Abstract
With advances in DNA sequencing technology, it is increasingly common and tractable to informatically look for genes of interest in the genomic databases of parasitic organisms and infer cellular states. Assignment of a putative gene function based on homology to functionally characterized genes in other organisms, though powerful, relies on the implicit assumption of functional homology, i.e. that orthology indicates conserved function. Eukaryotes reveal a dazzling array of cellular features and structural organization, suggesting a concomitant diversity in their underlying molecular machinery. Significantly, examples of novel functions for pre-existing or new paralogues are not uncommon. Do these examples undermine the basic assumption of functional homology, especially in parasitic protists, which are often highly derived? Here we examine the extent to which functional homology exists between organisms spanning the eukaryotic lineage. By comparing membrane trafficking proteins between parasitic protists and traditional model organisms, where direct functional evidence is available, we find that function is indeed largely conserved between orthologues, albeit with significant adaptation arising from the unique biological features within each lineage.
Keywords: Membrane-trafficking, protist, parasite, genomics, functional homology, endomembrane
1 Introduction
Genomics, the sequencing and analysis of genomes has empowered tremendous advances. Possessing a genome sequence for an organism, particularly one difficult to culture or genetically manipulate, allows the prediction of cellular organization, metabolism, gene expression mechanisms, and organellar complement, through in silico analysis of the corresponding predicted proteome.
This is essentially a comparative analysis, which at its heart relies on robust evidence of function in one or more organisms. Comparative genomics allows reconstruction of pan-eukaryotic complements of cellular components, including the cytoskeleton, nuclear transport, metabolism, and mitochondrion ([1], inter alia), providing evidence for the general or core aspects of cellular systems and which aspects are lineage-specific. This evidence is an important basis for understanding evolutionary mechanisms behind emergence of cellular complexity. Furthermore, the acceleration in understanding gained by the annotation of thousands of genes is invaluable, by producing initial hypotheses for expected interactions, pathways, and organellar roles that can be tested.
Inherent in comparative genomic studies is the assumption of functional homology, i.e. that orthologous genes retain equivalent function. Orthology is the relationship between two genes in distinct taxa that are directly related by vertical descent [2], and which may be considered as the “same gene”; the expectation is that such gene pairs retain equivalent properties and roles within the cell [3]. This assumption has been generally regarded as safe, based on a model of conservation of function rather than the widespread gain of novel functions or neofunctionalization and based on experimental validation of enzymes assayed heterologously or in vitro, where ‘function’ can be relatively readily defined. However, much of our understanding of eukaryotic cell biology is based on evidence from a small sample of true eukaryotic diversity and frequently from a restricted region of the eukaryotic tree. Given this sampling bias, to what extent can ‘function’ be reliably predicted across eukaryotic diversity based on sequence similarity alone?
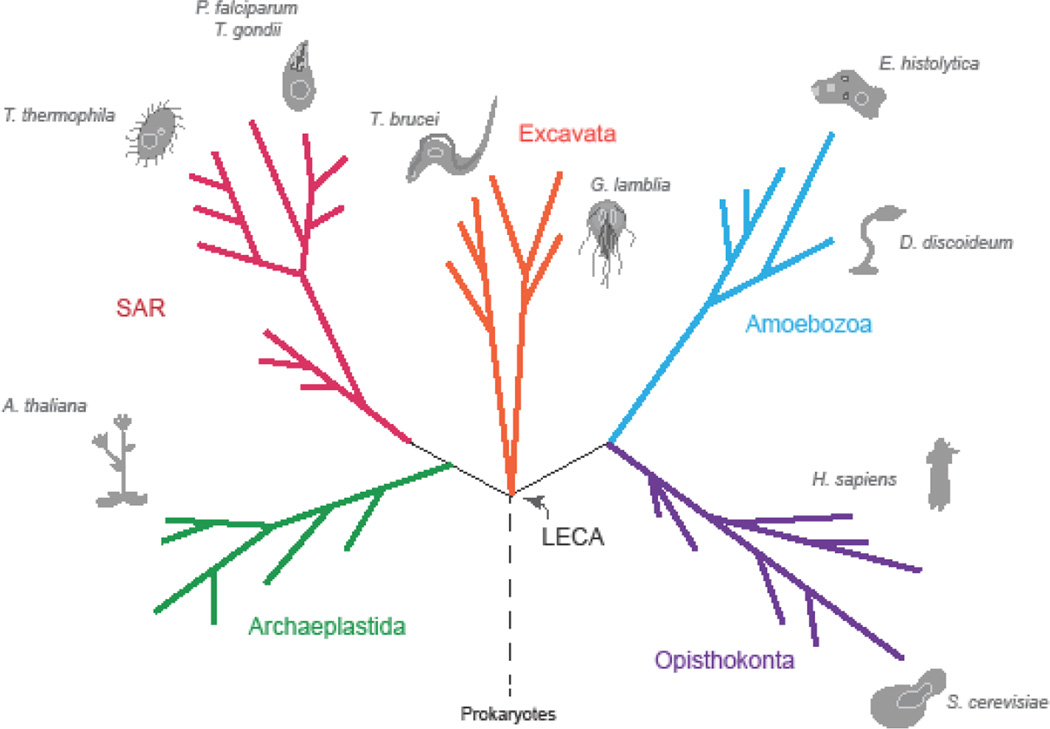
Testing the assumption of functional homology requires experimental evidence from organisms across a full taxonomic range of eukaryotes, and there are now fortunately tractable organisms from each of the major eukaryotic divisions or Supergroups (Figure 1). Here we have chosen a subset of non-metazoan organisms and assessed comparative data available for genes of the membrane trafficking system, a crucial cellular system underpinning pathogenic mechanisms in many parasitic protists, and which has been well studied. We not only assess the validity of the core assumption of functional homology in comparative studies of membrane trafficking genes, but also begin to identify the manner in which the endomembrane system is modified in individual parasitic lineages and which speaks directly to mechanisms of disease and the origins of parasitism.
Figure 1. Model Organisms Across Eukaryotes.
This figure demonstrates the distribution of model organisms across eukaryotic diversity. Colour-coded branches and corresponding labels denote eukaryotic Supergroups, with the branching order roughly corresponding to the organization of known diversity within each group. Model organisms are represented by greyscale illustrations and corresponding labels in italics. The position of the Last Eukaryotic Common Ancestor (LECA) is indicated. Though additional model organisms exist for each of these groups, they are excluded from this figure for simplicity.
1.1 The membrane-trafficking system: a modern molecular view
Membrane trafficking is the process by which proteins and other macromolecules are distributed throughout organelles of the endomembrane system, and released into, or internalized from, the extracellular environment. Trafficking is vital for metabolism, signaling, and interacting with the external environment. Transport vesicles act to transfer cargo molecules between the organelles of the endomembrane system, which possess discrete morphology, localization, and functions [4].
Anterograde trafficking involves movement from the endoplasmic reticulum (ER) through the Golgi complex, the trans-Golgi network (TGN), and on to the plasma membrane [5], whilst endocytosis begins at the plasma membrane where cargo is sorted by endosomes before recycling or targeting to acidic terminal organelles. During endocytosis organelles acidify, may acquire intralumenal vesicles (present in multi-vesicular bodies or MVBs), and modify their compositions [6]. In all trafficking pathways retrograde transport steps recycle selected components back to previous organelles for use in future rounds of trafficking.
Specialized protein complexes controlling vesicle budding, tethering, and fusion, many of which are large paralagous families, regulate transport. Arf/Sar family small GTPases and their regulators, cargo adaptors, and coat protein complexes are involved in vesicle formation/fission. Rab GTPases are involved in vesicle targeting, whilst coiled coil SNARE proteins are central to vesicle fusion [4]. Importantly, members of these multiple families act at discrete locations or trafficking pathways; the specificity of trafficking is in part encoded in the combinatorial interactions of these various players [7]. For example, COPII-coated vesicles mediate anterograde transport from the ER to the Golgi, while the corresponding retrograde transport step requires COPI vesicle formation [8]; clathrin-coated vesicles mediate multiple post-Golgi transport routes [9].
Our view of membrane trafficking is dominated by studies in animal and yeast cells. However, membrane trafficking is a central process underpinning growth, cell surface presentation and secretion. Thus it is critical to pathogenic mechanisms of many parasitic protists, for example by mediating host cell invasion [10] and immune system evasion [11]. It is therefore reasonable to ask what complement of membrane trafficking proteins is present across the broad diversity of eukaryotes and what we can infer about both evolution of the membrane trafficking system and the conserved set of eukaryotic membrane trafficking machinery, and how this has been modified in parasitic protists.
1.2 Evolution of membrane-trafficking: LECA complement and modern innovations
Comparative studies have allowed reconstruction of the gene complement of the last eukaryotic common ancestor, or LECA. The rationale is simple and powerful: if orthologues of a gene are identified in organisms covering the breadth of eukaryotic diversity, then parsimony dictates that gene was present in the LECA [1].
Three general patterns are observed. Some families, such as clathrin, retromer, COPI, and COPII are widely conserved and inferred present in the LECA; though few deviations from the ancestral complement of core machinery exist in extant organisms, some variability is seen in retention of accessory components [12–15]. Other families are more variable, for example the heterotetrameric adaptor protein complexes. The adaptor protein (AP) complexes 1 and 2 are well conserved, but AP-3 through AP-5 and TSET, a recently described member, while found in widely diverse taxa are frequently absent [16,17]. This is interpreted as ancestral presence in LECA and frequent subsequent loss of the latter complexes in extant eukaryotes. The third pattern, lineage-specific expansion, is exemplified by the Rab family, which reveals a patchy distribution in extant eukaryotes, but critically with new clades and paralogous expansion of conserved subfamilies arising in some lineages [18–20].
Hence, extant eukaryotes have gained and lost membrane trafficking machinery since diverging from LECA. Paralogous expansion and other lineage-specific features certainly provide machinery theoretically required for novel function and endomembrane specialization, but loss of machinery may also be involved in this process, and a full understanding necessitates comparison across eukaryotic diversity.
2 Emerging model organisms
Phylogenetics has resolved this eukaryotic diversity into five Supergroups, creating the necessary framework for comparative analyses (Figure 1). Despite increased knowledge of the taxonomic affiliation and cell biology of diverse eukaryotes, cell biological models remain biased towards the Supergroup Opisthokonta, namely humans and yeast (Figure 1, purple). Nonetheless, model organisms have been established across eukaryotes, including parasites, and many possess endomembrane features (proteins and organelles) not present in canonical models.
The multicellular plant Arabidopsis thaliana (Figure 1, green – Supergroup Archaeplastida) encodes a large genome with multiple paralogues for many membrane trafficking genes. A. thaliana has an endomembrane system largely similar to model opisthokonts. However, a key difference is the lack of a discrete early endosomal compartment, as internalized material is distributed to the TGN before being recycled or transiting the endosomal system for degradation in the vacuole [21–23].
The ciliate Tetrahymena thermophila (Figure 1, red – Supergroup SAR, which stands for Stramenopiles, Alveolates, and Rhizarians) is a ciliated heterotroph that engulfs prey in phagosomes that subsequently mature and undergo fission/fusion with other intracellular compartments before releasing their remaining contents. A prominent contractile vacuole is present for osmoregulation and dense core secretory granules underlie the plasma membrane. Canonical endomembrane compartments are present, though their intracellular location and arrangement differ from yeast and mammalian cells [24].
Also within the SAR Supergroup are the apicomplexan parasites Toxoplasma gondii and Plasmodium falciparum, causative agents of toxoplasmosis and malaria, respectively (Figure 1, red). These organisms possess a polarized endomembrane system including apical or “invasion” organelles, micronemes and rhoptries, to mediate host cell invasion and egress [25]. Apical organelles are likely divergent endo-lysosomes and other endo-lysosomal compartments, including an endosome-like compartment and vacuole, are also present, though the organization and identity of the endosomal system remains poorly understood [10,26,27].
Giardia lamblia, causative agent of giardiasis, is a member of the Supergroup Excavata possessing a reduced endomembrane system (Figure 1, brown). Giardia cells are bilaterally symmetric, possessing two diploid nuclei and four pairs of flagella. Aside from Golgi-like encystation-specific vesicles in encysting cells, the organism maintains only an ER and peripheral vacuoles, which perform functions associated with endo-lysosomes in model systems [28].
Another intensely studied group of excavates are the trypanosomatids (Figure 1, brown). Trypanosomes cause disease in humans, wild and domestic animals, insects, plants, and fish, as well as having free-living relatives, and hence have provided a wealth of data on genome evolution, cell biology, and mechanisms of interaction with, and adaptation to, their hosts [29]. Trypanosoma brucei is the organism of choice for dissection of tryapnosomatid cell biology, owing to the application of RNA interference and other technologies. Trypanosomes possess an endomembrane system similar to that in mammalian model systems, but differ in some aspects, such as restricting all endocytic uptake to a cellular region known as the flagellar pocket [30].
Entamoeba histolytica is a member of the Supergroup Amoebozoa (Figure 1, blue) with an unusual tubulovesicular endomembrane organization [31]. Consistent with its name, histolytica, this organism combines secreted virulence factors with cell killing via a specialized phagocytic process (trogocytosis) to induce host tissue damage and necrosis in the intestinal tract and liver [32]. Additionally, E. histolytica is capable of efficient whole-cell phagocytosis, but the exact mechanism is slightly different than in mammalian cells, involving fusion of nascent phagosomes with a pre-existing pre-phagosomal vacuole [33].
Dictyostelium discoideum (Figure 1, blue – Supergroup Amoebozoa) has a complex life cycle, encompassing unicellular amoebae that aggregate under starvation conditions to form transiently multicellular entities, first a bulbous slug, which then forms an elongated stalk structure known as a fruiting body from which to release spores [34]. The endomembrane system of D. discoideum is reminiscent of model organisms but also features non-acidic post-lysosomes and a prominent contractile vacuole [35]. Owing to ease of genetic manipulation, D. discoideum has contributed understanding to cellular processes including cell-cell adhesion, chemotactic signaling, cytoskeleton-dependent locomotion, cytokinesis, and, as a professional phagocyte, the formation and maturation of phagosomes as well [36].
3 Examining the case for functional homology
Prior to assessing functional homology it is worth defining our criteria, which we have divided into three categories of evidence.
Localization. Functional homology implies the gene product in question localizes to organelles or structures that are homologous in the respective cells.
Interactions. Functional homology implies that gene products should interact with homologous proteins, or in the case of other molecules, those of the same or similar molecular composition such as binding specific phosphoinositides or ions.
Genetic disruption. Functional homology implies that disruption should result in a similar phenotype between taxa. However, differences in cell physiology can make phenotypes difficult to directly compare and hence require careful interpretation.
4 Functional homology in trafficking machinery between divergent organisms
We have focused on proteins where broadly equivalent evidence from multiple organisms permits comparison of function in a relevant manner, including the adaptor proteins, ESCRT and retromer complexes, and finally select Rab GTPases.
4.1 Adaptor proteins
The adaptor protein complexes bind cargo proteins for inclusions into vesicular carriers that are then formed by the action of membrane-deforming coat proteins such as clathrin. There are five heterotetrameric adaptor complexes (AP-1 through AP-5) composed of two large (γ, α, δ, ε, ζ and β1–5), one medium (µ1–5), and one small subunit (σ1–5). They are related to other such complexes, including the coat-like TSET complex and the COPI coat [17]. We focus on AP-1 and AP-2, as the role of these complexes in mediating specific intracellular trafficking events together with clathrin is well established in model systems [9,37], and they are similarly the best-studied adaptor proteins in other organisms.
4.1.1 AP-1
In opisthokonts, the AP-1 complex is primarily localized to the TGN and early endosomes. It mediates transport between these organelles in both directions, but also mediates some trafficking between these organelles and the PM [38]. AP-1 interacts with clathrin and various monomeric adaptors, as well as trans-membrane receptors important for sorting biosynthetic endo-lysosomal cargo [39].
In A. thaliana AP-1 is primarily associated with the TGN/early endosome, as evidenced by co-localization with various markers for this organelle and correspondingly poor co-localization with markers of the Golgi or MVBs [40–42]. AP-1 subunits interact with clathrin heavy chain [40], the adaptor EPSIN1 [43], and two vacuolar sorting receptors [40,44]. Genetic disruption of AP-1 subunits results in defects in both vacuolar trafficking and TGN/early endosome to plasma membrane recycling [40–42].
Little is known about adaptor protein function in T. thermophila, but both AP-1µ subunit paralogues localize to distinct intracellular locations [45]. Early studies in T. gondii localized AP-1µ at the Golgi, endosome-like compartment, and rhoptries [46]. This is consistent with a recent study in P. falciparum showing the dynamic localization of tagged AP-1µ in puncta adjacent to the Golgi and rhoptries throughout the intracellular life cycle [47]. Expression of a dominant negative mu subunit in T. gondii causes mis-localization of the rhoptry protein ROP2 and impairs rhoptry formation, and AP-1µ both co-localizes, as well as interacts with, the vacuolar receptor TgSORTLR [46,48,49].
In G. lamblia, AP-1µ localizes to perinuclear regions and the cell periphery, in the latter case co-localizing with peripheral vacuole proteins, and can interact with clathrin [50]. AP-1µ also binds the vacuolar receptor Vps, and its knockdown by dsRNA induces degradation of Vps; this is specific to AP-1, as AP-2µ does not bind Vps [51]. Knockdown of AP-1µ also results in mis-localization of two peripheral vacuole proteins [50].
None of the AP complexes have been successfully localized in trypanosomes, and it is unclear why this may be so. AP-1 is involved in lysosomal delivery of p67, the major lysosomal glycoprotein, in T. brucei and there is evidence that this is developmentally regulated [52,53]. More recently AP-1 was implicated in sensitivity of T. brucei to suramin, an important frontline drug, and this appears to synergize with endocytosis of surface components, presumably to “condition” the lysosome in some manner to maintain sensitivity to the drug [52].
Though AP-1γ was identified in E. histolytica by proteomics to be associated with phagosomes, little else is currently known about its function [54]. In D. discoideum, AP-1γ localizes to phagosomes as well as multiple distinct intracellular puncta, some of which co-localize with the Golgi marker comitin [55,56]. Time course isolation of phagosomal membranes shows that AP-1 associates early and is subsequently lost over time [56]. As in model systems, AP-1 interacts with clathrin [55], but also the contractile vacuole protein Rh50 [57]. Consistent with these observations, knock out of AP-1µ results in secretion of unprocessed lysosomal enzymes, defects in phagocytosis and fluid phase uptake, and mis-localization of contractile vacuole markers [55,56].
4.1.2 AP-2
In animals and fungi, the AP-2 complex has a well-defined role in mediating clathrin-dependent endocytic uptake of specific cargo at the plasma membrane, often through interaction with other cargo adaptors [58].
The A. thaliana AP-2 complex is dynamically associated with the plasma membrane, as evidenced by a multitude of studies using tagged AP-2 subunits or specific antibodies [59–64]. Consistent with studies in model systems, various approaches indicate co-localization [59–62], and physical interactions [60–63], of AP-2 subunits with clathrin. In addition, AP-2a can interact with the C-terminal region of the monomeric clathrin adaptor AP180 [65]. Genetic disruption of AP-2 subunits, or use of chemical inhibitors of clathrin-mediated endocytosis, results in decreased endocytic uptake of specific plasma membrane cargo [59–62,64]. The severity of the resulting phenotype varies depending on the method of disruption, and this may be due to the role of the TPLATE/TSET complex in endocytosis in this lineage [64,66].
D. discoideum AP-2 localizes to distinct puncta near the cell surface which co-localize with clathrin; both AP-2 and clathrin also partially localize to the contractile vacuole network [67]. Similarly, the single beta subunit involved in both AP-1 and AP-2 complexes in D. discoideum localizes to the plasma membrane and also to intracellular structures [68]. Consistent with a role in endocytosis, AP-2 interacts with an Eps15-related protein [67], but also with the SNARE VAMP7 [69], which is known to associate with the contractile vacuole [70,71]. Oddly, knockout of AP-2 subunits does not affect the internalization of the contractile vacuole marker dajumin [67], or the localization of p25 or p80 endosomal markers [72]. Comparatively, knockout of the lone AP-1/2β subunit results in pleiomorphic defects, including impaired osmotic stress response [68], likely due to its function in both complexes.
Little is currently known regarding AP-2 function in other systems. T. thermophila AP-2µ co-localizes with a dynamin-related protein known to be important for endocytosis at the plasma membrane, as well as to contractile vacuole pores [45]. E. histolytica AP-2β was identified on isolated phagosomes by proteomics [54]. In G. lamblia, AP-2µ co-localizes with LysoTracker Red, which labels acidic organelles such as lysosomes, and also clathrin heavy chain, at peripheral vacuoles. Knockdown using dsRNA does not affect fluid phase uptake, but does impair receptor-mediated endocytosis [73]. AP-2 is absent in trypanosomatids that express the variant surface glycoprotein, which may represent an adaptation connected with very rapid endocytosis seen in African trypanosomes and critical for antigenic variation [11,74].
4.1.3 Functional homology in adaptor proteins
AP-1 mediates trafficking events between the Golgi, endosomes, and the PM, while AP-2 mediates endocytic uptake at the PM. Localization of these components in diverse eukaryotes is consistent with these roles: AP-1 and AP-2 in G. lamblia localize to peripheral vacuoles, which are thought to serve the function of endo-lysosomes, and potentially also the Golgi, and in both T. gondii and P. falciparum AP-1 localizes to the Golgi and endosomes. A role for AP-1 in phagosome function has been reported previously in murine macrophages [75], and this function may also be present in D. discoideum and E. histolytica. AP-1 and AP-2 in G. lamblia mediate trafficking to peripheral vacuoles from the ER and plasma membrane, respectively. Furthermore, interaction between Toxoplasma AP-1 and a vacuolar receptor, as well as a direct effect of AP-1 disruption on trafficking of rhoptry proteins, suggests AP-1 retains homologous function in Apicomplexa as well. AP-1 and AP-2 localize as expected in A. thaliana, and possess conserved roles in vacuolar trafficking and recycling, and endocytosis, respectively.
4.2 The ESCRT complexes
The endosomal sorting complexes required for transport (ESCRT) machinery mediate diverse processes from sorting of ubiquitylated cargo into intralumenal vesicles at MVBs to mediating cytokinesis and autophagy [76,77]. Of the five sub-complexes (ESCRTs 0,I,II,II,and IIIa), 0 is known to be opisthokont-specific while the others are found across eukaryotic diversity [78,79].
A. thaliana encodes all canonical ESCRT subunits, including multiple paralogues in many cases [80,81]. Specific antibodies against, or fluorescent fusions of, ESCRT-I [23,80] and ESCRT-II [23] components reveal primarily TGN/early endosome localization. C-terminal YFP fusions of ESCRT-III components partially co-localize with an MVB marker [82] and, although these fusions may not act in a physiological manner [82,83], additional work confirms an MVB localization for the ESCRT-IIIa component SKD1/Vps4 [82,84,85]. Hence, ESCRT components appear to be recruited sequentially during endosomal maturation. Functional disruption of ESCRT components results in aberrant vacuolar morphology, failure to degrade transmembrane vacuolar cargo, enlarged MVBs, impaired intralumenal vesicle formation, and impaired autophagy [82,84–88]. Additional plant-specific ESCRT components have been described [83,89– 93], the presence of which suggests that lineage-specific functional innovations are also present.
A lack of detailed characterization makes it unclear how a reduced ESCRT complement functions in Apicomplexa [78,94]. When expressed in either T. gondii or P. falciparum, the Plasmodium Vps4 orthologue is primarily cytosolic. Vps4 mutants predicted to be blocked in ATP binding or hydrolysis instead localize to distinct puncta, which co-localize with markers of the endosome-like compartment. Electron microscopy of these mutants reveal enlarged structures reminiscent of MVBs that are not observed in wild-type parasites [95].
G. lamblia encodes two paralogues of Vps46, one of which, Vps46A, localizes to the cytoplasm and shows intense signal near the plasma membrane, consistent with a possible role at peripheral vacuoles [96,97]. Furthermore, either paralogue is capable of restoring vacuolar sorting of carboxypeptidase S in a yeast Vps46 knockout [97], suggesting at least partial conservation of function between yeast and Giardia.
ESCRT components have been localized in trypanosomes, and as expected appear to be present at late endosomal compartments. This is consistent with the importance of ubiquitylation for turnover of surface molecules in T. brucei [78,98]. Whilst knockdowns suggest a role in trafficking of surface proteins in T. brucei, the impact is not strong, albeit this poor penetrance has also been observed in other eukaryotes. Although the absence of an endocytic blockade has been interpreted in trypanosomes as evidence for a divergent pathway for surface protein turnover [99], the paucity of data and clear soft phenotype obtained by knockdown at present make any firm conclusions unsafe.
In E. histolytica Vps4 localizes to small cytoplasmic puncta under normal conditions, but also surrounds ingested red blood cells following phagocytosis. An ATPase assay confirmed Vps4 ATPase activity, and overexpression of an enzymatically dead mutant impairs phagocytosis and the organism’s ability to cause hepatic abscesses in hamsters [100]. Three E. histolytica proteins contain a Bro1 domain, and thus may be homologues of Bro1/Vps31: ADH112, ADH112-like 1 and ADH112-like 2. Overexpressed ADH112 localizes to the plasma membrane and cytoplasmic vesicles and accumulates on MVBs, and can interact with the ESCRT subunit Vps32. Expression of exogenous Bro1 has a dominant negative effect on red blood cell phagocytosis [101], suggesting a possible role for ESCRT machinery in this process.
Tom1 has been proposed as an analogue of ESCRT 0 outside of opisthokonts, and in D. discoideum localizes to intracellular puncta distinct from p25 or p80 positive endosomes, and co-localizes with ubiquitin. It does interact with another ESCRT component Vps23/Tsg101, but also with ubiquitin, an Eps15-related protein, and clathrin [102]. Whereas Bro1/ALIX knockout cells cannot form spores or fruiting bodies [103], suggesting a possible function in differentiation or cytokinesis, Tom1 knockout cells do not show these defects, and display only mildly impaired fluid-phase uptake [102]. As such, the exact function of the ESCRT complexes in D. discoideum is currently unclear.
4.2.1 Functional homology in ESCRT complexes
Localization of Vps46 at peripheral vacuoles in Giardia is consistent with their putative homologous relationship to endo-lysosomes, and endo-lysosomal localization of ESCRT components in trypanosomes and A. thaliana has also been shown. The function of both Giardia Vps46 paralogues is sufficiently conserved to complement a yeast knockout, and ESCRT machinery in trypanosomes also appears to be functionally conserved. Functional conservation in A. thaliana has been convincingly demonstrated, as mutants fail to properly sort cargo and accumulate intralumenal vesicles that remain contiguous with the MVB bounding membrane. Localization of Entamoeba subunits Vps4 and ADH112 to both early and late phagosomal structures suggests some difference between E. histolytica and model systems, likely due to the unusual endomembrane organization in E. histolytica. Although alteration of Entamoeba Vps4 activity, or expression of exogenous Bro1, leads to defects in phagocytosis and pathogenicity, the exact function of the E. histolytica ESCRT machinery remains unclear. Further investigation into non-endocytic functions of ESCRT across eukaryotes may provide further insight into the patterns of subunit retention, for example in the Apicomplexa where conservation of ESCRT-III components may be due to a need for accurate cytokinesis and not be related to MVB formation.
4.3 Retromer
The retromer complex consists of a trimeric cargo-selective complex, comprising Vps26, Vps29, and Vps35, which interacts with sorting nexin (SNX) family proteins and other factors including Rab7 to mediate endosome-to-TGN and endosome-to-plasma membrane trafficking pathways [104,105]. One of the best-known functions of retromer, and that for which it was discovered, is recycling of the Vps10 cargo receptor [106].
A. thaliana encodes three copies of Vps35, two of Vps26, and a single copy of Vps29, together with SNX1, SNX2A, and SNX2B sorting nexins. The exact localization of retromer components has been disputed. VPS35, VPS29, and SNX2 co-localize with MVB/vacuole markers [107–113], while one study reported a primarily TGN localization for both VPS35 and SNX2A [114]. No VPS35 protein was detectable in Vps26 double mutants [110,115] while vps29 mutants have reduced levels of VPS35 [116], suggesting VPS35 stability is dependent on its presence in a complex. All three VPS35 genes can be disrupted, but triple null mutants are not viable; mutants in vps35a show different phenotypes from those in vps35b, suggesting sub-complexes exist with distinct functions [109,117,118]. Disruption of retromer function results in fragmented vacuoles, accumulation of vacuolar cargo precursors, and secretion of vacuolar cargo into the extracellular space, which in Arabidopsis constitutes a default pathway [109– 113,115,116,119]. Despite similarity in retromer trafficking compared to model systems, A. thaliana appears to possess a number of differences related to mechanisms of retromer subunit recruitment [110,113,115].
In T. thermophila only the Vps10 receptor has been investigated. Four Vps10/sortilin-like proteins, Sor1 through Sor4, are present. Sor4 stains cytoplasmic puncta distinct from secretory granules, but interacts with the secretory granule protein Grt1. Knockout of Sor4 causes mis-localization of two resident secretory granule proteins, as well as the aspartyl cathepsin protease CTH3, which is capable of processing secretory granule protein pro-domains [120,121].
The trimeric retromer complex in T. gondii co-localizes and interacts with the Vps10-like receptor TgSORTLR [48,49,122], and is involved in recycling between the endosome-like compartment and both the TGN and plasma membrane. In P. falciparum Vps29 and Vps35 localize to punctae throughout the intracellular lifecycle that are distinct from markers for the ER, Golgi, plastid, mitochondria, rhoptries, and micronemes [123]. Conversely, PfSORTLR co-localizes with the Golgi marker ERD2, indicating that the receptor is primarily present at the Golgi. Attempts to knockout retromer subunits in P. falciparum failed, suggesting the gene product is essential in intracellular parasites [123].
In G. lamblia Vps35 localizes to the cell periphery, consistent with peripheral vacuole localization, while Vps26 and Vps29 co-localize with the ER marker BiP; some partial co-localization between subunits is observed in a subset of peripheral vacuoles, and the observed localization patterns are further supported by sub-cellular fractionation. Vps35 co-localizes and interacts with the Vps10-like receptor Vps, and additionally interacts strongly with both Vps26 and Vps29 [124,125].
T. brucei encodes single orthologues of Vps26, Vps29, and Vps35, as well as a single SNX protein. Vps5 and Vps26 localize to the region between the nucleus and kinetoplast, consistent with endosomal localization. Additionally, Vps26 co-localizes with early endosomal markers including clathrin, Rab5A, Rab11, and EpsinR, and closely apposes signals for the MVB and lysosome. RNAi-mediated knockdown of these components exhibits mild defects in trafficking of p67 (lysosome) and ISG75 (plasma membrane), as well as Golgi fragmentation, suggesting a similar function of trypanosome retromer to that in mammalian and yeast systems [12].
In E. histolytica proteomic studies have identified Vps34, a PI-3-kinase known to regulate retromer function through generation of the phosphoinositide PI3P, on phagosomes [54]. Additionally, Vps26, Vps29, and Vps35 form a complex in vivo [126], and, together with Rab7A, retromer is likely involved in the maintenance of the pre-phagosomal vacuole [33,126]. These data point to a primary role for Entamoeba retromer in phagocytosis. Despite that D. discoideum possesses all retromer subunits [12], no functional data exist yet for retromer in this organism.
4.3.1 Functional homology in retromer
The localization of retromer across systems corresponds to its function in model organisms. Localization to pre-phagosomal vacuoles and phagosomes is consistent with their endo-lysosomal nature. However, differences in the localization of G. lamblia Vps35 and Vps26/Vps29 is at odds with their strong interaction and suggests a dynamic localization. Despite some studies indicating a primarily TGN localization of A. thaliana components, the bulk of evidence places retromer primarily at late endosomal compartments. The majority of evidence for retromer function in other non-model organisms is indirect, through characterization of the well-known retromer cargo Vps10/sortilin. Vps10 homologues mediate trafficking to secretory organelles in T. thermophila and Apicomplexa, and the G. lamblia homologue directly interacts with Vps35. Additionally, there is evidence for Vps10 homologues interacting with AP-1 in both G. lamblia and in T. gondii. This likely reflects AP-1 and retromer mediating distinct Vps10-dependent trafficking events, potentially anterograde and retrograde Golgi-endosome transport, respectively. In A. thaliana, where retromer has been better characterized, it appears to be important for vacuolar trafficking, as mutants secrete vacuolar cargo into the extracellular space via a default constitutive pathway.
4.4 Rab GTPases
While the above machinery is involved in vesicle formation, vesicle fusion machinery can similarly be assessed, perhaps most tractably the Rab GTPases. Like other GTPases Rabs cycle between GTP- and GDP-bound states. The state of the bound nucleotide has a direct effect on the conformation of the GTPase and regulates the ability of the GTPase to bind specific effector proteins [127]. Additional factors, e.g. guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), regulate the switch between bound nucleotide state, and can precisely regulate the intracellular location and concentration of GTP- and GDP-bound forms of specific GTPase proteins. Hence, Rabs are often referred to as “master regulators” or switches of processes, including membrane trafficking [128]. Three Rabs are well studied in many systems and have well-defined functions: Rab5, Rab7, and Rab11.
4.4.1 Rab5
In opisthokonts, Rab5 is present on early endosomal compartments and mediates the recruitment of effectors involved in the Rab5 to Rab7 switch important in endosome maturation [129,130]. Despite putative orthologues being present in their genomes, we could not find relevant characterization of Rab5 in either T. thermophila or D. discoideum, and a Rab5 orthologue has yet to be identified in G. lamblia [19].
A. thaliana encodes three Rab5 family proteins: RHA1/RABF2a, ARA7/RABF2b, and ARA6/RABF1. All three paralogues label endosomes, with RHA1 and ARA7 co-localizing, while ARA6 shows variable overlap with either RABF2 protein [131–137]. These likely represent endosomal populations, with RABF2 variants acting at MVBs and RABF1 at a variant of recycling endosomes. Constitutively active ARA6 localizes to the plasma membrane [131,133], and ARA6 co-localizes with endocytosed plasma membrane proteins [133], and yet, unlike RHA1 and ARA7, is not associated with vacuolar targeting of soluble cargo [133,138].
T. gondii and P. falciparum both encode three Rab5 paralogues, Rab5A, Rab5B, and Rab5C. Tagged versions of each paralogue in T. gondii revealed localization consistent with the endosome-like compartment [139–142], and overexpression of all three paralogues ablate parasite growth. However, only functional disruption of Rab5A or Rab5C result in mis-localization of a subset of microneme and rhoptry proteins [139]. Though Rab5B function is unknown, it is present in a retromer interactome [122].
In contrast, P. falciparum Rab5A is localized to haemoglobin-containing structures [143,144]. Expression of a constitutively active Rab5A increases haemoglobin uptake and food vacuole size, consistent with a role in endocytic uptake [143]. Rab5B, localizes to the plasma membrane and food vacuole of intracellular parasites [144]. Though Rab5B localization is consistent with an endocytic role, its function is currently unclear; it is essential in Plasmodium, despite the presence of both Rab5A and Rab5C paralogs, suggesting these paralogues do not possess redundant function [144].
All trypanosomatids encode two Rab5 paralogues, which represent a lineage-specific duplication. Both are essential, and critical for endocytosis of surface components in T. brucei [145,146]. Significantly, these two paralogues apparently mediate the trafficking of distinct cargo proteins [147], but the basis for the targeting of a molecule to a Rab5A or Rab5B-specific route, or the functional need for such a division, has remained elusive.
In E. histolytica Rab5 was identified on phagosomal membranes, albeit only at different time points and dependent on the material taken up [148,149], suggesting a similar association of Rab5 with phagosomes as seen in model systems, but also a potential for complex and dynamic regulation. Additionally, Rab5 associates with Rab7 in pre-phagosomal vacuoles in resting cells. Different from a model view of Rab5 localization though, assays using the fluid-phase marker FITC-dextran suggest that Rab5 does not localize to early endosomal structures in E. histolytica [33], in contrast to what has been observed in mammalian cells [150].
4.4.2 Rab7
In opisthokonts, Rab7 is present on mature endosomes, MVBs, and lysosomes, as well as on phagosomes. It is involved in recruitment of the HOPS tethering complex to ensure regulated fusion with the degradative compartment [151,152], as well as the retromer complex to ensure recycling of components prior to terminal degradation [105].
A. thaliana encodes eight putative Rab7 family proteins belonging to the RABG3 group, suggesting the potential for redundancy and/or novel functions. RABG3f primarily co-localizes with MVB and vacuole markers, and expression of a dominant negative version causes fragmentation of the vacuole and inhibits vacuolar trafficking [153]. RABG3b is involved in autophagic processes such as cell death and differentiation during growth and pathogen response [154,155]. Some functional redundancy likely exists, as various quintuple and sextuple mutants show phenotypic defects but remain viable [136].
Rab7 has not been extensively characterized in T. thermophila, but is present in a phagosome proteome [156], and tagged Rab7 is present both as bright puncta on phagosomes, as well as structures containing LysoTracker Red [157].
In T. gondii Rab7 localizes in the late secretory system of the parasite, and partially co-localizes with various markers of the endosome-like compartment and vacuole, but is distinct from both Rab5A and the Golgi protein GRASP [139,141,158]. Parasites overexpressing Rab7, or expressing constitutively active or dominant negative versions of Rab7, exhibit growth defects but no obvious trafficking defects [139]; this is at odds with an interaction between active Rab7 and the retromer component Vps26 [122]. Hence, the function of Rab7 in T. gondii is unclear.
P. falciparum Rab7 localizes primarily to distinct puncta throughout the intracellular life cycle that partially co-localize with the retromer component Vps35 but is distinct from Golgi-associated Rab6. Expression of constitutively active or dominant negative versions, similar to T. gondii, showed no appreciable trafficking defect [123].
As with Rab5, we could not find report of a Rab7 orthologue in G. lamblia. Trypanosomes retain a single Rab7 paralogue, which closely juxtaposes to the lysosome. Knockdown of TbRab7 impairs uptake of a subset of endocytic cargo, but does not appear to affect the delivery of biosynthetic lysosomal cargo [159].
E. histolytica has multiple Rab7 paralogues. Rab7A through Rab7E are present by proteomic analysis on phagosomal membranes at multiple time points [148], and, as previously mentioned, Rab7 associates with Rab5 at pre-phagosomal vacuoles and interacts with the retromer complex [33,126]. Overexpression of Rab7 results in enlarged intracellular vesicles, and an overall increase in cell acidity, but no apparent defect in phagocytosis or endocytosis [126]. Though four Rab7 paralogues are present in a cell surface proteome their localization and function has yet to be fully elucidated [160].
In D. discoideum Rab7 has been localized to phagosomes by proteomics of isolated organelles [70,161,162]. By microscopy, Rab7 localizes to phagosomes, macropinosomes, lysosomes, and post-lysosomes [163–165]. Expression of a dominant negative Rab7 inhibits macropinocytosis and phagocytosis [163,165], and prevents delivery of endo-lysosomal components, yet enhances the delivery of unprocessed proteases and sugar-linked proteins, to maturing phagosomes [164].
4.4.3 Rab11
In opisthokonts Rab11 is primarily involved in recycling of cell surface proteins, but also plays a role in other cellular processes including innate immune responses, delivery of components to the cleavage furrow during cytokinesis, and ciliogenesis, at least in mammalian cells [166,167].
The Rab11 subfamily is highly expanded in A. thaliana, with 26 putative members divided into six sub-groups, RABA1 through RABA6. RABA1 members display dynamic localization between the TGN, endosomes, and plasma membrane [168–170], suggestive of a possible recycling function; consistent with this, RABA1b mutants show hypersensitive intracellular aggregation of plasma membrane proteins in response to Brefeldin A [168], and the RABA1 quadruple mutant is sensitive to salinity stress [170,171]. All RABA2 and RABA3 members appear to localize to the same compartment, which is distinct from the Golgi and late endosomes, but does overlap with markers of the TGN and other Rab11 members [168,172]. During cell division, various RABA members re-locate to the cell plate, where they co-localize with KNOLLE, a SNARE involved in cytokinesis [172]. Consistent with this, cell wall analysis revealed a decrease in specific constituents in rabA2b, rabA2d, and three rabA4 mutants [173]. Additionally, RABA4 members localize to the tip area of growing cells [174–177], where they interact with PI-4-kinases and phosphatases [174–178] to mediate polarized growth; RABA4c also plays a role in recycling of plasma membrane receptors [169].
T. thermophila encodes multiple Rab11 paralogues, one of which, Rab11A, labels posterior to anteriorly directed vesicles, which may represent recycling endosomes, and also partially labels the contractile vacuole [157].
A proteomic study of isolated rhoptries in T. gondii revealed the presence of Rab11A in this compartment [179]. Confirming this, Rab11A partially co-localizes with the rhoptry protein ROP5, but also with endosome-like compartment markers. Expression of a dominant negative Rab11A does not affect invasion organelles, endosymbiotic organelles, or the Golgi, but prevents delivery of late stage components of a plasma membrane-associated complex termed the IMC, and results in defective cell division [180]. Rab11B, the other Rab11 paralogue, co-localizes with a Golgi marker in resting parasites, but relocates to the IMC in developing daughter cells. Expression of a dominant negative Rab11B shows a similar defect in cell division as Rab11A, albeit due to distinct trafficking pathways with different timing [181], and Rab11B is also present in a retromer interactome [122].
Similar to T. gondii, Rab11A was found to localize in discrete puncta throughout the intracellular lifecycle of P. falciparum, some of which co-localize with the resident rhoptry protein Rhop2 and the IMC protein GAP45 [180].
The single Rab11 in G. lamblia is present in puncta or stacks in cells preparing to encyst, and at the cell periphery in mature cysts, where it co-localizes with the cyst wall protein CWP1. Ribozyme-mediated knockdown results in a decrease in CWP1 present in encystation-specific vesicles, instead being present in numerous cytoplasmic puncta, suggesting a trafficking defect [182].
Rab11 is a major regulator of recycling pathways in African trypanosomes. Turnover of surface proteins in T. brucei is strongly influenced by Rab11, while extensive disruption of endocytic pathways follows Rab11 knockdown. Furthermore the underlying interactome for Rab11 is divergent between trypanosomes and mammalian cells; FIP proteins that mediate Rab11 function in mammalian cells are absent, and at least one trypanosome-specific interacting protein has been identified [183]. In T cruzi Rab11 mediates an unusual pathway that traffics the critical trans-sialidase surface protein family to the surface, but which is via the contractile vacuole [184]. This suggests that the diversification of function within trypanosomes is often cryptic, and as discussed above, can depend on the precise cellular configuration.
In E. histolytica Rab11 is enriched in endosomal fractions [185], but microscopy revealed localization in small cytoplasmic vesicles, and a lack of co-localization with phagocytosed E. coli, endocytosed transferrin, or markers of the ER or Golgi [186]. Similarly, Rab11B is associated with non-acidified compartments that are distinct from the ER, early endosomes, and lysosomes. Rab11B overexpression enhances exocytosis of fluid phase markers, intracellular and secreted cysteine protease activity, and improves killing efficiency, suggesting a potential role in recycling and release of pathogenesis factors [187].
Multiple Rab11 paralogues exist in D. discoideum. Rab11A localizes to the contractile vacuole network, and also co-localizes, as well as interacts with, the contractile vacuole-associated ion channel P2XA [188]. A previous study identified Rab11 in contractile vacuole-associated fractions by blotting, and co-localized Rab11 with other markers of the contractile vacuole network [189]. Overexpression, or expression of a dominant negative version, of Rab11 results in aberrant contractile vacuole morphology and impaired osmotic stress response [188,189]. Correlative data suggests that Rab11A and Rab11C may be involved in delivery of a V-ATPase to phagosomes [190], which is consistent with their identification in a proteomic analysis of purified phagosomes [162].
4.4.4 Functional homology in Rab GTPases
Rab5 and Rab7 have well defined localisations and functions in model systems, and the Rab5 to Rab7 switch is a paradigm for dynamic protein association during organelle maturation. The localization and function of Rab5 in trypanosomes is consistent with a canonical role, while the role of Rab5A and Rab5C in trafficking to T. gondii apical organelles is conserved when these organelles are viewed as derived endo-lysosomes. Similarly, Rab7 performs the expected function in trypanosomes, and its localization in Apicomplexa to compartments homologous to late endosomes/lysosomes, is also consistent with model systems. Paralogous expansion of both Rab5 and Rab7 in A. thaliana complicates assessment of functional homology, including the role of ARA6 in recycling traffic, though overall localization and function imply conservation. Studies in D. discoideum and E. histolytica suggest that Rab5 and Rab7 maintain a conserved role in the function and maturation of compartments derived from internalization of extracellular material
Rab11 primarily mediates trafficking through recycling endosomes. Entamoeba Rab11 is present at compartments distinct from early and late endosomes, potentially in a recycling endosome, which is consistent with the increased exocytosis noted in cells overexpressing Rab11B. Similarly, T. brucei Rab11 is important for recycling traffic. The primary role of Rab11 in G. lamblia, T. gondii, and P. falciparum can generally be described as delivery of cargo to structures adjacent to the plasma membrane. The unique mechanisms by which apicomplexan parasites undergo cell division (endodyogeny in T. gondii and schizogeny in P. falciparum) are important when assessing functional homology. In these organisms progeny emerge from within the mother cell, mediated in part through the specific and timely IMC formation [191–193], which is mediated by both Rab11 paralogues. This is reminiscent of the regulatory role for Rab11 in animal cell cytokinesis, together with exocyst [194]. The extensive diversification of the Rab11 family in A. thaliana is unprecedented in other eukaryotes, but some members possess functions such as recycling and trafficking of plasma membrane and cell wall constituents during cell division and polarized cell growth.
Rab11 may be involved in contractile vacuole function in both D. discoideum and T. thermophila. The contractile vacuole is an enigmatic organelle present in a subset of organisms across eukaryotic diversity though it is not yet established whether these are homologous or analogous. A role for Rab11 in the function of this compartment is consistent with exocyst involvement in the contractile vacuole of D. discoideum, as well as the unicellular archaeplastid Chlamydomonas reinhardtii [195–197]. Additionally, Rab11 has been identified in proteomic studies of the contractile vacuole in T. cruzi [198], and recycling traffic appears to transit this organelle [184]. Finally, though current evidence is limited, Rab11 also appears to play a role in trafficking to phagosomes in D. discoideum. This is consistent with recent studies suggesting such a role for Rab11 and exocyst in phagosome maturation in endothelial cells [199].
5 Discussion
5.1 Overview
With the increasing ease and prevalence of comparative genomics, the validity of assuming functional homology is both critical to assess and fruitful to explore. First and foremost, the simple conclusion from our comparative survey is that yes, orthology does appear to translate into functional homology. However, this is complicated by many factors, and needs to be taken as a first foray into this kind of assessment, and not a question laid to rest.
Firstly, despite considerable efforts to expand experimental investigation into non-model eukaryotes, there are still large gaps in our knowledge base, as evidenced by the fact that we were only able to find comparable molecular cell biological data for a small set of membrane-trafficking genes, essentially all within the endocytic system. Future studies expanding into the secretory system and encompassing machinery identified in diverse eukaryotes but that is absent or diverged in opisthokont taxa, for example the TSET complex and novel ArfGAP subfamily ArfGAPC2 [17,200], will aid in correcting the asymmetrical bias on opisthokonts in our models of membrane-trafficking.
Nonetheless, this basic position of functional homology enables hypotheses to be generated and tested to better understand the effect of paralogous expansion and accretion of novel factors. Additionally, our comparative analysis indicates that considering differences in endomembrane organization and trafficking pathways (e.g. the presence of unique organelles or expanded trafficking pathways), is essential to assessment of both functional homology and novelty among lineages.
5.2 Functional homology of trafficking machinery in diverse eukaryotes
Our pan-eukaryotic comparisons highlight the plasticity of the endomembrane system, not only in parasites, which possess modifications concurrent with their unique pathogenic mechanisms, but also in free-living taxa, and this plasticity must be considered in order to properly assess functional homology.
Perhaps the best example is G. lamblia, where the peripheral vacuoles correspond to, and encompass the function of, diverse endo-lysosomes present in model systems. Hence, localization of a plethora of machinery, including AP-1, AP-2, ESCRT, and retromer to these structures is consistent with conserved function, though coincident localization of all these factors in other cells would be unusual.
Understanding trafficking in higher plants requires consideration of the unique organization of their endocytic system, namely that of a combined TGN/early endosome. Some phenotypes, such as aggregation of plasma membrane receptors in response to Brefeldin A, make sense only in the context of this feature. Additionally, the endosomal system in these organisms is likely more complex than has been fully appreciated in previous studies: MVBs appear to bud directly from the TGN/early endosome [23], incomplete co-localization of endosomal markers suggests existence of sub-populations, and a recent study has suggested at least three distinct pathways exist for the movement of cargo from the TGN/early endosome to the vacuole [136].
The organization of the apicomplexan endomembrane system shows significant lineage-specific divergence. The role of a Vps10-like receptor, Rab5A and Rab5C, AP-1, and retromer in mediating apical organelle biogenesis appears at odds with canonical functions for these proteins. However, apical organelles are homologous to endo-lysosomes, and some evidence points to a plant-like organization for the TGN/endosome-like compartment. Hence, these factors can be understood to mediate both anterograde and retrograde transport through an intermediate compartment within the endosomal system, and their function is thus conserved.
In E. histolytica, as in humans, Rab5 and Rab7 are involved in phagocytosis, yet Rab5 does not appear to be involved in endocytosis. Subunits of the AP-1 and AP-2 complexes, as well as retromer, are found at phagosomes, and, while this may seem superficially like a case of neofunctionalization, is consistent with a role for AP-1 in phagocytosis in murine macrophages [75], and evidence for roles for both AP-2 and retromer in phagocytic clearance of apoptotically killed cells in Caenorhabditis elegans [201,202]. Therefore, many seemingly non-canonical functions of trafficking factors in E. histolytica may represent specialization common to professional phagocytic cells.
5.3 Evolutionary precedent of conserved and novel features
The cell biological complement in the LECA served as initial building blocks for environmental adaptation during eukaryotic radiation, including in parasites. It is likely that drastic alterations from an established state would be selected against, unless the environment was radically different than that encountered by previous generations. This both explains the gross underlying pattern of functional homology and provides a precedent for trafficking system modification.
In many cases, such as Giardia, apicomplexans and to a lesser extent kinetoplastids, parasites have reduced their membrane-trafficking gene complements [29,94,203], often interpreted as jettisoning unnecessary or redundant pathways. Further experimental characterization will be needed to determine the extent to which this interpretation bears out. By contrast, other taxa, such as Entamoeba, Dictyostelium and Tetrahymena, have expanded their complements. In cases where multiple paralogues exist, some may possess a similar basic function, but may do so only in specific life cycle stages, or only in a restricted region of the cell, allowing for polarized trafficking and specialization.
We argue that this latter mode of innovation in the trafficking system is best viewed as an extension of the Organelle Paralogy Hypothesis [204]. Just as the process of gene duplication and co-evolution of identity encoding machinery is proposed to have given rise to the basic set of membrane-trafficking organelles prior to the LECA [205], the same process should continue to act in extant eukaryotes. Hence new organelles may arise from an ancestral compartment through concurrent duplication and co-evolution of the underlying identity-encoding trafficking machinery, such that the machinery acquires specific features for this role. This may include specific trafficking signals, the ability to bind to specific proteins or phosphoinositides, and additionally they may be further regulated by specific factors such as GEF and GAP proteins.
By extending this to descendants of a lineage in which the organelle arose, particularly when the homologous organelle is present and its function required, some paralogues that arose concurrently with it would be maintained and constrained to performing required functions for organelle biogenesis and/or maintenance, and hence will be functionally homologous. However, in descendants no longer possessing the organelle or its required function, or in cases of further expansion, regardless of the presence or absence of a homologous organelle, paralogues are unconstrained and may acquire new function. Hence, despite a conserved set of organelles and machinery inferred in the LECA, extant eukaryotes display an array of unique features. This not only applies to the endomembrane system, as we have described here, but also likely extends across cellular systems.
Although we can be relatively optimistic in assuming functional homology within the membrane trafficking system, equivalent assessments may or may not show the same thing in other cellular systems; the question is certainly worth asking.
5.4 Conclusions and future perspectives
In conclusion, despite considerable divergence in cellular systems among diverse eukaryotes since the LECA, efforts to map function on the basis of comparative genomic data appear to be well founded. Our literature review revealed that functional homology is present in membrane trafficking system machinery in several taxa spanning eukaryotic diversity and encompassing both free-living and parasitic organisms. This allows for some further degree of confidence in continued molecular evolutionary and comparative genomic analysis as well as providing a lens through which to view the unique cell biological adaptation present in each organism in order to fully appreciate how these systems may differ. In particular, expanding this analysis across systems between parasites and their hosts can be expected to provide valuable insight into the complex interactions between them.
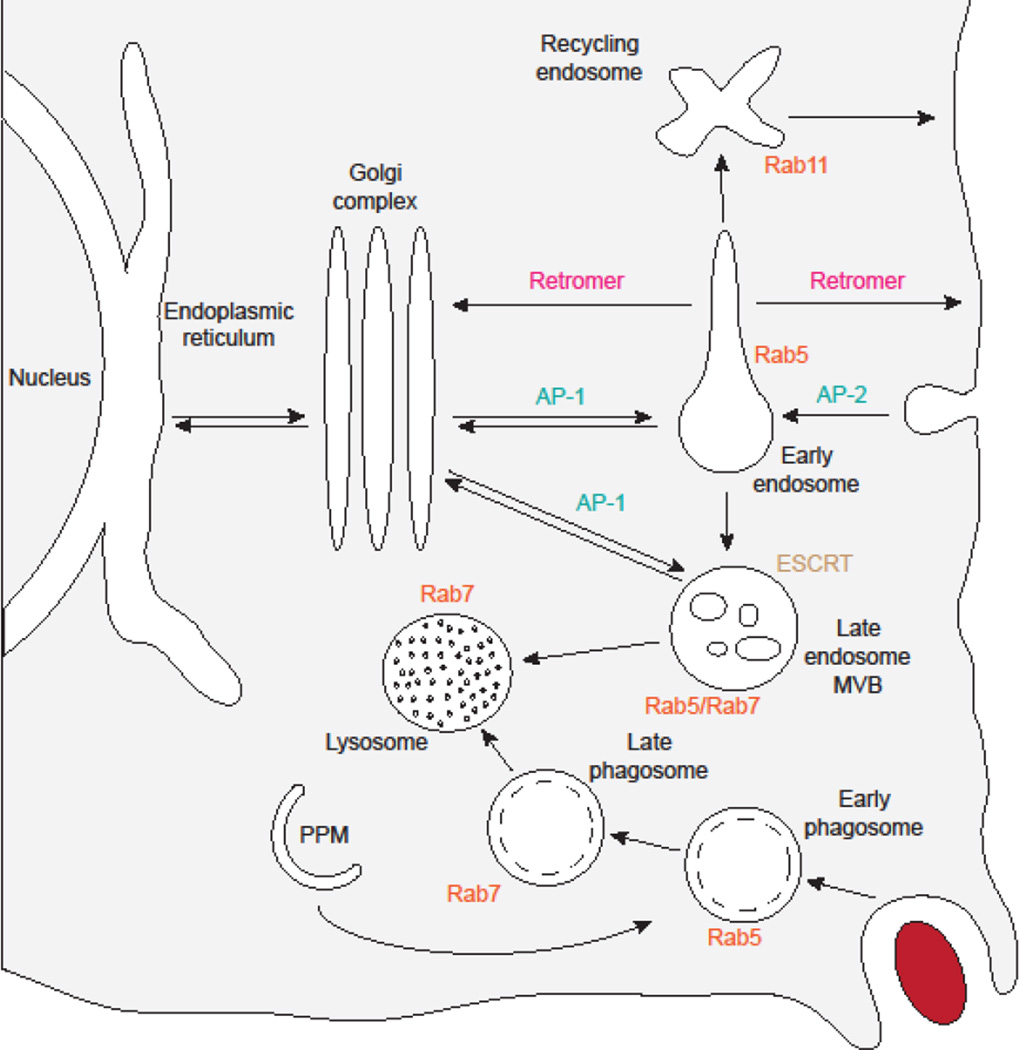
Figure 2. Function of select membrane-trafficking machinery in a model endomembrane system.
This figure depicts roles for membrane-trafficking system machinery under discussion in a generalized eukaryotic cell, based on studies primarily in yeast and mammalian systems. Components are colour-coded, with adaptor proteins (AP, teal), ESCRT (brown), retromer (magenta), and Rab GTPases (orange). Organelles are depicted based on common morphology and labeled in plain text. Arrows, including the directionality of each step, indicate trafficking between organelles. The presence of a dotted line in the interior of phagosomes represents the presence of either a single bounding membrane (phagosomes), or two bounding membranes (autophagosomes). The red oval represents a particle to be phagocytosed. Additional machinery is required for each trafficking event shown, but for simplicity is not included in this diagram. Note that not all organisms perform the illustrated trafficking events (eg. phagocytosis has not yet been reported in apicomplexans or kinetoplastids), and other events occur that are not depicted in this diagram.
Table 1.
Functional homology across model systems.
| A. thaliana | T. thermophila | T. gondii & P. falciparum |
G. lamblia | T. brucei | E. histolytica | D. discoideum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Com | Evi | Des | Ref | Des | Ref | Des | Ref | Des | Ref | Des | Ref | Des | Ref | Des | Ref |
| AP-1 | Loc | TGN, endosomes |
[40–42] | Puncta | [45] | Golgi, ELC | [46,47] | PV | [50] | Phagosomes | [54] | Phagosomes, Golgi |
[55, 56] |
||
| Fxn | Vacuolar delivery, PM recycling |
[40–42] | Mic/Rhop biogenesis |
[46] | PV trafficking |
[50, 51] |
Lysosomal delivery |
[52,53] | Phagocytosis, CV, lysosomal delivery |
[55– 57] |
|||||
| AP-2 | Loc | PM, puncta | [59–64] | CV, basal bodies |
[45] | PV | [73] | Phagosomes | [54] | PM, CV, puncta | [67, 68] |
||||
| Fxn | Endocytosis | [59–62,64] | Endocytosis, cyst formation |
[73] | |||||||||||
| ESCRT | Loc | TGN, endosomes, MVBs |
[23,80, 82–85] |
Vps4 cytosolic |
[95] | PV | [96, 97] |
LE/MVB | [78] | Phagosomes, MVBs |
[100, 101] |
Intracellular puncta |
[102] | ||
| Fxn | Vacuolar delivery, autophagy |
[82,84–88] | Vacuolar delivery |
[78] | Phagocytosis | [101] | Differentiation | [103] | |||||||
| Retromer | Loc | TGN, endosomes |
[107–114] | Vps10- like puncta |
[120] | TGN,ELC | [122, 123] |
PV, ER | [124, 125] |
Endosomes | [12] | ||||
| Fxn | Vacuolar delivery |
[109– 113,115, 116,119] |
DCG biogenesis |
[120, 121] |
ELC to TGN and PM recycling |
[122] | Vacuolar delivery |
[12] | PPV maintenance |
[33, 126] |
|||||
| Rab5 | Loc | Endosomes | [131–137] | ELC, PM | [139–144] | Endosomes | [145– 147] |
Phagosomes, PPVs |
[33, 148, 149] |
||||||
| Fxn | Vacuolar delivery, recycling |
[131,133, 138] |
Mic/Rhop and DV trafficking |
[139, 143, 144] |
Endocytosis | [145– 147] |
PPV maintenance |
[33] | |||||||
| Rab7 | Loc | Endosomes, vacuole |
[153] | Phagosomes | [156, 157] |
ELC, VAC | [122,123, 139,141, 158] |
LE/MVB | [159] | Phagosomes, PPVs |
[33, 148] |
Phagsosomes, late endocytic |
[70, 161– 165] |
||
| Fxn | Vacuolar delivery, autophagy |
[153–155] | Vacuolar delivery |
[159] | PPV maintenance |
[33, 126] |
Phagocytosis, lysosomal delivery |
[163– 165] |
|||||||
|
Rab 11 |
Loc | PM,TGN, endosomes |
[168– 170,172, 174–177] |
Endosomes | [157] | Rhops, PM (IMC) |
[179–181] | Puncta, PM |
[182] | Endosomes | [183, 184] |
Endosomes, puncta |
[185– 187] |
CV, phagosomes |
[162, 188, 189] |
| Fxn | Cytokinesis, PM trafficking |
[168–178] | Cell division | [180, 181] |
Cyst formation |
[182] | Recycling | [183, 184] |
Recycling | [187] | CV function, osmotic stress |
[188, 189] |
|||
This table provides a brief summary of the evidence for functional homology for select membrane trafficking components across discussed model organisms. Trafficking machinery is listed by row and organisms by column. For each component listed, the major localization and presumed function are listed, with appropriate references for each; for more extensive description of the underlying evidence please see the relevant main text section(s).
Abbreviations: Com, component; Evi, evidence; Des, description; Ref, references; Loc, localization; Fxn, function; PM, plasma membrane; TGN, trans-Golgi network; MVB, multi-vesicular body; LE, late endosome; CV, contractile vacuole; DCG, dense core granule; ELC, endosome-like compartment; Mic, microneme; Rhop, rhoptry; VAC, vacuolar compartment; DV, digestive vacuole; IMC, inner membrane complex; PPV, pre-phagosomal vacuole. Blank cells are present where components are either unknown or no evidence exists.
Highlights.
-
-
Genomics enables powerful advances in molecular and evolutionary parasitology
-
-
Diverse model parasites allows for comparison of membrane-trafficking proteins
-
-
Functional homology is largely observed in the membrane-trafficking system
-
-
Endomembrane organization in poorly studied eukaryotes can be confidently inferred
-
-
Unusual endomembrane organelles can be understood through relationships with canonical ones
Acknowledgments
We wish to thank members of the Dacks lab for fruitful discussions. Work in the Dacks lab is supported by a Discovery Grant RES0021028 from the Natural Sciences and Engineering Research Council. JBD is the Canada Research Chair in Evolutionary Cell Biology. CMK is funded by an Alberta Innovates Health Solution Fulltime Studentship and a Canada Vanier Graduate Scholarship. His research has been funded in part by the generosity of the Stollery Children’s Hospital Foundation and supporters of the Lois Hole Hospital for Women through the Women and Children’s Health Research Institute. EKH is funded by an Alberta Innovates Health Solution Fulltime Studentship and a Canada Vanier Graduate Scholarship. Work in the Turkewitz Lab is funded by NIH grant NIH-RO1 GM105783.
Abbreviations
- AP
Adaptor protein
- ESCRT
Endosomal sorting complexes required for transport
- SNARE
Soluble N-ethyl-maleimide-sensitive factor attachment protein receptors
- Rab
Ras from brain
- Vps
Vacuolar protein sorting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koumandou VL, Wickstead B, Ginger ML, van der Giezen M, Dacks JB, Field MC. Molecular paleontology and complexity in the last eukaryotic common ancestor. Crit. Rev. Biochem. Mol. Biol. 2013;48:373–396. doi: 10.3109/10409238.2013.821444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitch WM. Distinguishing homologous from analogous proteins. Syst. Zool. 1970;19:99–113. [PubMed] [Google Scholar]
- 3.Koonin EV. Orthologs, Paralogs, and Evolutionary Genomics. Annu. Rev. Genet. 2005;39:309–338. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 5.Barlowe CK, Miller EA. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193:383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Linero AM, Muñiz M. Membrane trafficking: Returning to the fold(ER) Curr. Biol. 2015;25:R288–R290. doi: 10.1016/j.cub.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Robinson MS. Forty Years of Clathrin-coated Vesicles. Traffic. 2015;16:1210–1238. doi: 10.1111/tra.12335. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Ruiz E, Morlon-Guyot J, Daher W, Meissner M. Vacuolar protein sorting mechanisms in apicomplexan parasites. Mol. Biochem. Parasitol. 2016:1–8. doi: 10.1016/j.molbiopara.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manna PT, Boehm C, Leung KF, Natesan SK, Field MC. Life and times: synthesis, trafficking, and evolution of VSG. Trends Parasitol. 2014;30:251–258. doi: 10.1016/j.pt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koumandou VL, Klute MJ, Herman EK, Nunez-Miguel R, Dacks JB, Field MC. Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J. Cell Sci. 2011;124:1496–1509. doi: 10.1242/jcs.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlacht A, Dacks JB. Unexpected ancient paralogues and an evolutionary model for the COPII coat complex. Genome Biol. Evol. 2015;7:1098–1109. doi: 10.1093/gbe/evv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field MC, Sali A, Rout MP. Evolution: On a bender-BARs, ESCRTs, COPs, and finally getting your coat. J. Cell Biol. 2011;193:963–972. doi: 10.1083/jcb.201102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field MC, Gabernet-Castello C, Dacks JB. Reconstructing the evolution of the endocytic system: insights from genomics and molecular cell biology. Adv. Exp. Med. Biol. 2007;607:84–96. doi: 10.1007/978-0-387-74021-8_7. [DOI] [PubMed] [Google Scholar]
- 16.Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MNJ, Dacks JB, et al. The fifth adaptor protein complex. PLoS Biol. 2011;9:e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirst J, Schlacht A, Norcott JP, Traynor D, Bloomfield G, Antrobus R, et al. Characterization of TSET, an ancient and widespread membrane trafficking complex. Elife. 2014;3:e02866. doi: 10.7554/eLife.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias M, Patron NJ, Keeling PJ. The RAB family GTPase Rab1A from Plasmodium falciparum defines a unique paralog shared by chromalveolates and rhizaria. J. Eukaryot. Microbiol. 2009;56:348–356. doi: 10.1111/j.1550-7408.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 19.Elias M, Brighouse A, Gabernet-Castello C, Field MC, Dacks JB. Sculpting the endomembrane system in deep time: high resolution phylogenetics of Rab GTPases. J. Cell Sci. 2012;125:2500–2508. doi: 10.1242/jcs.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diekmann Y, Seixas E, Gouw M, Tavares-Cadete F, Seabra MC, Pereira-Leal JB. Thousands of rab GTPases for the cell biologist. PLoS Comput. Biol. 2011;7:e1002217. doi: 10.1371/journal.pcbi.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase Activity Is Required for Endocytic and Secretory Trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viotti C, Bubeck J, Stierhof Y-D, Krebs M, Langhans M, van den Berg W, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, et al. Multivesicular Bodies Mature from the Trans-Golgi Network/Early Endosome in Arabidopsis. Plant Cell. 2011;23:3463–3481. doi: 10.1105/tpc.111.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briguglio JS, Turkewitz AP. Tetrahymena thermophila: A divergent perspective on membrane traffic. J. Exp. Zool. B. Mol. Dev. Evol. 2014;322:500–516. doi: 10.1002/jez.b.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum J, Gilberger T-W, Frischknecht F, Meissner M. Host-cell invasion by malaria parasites: insights from Plasmodium and Toxoplasma. Trends Parasitol. 2008;24:557–563. doi: 10.1016/j.pt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Ngô HM, Yang M, Joiner KA. Are rhoptries in Apicomplexan parasites secretory granules or secretory lysosomal granules? Mol. Microbiol. 2004;52:1531–1541. doi: 10.1111/j.1365-2958.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- 27.Klinger CM, Nisbet RE, Ouologuem DT, Roos DS, Dacks JB. Cryptic organelle homology in apicomplexan parasites: insights from evolutionary cell biology. Curr. Opin. Microbiol. 2013;16:424–431. doi: 10.1016/j.mib.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faso C, Hehl AB. Membrane trafficking and organelle biogenesis in Giardia lamblia: use it or lose it. Int. J. Parasitol. 2011;41:471–480. doi: 10.1016/j.ijpara.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Jackson AP, Otto TD, Aslett M, Armstrong SD, Bringaud F, Schlacht A, et al. Kinetoplastid Phylogenomics Reveals the Evolutionary Innovations Associated with the Origins of Parasitism. Curr. Biol. 2016;26:161–172. doi: 10.1016/j.cub.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field MC, Carrington M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira JE, Huston CD. Evidence of a continuous endoplasmic reticulum in the protozoan parasite Entamoeba histolytica. Eukaryot. Cell. 2008;7:1222–1226. doi: 10.1128/EC.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ralston KS. Taking a bite: Amoebic trogocytosis in Entamoeba histolytica and beyond. Curr Opin Microbiol. 2015;28:26–35. doi: 10.1016/j.mib.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito-Nakano Y, Yasuda T, Nakada-Tsukui K, Leippe M, Nozaki T. Rab5-associated vacuoles play a unique role in phagocytosis of the enteric protozoan parasite Entamoeba histolytica. J. Biol. Chem. 2004;279:49497–49507. doi: 10.1074/jbc.M403987200. [DOI] [PubMed] [Google Scholar]
- 34.Tarnita CE, Washburne A, Martinez-Garcia R, Sgro AE, Levin SA. Fitness tradeoffs between spores and nonaggregating cells can explain the coexistence of diverse genotypes in cellular slime molds. Proc. Natl. Acad. Sci. U. S. A. 2015;112:2776–2781. doi: 10.1073/pnas.1424242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuhaus EM, Almers W, Soldati T. Morphology and Dynamics of the Endocytic Pathway in Dictyostelium discoideum. Mol. Biol. Cell. 2002;13:1390–1407. doi: 10.1091/mbc.01-08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller-Taubenberger A, Kortholt A, Eichinger L. Simple system - substantial share: The use of Dictyostelium in cell biology and molecular medicine. Eur. J. Cell Biol. 2013;92:45–53. doi: 10.1016/j.ejcb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Bonifacino JS. Adaptor proteins involved in polarized sorting. J. Cell Biol. 2014;204:7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirst J, Borner GHH, Antrobus R, Peden AA, Hodson NA, Sahlender DA, et al. Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 2012;22:1711–1716. doi: 10.1016/j.cub.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park M, Song K, Reichardt I, Kim H, Mayer U, Stierhof Y-D, et al. Arabidopsis µ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10318–10323. doi: 10.1073/pnas.1300460110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teh OK, Shimono Y, Shirakawa M, Fukao Y, Tamura K, Shimada T, et al. The AP-1 µ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis. Plant Cell Physiol. 2013;54:838–847. doi: 10.1093/pcp/pct048. [DOI] [PubMed] [Google Scholar]
- 42.Wang J-G, Li S, Zhao X-Y, Zhou L-Z, Huang G-Q, Feng C, et al. HAPLESS13, the Arabidopsis µ1 adaptin, is essential for protein sorting at the trans-Golgi network/early endosome. Plant Physiol. 2013;162:1897–1910. doi: 10.1104/pp.113.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song J, Lee MH, Lee G-J, Yoo CM, Hwang I. Arabidopsis EPSIN1 Plays an Important Role in Vacuolar Trafficking of Soluble Cargo Proteins in Plant Cells via Interactions with Clathrin, AP-1, VTI11, and VSR1. Plant Cell. 2006;18:2258–2274. doi: 10.1105/tpc.105.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura K, Matsunami E, Yoshida S, Kohata S, Yamauchi J, Jisaka M, et al. The tyrosine-sorting motif of the vacuolar sorting receptor VSR4 from Arabidopsis thaliana, which is involved in the interaction between VSR4 and AP1M2,µ1-adaptin type 2 of clathrin adaptor complex 1 subunits, participates in the post-Golgi sorting of VS. Biosci. Biotechnol. Biochem. 2016;80:694–705. doi: 10.1080/09168451.2015.1116925. [DOI] [PubMed] [Google Scholar]
- 45.Elde NC, Morgan G, Winey M, Sperling L, Turkewitz AP. Elucidation of clathrin-mediated endocytosis in tetrahymena reveals an evolutionarily convergent recruitment of dynamin. PLoS Genet. 2005;1:e52. doi: 10.1371/journal.pgen.0010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngô HM, Yang M, Paprotka K, Pypaert M, Hoppe H, Joiner KA. AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J. Biol. Chem. 2003;278:5343–5352. doi: 10.1074/jbc.M208291200. [DOI] [PubMed] [Google Scholar]
- 47.Kibria KMK, Rawat K, Klinger CM, Datta G, Panchal M, Singh S, et al. A role for adaptor protein complex 1 in protein targeting to rhoptry organelles in Plasmodium falciparum. Biochim. Biophys. Acta - Mol. Cell Res. 2015;1853:699–710. doi: 10.1016/j.bbamcr.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Sloves P-J, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, Hovasse A, et al. Toxoplasma sortilin-like receptor regulates protein transport and is essential for apical secretory organelle biogenesis and host infection. Cell Host Microbe. 2012;11:515–527. doi: 10.1016/j.chom.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Tomavo S, Slomianny C, Meissner M, Carruthers VB. Protein trafficking through the endosomal system prepares intracellular parasites for a home invasion. PLoS Pathog. 2013;9:e1003629. doi: 10.1371/journal.ppat.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Touz MC, Kulakova L, Nash TE. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol. Biol. Cell. 2004;15:3053–3060. doi: 10.1091/mbc.E03-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivero MR, Miras SL, Feliziani C, Zamponi N, Quiroga R, Hayes SF, et al. Vacuolar protein sorting receptor in Giardia lamblia. PLoS One. 2012;7:e43712. doi: 10.1371/journal.pone.0043712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoltner M, Leung KF, Alsford S, Horn D, Field MC. Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes. PLoS Pathog. 2015;11:e1005236. doi: 10.1371/journal.ppat.1005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tazeh NN, Silverman JS, Schwartz KJ, Sevova ES, Sutterwala SS, Bangs JD. Role of AP-1 in developmentally regulated lysosomal trafficking in Trypanosoma brucei. Eukaryot. Cell. 2009;8:1352–1361. doi: 10.1128/EC.00156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marion S, Laurent C, Guillén N. Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: A proteomic approach. Cell. Microbiol. 2005;7:1504–1518. doi: 10.1111/j.1462-5822.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 55.Lefkir Y, de Chassey B, Dubois A, Bogdanovic A, Brady RJ, Destaing O, et al. The AP-1 Clathrin-adaptor Is Required for Lysosomal Enzymes Sorting and Biogenesis of the Contractile Vacuole Complex in Dictyostelium Cells. Mol. Biol. Cell. 2003;14:1835–1851. doi: 10.1091/mbc.E02-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefkir Y, Malbouyres M, Gotthardt D, Ozinsky A, Cornillon S, Bruckert F, et al. Involvement of the AP-1 Adaptor Complex in Early Steps of Phagocytosis and Macropinocytosis. Mol. Biol. Cell. 2004;15:861–869. doi: 10.1091/mbc.E03-06-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercanti V, Blanc C, Lefkir Y, Cosson P, Letourneur F. Acidic clusters target transmembrane proteins to the contractile vacuole in Dictyostelium cells. J. Cell Sci. 2006;119:837–845. doi: 10.1242/jcs.02808. [DOI] [PubMed] [Google Scholar]
- 58.Reider A, Wendland B. Endocytic adaptors - social networking at the plasma membrane. J. Cell Sci. 2011;124:1613–1622. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bashline L, Li S, Anderson CT, Lei L, Gu Y. The endocytosis of cellulose synthase in Arabidopsis is dependent on µ2, a clathrin-mediated endocytosis adaptin. Plant Physiol. 2013;163:150–160. doi: 10.1104/pp.113.221234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Rubbo S, Irani NG, Kim SY, Xu Z-Y, Gadeyne A, Dejonghe W, et al. The Clathrin Adaptor Complex AP-2 Mediates Endocytosis of BRASSINOSTEROID INSENSITIVE1 in Arabidopsis. Plant Cell. 2013;25:2986–2997. doi: 10.1105/tpc.113.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan L, Hao H, Xue Y, Zhang L, Song K, Ding Z, et al. Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development. 2013;140:3826–3837. doi: 10.1242/dev.095711. [DOI] [PubMed] [Google Scholar]
- 62.Kim SY, Xu Z-Y, Song K, Kim DH, Kang H, Reichardt I, et al. Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell. 2013;25:2970–2985. doi: 10.1105/tpc.113.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaoka S, Shimono Y, Shirakawa M, Fukao Y, Kawase T, Hatsugai N, et al. Identification and Dynamics of Arabidopsis Adaptor Protein-2 Complex and Its Involvement in Floral Organ Development. Plant Cell. 2013;25:2958–2969. doi: 10.1105/tpc.113.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Hu T, Yan X, Meng T, Wang Y, Wang Q, et al. Differential Regulation of Clathrin and Its Adaptor Proteins, AP-2 and the TPLATE Complex, during Their Membrane Recruitment in Arabidopsis. Plant Physiol. 2015:01716. doi: 10.1104/pp.15.01716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barth M, Holstein SEH. Identification and functional characterization of Arabidopsis AP180, a binding partner of plant alphaC-adaptin. J. Cell Sci. 2004;117:2051–2062. doi: 10.1242/jcs.01062. [DOI] [PubMed] [Google Scholar]
- 66.Gadeyne A, Sánchez-Rodríguez C, Vanneste S, Di Rubbo S, Zauber H, Vanneste K, et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell. 2014;156:691–704. doi: 10.1016/j.cell.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 67.Macro L, Jaiswal JK, Simon SM. Dynamics of clathrin-mediated endocytosis and its requirement for organelle biogenesis in Dictyostelium. J. Cell Sci. 2012;125:5721–5732. doi: 10.1242/jcs.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sosa TR, Weber MM, Wen Y, O’Halloran TJ. A Single β Adaptin Contributes to AP1 and AP2 Complexes and Clathrin Function in Dictyostelium. Traffic. 2012;13:305–316. doi: 10.1111/j.1600-0854.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennett N, Letourneur F, Ragno M, Louwagie M. Sorting of the v-SNARE VAMP7 in Dictyostelium discoideum: a role for more than one Adaptor Protein (AP) complex. Exp. Cell Res. 2008;314:2822–2833. doi: 10.1016/j.yexcr.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Gotthardt D, Warnatz HJ, Henschel O, Brückert F, Schleicher M, Soldati T. High-Resolution Dissection of Phagosome Maturation Reveals Distinct Membrane Trafficking Phases. Mol. Biol. Cell. 2002;13:3508–3520. doi: 10.1091/mbc.E02-04-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen Y, Stavrou I, Bersuker K, Brady RJ, De Lozanne A, O’Halloran TJ. AP180-Mediated Trafficking of Vamp7B Limits Homotypic Fusion of Dictyostelium Contractile Vacuoles. Mol. Biol. Cell. 2009;20:4278–4288. doi: 10.1091/mbc.E09-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charette SJ, Mercanti V, Letourneur F, Bennett N, Cosson P. A role for adaptor protein-3 complex in the organization of the endocytic pathway in Dictyostelium. Traffic. 2006;7:1528–1538. doi: 10.1111/j.1600-0854.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 73.Rivero MR, V Vranych C, Bisbal M, Maletto BA, Ropolo AS, Touz MC. Adaptor protein 2 regulates receptor-mediated endocytosis and cyst formation in Giardia lamblia. Biochem. J. 2010;428:33–45. doi: 10.1042/BJ20100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manna PT, Kelly S, Field MC. Adaptin evolution in kinetoplastids and emergence of the variant surface glycoprotein coat in African trypanosomatids. Mol. Phylogenet. Evol. 2013;67:123–128. doi: 10.1016/j.ympev.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braun V, Deschamps C, Raposo G, Benaroch P, Benmerah A, Chavrier P, et al. AP-1 and ARF1 Control Endosomal Dynamics at Sites of FcR-mediated Phagocytosis. Mol. Biol. Cell. 2007;18:4921–4931. doi: 10.1091/mbc.E07-04-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev. Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Manil-Segalén M, Lefebvre C, Culetto E, Legouis R. Need an ESCRT for autophagosomal maturation? Commun. Integr. Biol. 2012;5:566–571. doi: 10.4161/cib.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung KF, Dacks JB, Field MC. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic. 2008;9:1698–1716. doi: 10.1111/j.1600-0854.2008.00797.x. [DOI] [PubMed] [Google Scholar]
- 79.Herman EK, Walker G, van der Giezen M, Dacks JB. Multivesicular bodies in the enigmatic amoeboflagellate Breviata anathema and the evolution of ESCRT 0. J. Cell Sci. 2011;124:613–621. doi: 10.1242/jcs.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spitzer C, Schellmann S, Sabovljevic A, Shahriari M, Keshavaiah C, Bechtold N, et al. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development. 2006;133:4679–4689. doi: 10.1242/dev.02654. [DOI] [PubMed] [Google Scholar]
- 81.Winter V, Hauser MT. Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 2006;11:115–123. doi: 10.1016/j.tplants.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai Y, Zhuang X, Gao C, Wang X, Jiang L. The Arabidopsis Endosomal Sorting Complex Required for Transport III Regulates Internal Vesicle Formation of the Prevacuolar Compartment and Is Required for Plant Development. Plant Physiol. 2014;165:1328–1343. doi: 10.1104/pp.114.238378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katsiarimpa A, Kalinowska K, Anzenberger F, Weis C, Ostertag M, Tsutsumi C, et al. The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell. 2013;25:2236–2252. doi: 10.1105/tpc.113.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haas TJ, Sliwinski MK, Martínez DE, Preuss M, Ebine K, Ueda T, et al. The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell. 2007;19:1295–1312. doi: 10.1105/tpc.106.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahriari M, Keshavaiah C, Scheuring D, Sabovljevic A, Pimpl P, Häusler RE, et al. The AAA-type ATPase AtSKD1 contributes to vacuolar maintenance of Arabidopsis thaliana. Plant J. 2010;64:71–85. doi: 10.1111/j.1365-313X.2010.04310.x. [DOI] [PubMed] [Google Scholar]
- 86.Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, Otegui MS. The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell. 2009;21:749–766. doi: 10.1105/tpc.108.064865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spitzer C, Li F, Buono R, Roschzttardtz H, Chung T, Zhang M, et al. The Endosomal Protein CHARGED MULTIVESICULAR BODY PROTEIN1 Regulates the Autophagic Turnover of Plastids in Arabidopsis. Plant Cell. 2015;27:391–402. doi: 10.1105/tpc.114.135939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardona-López X, Cuyas L, Marín E, Rajulu C, Irigoyen ML, Gil E, et al. ESCRT-III-Associated Protein ALIX Mediates High-Affinity Phosphate Transporter Trafficking to Maintain Phosphate Homeostasis in Arabidopsis. Plant Cell. 2015;27:2560–2581. doi: 10.1105/tpc.15.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isono E, Katsiarimpa A, Müller IK, Anzenberger F, Stierhof Y-D, Geldner N, et al. The deubiquitinating enzyme AMSH3 is required for intracellular trafficking and vacuole biogenesis in Arabidopsis thaliana. Plant Cell. 2010;22:1826–1837. doi: 10.1105/tpc.110.075952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao C, Luo M, Zhao Q, Yang R, Cui Y, Zeng Y, et al. A Unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 2014;24:2556–2563. doi: 10.1016/j.cub.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Gao C, Zhuang X, Cui Y, Fu X, He Y, Zhao Q, et al. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:1886–1891. doi: 10.1073/pnas.1421271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolb C, Nagel M-K, Kalinowska K, Hagmann J, Ichikawa M, Anzenberger F, et al. FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol. 2015;167:1361–1373. doi: 10.1104/pp.114.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reyes FC, Buono RA, Roschzttardtz H, Di Rubbo S, Yeun LH, Russinova E, et al. A novel endosomal sorting complex required for transport (ESCRT) component in Arabidopsis thaliana controls cell expansion and development. J. Biol. Chem. 2014;289:4980–4988. doi: 10.1074/jbc.M113.529685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woo YH, Ansari H, Otto TD, Klinger CM, Kolisko M, Michálek J, et al. Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. Elife. 2015;4:e06974. doi: 10.7554/eLife.06974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang M, Coppens I, Wormsley S, Baevova P, Hoppe HC, Joiner KA. The Plasmodium falciparum Vps4 homolog mediates multivesicular body formation. J. Cell Sci. 2004;117:3831–3838. doi: 10.1242/jcs.01237. [DOI] [PubMed] [Google Scholar]
- 96.Wampfler PB, Tosevski V, Nanni P, Spycher C, Hehl AB. Proteomics of secretory and endocytic organelles in Giardia lamblia. PLoS One. 2014;9:e94089. doi: 10.1371/journal.pone.0094089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dutta S, Saha N, Ray A, Sarkar S. Significantly Diverged Did2 / Vps46 Orthologues from the Protozoan Parasite Giardia lamblia. Curr. Microbiol. 2015;71:333–340. doi: 10.1007/s00284-015-0844-4. [DOI] [PubMed] [Google Scholar]
- 98.Chung W-L, Leung KF, Carrington M, Field MC. Ubiquitylation is required for degradation of transmembrane surface proteins in trypanosomes. Traffic. 2008;9:1681–1697. doi: 10.1111/j.1600-0854.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 99.Silverman JS, Muratore KA, Bangs JD. Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei. Traffic. 2013;14:1078–1090. doi: 10.1111/tra.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.López-Reyes I, García-Rivera G, Bañuelos C, Herranz S, Vincent O, López-Camarillo C, et al. Detection of the endosomal sorting complex required for transport in Entamoeba histolytica and characterization of the EhVps4 protein. J. Biomed. Biotechnol. 2010;2010:890674. doi: 10.1155/2010/890674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bañuelos C, García-Rivera G, López-Reyes I, Mendoza L, González-Robles A, Herranz S, et al. EhADH112 Is a Bro1 Domain-Containing Protein Involved in the Entamoeba histolytica Multivesicular Bodies Pathway. J. Biomed. Biotechnol. 2012;2012:657942. doi: 10.1155/2012/657942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanc C, Charette SJ, Mattei S, Aubry L, Smith EW, Cosson P, et al. Dictyostelium Tom1 participates to an ancestral ESCRT-0 complex. Traffic. 2009;10:161–171. doi: 10.1111/j.1600-0854.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 103.Mattei S, Klein G, Satre M, Aubry L. Trafficking and developmental signaling: Alix at the crossroads. Eur. J. Cell Biol. 2006;85:925–936. doi: 10.1016/j.ejcb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 104.Seaman MNJ. The retromer complex - endosomal protein recycling and beyond. J. Cell Sci. 2012;125:4693–702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rojas R, Van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seaman MNJ, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, et al. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell. 2006;18:1239–1252. doi: 10.1105/tpc.105.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17812–17817. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, et al. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 2008;49:142–156. doi: 10.1093/pcp/pcn006. [DOI] [PubMed] [Google Scholar]
- 110.Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, et al. Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J. Biol. Chem. 2013;288:8815–8825. doi: 10.1074/jbc.M112.440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munch D, Teh O-K, Malinovsky FG, Liu Q, Vetukuri RR, El Kasmi F, et al. Retromer Contributes to Immunity-Associated Cell Death in Arabidopsis. Plant Cell. 2015;27:463–479. doi: 10.1105/tpc.114.132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T. The Retromer Protein VPS29 Links Cell Polarity and Organ Initiation in Plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 113.Pourcher M, Santambrogio M, Thazar N, Thierry A-M, Fobis-Loisy I, Miège C, et al. Analyses of sorting nexins reveal distinct retromer-subcomplex functions in development and protein sorting in Arabidopsis thaliana. Plant Cell. 2010;22:3980–3991. doi: 10.1105/tpc.110.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niemes S, Langhans M, Viotti C, Scheuring D, San Wan Yan M, Jiang L, et al. Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 2009;61:107–121. doi: 10.1111/j.1365-313X.2009.04034.x. [DOI] [PubMed] [Google Scholar]
- 115.Zelazny E, Santambrogio M, Gaude T. Retromer association with membranes: plants have their own rules! Plant Signal. Behav. 2013;8:e25312. doi: 10.4161/psb.25312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M, et al. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 2006;47:1187–1194. doi: 10.1093/pcp/pcj103. [DOI] [PubMed] [Google Scholar]
- 117.Hashiguchi Y, Niihama M, Takahashi T, Saito C, Nakano A, Tasaka M, et al. Loss-of-function mutations of retromer large subunit genes suppress the phenotype of an Arabidopsis zig mutant that lacks Qb-SNARE VTI11. Plant Cell. 2010;22:159–172. doi: 10.1105/tpc.109.069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nodzynski T, Feraru MI, Hirsch S, De Rycke R, Niculaes C, Boerjan W, et al. Retromer subunits VPS35A and VPS29 mediate prevacuolar compartment (PVC) function in Arabidopsis. Mol. Plant. 2013;6:1849–1862. doi: 10.1093/mp/sst044. [DOI] [PubMed] [Google Scholar]
- 119.Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, et al. Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell. 2007;19:597–609. doi: 10.1105/tpc.106.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Briguglio JS, Kumar S, Turkewitz AP. Lysosomal sorting receptors are essential for secretory granule biogenesis in Tetrahymena. J. Cell Biol. 2013;203:537–550. doi: 10.1083/jcb.201305086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar S, Briguglio JS, Turkewitz AP. An aspartyl cathepsin, CTH3, is essential for proprotein processing during secretory granule maturation in Tetrahymena thermophila. Mol. Biol. Cell. 2014;25:2444–2460. doi: 10.1091/mbc.E14-03-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sangaré LO, Alayi TD, Westermann B, Hovasse A, Sindikubwabo F, Callebaut I, et al. Unconventional endosome-like compartment and retromer complex in Toxoplasma gondii govern parasite integrity and host infection. Nat. Commun. 2016;7:11191. doi: 10.1038/ncomms11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krai P, Dalal S, Klemba M. Evidence for a Golgi-to-endosome protein sorting pathway in Plasmodium falciparum. PLoS One. 2014;9:e89771. doi: 10.1371/journal.pone.0089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Touz MC, Rivero MR, Miras SL, Bonifacino JS. Lysosomal protein trafficking in Giardia lamblia: common and distinct features. Front. Biosci. 2012;4:1898–1909. doi: 10.2741/511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miras SL, Merino MC, Gottig N, Rópolo AS, Touz MC. The giardial VPS35 retromer subunit is necessary for multimeric complex assembly and interaction with the vacuolar protein sorting receptor. Biochim. Biophys. Acta. 2013;1833:2628–2638. doi: 10.1016/j.bbamcr.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol. Biol. Cell. 2005;16:5294–5303. doi: 10.1091/mbc.E05-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gillingham AK, Sinka R, Torres IL, Lilley KS, Munro S. Toward a Comprehensive Map of the Effectors of Rab GTPases. Dev. Cell. 2014;31:358–373. doi: 10.1016/j.devcel.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 129.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 130.Nordmann M, Cabrera M, Perz A, Bröcker C, Ostrowicz C, Engelbrecht-Vandré S, et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr. Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Ueda T, Yamaguchi M, Uchimiya H, Nakano A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 2001;20:4730–4741. doi: 10.1093/emboj/20.17.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee G-J, Sohn EJ, Lee MH, Hwang I. The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol. 2004;45:1211–1220. doi: 10.1093/pcp/pch142. [DOI] [PubMed] [Google Scholar]
- 133.Ebine K, Fujimoto M, Okatani Y, Nishiyama T, Goh T, Ito E, et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011;13:853–859. doi: 10.1038/ncb2270. [DOI] [PubMed] [Google Scholar]
- 134.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-Temporal Cellular Dynamics of the Arabidopsis Flagellin Receptor Reveal Activation Status-Dependent Endosomal Sorting. Plant Cell. 2012;24:4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh MK, Krüger F, Beckmann H, Brumm S, Vermeer JEM, Munnik T, et al. Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr. Biol. 2014;24:1383–1389. doi: 10.1016/j.cub.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Ebine K, Inoue T, Ito J, Ito E, Uemura T, Goh T, et al. Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr. Biol. 2014;24:1375–1382. doi: 10.1016/j.cub.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 137.Ueda T, Uemura T, Sato MH, Nakano A. Functional differentiation of endosomes in Arabidopsis cells. Plant J. 2004;40:783–789. doi: 10.1111/j.1365-313X.2004.02249.x. [DOI] [PubMed] [Google Scholar]
- 138.Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim Y-W, et al. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell. 2003;15:1057–1070. doi: 10.1105/tpc.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, Mahler S, et al. An overexpression screen of Toxoplasma gondii Rab-GTPases reveals distinct transport routes to the micronemes. PLoS Pathog. 2013;9:e1003213. doi: 10.1371/journal.ppat.1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harper JM, Huynh M-H, Coppens I, Parussini F, Moreno S, Carruthers VB. A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol. Biol. Cell. 2006;17:4551–4563. doi: 10.1091/mbc.E06-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol. Microbiol. 2010;76:1340–1357. doi: 10.1111/j.1365-2958.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Robibaro B, Stedman TT, Coppens I, Ngô HM, Pypaert M, Bivona T, et al. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol. 2002;4:139–152. doi: 10.1046/j.1462-5822.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- 143.Elliott DA, McIntosh MT, Hosgood HD, Chen S, Zhang G, Baevova P, et al. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2463–2468. doi: 10.1073/pnas.0711067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, et al. Plasmodium falciparum Rab5B is an N-terminally myristoylated Rab GTPase that is targeted to the parasite’s plasma and food vacuole membranes. PLoS One. 2014;9:e87695. doi: 10.1371/journal.pone.0087695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pal A, Hall BS, Jeffries TR, Field MC. Rab5 and Rab11 mediate transferrin and anti-variant surface glycoprotein antibody recycling in Trypanosoma brucei. Biochem. J. 2003;374:443–451. doi: 10.1042/BJ20030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hall B, Allen CL, Goulding D, Field MC. Both of the Rab5 subfamily small GTPases of Trypanosoma brucei are essential and required for endocytosis. Mol. Biochem. Parasitol. 2004;138:67–77. doi: 10.1016/j.molbiopara.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 147.Pal A, Hall BS, Nesbeth DN, Field HI, Field MC. Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J. Biol. Chem. 2002;277:9529–9539. doi: 10.1074/jbc.M110055200. [DOI] [PubMed] [Google Scholar]
- 148.Okada M, Huston CD, Oue M, Mann BJ, Petri WA, Jr, Kita K, et al. Kinetics and strain variation of phagosome proteins of Entamoeba histolytica by proteomic analysis. Mol Biochem Parasitol. 2006;145:171–183. doi: 10.1016/j.molbiopara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 149.Okada M, Nozaki T. New insights into molecular mechanisms of phagocytosis in Entamoeba histolytica by proteomic analysis. Arch. Med. Res. 2006;37:244–252. doi: 10.1016/j.arcmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 150.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of Low-Molecular-Weight Gtp Binding-Proteins to Exocytic and Endocytic Compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 151.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cui Y, Zhao Q, Gao C, Ding Y, Zeng Y, Ueda T, et al. Activation of the Rab7 GTPase by the MON1-CCZ1 Complex Is Essential for PVC-to-Vacuole Trafficking and Plant Growth in Arabidopsis. Plant Cell. 2014;26:2080–2097. doi: 10.1105/tpc.114.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Il Kwon S, Cho HJ, Jung JH, Yoshimoto K, Shirasu K, Park OK. The Rab GTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. Plant J. 2010;64:151–164. doi: 10.1111/j.1365-313X.2010.04315.x. [DOI] [PubMed] [Google Scholar]
- 155.Il Kwon S, Cho HJ, Kim SR, Park OK. The Rab GTPase RabG3b Positively Regulates Autophagy and Immunity-Associated Hypersensitive Cell Death in Arabidopsis. Plant Physiol. 2013;161:1722–1736. doi: 10.1104/pp.112.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacobs ME, DeSouza LV, Samaranayake H, Pearlman RE, Siu KWM, Klobutcher LA. The Tetrahymena thermophila phagosome proteome. Eukaryot. Cell. 2006;5:1990–2000. doi: 10.1128/EC.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bright LJ, Kambesis N, Nelson SB, Jeong B, Turkewitz AP. Comprehensive analysis reveals dynamic and evolutionary plasticity of Rab GTPases and membrane traffic in Tetrahymena thermophila. PLoS Genet. 2010;6:e1001155. doi: 10.1371/journal.pgen.1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miranda K, Pace DA, Cintron R, Rodrigues JCF, Fang J, Smith A, et al. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol. Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Silverman JS, Schwartz KJ, Hajduk SL, Bangs JD. Late endosomal Rab7 regulates lysosomal trafficking of endocytic but not biosynthetic cargo in Trypanosoma brucei. Mol. Microbiol. 2011;82:664–678. doi: 10.1111/j.1365-2958.2011.07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Biller L, Matthiesen J, Kühne V, Lotter H, Handal G, Nozaki T, et al. The cell surface proteome of Entamoeba histolytica. Mol. Cell. Proteomics. 2014;13:132–144. doi: 10.1074/mcp.M113.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Srinivasan S, Traini M, Herbert B, Sexton D, Harry J, Alexander H, et al. Proteomic analysis of a developmentally regulated secretory vesicle. Proteomics. 2001;1:1119–1127. doi: 10.1002/1615-9861(200109)1:9<1119::AID-PROT1119>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 162.Gotthardt D, Blancheteau V, Bosserhoff A, Ruppert T, Delorenzi M, Soldati T. Proteomics fingerprinting of phagosome maturation and evidence for the role of a Galpha during uptake. Mol. Cell. Proteomics. 2006;5:2228–2243. doi: 10.1074/mcp.M600113-MCP200. [DOI] [PubMed] [Google Scholar]
- 163.Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol. Biol. Cell. 1997;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rupper A, Grove B, Cardelli J. Rab7 regulates phagosome maturation in Dictyostelium. J. Cell Sci. 2001;114:2449–2460. doi: 10.1242/jcs.114.13.2449. [DOI] [PubMed] [Google Scholar]
- 165.Rupper A, Lee K, Knecht D, Cardelli J. Sequential activities of phosphoinositide 3-kinase, PKB/Aakt, and Rab7 during macropinosome formation in Dictyostelium. Mol. Biol. Cell. 2001;12:2813–2824. doi: 10.1091/mbc.12.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24:407–415. doi: 10.1016/j.tcb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 167.Knödler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feraru E, Feraru MI, Asaoka R, Paciorek T, De Rycke R, Tanaka H, et al. BEX5/RabA1b Regulates trans-Golgi Network-to-Plasma Membrane Protein Trafficking in Arabidopsis. Plant Cell. 2012;24:3074–3086. doi: 10.1105/tpc.112.098152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Choi S-W, Tamaki T, Ebine K, Uemura T, Ueda T, Nakano A. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor. Plant Cell. 2013;25:1174–1187. doi: 10.1105/tpc.112.108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Asaoka R, Uemura T, Ito J, Fujimoto M, Ito E, Ueda T, et al. Arabidopsis RABA1 GTPases are involved in transport between the trans-Golgi network and the plasma membrane, and are required for salinity stress tolerance. Plant J. 2013;73:240–249. doi: 10.1111/tpj.12023. [DOI] [PubMed] [Google Scholar]
- 171.Asaoka R, Uemura T, Nishida S, Fujiwara T, Ueda T, Nakano A. New insights into the role of Arabidopsis RABA1 GTPases in salinity stress tolerance. Plant Signal. Behav. 2013;8:e25377. doi: 10.4161/psb.25377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chow C-M, Neto H, Foucart C, Moore I. Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell. 2008;20:101–123. doi: 10.1105/tpc.107.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lunn D, Gaddipati SR, Tucker GA, Lycett GW. Null Mutants of Individual RABA Genes Impact the Proportion of Different Cell Wall Components in Stem Tissue of Arabidopsis thaliana. PLoS One. 2013;8:e75724. doi: 10.1371/journal.pone.0075724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E. The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell. 2004;16:1589–1603. doi: 10.1105/tpc.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thole JM, Vermeer JEM, Zhang Y, Gadella TWJ, Nielsen E. ROOT HAIR DEFECTIVE4 Encodes a Phosphatidylinositol-4-Phosphate Phosphatase Required for Proper Root Hair Development in Arabidopsis thaliana. Plant Cell. 2008;20:381–395. doi: 10.1105/tpc.107.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kang BH, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA. Electron Tomography of RabA4b- and PI-4Kβ1-Labeled Trans Golgi Network Compartments in Arabidopsis. Traffic. 2011;12:313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 177.Szumlanski AL, Nielsen E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell. 2009;21:526–544. doi: 10.1105/tpc.108.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 180.Agop-Nersesian C, Naissant B, Ben Rached F, Rauch M, Kretzschmar A, Thiberge S, et al. Rab11A–controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis. PLoS Pathog. 2009;5:e1000270. doi: 10.1371/journal.ppat.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agop-Nersesian C, Egarter S, Langsley G, Foth BJ, Ferguson DJP, Meissner M. Biogenesis of the inner membrane complex is dependent on vesicular transport by the alveolate specific GTPase Rab11B. PLoS Pathog. 2010;6:e1001029. doi: 10.1371/journal.ppat.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Castillo-Romero A, Leon-Avila G, Wang CC, Perez Rangel A, Camacho Nuez M, Garcia Tovar C, et al. Rab11 and actin cytoskeleton participate in Giardia lamblia encystation, guiding the specific vesicles to the cyst wall. PLoS Negl. Trop. Dis. 2010;4:e697. doi: 10.1371/journal.pntd.0000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gabernet-Castello C, DuBois KN, Nimmo C, Field MC. Rab11 function in trypanosoma brucei: Identification of conserved and novel interaction partners. Eukaryot. Cell. 2011;10:1082–1094. doi: 10.1128/EC.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Niyogi S, Mucci J, Campetella O, Docampo R. Rab11 Regulates Trafficking of Trans-sialidase to the Plasma Membrane through the Contractile Vacuole Complex of Trypanosoma cruzi. PLoS Pathog. 2014;10:e1004224. doi: 10.1371/journal.ppat.1004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Temesvari LA, Harris EN, Stanley SL, Cardelli JA. Early and late endosomal compartments of Entamoeba histolytica are enriched in cysteine proteases, acid phosphatase and several Ras-related Rab GTPases. Mol. Biochem. Parasitol. 1999;103:225–241. doi: 10.1016/s0166-6851(99)00133-4. [DOI] [PubMed] [Google Scholar]
- 186.McGugan GC, Temesvari LA. Characterization of a Rab11-like GTPase, EhRab11, of Entamoeba histolytica. Mol. Biochem. Parasitol. 2003;129:137–146. doi: 10.1016/s0166-6851(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 187.Mitra BN, Saito-Nakano Y, Nakada-Tsukui K, Sato D, Nozaki T. Rab11B small GTPase regulates secretion of cysteine proteases in the enteric protozoan parasite Entamoeba histolytica. Cell. Microbiol. 2007;9:2112–2125. doi: 10.1111/j.1462-5822.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 188.Parkinson K, Baines AE, Keller T, Gruenheit N, Bragg L, North RA, et al. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat. Cell Biol. 2014;16:87–98. doi: 10.1038/ncb2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Harris E, Yoshida K, Cardelli J, Bush J. Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in dictyostelium. J. Cell Sci. 2001;114:3035–3045. doi: 10.1242/jcs.114.16.3035. http://www.ncbi.nlm.nih.gov/pubmed/11686306. [DOI] [PubMed] [Google Scholar]
- 190.Dieckmann R, Gueho A, Monroy R, Ruppert T, Bloomfield G, Soldati T. The Balance in the Delivery of ER Components and the Vacuolar Proton Pump to the Phagosome Depends on Myosin IK in Dictyostelium. Mol. Cell. Proteomics. 2012;11:886–900. doi: 10.1074/mcp.M112.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Francia ME, Striepen B. Cell division in apicomplexan parasites. Nat. Rev. Microbiol. 2014;12:125–136. doi: 10.1038/nrmicro3184. [DOI] [PubMed] [Google Scholar]
- 192.Ouologuem DT, Roos DS. Dynamics of the Toxoplasma gondii inner membrane complex. J. Cell Sci. 2014;127:3320–3330. doi: 10.1242/jcs.147736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Harding CR, Egarter S, Gow M, Jiménez-Ruiz E, Ferguson DJP, Meissner M. Gliding Associated Proteins Play Essential Roles during the Formation of the Inner Membrane Complex of Toxoplasma gondii. PLOS Pathog. 2016;12:e1005403. doi: 10.1371/journal.ppat.1005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Giansanti MG, Vanderleest TE, Jewett CE, Sechi S, Frappaolo A, Fabian L, et al. Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis in Drosophila. PLoS Genet. 2015;11:e1005632. doi: 10.1371/journal.pgen.1005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Essid M, Gopaldass N, Yoshida K, Merrifield C, Soldati T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol. Biol. Cell. 2012;23:1267–1282. doi: 10.1091/mbc.E11-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Komsic-Buchmann K, Stephan LM, Becker B. The SEC6 protein is required for contractile vacuole function in Chlamydomonas reinhardtii. J. Cell Sci. 2012;125:2885–2895. doi: 10.1242/jcs.099184. [DOI] [PubMed] [Google Scholar]
- 197.Komsic-Buchmann K, Wöstehoff L, Becker B. The contractile vacuole as a key regulator of cellular water flow in Chlamydomonas reinhardtii. Eukaryot. Cell. 2014;13:1421–1430. doi: 10.1128/EC.00163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ulrich PN, Jimenez V, Park M, Martins VP, Atwood J, Moles K, et al. Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS One. 2011;6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rauch L, Hennings K, Aepfelbacher M. A role for exocyst in maturation and bactericidal function of staphylococci-containing endothelial cell phagosomes. Traffic. 2014;15:1083–1098. doi: 10.1111/tra.12189. [DOI] [PubMed] [Google Scholar]
- 200.Schlacht A, Mowbrey K, Elias M, Kahn RA, Dacks JB. Ancient complexity, opisthokont plasticity, and discovery of the 11th subfamily of Arf GAP proteins. Traffic. 2013;14:636–649. doi: 10.1111/tra.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen D, Jian Y, Liu X, Zhang Y, Liang J, Qi X, et al. Clathrin and AP2 Are Required for Phagocytic Receptor-Mediated Apoptotic Cell Clearance in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003517. doi: 10.1371/journal.pgen.1003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen D, Xiao H, Zhang K, Wang B, Gao Z, Jian Y, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- 203.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 204.Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 2007;120:2977–2985. doi: 10.1242/jcs.013250. [DOI] [PubMed] [Google Scholar]
- 205.Dacks JB, Poon PP, Field MC. Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc. Natl. Acad. Sci. U. S. A. 2008;105:588–593. doi: 10.1073/pnas.0707318105. [DOI] [PMC free article] [PubMed] [Google Scholar]