Abstract
Background
Novel tobacco products entering the US market include electronic cigarettes (ECIGs) and products advertised to “heat, not burn” tobacco. There is a growing literature regarding the acute effects of ECIGs. Less is known about “heat, not burn” products. This study’s purpose was to expand existing clinical laboratory methods to examine, in cigarette smokers, the acute effects of a “heat, not burn” “loose-leaf tobacco vaporizer” (LLTV).
Methods
Plasma nicotine and breath carbon monoxide (CO) concentration and tobacco abstinence symptom severity were measured before and after two 10-puff (30-sec interpuff interval) product use bouts separated by 60 minutes. LLTV effects were compared to participants’ own brand (OB) cigarettes and an ECIG (3.3 V; 1.5 Ohm; 18 mg/ml nicotine).
Results
Relative to OB, LLTV increased plasma nicotine concentration to a lesser degree, did not increase CO, and appeared to not reduce abstinence symptoms as effectively. Relative to ECIG, LLTV nicotine and CO delivery and abstinence symptom suppression did not differ. Participants reported that both the LLTV and ECIG were significantly less satisfying than OB.
Conclusions
Results demonstrate that LLTVs are capable of delivering nicotine and suppressing tobacco abstinence symptoms partially; acute effects of these products can be evaluated using existing clinical laboratory methods. Results can inform tobacco product regulation and may be predictive of the extent that these products have the potential to benefit or harm overall public health.
Keywords: loose-leaf tobacco vaporizer, heat-not-burn products, electronic cigarette, plasma nicotine, carbon monoxide
1. INTRODUCTION
Since the late 1990s, the United States has seen a steady rise in the number of novel tobacco products (including electronic cigarettes; ECIGs) introduced to the market. Some of these novel products have been advertised to “heat, not burn” tobacco, and little data exist regarding the acute physiological and subjective effects of product use. In May, 2016, the Food and Drug Administration (FDA) announced that it will extend its authority to regulate tobacco products to include ECIGs and other personal vaporizers (“heat, not burn” products fall within these categories; Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act, 2016). The FDA needs data that reveal product effects so that subsequent regulatory action is science-based.
The term “heat, not burn” implies an absence of tobacco combustion, and several products that meet this description have been marketed under a variety of names and with different features. For example, one such product (no longer marketed in the U.S.) was the Accord, a handheld, puff-activated chamber system with specially designed cigarettes made from pressed tobacco that were heated electronically and were consumed in 8 puffs (Buchhalter and Eissenberg, 2000). Compared to conventional tobacco cigarettes, Accord use resulted in significantly less nicotine and CO delivery (Breland et al., 2002), and incomplete suppression of tobacco abstinence symptoms (Buchhalter et al., 2001; Breland et al., 2002; Hughes and Keely, 2004). The Accord also reduced, but did not substitute fully for own brand cigarette consumption (Hughes and Keely, 2004). Specifically, even when participants who smoked an average of 27 cigarettes per day at baseline were using 15 Accord products/day, they were also smoking on average, 18 cigarettes per day. Importantly, the failure of this product to substitute completely for tobacco cigarettes was predicted based on clinical laboratory results (e.g., Breland et al., 2002). Interestingly, a new version of this product is being marketed in Japan and the European Union (Protano et al., 2016; Tabuchi et al., 2016), though no published reports of its acute or longer-term effects are available to our knowledge.
Another “heat, not burn” product was the Eclipse (also no longer marketed in the U.S.), a paper-encased tobacco plug heated by a carbon element (Breland et al., 2002). Many smokers believed that this product was a safer alternative to conventional tobacco cigarettes (Hughes et al., 2005; Shiffman et al., 2004), and could aid in quitting attempts (Caraballo et al., 2006; Hughes et al., 2005; Shiffman et al., 2004). Clinical laboratory studies revealed that the product could suppress abstinence symptoms fully (Breland et al., 2002), reduce exposure to the carcinogen NNK (Breland et al., 2006), deliver nicotine (although significantly less than participants’ own brand of cigarettes; Breland et al., 2002, 2006), and increase expired air CO concentration (Breland et al., 2002, 2006; Fagerström et al., 2000, 2002). Product use also decreased the number of conventional cigarettes smoked per day, though the increased expired air CO concentration associated with its use caused concern (Fagerström et al., 2000, 2002). More recently, tobacco industry scientists report a “carbon-heated tobacco product” that, over 5 days of use, reduces user exposure to some toxicants, compared to conventional cigarettes (Ludicke et al., 2016).
Another class of “heat, not burn” product is the loose-leaf tobacco vaporizer (LLTV) that involves placing loose-leaf tobacco (also used in “roll your own” cigarettes) into a chamber that is heated by an electrically-powered element. One example is the Pax (Ploom), advertised to “…not heat to combustion and there is no smoke nor secondhand smoke” (https://www.paxvapor.com/pax/#pax-accessories). While the Pax is marketed as a LLTV, it is sometimes used for cannabis consumption (www.reddit.com/r/vaporents; forum.grasscity.com/forums/vaporizers.723/; Stevenson, 2014). The LLTV thus differs substantially from previous “heat, not burn” products in that the user determines the plant material to place in it, and no published research exists that describes its effects on user plasma nicotine or expired air CO concentration or tobacco abstinence symptom suppression.
In contrast to the little data available for these “heat, not burn” products, there is a rapidly growing literature related to the effects of electronic cigarettes (ECIGs; Breland et al., 2016; Grana et al., 2014, Hajek et al., 2013). Some of this literature includes clinical laboratory evaluations of ECIG effects on plasma nicotine, expired air CO, and tobacco abstinence symptoms in tobacco cigarette smokers (Lopez et al., 2016), using participants’ own brand cigarettes as a positive control (Vansickel et al., 2012). Most recent studies demonstrate that ECIGs can deliver physiologically active doses of nicotine to the user, do not deliver CO, and can suppress tobacco abstinent symptoms (Lopez et al., 2016; Ramôa et al., 2016; Spindle et al., 2015; St. Helen et al., 2016). These outcomes are relevant to tobacco product regulation, as they address dependence potential, toxicant exposure, and the ability of a product to supplement or substitute for conventional cigarette use. Moreover, the results of clinical laboratory evaluations of tobacco products demonstrably are reliable across studies for some products (e.g., Breland et al., 2016) but few studies of ECIG effects have replicated methods (particularly with respect to product/nicotine liquid used) so reliability of these methods with ECIGs is, as yet, uncertain. The primary purpose of this study was to compare, in cigarette smokers, the effects of short-term LLTV use on plasma nicotine and expired air CO concentration and abstinence symptom suppression with the effects of participants’ own brand cigarettes.
2. METHOD
2.1 Participants
This study was approved by Virginia Commonwealth University’s (VCU’s) institutional review board. Cigarette smokers (≥ 10 cigarettes/day) were recruited by advertisements and word of mouth and were eligible to participate if they reported being healthy, aged 18–55, and had used an ECIG ≤ 20 times and a LLTV < 5 times in their lifetime. Individuals were excluded from participation if they reported history of chronic disease or psychiatric condition, regular prescription medication use (aside from birth control), marijuana use > 10 days and alcohol use > 25 days in the past 30, use of a vaporizer for marijuana > 5 times in their lifetime, and any illicit drug use (e.g., cocaine, opioids, benzodiazepines, and methamphetamine) in the past 30 days. Women were excluded if they tested positive for pregnancy (by urinalysis) at screening.
2.2 Procedures
Participants completed each of the three, Latin-square ordered, ~2.5-hour sessions at Virginia Commonwealth University’s (VCU) Clinical Behavioral Pharmacology Laboratory. Sessions were separated by a minimum of 48 hours and differed by the product used: own brand (OB), LLTV, or ECIG. Participants were instructed to abstain from nicotine/tobacco for ≥ 12 hours prior to each session. Abstinence from combustible tobacco was verified via participants’ expired air CO concentration prior to the start of the session (≤ 10 ppm). In each session, participants completed two, 10-puff product use bouts (with 30 second inter-puff-intervals), separated by 60 minutes (as in Lopez et al., 2016). An intravenous catheter was used to sample blood (~7 ml) 10 times in each session (10 min prior to and 5, 15, 30, 45, and 55 min after bout 1, and 5, 15, 30, and 45 min after bout 2). Subjective questionnaires were administered immediately following each of the 10 blood samples and CO was measured at the start of the session and again 5 and 55 min after bout 1 and 5 and 45 min after bout 2. Physiological monitoring occurred throughout each session.
2.3 Materials
Participants used either their OB cigarettes, the LLTV, “Pax” (Ploom, CA), or an “eGo”, 3.3 V, 1000 mAh ECIG battery attached to a 1.5 Ohm, dual coil, 510-style cartomizer (produced by SmokTech; Shenzhen, China). OB cigarettes were purchased by study staff and provided to the participant in the appropriate condition. In the LLTV condition, the Pax vaporizer was prefilled with 1 gram of loose leaf tobacco (Zig Zag brand, National Tobacco Company, Louisville, Kentucky; no information on the nicotine content of this product was available). In the ECIG condition, the cartomizer was pre-loaded with approximately 1 ml of 18 mg/ml nicotine liquid that was approximately 70% propylene glycol and 30% vegetable glycerin (AVAIL Vapor, Richmond, VA). Loose leaf tobacco and ECIG liquid flavor (tobacco or menthol) was selected and matched to the participants’ preferred OB flavor. Ultimately, six participants used menthol and nine used tobacco flavored loose leaf tobacco/ECIG liquid based on the flavor of the OB cigarettes.
2.4 Outcome Measures
2.4.1 Physiological Measures
All blood samples were centrifuged, stored at −70°C, and sent to VCU’s Bioanalytical Analysis Core Laboratories for analysis of nicotine concentration with a limit of quantitation (LOQ) of 2 ng/ml (see Breland et al., 2006). Heart rate was monitored every 20 seconds using Criticare Systems model 507 (fitted with pulse oximeter). Participants’ expired air CO was measured via a BreathCO monitor (Vitalograph, Lenexa, KS).
2.4.2 Subjective Questionnaires
Four subjective measures were administered via computer at ten separate time points. Three of these measures were in the form of visual analog scale (VAS) items, consisting of a word or phrase centered on a horizontal line with “not at all” on the left and “extremely” on the right. Responses were recorded by participants moving a mouse cursor and clicking at any point on the horizontal line with scores being expressed as a percentage of the total line length (0–100). These VAS measures were the 10-item Direct Effects of Nicotine Questionnaire (Evans et al., 2006), the modified Hughes-Hatsukami withdrawal scale (11 items, omitting two items from the original: “Increased eating,” and “Insomnia/Disturbed sleep”), and The Direct Effects of Product scale. The Direct Effects of Product scale was adapted from the Direct Effects of Tobacco scale, modified such that when the word “cigarette” appeared in the original scale the word “product” appeared in this study (e.g., Foulds et al., 1992; Pickworth et al., 1994; see Breland et al., 2006). The fourth measure was the 10-item Tiffany-Drobes Questionnaire of Smoking Urges Brief; scores on individual items were used to create two factors, one measuring intention to smoke (0 – 30), and the other measuring anticipation of relief from withdrawal symptoms (0–24; QSU Brief; Cox et al., 2001).
2.5 Data Preparation and Analysis
As reported in previous work, plasma nicotine values below the LOQ were replaced with 2 ng/ml as this is a more conservative approach than assuming that values below the LOQ are zero (Lopez et al., 2016; Spindle et al., 2015; Vansickel et al., 2010). Heart rate values were averaged for three periods: five minutes before product use, and during product use for bouts 1 and 2. These three periods were chosen to reduce the possible interference of other activities (blood draws) on heart rate that might occur during other time periods.
Condition (three levels) by time repeated measures analysis of variance (ANOVA) was used to examine plasma nicotine (10 levels of time), heart rate (3 levels of time), and expired CO data (10 levels of time), and for all subjective measures that also had 10 levels of time except for the Direct Effects of Product scale, where the baseline time point was omitted from the analysis because participants had not sampled the product at baseline (thus, 9 levels for time). For plasma nicotine, one participant had one missing data point (at the 8th time point, in the Pax condition); this missing data point was imputed by taking the mean of the surrounding data points for that participant. For all ANOVAs, Huynh-Feldt corrections were used to adjust for potential violations of the sphericity assumption. To measure effect size for the F tests, eta-squared was calculated.
To compare means within products, a set of a priori comparisons were conducted using dependent samples t tests at baseline and immediately after both product use bouts only (during product use for heart rate data). These comparisons used the same baseline value twice and were therefore non-orthogonal within-product, so a Bonferroni correction was used (i.e., initial α < 0.05/2 comparisons = α < 0.025 for each comparison; Keppel, 1991). No comparisons across time were conducted for the Direct Effects of Product scale, as there was no true baseline time point. To compare across products, a set of a priori comparisons were conducted using dependent samples t tests at time points immediately after product use: OB compared to LLTV and ECIG, and LLTV compared to ECIG. These three comparisons were non-orthogonal, therefore, a Bonferroni correction was used (i.e., initial α < 0.05/3 comparisons = α < 0.017 for each comparison; Keppel, 1991). To measure effect size for these comparisons, Cohen’s d was calculated.
3. RESULTS
3.1 Participant Characteristics
Forty participants provided informed consent for this study, but 25 did not complete it. Sixteen did not meet eligibility criteria and 9 were discontinued because: they failed to keep scheduled appointments (n = 2), they failed to comply with study restrictions (n = 3), they became nauseous after using a study product (n=1), or staff could not achieve venous access (n = 3). In total, 15 individuals (3 women; 7 white or Caucasian, 6 Black/African American, 1 Asian, and 1 of unknown race and ethnicity) completed all three study conditions and were included in the final analyses. Mean (SD) age was 33.6 (11.8) years, participants smoked 16.1 (4.5) cigarettes/day on average, and had been smoking for an average of 10.2 (8.4) years. Mean ever ECIG use was 4.1 (5.2) times and mean LLTV use was 0.5 (1.1) times. At screening, mean Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) score was 5.1 (2.4) indicating moderate nicotine dependence, and mean expired air CO concentration was 21.1 (4.8) ppm.
3.2 Plasma Nicotine
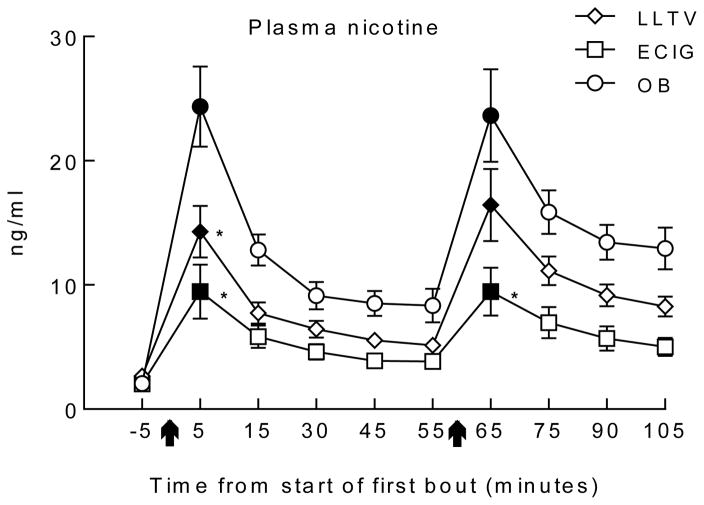
Figure 1 shows the time course of plasma nicotine concentration by each product use session, and analysis revealed a significant interaction of condition by time [F(18, 252) = 4.8, p < 0.01]. Immediately after bout 1, mean (SD) plasma nicotine concentration increased significantly from baseline in all conditions: mean plasma nicotine level in the OB condition was 24.4 (12.6) ng/ml (d = 2.5), in the LLTV condition was 14.3 (8.1) ng/ml (d = 1.2) and in the ECIG condition was 9.5 (8.5) ng/ml (d = 2.0) [ts(14) < −3.2, ps <.025]. Immediately after bout 2, mean (SD) plasma nicotine concentration also increased significantly (from baseline) in all conditions: for the OB condition plasma nicotine concentration was 23.7 (14.5) ng/ml (d = 2.1 ), for LLTV it was 16.4 (11.3) ng/ml (d = 1.7) and for ECIG it was 9.5 (7.5) ng/ml (d =1.4 ) [ts(14) < −3.7, ps <.025] After bout 1, mean plasma nicotine concentrations in the OB condition were significantly higher than means in the LLTV (d = 1.0) and ECIG conditions (d = 1.4) although LLTV and ECIG mean concentrations were not significantly different [ts(14) > 2.8, ps < 0.017; d = 0.6]. After bout 2, mean plasma concentration for the ECIG condition was significantly lower than OB (d = 1.2), but LLTV and ECIG means did not differ significantly [t(14) = 3.3, p < .017; d = 0.5].
Figure 1.
Mean ±SEM plasma nicotine concentrations for 15 participants who completed three conditions; own brand (OB), loose-leaf tobacco vaporizer (LLTV), and an electronic cigarette (ECIG). Arrows indicate the onset of each 10-puff use bout (30 second inter-puff interval). Filled symbols indicate significant difference from baseline (only conducted on time points immediately post-bout. For these non-orthogonal comparisons, all ps <.025). Asterisk indicates a significant difference from OB using paired-samples t tests (only conducted on time points immediately post-bout (for these non-orthogonal comparisons, all ps used < .017).
3.3 Heart Rate
Table 1 shows the statistical analysis results for heart rate. There was a significant interaction of condition by time [F(4, 56) = 19.1, p < .001]. During bout 1, mean (SD) heart rate increased significantly from baseline in all conditions: for the OB condition, mean heart rate was 80.7 (14.4) bpm (d = 1.7) for LLTV it was 78.3 (11.7) bpm (d = 1.4) and for ECIG it was 73.8 (11.7) bpm (d = 0.5) [ts(14) < −3.3, ps <.025]. During bout 2, mean (SD) heart rate increased significantly in the OB condition where it was 76.6 (11.5) bpm (d = 0.9) and LLTV condition where it was 74.6 (10.9) bpm (d = 1.2) [ts(14) < −4.5, ps <.025]. Across conditions, there were significant differences between OB and ECIG (d = 0.4, −0.2), and between LLTV and ECIG (d = 0.4, 0.4), during both bouts [ts(14) < −3.0, ps < .017]. There were no significant differences between OB and LLTV.
Table 1.
Results for statistical analyses for measures assessing acute use of an own brand cigarette, a loose-leaf tobacco vaporizer, and an ECIG (3.3 V, 1.5 ohms, filled with 18 mg/ml liquid nicotine concentration), over time.
| Condition | Time | Condition*Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Eta2 | F | p | Eta2 | F | p | Eta2 | |
| Plasma Nicotinea | 14.17 | <0.001 | 0.17 | 35.85 | <0.001 | 0.32 | 4.75 | <0.01 | 0.06 |
| Carbon Monoxideb | 69.94 | <0.001 | 0.60 | 23.42 | <0.001 | 0.05 | 64.58 | <0.001 | 0.19 |
| Heart Ratec | 2.64 | n.s. | 0.04 | 40.06 | <0.001 | 0.45 | 19.06 | <0.001 | 0.08 |
| Tiffany Drobes QSUa | |||||||||
| Factor 1: Intention | 5.34 | <0.05 | 0.12 | 17.74 | <0.001 | 0.20 | 4.16 | <0.001 | 0.05 |
| Factor 2: Anticipation | 3.23 | n.s. | 0.09 | 9.54 | <0.001 | 0.12 | 5.62 | <0.001 | 0.06 |
| Hughes-Hatsukamia | |||||||||
| Anxious | 1.57 | n.s. | 0.05 | 1.20 | n.s. | 0.01 | 1.54 | n.s. | 0.03 |
| Craving | 1.24 | n.s. | 0.03 | 5.83 | <0.001 | 0.10 | 2.10 | n.s. | 0.04 |
| Depression | 0.35 | n.s. | 0.01 | 0.90 | n.s. | 0.01 | 1.58 | n.s. | 0.03 |
| Difficultly Concentrating | 1.58 | n.s. | 0.04 | 1.27 | n.s. | 0.02 | 1.52 | n.s. | 0.03 |
| Drowsy | 0.45 | n.s. | 0.01 | 0.59 | n.s. | 0.01 | 1.69 | n.s. | 0.05 |
| Hunger | 1.64 | n.s. | 0.06 | 2.83 | <0.05 | 0.03 | 0.62 | n.s. | 0.01 |
| Impatient | 0.19 | n.s. | 0.01 | 3.32 | <0.05 | 0.04 | 1.20 | n.s. | 0.02 |
| Irritable | 1.25 | n.s. | 0.03 | 2.28 | n.s. | 0.03 | 1.15 | n.s. | 0..03 |
| Restless | 0.74 | n.s. | 0.02 | 2.94 | <0.05 | 0.06 | 1.17 | n.s. | 0.02 |
| Sweets | 0.38 | n.s. | 0.01 | 2.63 | <0.05 | 0.03 | 1.26 | n.s. | 0.03 |
| Urge | 3.02 | n.s. | 0.06 | 10.19 | <0.001 | 0.13 | 1.92 | n.s. | 0.04 |
| Direct Effects of Nicotinea | |||||||||
| Confused | 0.99 | n.s. | 0.01 | 1.69 | n.s. | 0.05 | 1.69 | n.s. | 0.05 |
| Dizzy | 2.43 | n.s. | 0.01 | 9.32 | <0.001 | 0.17 | 2.67 | <0.05 | 0.08 |
| Headache | 2.57 | n.s. | 0.01 | 0.54 | n.s. | 0.02 | 1.18 | n.s. | 0.03 |
| Heart pound | 2.17 | n.s. | 0.02 | 3.39 | <0.05 | 0.07 | 2.55 | <0.05 | 0.07 |
| Lightheaded | 2.59 | n.s. | 0.02 | 10.76 | <0.001 | 0.19 | 3.69 | <0.01 | 0.09 |
| Nauseous | 0.51 | n.s. | 0.01 | 2.11 | n.s. | 0.05 | 1.03 | n.s. | 0.03 |
| Nervous | 1.60 | n.s. | 0.01 | 0.45 | n.s. | 0.01 | 0.85 | n.s. | 0.03 |
| Salivation | 4.57 | <0.05 | 0.03 | 3.79 | <0.05 | 0.09 | 0.96 | n.s. | 0.03 |
| Sweaty | 0.02 | n.s. | 0.00 | 1.11 | n.s. | 0.05 | 0.89 | n.s. | 0.01 |
| Weak | 0.93 | n.s. | 0.01 | 1.13 | n.s. | 0.03 | 0.95 | n.s. | 0.03 |
| Direct Effects of Productd | |||||||||
| Awake | 8.17 | <0.01 | 0.17 | 7.99 | <0.001 | 0.13 | 0.85 | n.s. | 0.01 |
| Calm | 11.96 | <0.001 | 0.19 | 11.54 | <0.001 | 0.16 | 1.69 | n.s. | 0.02 |
| Concentrate | 5.77 | <0.05 | 0.16 | 8.02 | <0.001 | 0.09 | 1.07 | n.s. | 0.01 |
| Dizzy | 5.72 | <0.01 | 0.10 | 10.08 | <0.001 | 0.12 | 2.69 | <0.05 | 0.23 |
| Pleasant | 11.74 | <0.001 | 0.32 | 4.57 | <0.05 | 0.12 | 0.87 | n.s. | 0.06 |
| Reduce hunger | 8.62 | <0.01 | 0.15 | 3.83 | <0.05 | 0.08 | 1.63 | n.s. | 0.03 |
| Right now | 1.89 | n.s. | 0.07 | 7.51 | <0.001 | 0.05 | 3.28 | <0.01 | 0.04 |
| Satisfying | 12.86 | <0.001 | 0.35 | 6.09 | <0.01 | 0.06 | 0.67 | n.s. | 0.00 |
| Sick | 0.83 | n.s. | 0.03 | 0.75 | n.s. | 0.01 | 0.88 | n.s. | 0.01 |
| Taste good | 11.46 | <0.001 | 0.34 | 4.55 | <0.05 | 0.04 | 0.98 | n.s. | 0.00 |
df Condition = (2,28); df Time = (9,126); df Condition x Time = (18,252)
df Condition = (2,28); df Time = (4,56); df Condition x Time = (8,112)
df Condition = (2,28); df Time = (2,28); df Condition x Time = (4,56)
df Condition = (2,28); df Time = (8,112); df Condition x Time = (16,224)
3.4 Expired Breath CO
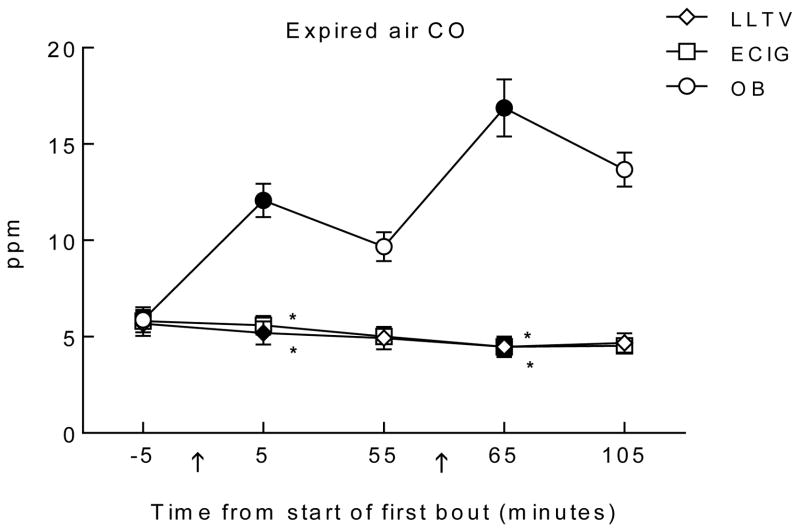
Figure 2 shows the time course of expired breath CO by each product use session and analysis revealed a significant interaction of condition by time [F(8, 112) = 64.6, p < 0.001]. Immediately after bout 1, mean (SD) expired breath CO concentration for OB increased significantly to 12.1 (3.4) ppm (d = 2.1), and also increased (from baseline) after bout 2 to 16.9 (5.8) ppm (d = 2.5) [ts(14) < −8.3; ps <0.025). CO concentration for LLTV decreased significantly after both bouts (d = −0.2, −0.5), and CO levels for ECIG significantly decreased after bout 2 [ts(14) > 3.4; ps < 0.025; d = −0.7]. Significant differences were observed between OB and LLTV (d = 2.4) and between OB and ECIG (d = 2.4) after bout 1 [ts(14) > 7.7, ps < .017]. Immediately after bout 2, mean (SD) CO concentration for OB was 16.9 (5.8) ppm, for LLTV was 4.5 (2.1) ppm, and for ECIG was 4.5 (1.7) ppm, with significant differences between OB and LLTV (d = 2.9) and between OB and ECIG (d = 2.9) [ts(14) > 9.0, ps < .017]. LLTV and ECIG did not differ significantly from each other at either time point.
Figure 2.
Mean ±SEM expired carbon monoxide (CO) levels for 15 participants who completed three conditions; own brand (OB), loose-leaf tobacco vaporizer (LLTV), and an electronic cigarette (ECIG). In all other respects the figure is identical to Figure 1.
3.5 Abstinence Symptom Suppression
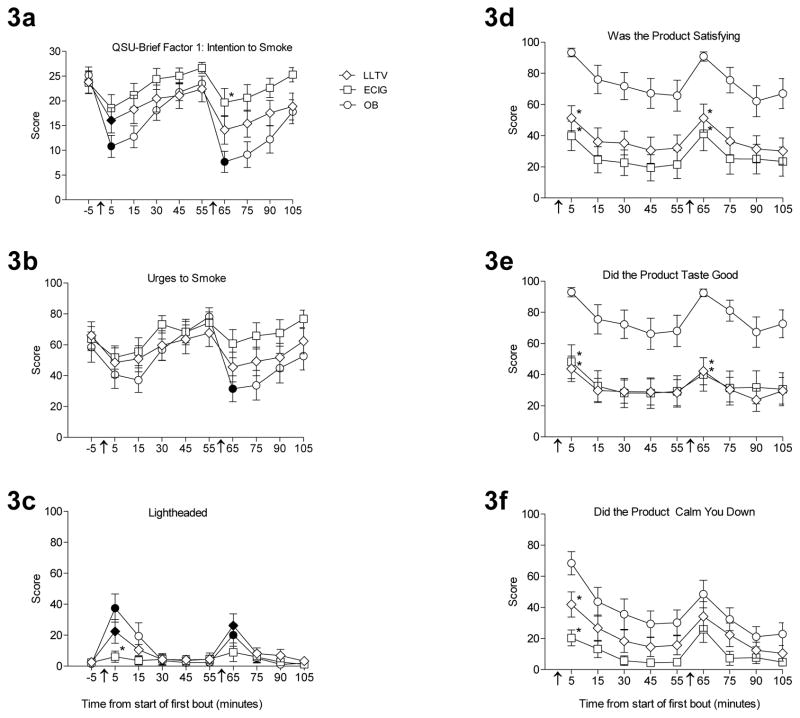
Statistical analysis results of the subjective measures are shown in Table 1. There were significant interactions of condition by time for both QSU factors [Fs (18, 252) > 4.1, ps < 0.001], with each factor showing decreases relative to baseline following product administration. For example, Figure 3a displays data from QSU Factor 1- Intention to Smoke, on which scores significantly decreased after bout 1, relative to baseline, for both OB (d = 2.0 ) and LLTV (d = 0.8). Specifically, in the OB condition, scores decreased from 25.2 (6.4) to 10.8 (8.6) after bout 1; in the LLTV condition, scores decreased from 23.8 (8.7) to 16.1 (9.9; [ts(14) > 3.0, ps <0.025). Scores significantly decreased (from baseline) after bout 2 for the OB condition only [t(14) = 6.4; p < 0.025; d = 2.4]. While there were no differences between the conditions immediately following bout 1, OB had a significantly lower mean score of 7.7 (8.3) compared to 19.7 (10.9) for the ECIG condition immediately following bout 2 [ts(14) = − 3.4, p < 0.017; d = −1.2].
Figure 3.
Mean ±SEM ratings for six visual analog scale items from 15 participants before and after product use over three sessions that differed by product; own brand (OB), loose-leaf tobacco vaporizer (LLTV), and an electronic cigarette (ECIG). “Intention to smoke a cigarette” from the QSU-Brief Factor 1 score (Figure 3a); “Urges to Smoke” from the Hughes-Hatsukami withdrawal scale (Figure 3b); “Lightheaded” from the Direct Effects of Nicotine scale (Figure 3c); “Was the product satisfying” (Figure 3d), “Did the product taste good” (Figure 3e), and “Did the product calm you down” (Figure 3f) from the Direct Effects of Product Use scale. No comparisons across time were conducted for figures 3d, 3e, or 3f, as these questions did not have a true baseline time point. In all other respects the figure is identical to Figure 1.
While the analysis revealed no significant interaction for any of the Hughes-Hatsukami items, main effects of time were observed for six of them [Fs (9, 126) > 2.6, ps < 0.05], with general patterns of decreasing mean scores following product administration. For example, Figure 3b displays data from the “Urges to Smoke” item. A significant decrease on scores for this item was observed only for the OB condition, after bout 2. More specifically, in the OB condition, during the second bout, the mean baseline score was 58.5 (38.0), and decreased to 31.5 (32.4) after OB use (d = 0.8). There were no significant differences between any of the conditions immediately following either bout.
A significant interaction was observed for the items “Dizzy”, “Heart Pound”, and “Lightheaded” from the Direct Effects of Nicotine questionnaire [Fs (18, 252) > 2.5, ps < 0.05]. For example, Figure 3c displays data from the “Lightheaded” item, for which mean scores significantly increased relative to baseline for both the OB and LLTV conditions immediately following bout 1 (d = 1.4, 0.9). That is, scores in the OB condition significantly increased from 1.5 (3.3) to 37.5 (35.2), and scores in the LLTV condition significantly increased from 2.60 (8.2) to 22.5 (29.8) [ts (14) < −2.8, ps < 0.025]. A similar pattern immediately following bout 2 for both OB and LLTV relative to baseline was also apparent [ts(14) < −2.5, ps < 0.025; d = 0.9, 1.1]. Furthermore, there was a significant difference in mean scores between the OB and ECIG conditions at the time point following bout 1, with the OB condition score of 37.5 (35.2) being significantly higher than the ECIG condition score of 6.1 (14.0; [t(14) = − 3.4, p < 0.017; d = 1.2]). However, there were no differences between the OB and LLTV conditions at that same time point. There were no significant differences between any of the conditions immediately following bout 2.
There were two items of the Direct Effects of Product Scale that showed significant interactions of condition by time: “Did the product make you dizzy?” and “Would you like to use another product RIGHT NOW?” [Fs (16, 224) > 2.6, ps < 0.05] with significant main effects of time observed for seven additional items [Fs (8, 112) > 3.8, ps < 0.05]. For example, Figure 3d displays data from the “Was the product satisfying?” item. Immediately following bout 1, the mean score for the OB condition of 93.3 (10.51) was significantly higher compared to the scores of 51.2 (30.9) for the LLTV condition (d = 1.8) and 39.9 (36.7) for the ECIG condition (d = 2.0) [ts (14) > 4.1, ps < 0.017]. There was a similar pattern following bout 2. Similarly, figure 3e displays data from the “Did the product taste good?” item: immediately following bout 1, the mean score for the OB condition of 92.9 (11.4) was significantly higher compared to the score of 43.7 (31.8) for the LLTV condition [t(14) = 5.2, p < 0.017; d = 2.1 ] and also significantly higher compared to the score of 48.2 (42.2) for the ECIG condition [t(14) = −4.0, ps < 0.017; d = 1.5]. For the item “Did the product calm you down?” (Figure 3f), immediately following bout 1, the mean score for the OB condition of 68.4 (28.9) was significantly higher compared to the LLTV score of 41.8 (31.2; [t(14) = 4.1, p < 0.017; d = 0.9]) and the ECIG score of 20.3 [19.71; t(14) = −5.6, ps < 0.017; d = 1.9]. There were no significant differences between any of the conditions immediately following bout 2.
4. DISCUSSION
This study is the first to reveal LLTV effects on measures of nicotine and CO delivery and abstinence symptom suppression. In this short-term, laboratory study, LLTV use significantly increased plasma nicotine concentration, did not significantly increase expired air CO concentration, and significantly reduced abstinence symptom severity in tobacco cigarette smokers. These effects did not differ on most measures from those observed with the particular ECIG/liquid combination used here, and were less pronounced than those observed for participants’ own brand of combustible tobacco cigarette. Participants reported that LLTV and ECIG were significantly less satisfying than their own brand of cigarettes.
These results have several implications. First, the low CO delivery associated with LLTV and ECIG use suggests that smokers who switch to these products exclusively could reduce their risk of CO-induced cardiovascular dysfunction (Papathanasiou et al., 2014). Second, as both the LLTV and ECIG delivered nicotine, both products have the potential for creating and/or maintaining nicotine dependence in the user. However, to the extent that matching the nicotine delivery of a tobacco cigarette is important in facilitating a complete switch to LLTV or ECIG, these results suggest that the products and conditions reported here may be inadequate. Third, the reduced abstinence symptom suppression associated with both the LLTV and the ECIG, relative to participants’ own brand, may suggest that complete substitution of these products (under these use conditions) for tobacco cigarettes may be less likely. Indeed, another “heat, not burn product” (Accord) may have failed commercially in part due to its inability to suppress withdrawal and/or deliver nicotine as effectively as a combustible tobacco cigarette. One concern with incomplete abstinence suppression/nicotine delivery is that users of such products who are also tobacco cigarette smokers may supplement their tobacco cigarette use with these products (i.e., become “dual users”; Kalkhoran et al., 2015; Weaver et al., 2015; Wills et al., 2015).
A fourth implication of this work is that clinical laboratory methods such as those described here and also used to evaluate other novel tobacco products (Buchhalter et al., 2001; Breland et al., 2002; Cobb et al., 2015, 2010; Gray et al., 2008; Lopez et al., 2016) are valuable for revealing important features of these products, including toxicant delivery and physiological and subjective effect profile. Advantages of these methods are that they are time- and cost-efficient relative to longer-term studies, and can be predictive of the results of those studies (e.g., Breland et al., 2002; Fagerström et al., 2000, 2002; Hughes and Keely, 2004). The effects reported in these studies are also reliable, as has been demonstrated previously for a “heat, not burn” product (Buchhalter and Eissenberg, 2000; Buchhalter et al., 2001; Breland et al., 2002) and for tobacco smoking using a waterpipe (Blank et al., 2011; Cobb et al., 2015, 2011) and as highlighted by comparing the ECIG effects reported here with those that were reported elsewhere using an identical device/liquid combination (Lopez et al., 2016). That is, in this study and that reported by Lopez et al. (2016), cigarette-smoking participants completed two, 10-puff (30 sec interpuff interval) use bouts with an ECIG composed of a 3.3 V battery attached to a 1.5 Ohm dual-coil heating element loaded with approximately 1 ml of a 18 mg/ml nicotine liquid made of approximately 70% propylene glycol and 30% vegetable glycerin. Mean (SD) plasma nicotine “boost” (plasma nicotine concentration immediately after the bout minus the concentration immediately before the bout) in this study (N=15) was 7.4 ng/ml (8.6) compared to 9.8 ng/ml (13.0; N=16, Lopez et al., 2016) for bout 1 and 5.6 ng/ml (5.8) compared to 7.7 ng/ml (12.4) for bout 2. Independent samples t-tests revealed no significant differences between the means for the two studies. The ability of acute laboratory evaluations to predict the outcome of longer-term, real-world studies accurately suggests validity, and reproducible results with the same product across different samples suggests reliability. In a context where novel tobacco products are proliferating within a nascent regulatory framework, time- and cost-efficient, valid, and reliable methods like those reported here are critical to inform science-based policies designed to protect individual and public health.
Future research on LLTV should include measurement of exposure to other toxicants in addition to nicotine and CO. While exclusive ECIG use may be associated with reduced exposure to CO and many of the carcinogens associated with combustible tobacco (e.g., Hecht et al., 2015), no information regarding LLTV carcinogen delivery or emissions has been published to date. In addition, the extent to which LLTV users use this product as a substitute for combustible tobacco cigarettes or a supplement to them is important to understand. Finally, the extent to which LLTV users use marijuana and/or other substances in the product, and the effects of that use needs to be determined.
This study has several limitations. First, puffing behavior (topography) was not measured, as existing equipment could not be modified easily to fit the LLTV mouthpiece. Measuring participants’ LLTV use topography, and comparing it to topography for cigarettes and other products such as ECIGs, could help researchers understand the relationship between user behavior and toxicant yield, delivery, and subjective response. This same issue has been noted for ECIGs (Spindle et al., in press). There is an urgent need for a topography device that is less dependent on the shape of a product’s mouthpiece. Second, participant use behavior was constrained to a specific puffing pattern (10 puffs, 30 sec interpuff interval). This constraint likely influenced the nicotine delivery and subjective effect profile described here. Allowing participants to puff “ad libitum” within a clinical laboratory study (i.e., as in Spindle et al., in press; Farsalinos et al., 2015) may be an important adjunct to the methods reported here. Third, participants were current smokers with no previous use with LLTVs and only became familiar with the LLTV during the two use bouts in this study. This lack of previous experience could have influenced the results (i.e., participants’ use behavior, nicotine levels, and subjective answers). However, based on the authors’ experience recruiting a variety of tobacco product users, it appears that very few experienced LLTV users live in the Richmond, VA area (and no epidemiological data is available, to the authors’ knowledge); thus, experienced LLTV users would have been a difficult population to recruit.
In sum, the results from this and other clinical laboratory studies can inform tobacco control policy with respect to toxicant exposure and physiological and subjective effect profile. All new tobacco products, including “heat, not burn” products, should be evaluated on these and other health-related outcomes prior to marketing them to consumers. Until they are, and until product regulation and advertising are based on the results of these evaluations, tobacco cigarette smokers who want to reduce their exposure to disease-causing tobacco toxicants are likely to be informed and influenced by industry marketing that may be more concerned with profit than it is individual and public health.
Highlights.
This is the first human laboratory study of a loose-leaf tobacco vaporizer (LLTV).
LLTV use significantly increased plasma nicotine concentration, but not CO.
LLTV use reduced tobacco abstinence symptom severity, but less so than a cigarette.
Both the LLTV and an ECIG were significantly less satisfying than cigarettes.
Laboratory methods can be expanded to “heat-not-burn” products.
Acknowledgments
Role of Funding This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
The authors thank Janet Austin, Barbara Kilgalen, Tory Spindle, Hannah Mayberry, Kathleen Osei, Kendall Pettaway, and Caroline Smith for their help with protocol development, data collection and data management.
Footnotes
Contributors Alexa Lopez, Marzena Hiler, Sarah Maloney, Thomas Eissenberg, Alison Breland
Conflict of Interest No conflict declared.
Contributors
Alexa Lopez, Marzena Hiler, Thomas Eissenberg, and Alison Breland designed the study and developed the protocol. A. Lopez conducted most of the statistical analysis and wrote the first draft of the manuscript. M. Hiler and S. Maloney helped to collect study data. All authors contributed to a final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, Eissenberg T. Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study. Drug Alcohol Depend. 2011;116:102–109. doi: 10.1016/j.drugalcdep.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Kleykamp B, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Breland A, Buchhalter A, Evans S, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res. 2002;4:131–140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Buchhalter A, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Buchhalter A, Eissenberg T. Preliminary evaluation of a novel smoking system: effects on subjective and physiological measures and on smoking behavior. Nicotine Tob Res. 2000;2:39–43. doi: 10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- Caraballo R, Pederson L, Gupta N. New tobacco products: do smokers like them? Tob Control. 2006;15:39–44. doi: 10.1136/tc.2005.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Blank MD, Morlett A, Shihadeh A, Jaroudi E, Karaoghlanian N, Kilgalen B, Austin J, Weaver MF, Eissenberg T. Comparison of puff topography, toxicant exposure, and subjective effects in low-and high-frequency waterpipe users: a double-blind, placebo-control study. Nicotine Tob Res. 2015;17:667–74. doi: 10.1093/ntr/ntu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Shihadeh A, Weaver MF, Eissenberg T. Waterpipe tobacco smoking and cigarette smoking: a direct comparison of toxicant exposure and subjective effects. Nicotine Tob Res. 2011;13:78–87. doi: 10.1093/ntr/ntq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L, Tiffany S, Christen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act, 2016. 81 FR 28973 (May 10, 2016). Docket No. FDA-2014-N-0189
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO, Hughes JR, Callas PW. Long-term effects of the eclipse cigarette substitute and the nicotine inhaler in smokers not interested in quitting. Nicotine Tob Res. 2002;4:141–145. doi: 10.1080/1462220021000032771. [DOI] [PubMed] [Google Scholar]
- Fagerström K, Hughes J, Rasmussen T, Callas P. Randomised trial investigating effect of a novel nicotine delivery device (eclipse) and a nicotine oral inhaler on smoking behaviour, nicotine and carbon monoxide exposure, and motivation to quit. Tob Control. 2000;9:327–333. doi: 10.1136/tc.9.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, … Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers) Sci Rep. 2015:5. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MAH. Effects of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology. 1992;106:421–427. doi: 10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hanson K, Briggs A, Parascandola M, Genkinger JM, O’Connor R, Shields PG. Clinical trials methods for evaluation of potential reduced exposure products. Cancer Epidemiol Biomark. 2009;18:3143–3195. doi: 10.1158/1055-9965.EPI-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hecht S, Carmella S, Kotandeniya D, Pillsbury M, Chen M, Ransom B, Vogel R, Thompson E, Murphy S, Hatsukami D. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17:704–709. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Callas PW. Ever users versus never users of a “less risky” cigarette. Psycholol Addict Behav. 2005;19:439–442. doi: 10.1037/0893-164X.19.4.439. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP. The effect of a novel smoking system-Accord- on ongoing smoking and toxin exposure. Nicotine Tob Res. 2004;6:1021–1027. doi: 10.1080/14622200412331296011. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis, a Researcher’s Handbook. Pearson, Englewood; New Jersey: 1991. [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, Breland AB, Shihadeh AL, Eissenberg T. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob Res. 2016;18:720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdicke F, Haziza C, Weitkunat R, Magnette J. Evaluation of biomarkers of exposure in smokers switching to a carbon-heated tobacco product: a controlled, randomized, open-label 5-day exposure study. Nicotine Tob Res. 2016;18:1606–1613. doi: 10.1093/ntr/ntw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pax Labs, Inc. [Accessed May 30, 2016];Pax Ploom’s website for vaporizers and accessories. www.paxvapor.com/pax/#pax-accessories.
- Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Effects of smoking on cardiovascular function: the role of nicotine and carbon monoxide. Health Sci J. 2014;8:274–290. [Google Scholar]
- Pickworth WB, Bunker EB, Henningfield JE. Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology. 1994;115:9–14. doi: 10.1007/BF02244745. [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, Breland AB, Shihadeh A, Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Di Marino ME. Smoker and ex-smoker reactions to cigarettes claiming reduced risk. Tob Control. 2004;13:78–84. doi: 10.1136/tc.2003.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle T, Hiler M, Breland A, Karaoghlanian N, Shihadeh AL, Eissenberg T. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine Tob Res. 2016:ntw174. doi: 10.1093/ntr/ntw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17:142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111:535–544. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson S. A High-Tech High: How The Rise Of The Vaporizer Is Changing Pot Culture. Slate. 2014 Jul 2; Retrieved from http://www.slate.com/articles/technology/technology/2014/07/vaporizers_how_the_new_high_tech_devices_are_changing_marijuana_culture.html.
- Tabuchi T, Kiyohara K, Hoshino T, Bekki K, Inaba Y, Kunugita N. Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan. Addiction. 2016;111:706–713. doi: 10.1111/add.13231. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomark. 2010;19:1945–53. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]