naughton GA, Silburn PA, Cole MH. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS One. 2015;10:1–22. doi: 10.1371/journal.pone.0123705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maetzler W, Klucken J, Horne MK. A clinical view on the development of technology-based tools in managing Parkinson’s disease. Mov Disord. 2016 Sep;31(9):1263–71. doi: 10.1002/mds.26673. Epub 2016 Jun 7. [DOI] [PubMed] [Google Scholar]
- 14.Crenna P, Carpinella I, Rabuffetti M, Rizzone M, Lopiano L, Lanotte M, Ferrarin M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp Brain Res. 2006;172:519–532. doi: 10.1007/s00221-006-0360-7. [DOI] [PubMed] [Google Scholar]
- 15.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–15. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]