Abstract
Background
The retroantral ethmoid cell (RAEC) is defined as a posterior ethmoid cell that pneumatizes inferolaterally behind the posterior wall of the maxillary sinus. The RAEC can present a challenge to otolaryngologists during endoscopic ethmoidectomy due to its concealed location. It is also encountered during the endoscopic transpterygoid approach to the skull base, which requires dissection behind the posterior wall of the maxillary sinus. Because the RAEC is not described in the literature, this study aims to better characterize this anatomic variant.
Methods
This is a retrospective review of 58 consecutive patients who underwent revision functional endoscopic sinus surgery (FESS) within a 2-year period at a tertiary referral center. Sinus CT scans for this cohort (116 sides total) were reviewed independently by three authors to determine the incidence of the RAEC and the degree of surgical dissection during prior surgery.
Results
Of the 116 sides included in the study, RAEC was identified in 19 (16%). Furthermore, 14/19 (74%) cells were diseased with evidence mucosal thickening or neo-osteogenesis. Of the 12 sides with RAEC that had evidence of previous posterior ethmoidectomy, 4 (33%) cells were not opened, 6 (50%) were partially opened, and only 2 (17%) were completely opened.
Conclusions
This study demonstrates the relatively high prevalence of the RAEC in our patient population. The majority of RAECs showed both evidence of disease and that they were not completely opened during previous surgery. Recognition of this anatomic entity may allow for more thorough ethmoidectomy.
Keywords: ethmoid cell, revision surgery, endoscopic sinus surgery, FESS, ethmoidectomy, anatomic variant, CT imaging
Introduction
Introduction of functional endoscopic sinus surgery (FESS) has revolutionized the treatment of medically refractory chronic rhinosinusitis, with now over 500,000 cases per year being performed by otolaryngologists in the United States alone.1 However, despite major advancements, as many as 10 to 15% of patients will go on to have recurrent disease and require revision surgery.2 A multitude of factors have been proposed as contributing to initial failure, with incomplete surgery being a common cause.3,4 For example, prevalence of residual ethmoid cells seen in revision FESS patients was reported to be 65% for anterior, and 75% for posterior ethmoid cells.5 Even with the use of CT image guidance, a thorough ethmoidectomy is still difficult to achieve, with numbers of residual anterior ethmoid and posterior ethmoid air cells reported to be 1.4 and 1.2, respectively.6
The retroantral ethmoid cell (RAEC) can be defined as a posterior ethmoid cell that pneumatizes inferolaterally behind the posterior wall of the maxillary sinus. As far as we know, this anatomic variant has not been described in the literature. From our experience, the RAEC can present a challenge during endoscopic ethmoidectomy due to its relatively hidden location and incomplete description in the literature. We hypothesize that the RAEC is frequently missed during primary FESS. This study aims to better characterize this anatomic variant and understand its significance in patients undergoing FESS.
Methods
This study was granted approval by the Duke University Medical Center IRB. This is a retrospective review of fifty-eight consecutive patients who underwent revision FESS at an academic tertiary care center between December 1, 2012 and December 1, 2014. Sinus CT scans for this cohort were identified through the chart review and examined independently by three authors, including the senior author, to identify the overall prevalence of the RAEC. 60 patients were initially identified, but 2 were excluded because preoperative imaging was not available, yielding a total of 58 patients. Each side was examined independently, with a total of 116 sides analyzed. For study purposes, the criterion for inferior extension was at least 5 mm below the maxillary sinus roof, and criterion for lateral extension was at least 5 mm lateral to the junction of the medial maxillary wall and the floor of nose. For patients who had an RAEC, we assessed the extent to which the cell was opened during previous surgery. These were categorized as not opened, partially opened, or completely opened. “Partially opened” was defined as a cell in which only a single wall of the cell or less was removed. “Completely opened” was defined as a cell in which both medial and anterior walls were removed.
Results
In this study, we present extensive radiographic characterization of this anatomic variant (Figure 1, 2, 3). The RAEC was defined as a posterior ethmoid cell that pneumatizes inferolaterally behind the posterior wall of the maxillary sinus. It was consistently bounded by the orbital floor superiorly, lateral wall of the maxilla laterally, posterior wall of the maxillary sinus anteriorly, and the anterior wall of the pterygopalatine fossa posteriorly. The medial aspect of the RAEC was adjacent to the remainder of the posterior ethmoid cavity. By definition, it was always posterior to the basal lamella, in the posterior ethmoid cavity. Of the 58 revision FESS patients identified, the average age was 52 years (range 14–77). 47 patients (81%) were Caucasian and 32 (55%) were male. Upon review of all 116 sides, a total of 19 (16%) RAECs were identified. This consisted of 8 RAECs on the right, and 11 on the left. Of these cells, 14 (74%) had evidence of disease: 7 with mucosal thickening, 2 with neo-osteogenesis, and 5 with both. Furthermore, 12 of 19 had evidence of prior posterior ethmoidectomy (63%). Of these, 4 (33%) cells were not opened, 6 (50%) were partially opened, and only 2 (17%) were completely opened during previous ethmoidectomy (Table 1).
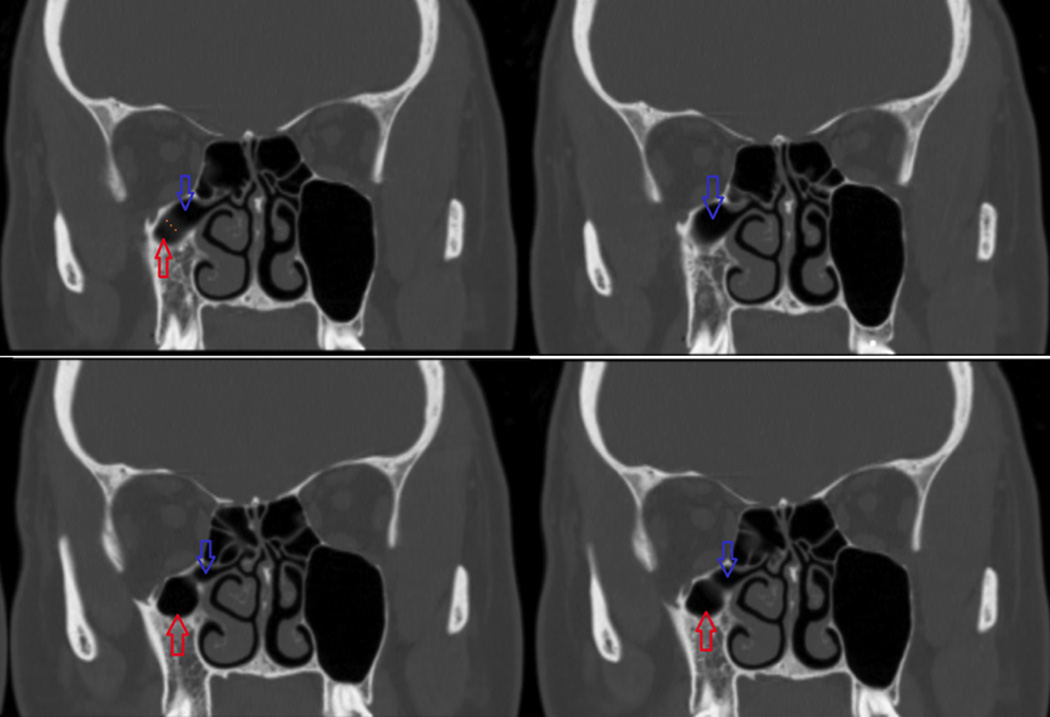
Figure 1.
Coronal CT image series illustrating a patient with a right RAEC. Red arrow = right maxillary sinus. Blue arrow = RAEC.
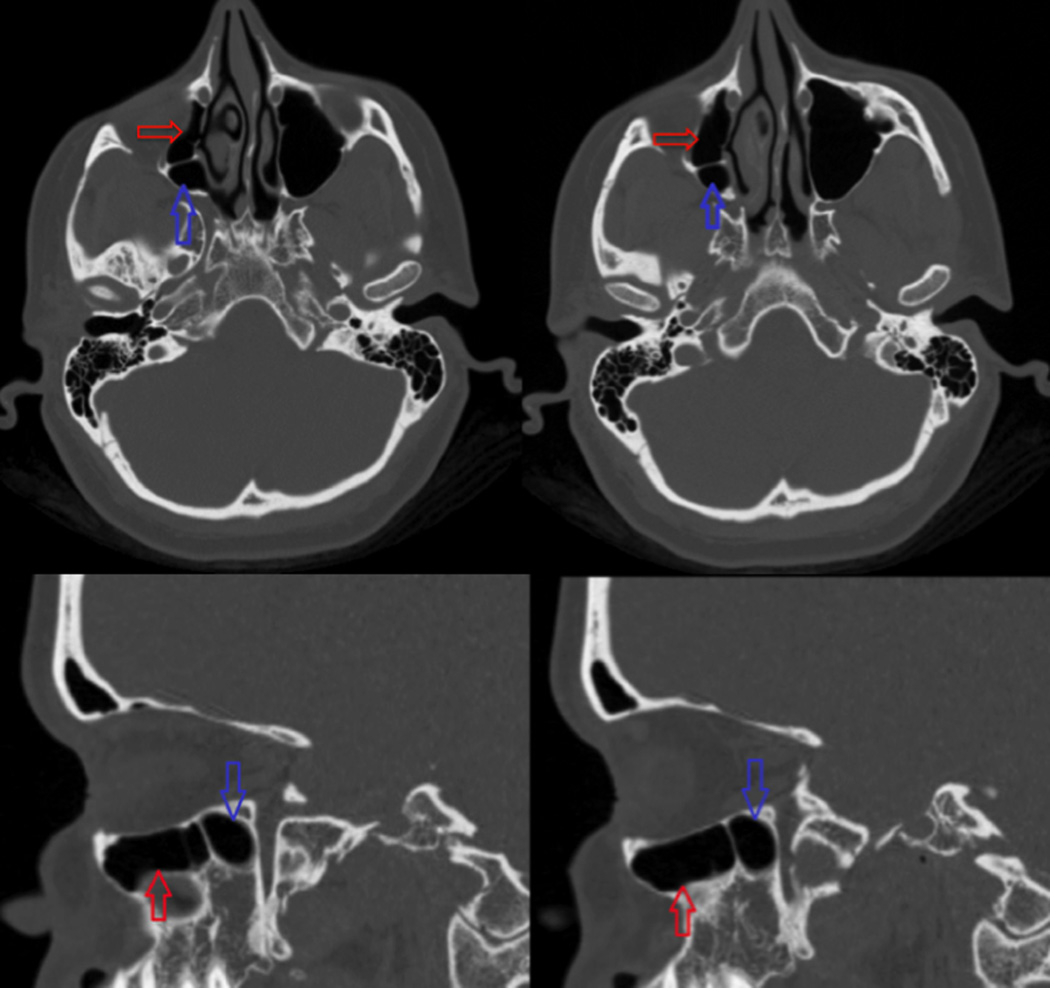
Figure 2.
Axial and sagittal cuts of CT scan from Figure 1 showing RAEC extending behind the posterior wall of the maxillary sinus. Red arrow = right maxillary sinus. Blue arrow = RAEC.
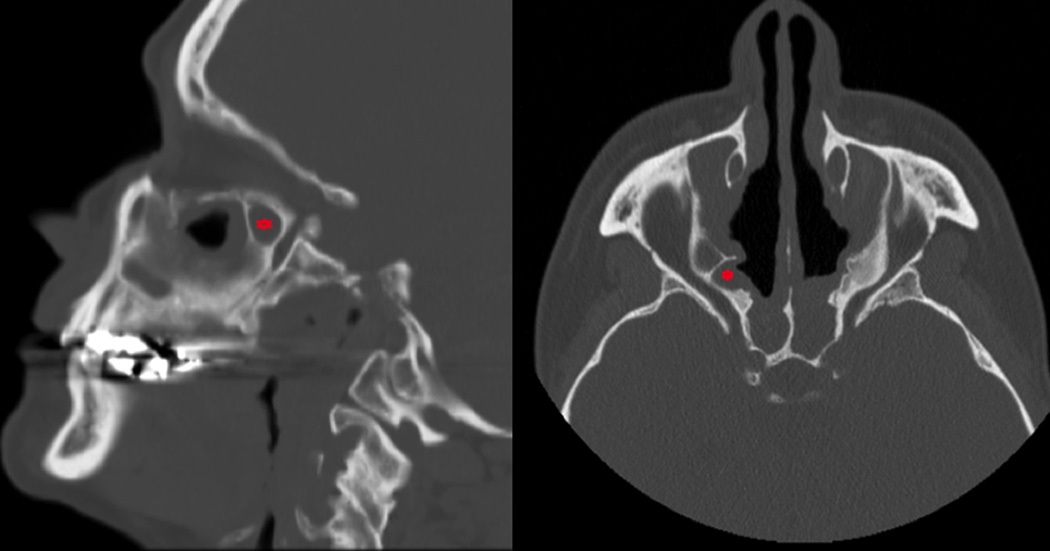
Figure 3.
Sagittal and axial images of a patient with an opacified right RAEC (indicated with asterisk).
Table 1.
Incidence of RAEC with disease status and extent of surgical opening
| Number of Sinus CT Sides (%) | |
|---|---|
| Total sides | 116 |
| No. of RAECs | 19/116 (16%) |
| Diseased RAECs | 14/19 (74%) |
| RAECs w/prior PE | 12/19 (63%) |
| Unopened | 4/12 (33%) |
| Partially Opened | 6/12 (50%) |
| Completely Opened | 2/12 (17%) |
RAEC: retroantral ethmoid cell, PE: posterior ethmoidectomy
Discussion
We defined the retroantral ethmoid cell (RAEC) as an ethmoid cell that pneumatizes inferolaterally behind the posterior wall of the maxillary sinus. Figure 1 and 2 illustrate its radiographic anatomic location with respect to the maxillary sinus and other ethmoid cells. Figure 3 provides additional sagittal and axial views of an opacified RAEC. For purposes of this study, we labeled a cell as an RAEC only if it extended 5mm laterally past the junction of medial maxillary wall and floor of nose, and extended 5mm inferior to the maxillary roof. However, this criterion was somewhat arbitrary, as extension less than 5mm was seen but not included.
The results demonstrate the relatively high prevalence (16%) of the RAEC in our patient population requiring revision FESS. However, one weakness of this study is that normal CT scans were not analyzed as a control group, and therefore we recognize that the true incidence in the general population may vary. Of the RAECs we identified, 74% had radiographic evidence of disease defined as mucosal thickening or neo-osteogenesis. More importantly, when analyzing only patients that have undergone prior posterior ethmoidectomy, we found that 83% of the time, the RAEC was unopened or only partially opened during primary or revision surgery (Table 1). Because CT scans at the time of initial surgery were not available, we cannot comment on whether the RAEC should have been opened initially. However, our findings suggest that the location of the RAEC posterior to the maxillary sinus makes endoscopic visualization challenging using a zero degree scope, and it can therefore easily be missed during posterior ethmoidectomy. We reiterate the importance of reviewing the sinus CT scan pre-operatively, which may assist with identification of the presence of such a cell and therefore guide the need for surgically addressing the cell.
As suggested in prior literature, incomplete dissection of the ethmoid cavity is not uncommon and can lead to persistent symptoms and suboptimal outcomes.2,7 While success rates for primary surgery vary between 76–98%, the rate for revision procedures decrease to 65–78%, and is associated with increased complications.3 Furthermore, patient history of prior ethmoid surgery in itself was found to be a poor prognostic outcome in repeat FESS.8 We suggest that increased recognition of the RAEC as an anatomic variant may help surgeons identify and fully open this cell. The RAEC location posterior to the maxillary sinus makes endoscopic visualization of RAEC challenging using a zero degree scope. However, it can be better visualized by removing the common partition between it and the maxillary sinus with an angled through-cutting forceps. Figure 4 demonstrates an endoscopic view of the RAEC following removal of the common partition between the cell and the maxillary sinus and patient’s corresponding pre-operative sinus CT imaging.
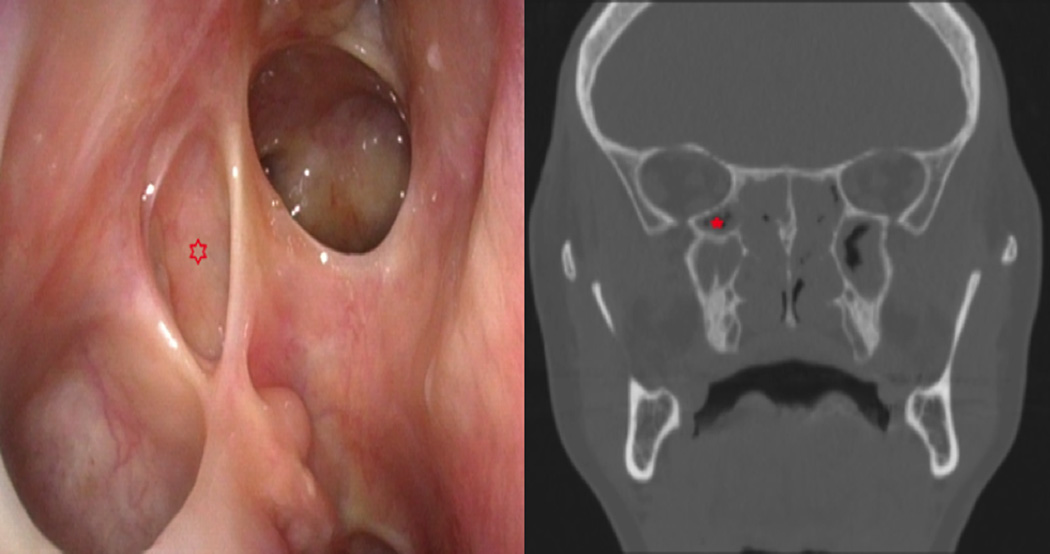
Figure 4.
Postoperative endoscopic image of right RAEC (indicated with asterisk) following removal of the common partition between it and the maxillary sinus with corresponding pre-operative coronal sinus CT.
Conclusion
In conclusion, this radiographic study describes a common anatomic variant of the posterior ethmoid cavity that is often incompletely opened during primary sinus surgery and can be a site of persistent disease. Increased recognition of the RAEC may allow for more thorough ethmoid surgery.
Acknowledgments
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR001116. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interests: The authors have no conflicts of interests, financial or otherwise to disclose.
References
- 1.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22(3):297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 2.Krings JG, Kallogjeri D, Wineland A, et al. Complications of primary and revision functional endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2014;124(4):838–845. doi: 10.1002/lary.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musy PY, Kountakis SE. Anatomic findings in patients undergoing revision endoscopic sinus surgery. Am J Otolaryngol. 2004;25(6):418–422. doi: 10.1016/j.amjoto.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Sillers MJ, Lay KF. Principles of revision functional endoscopic sinus surgery. Operative Techniques in Otolaryngology. 2006;17:6–12. [Google Scholar]
- 5.Gore MR, Ebert CS, Jr, Zanation AM, Senior BA. Beyond the "central sinus": radiographic findings in patients undergoing revision functional endoscopic sinus surgery. Int Forum Allergy Rhinol. 2013;3(2):139–146. doi: 10.1002/alr.21079. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings BA, Han JK. Level of complete dissection of the ethmoid sinuses with a computed tomographic image guidance system. Ann Otol Rhinol Laryngol. 2010;119(1):17–21. doi: 10.1177/000348941011900103. [DOI] [PubMed] [Google Scholar]
- 7.Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1999;109(1):27–29. doi: 10.1097/00005537-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Smith TL, Litvack JR, Hwang PH. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142(1):55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]