Abstract
Background
Patterns of smoking vary as a function of age and race. The goals of this study were to identify trajectories of maternal cigarette use over a 17-year span, and to determine if maternal age at first birth and race were associated with smoking trajectories.
Methods
Pregnant women (N = 690) were recruited at an urban prenatal clinic. The women (13–42 years old; 62% African-American, 38% White) were interviewed about cigarette use during pregnancy and 6, 10, 14, and 16 years postpartum. Growth mixture modeling (GMM) was used to identify trajectories. Regressions were used to determine if maternal age at first birth and race predicted trajectory class membership.
Results
A GMM of maternal cigarette use delineated 5 groups: none/unlikely to use (33%), decreasing likelihood of use (6%), late desistance (5%), increasing likelihood of use (17%), and chronic use (39%). Women who became mothers at a younger age were more likely to be classified as late desisters or increasingly likely to smoke. White mothers were more likely to be chronic smokers. Different smoking trajectories and predictors of trajectories were identified for the African-American and White mothers. Covariates including prenatal substance use, hostility, education, and economic hardship also differentiated smoking trajectories.
Conclusions
Both prevention and treatment of smoking should be targeted to specific groups by age of first pregnancy and race. Pregnant smokers should be provided with more information and resources to help them avoid cigarettes during pregnancy and maintain abstinence after pregnancy.
Keywords: tobacco, smoking, maternal, mother, women, desistance
Graphical Abstract
1. Introduction
Maternal smoking significantly affects the health of mothers and their children, predicting decreased fetal growth, altered brain development, and physical and behavioral health problems in offspring (Bernstein et al., 2005; Cornelius & Day, 2009; El Marroun et al., 2014; England et al., 2001; Lindell et al., 2012; Lux et al., 2000). In addition, mothers who smoke expose their children to secondhand smoke, and these offspring are more likely to smoke and use other substances (Cornelius et al., 2000; 2005; De Genna et al., 2016; Goldschmidt et al., 2012; Melchior et al., 2010).
1.1 Developmental Trajectories of Cigarette Smoking
Researchers have identified varying patterns of smoking in different populations, although most studies were conducted on data from predominantly White samples. However, these 3 groups are consistently identified: non/experimental smokers, chronic smokers, and individuals that desist over time. In a large Midwestern community sample (N = 8,566), Chassin and colleagues (2000) identified these three groups, bu removed abstainers and experimental smokers from trajectory analysis a priori. When these groups were included, Brooke et al. (2008) reported 5 groups: the main 3 groups, experimenters and abstainers, and late starters. Macy et al. (2015) also reported 5 groups but considered age of onset as a factor in all smoker groups identifying nonsmokers, late-onset experimental smokers, early onset with cessation, delayed onset with cessation, and chronic smokers.
1.2 Race/ethnicity and Cigarette Use
Studies with more diverse samples highlight racial differences in patterns of cigarette use. White et al. (2004) found more African-American males in the non-smoker group, and more White males in the chronic smoker group. Similarly, there were more White mothers in the smoking trajectories and more African-American mothers in the non-smoking trajectory in the Early Childhood Longitudinal Study (ECLS-B: Mumford et al., 2014; 2015). There may also be an interaction or —crossover effect for race and age: White adolescents are more likely to smoke, but African-Americans who smoke are less likely to desist in adulthood (Evans-Polce et al., 2015; Geronimus et al., 1993; Kandel et al., 2011; Lawrence et al., 2014; Yuan, 2011). As a result, differences in the prevalence of cigarette use between White and African-American youth peak at age 18 and are much lower by age 29 (Evans-Polce et al., 2015, Keyes et al., 2015).
1.3 Maternal Age and Cigarette Use
Individuals change their patterns of health behavior during major life transitions such as the transition to motherhood (Elder, 1975). Motherhood in adult women is correlated with a reduction in cigarette use (McDermott et al., 2004; 2009). However, for adolescents, pregnancy is associated with smoking (Ellickson et al., 2001; Graham et al., 2010; McGee & Williams, 2006; Tucker et al., 2006) and teen mothers have higher rates of perinatal smoking than older women (Cornelius et al., 1994; De Genna et al., 2009; Graham et al., 2010). Only two prior studies have examined the role of maternal age in patterns of smoking over time, finding more smoking overall and more heterogeneity in patterns of smoking among the younger mothers (Mumford & Liu, 2015; Tucker et al., 2006).
1.4 Current Study
This study on longitudinal patterns of maternal smoking is targeted to the following audiences: public health officials concerned with smoking in women, clinicians (women’s primary health care providers and pediatricians), and researchers investigating developmental patterns of women’s smoking, racial disparities in smoking, and maternal and child health. This study addresses several gaps in the current literature. No study has examined maternal age at first birth as a predictor of smoking trajectories in women from a wide age range over a 17-year span. This is also the first study to examine the role of maternal age in patterns of smoking in women separately by race for African-American and White mothers. Other substances used during pregnancy, also measured prospectively, were included as covariates because substance use co-occurs with smoking and is associated with maternal age and race (De Genna et al., 2009; De Genna et al., 2015; Liu et al., 2015). We hypothesized that younger maternal age would be associated with more problematic patterns of maternal smoking (such as chronic cigarette use compared to abstinence or patterns of desistance) after controlling for socioeconomic status and mental health. We also expected to find different trajectories and predictors of patterns of smoking in White and African-American mothers, although this hypothesis is exploratory as there are no other studies of separate smoking trajectories for White and African-American mothers.
2. Material and methods
2.1 Participants
Data from three birth cohorts that are part of a consortium of studies on the effects of substance use on offspring physical and neurobehavioral development were merged for these analyses. Data were collected for NIH-funded studies on substance use in adolescent (under 19) and adult mothers (over 18). Integrative data analysis is vulnerable to bias from between-subject heterogeneity (Curran & Hussong, 2009). However, these participants were all drawn from the same prenatal clinic, with the same measures, personnel, and follow-up time periods.
Participants of the adult mother cohorts were from two studies of adult women who attended the prenatal clinic from 1982–1985 and enrolled at their 4th prenatal month clinic visit. Eighty-five percent of the women agreed to participate. There were no differences in age, income, or race between those who participated and those who refused. Two cohorts were selected from the initial sample, one to study the effects of prenatal alcohol use and another to study the effects of prenatal marijuana use (combined n = 763). Women were selected for the alcohol study if they drank 3 or more alcoholic drinks per week, along with the next pregnant adult woman who drank less often or not at all (AA06390: PI N. Day). Women were selected for the marijuana study if they used 2 or more joints per month, along with the next woman who less often or not at all (DA03874: PI N. Day). At the 16-year follow-up, 574 of the women from the adult mother cohorts were assessed (75% of the birth sample).
For the adolescent mother study, pregnant adolescents were recruited from 1990–1994 and interviewed during their 4th prenatal month visit and again at delivery (AA08284: DA09275: PI M. Cornelius). Ninety-nine percent agreed to participate (n = 413). By the 16-year follow-up, a total of 326 women were assessed: 79% of the birth cohort.
At birth, the combined sample size was 1,176 mothers. The focus of the parent studies was the effect of prenatal exposures on offspring, so mothers were not re-assessed if children died or were placed for adoption. By 16 years postpartum, 13 children had died, 15 were adopted or institutionalized, and 34 mothers refused further contact. Mothers were not included in the present analysis if they had lost custody of their child, moved out of the area, were lost to follow up, or were missing 2 or more postpartum drug and tobacco assessments (n = 486). This resulted in an analytical sample of 690 mothers. There were significant differences in race and prenatal cigarette smoking between those included in the analyses (n = 690) and those who did not participate (n = 486): 38% of the subjects who participated at 16 years were White compared to 50% among the non-participants, and 48% of study participants smoked cigarettes during the 1st trimester of pregnancy compared to 57% among the excluded. There were no significant differences in maternal age, education or other prenatal substance use between these two groups.
Mothers ranged in age from 13–42 years old (M = 20.75, SD = 4.7), and were 62% African-American and 38% White. On average, they were 18.9 years old (SD = 3.2, range = 13–31) when their first child was born. At baseline, 21% were married, and 35% were married at the 16 years post-partum phase. Mean educational attainment 16 years post-partum was 12.5 years (SD = 1.9: range = 6–18 years).
2.2. Procedure
Participants were recruited during the fourth or fifth month prenatal visit and interviewed about tobacco and other drug use during the first trimester. Mothers were seen within 36 hours after delivery, and interviewed about third trimester use. At the 6-, 10-, 14- and 16-year follow-up visits, mothers provided information about their tobacco and other substance use, demographic and psychological status. A federal Certificate of Confidentiality was obtained to protect participant confidentiality. The Institutional Review Board of the Magee-Womens Hospital approved the prenatal and delivery phases of the cohort studies, and the University IRB approved all later phases.
2.3 Measures
2.3.1. Maternal age at first birth (independent variable)
Baseline demographic characteristics were recorded during the first prenatal visit, including maternal date of birth and age at the birth of their first child if not primiparous. Age at first birth was the predictor entered in the analyses rather than current maternal age, because the variable of interest was age at transition to motherhood.
2.3.2. Maternal cigarette use (dependent variable)
Mothers were interviewed in a private setting by interviewers who were comfortable discussing substance use in a non-judgmental manner, trained to use the instruments reliably, and to accurately identify the substances used as well as the amount of use. Mothers were asked about the number of cigarettes they smoked on a typical day during the first trimester, third trimester, and 6, 10, 14, and 16 years postpartum. Cigarette use was dichotomized to use/no use.
2.3.3. Covariates
Demographic characteristics
Maternal race was recorded during the first prenatal visit. A summary variable capturing maternal economic hardship was constructed from monthly family income, mother’s ability to handle bills, and financial strain 6 years postpartum, representing the mid-point of the study (Hardaway & Cornelius, 2014). Financial strain was measured with three questions in the maternal interview: how often the mother was short of money at the end of the month, how often the mother could not buy essential things for her child, and how often the mother could not do extra things for her child (alpha = .73). Maternal educational attainment was measured at the 16-yearpost-partum assessment.
Other substance use during pregnancy
Mothers were asked about the quantity and frequency of alcohol and marijuana they used during the first and third trimesters. Average daily use of alcohol and marijuana for each trimester were calculated based on the quantity and frequency of use reported by the mothers.
Maternal psychological status
Maternal levels of depression and hostility were measured at all phases of the study. Maternal depression was assessed using the Center for Epidemiological Studies Depression scale (CES-D: Radloff, 1977), a measure of depression that is widely used in large studies (Eaton & Kessler, 1981; Murphy, 2002; Radloff & Locke, 1986). A summary score capturing chronicity of depression was created by adding a point for each phase that the mothers scored higher than or equal to 21 on the CES-D, indicating a clinically significant depressive episode in the past year (Radloff, 1977). Maternal dispositional anxiety and hostility were assessed using the State Trait Anxiety Index (STAI: Spielberger et al., 1970). Similarly, a summary score capturing chronicity of hostility was created by adding a point for each phase that the mothers scored higher than or equal to 18 on the hostility subscale of the STAI, placing them in the top quartile for hostility.
2.4 Statistical analysis
A growth mixture model (GMM) was applied to maternal cigarette use measured at all phases to identify trajectories of use over time. GMM allows variation in trajectories across individuals, and at the same time, estimates mean parameters for each trajectory (Muthén & Muthén, 2000). Cubic curves were fitted to identify trajectory classes of maternal cigarette use. The number of classes that best fit the data was determined using several statistics: relatively lower Bayes Information Criterion (BIC), non-significant likelihood ratio test, and relatively higher entropy value (Nylund et al., 2007). The posterior probabilities for each class were also screened to ascertain lack of ambiguity in assigning individuals to different classes. GMM was first applied to the entire sample, and then separately for African-American and White mothers. Polytomous logistic regressions were then used to test the association between maternal age at first pregnancy and maternal cigarette use trajectories. The same set of covariates was used for all regressions (except race was omitted in the separate regressions conducted on the predictors of the different trajectories for African-American and White mothers). Regressions were carried in a stepwise manner, with Tables 2–4 listing the covariates that were statistically significant in the final models.
Table 2.
Regression results predicting latent cigarette use trajectory classes in full sample (reference group = non-smokers)
| Latent Class Contrast vs. Non-smokers: Odds Ratio (confidence intervals) | ||||
|---|---|---|---|---|
| Predictor | Increasingly Likely (17%) | Decreasingly Likely (6%) | Late Desistance (5%) | Chronic Smokers (39%) |
| Age at first birth | 0.9* (0.84–0.98) | 1.0 (0.92–1.1) | 0.79* (0.68–0.92) | 0.95 (0.89–1.0) |
| White Race | 0.65 (0.38–1.1) | 1.6 (0.74–3.3) | 0.99 (0.42–2.3) | 2.00* (1.3–3.0) |
| Education | 0.87* (0.76–0.99) | 0.80* (0.66–0.97) | 0.84 (0.68–1.0) | 0.79* (0.71–0.89) |
| Economic hardship | 0.95 (0.87–1.0) | 1.0 (0.89–1.2) | 1.0 (0.87–1.1) | 0.90 (0.84–0.97) |
| Chronic hostility | 1.3* (1.1–1.6) | 0.89 (0.66–1.2) | 1.3* (1.01–1.7) | 1.10 (0.98–1.3) |
| Prenatal alcohol use | 1.0 (0.63–1.7) | 2.0* (1.4–2.9) | 1.8* (1.2–2.8) | 1.90* (1.4–2.7) |
| Prenatal marijuana use | 1.2 (0.77–1.8) | 1.6* (1.01–2.4) | 1.5 (0.93–2.4) | 1.60* (1.2–2.2) |
Note.
p < .05
Table 4.
Regression predicting latent cigarette use trajectory classes in White mothers (reference group = non-smokers)
| Latent Class Contrast vs. Non-smokers: Odds Ratio (confidence intervals) | |||
|---|---|---|---|
|
| |||
| Increasingly Likely to Smoke | Decreasingly Likely to Smoke | Chronic Smokers | |
| Education | 0.93* (0.73–1.2) | 0.77* (0.62–0.96) | 0.76* (0.65–0.89) |
| Prenatal alcohol use | 1.3 (0.63–2.6) | 0.95 (0.39–2.3) | 1.9* (1.2–3.1) |
Note.
p < .05
3. Results
3.1. Maternal smoking in the entire sample
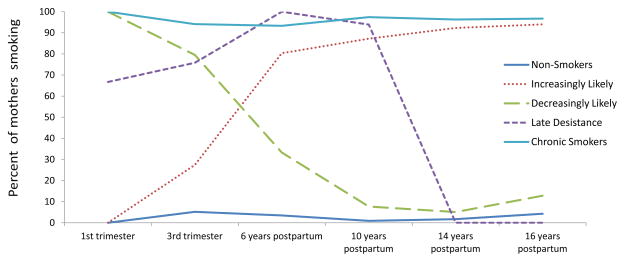
Table 1 presents the results of the growth mixture modeling (GMM). As seen in section A of the table, a 5-class model had the lowest BIC and best fit the data for the entire sample (entropy = 0.953). The trajectories for this model are presented in Figure 1. The —non-smoker class had a stable low trajectory: mothers in this class were the least likely to smoke at any time point. In contrast, 93% or more of the mothers in the —chronic smoker class used cigarettes at each wave of testing (including pregnancy). An —increasingly likely to smoke class included mothers who did not smoke early in their pregnancy, but became increasingly likely to smoke as time went on: 27% smoked by the third trimester, and 80%, 87%, 92% and 94% smoked 6, 10, 14 and 16 years post-partum. A —decreasingly likely to smoke class of mothers included women who all smoked during their first trimester, but were likely to quit smoking as time progressed. Although 80% of the women in this trajectory smoked during their third trimester, only 33%, 8%, 5% and 13% were smokers at 6, 10, 14, and 16 years postpartum, respectively. A —late desistance class included women who smoked during pregnancy (67% and 76% in their first and third trimesters) and continued to smoke at 6 (100%) and 10 (94%) years post-partum. However, all of the women in the —late desistance group had quit by 14 years post-partum and stayed quit 16 years post-partum.
Table 1.
Criteria for Class Formations for the Growth Mixture Models of Maternal Cigarette Use
|
A. Entire sample (n = 690)
| ||||||
|---|---|---|---|---|---|---|
| Number of classes | LRχ2 | df | p | BIC | LMR LRT | Entropy |
| 3 | 270.4 | 49 | 0.00 | 3633 | 218.2 | 0.94 |
|
| ||||||
| 4 | 79.0 | 44 | <0.001 | 3527 | 165.7 | 0.932 |
| 5 | 44.7 | 39 | NS | 3526 | 33.2 | 0.953 |
| 6 | 38.0 | 34 | NS | 3552 | 32.5 | 0.853 |
|
B. African-American mothers (n = 430)
| ||||||
|---|---|---|---|---|---|---|
| Number of classes | LRχ2 | df | p | BIC | LMR LRT | Entropy |
| 3 | 213.9 | 49 | 0.00 | 2343 | 135.7 | 0.958 |
|
| ||||||
| 4 | 72.4 | 44 | < 0.01 | 2232 | 132.4 | 0.95 |
| 5 | 47.8 | 39 | NS | 2238 | 31.1 | 0.964 |
| 6 | 44.5 | 34 | NS | 2265 | 23.7 | 0.919 |
|
C. White mothers (n = 260)
| ||||||
|---|---|---|---|---|---|---|
| Number of classes | LRχ2 | df | p | BIC | LMR LRT | Entropy |
| 3 | 102.3 | 49 | 0.00 | 1325 | 67.2 | 0.92 |
|
| ||||||
| 4 | 42.3 | 44 | NS | 1293 | 67.4 | 0.934 |
| 5 | 35.0 | 39 | NS | 1314 | 7.1 | 0.91 |
| 6 | 29.9 | 34 | NS | 1336 | 6.6 | 0.935 |
Note. LR = likelihood ratio; df = degrees of freedom; BIC = Bayesian information criterion; LMR LRT = Lo-Mendell-Rubin likelihood ratio test.
Figure 1.
Smoking trajectories (all mothers)
All mothers (n = 690)
Maternal mean age at first birth for the smoking trajectory classes described above was 19.1, 19.1, 18.2, 19.5, and 17.4 (p< .05), respectively. The increasing and the late desistance classes included the youngest mothers. Table 2 presents the results of the polytomous logistic regression on the entire sample predicting latent cigarette use trajectory classes compared to non-smokers, the referent group. Maternal age at first birth continued to significantly distinguish between the cigarette trajectories after controlling for other covariates. Compared to non-smokers, mothers who were increasingly likely to smoke were younger, less educated, and more hostile. Mothers who were decreasingly likely to smoke were less educated and used more alcohol and marijuana while pregnant than the non-smokers. Late desistance smokers were younger, used more alcohol prenatally, and were more hostile over time than non-smokers. Chronic smokers were more likely to be White, less educated, report more economic hardship and more alcohol and marijuana use during pregnancy than non-smokers.
3.2 Smoking in the African-American mothers
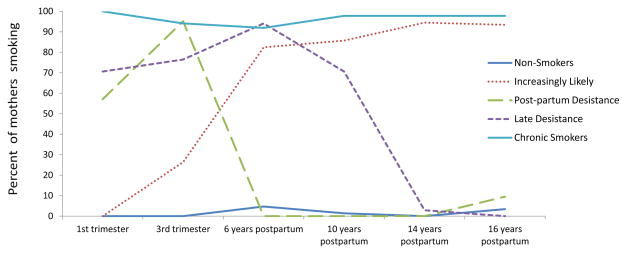
A 5-class model had relatively low BIC, the highest entropy (=0.964), and a non-significant LR test and hence best fit the data (Table 1, section B). These 5 classes of smoking in the African-American mothers are illustrated in Figure 2: non-smokers (34%), chronic smokers (32%), increasingly likely to smoke (21%), post-partum quitters who had quit by 6 years-post-partum, (5%), and late desistance smokers who continued to smoke at 10 years post-partum (8%). Maternal average age at first birth for these classes was 18.8, 18.7, 17.9, 17.4, and 17.9 (p < .10), respectively. Although the non-smokers were relatively older than other classes, the difference was only marginally significant. Table 3 depicts the regression results predicting latent cigarette use trajectory classes for the African-American mothers with the non-smoking mothers as the referent group. Economic hardship differentiated the post-partum quitters from the non-smokers in this subsample. Hostility differentiated all smoking groups from the non-smokers except for the late desistance smokers, who were no more hostile than the non-smokers. Higher levels of prenatal alcohol use differentiated all smoking groups from the non-smokers except for the increasingly likely to smoke group, who used no more alcohol than the non-smokers. Higher levels of prenatal marijuana use only differentiated the chronic smokers from the non-smokers.
Figure 2.
Smoking trajectories in the African-American mothers
African-American mothers (n = 430)
Table 3.
Regression predicting latent cigarette use trajectory classes In African-American mothers (reference group = non-smokers)
| Latent Class Contrast vs. Non-smokers: Odds Ratio (confidence intervals) | ||||
|---|---|---|---|---|
|
| ||||
| Increasingly Likely to Smoke | Post-partum Quitters | Late Desistance | Chronic Smokers | |
| Economic hardship | 1.0 (0.93–1.1) | 1.3* (1.1–1.6) | 0.99 (0.86–1.1) | 0.93 (0.85–1.0) |
| Chronic hostility | 1.6* (1.3–2.0) | 1.5* (1.01–2.2) | 1.3 (0.92–1.7) | 1.3* (1.01–1.6) |
| Prenatal alcohol use | 0.90 (0.48–1.7) | 1.9* (1.1–3.1) | 2.0* (1.2–3.1) | 1.9* (1.2–2.5) |
| Prenatal marijuana use | 1.1 (0.70–1.8) | 1.4 (0.82–2.6) | 1.5 (0.94–2.4) | 1.7* (1.2–2.5) |
Note.
p < .05
3.3 Smoking in the White mothers
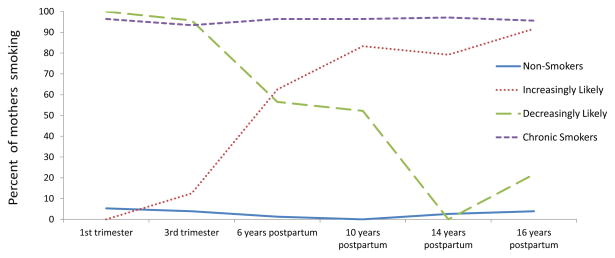
The results of the GMM for the White mothers are seen in section C of Table 2, with a 4-class solution best fitting the data (entropy = 0.934). As shown in Figure 3, the 4 groups of maternal cigarette use included non-smokers (29%), chronic smokers (53%), mothers who were increasingly likely to smoke over time (9%) and mothers who were decreasingly likely to smoke (9%). Maternal average age of first birth for non-smokers, chronic, increasing, and decreasing classes was 20.1, 19.5, 19.3, and 19.5 (p=.6), respectively. As was the case for the African-American mothers, age differences among the groups were not statistically significant, even though the nonsmokers included the oldest mothers. Table 4 depicts the regression results predicting latent cigarette use trajectory classes for the White mothers compared to non-smoking White mothers, the referent group. Lower education differentiated all 3 smoker groups from the non-smokers in this sub-sample. Higher prenatal alcohol use only differentiated the chronic smokers from the non-smokers.
Figure 3.
Smoking trajectories in the White mothers
White mothers (n = 260)
4. Discussion
We found 5 distinct trajectories of maternal smoking over a 17-year span, as did other investigators of smoking trajectories of men and women followed for a similarly long period (e.g., Brook et al., 2008; Chassin et al., 2000; Macy et al., 2015). However, our study focused specifically on mothers, and our sample was more diverse. Nonetheless, our findings converge with the results of those studies: 5 classes were identified and 3 of the same patterns of smoking (non/experimental smokers, a chronic smoker group, and a group that desisted use over time) were observed in all groups. Compared to the African-American mothers, more of the White mothers were classified in the chronic smoker trajectory (52% vs. 32%) and fewer were in the non-smoker trajectory (29% vs. 34%), consistent with previous findings among men (White et al., 2004). In the Early Childhood Longitudinal Study (ECLS-B), White mothers were also more likely than African-American mothers to be in one of the smoking trajectories rather than in the non-smoking class, regardless of their age (Mumford & Liu, 2015).
Younger first-time mothers were more likely to increase their smoking over time or to be late desisters, consistent with our first hypothesis. However, maternal age at first birth did not predict chronic cigarette use. Similarly, Mumford and colleagues (2014) reported that the women from the ECLS-B who became mothers by age 15 were more likely to be delayed initiators or temporary quitters than non-smokers, but not more likely to be persistent smokers. Maternal age at first birth was only a predictor of smoking trajectories in the sample as a whole, and was not a significant predictor of smoking trajectories in the separate analyses on White and African-American mothers. African-American mothers were younger and were more often classified in the —increasingly likely to smoke group, which may explain the significant association with age in the total sample but not in the race-specific analyses. Alternately, maternal age may be less important for maternal cigarette use over time than race-specific predictors such as chronic hostility and maternal education. Also, there was less power to detect effects in the race-specific analyses.
The race-specific trajectory analyses revealed interesting differences in patterns of maternal smoking over time. A majority (62%) of White mothers smoked during pregnancy, compared to 32% of the African-American mothers. However, more than twice as many African-American mothers were in the —increasingly likely to smoke group than were White mothers (21% vs. 9%). Thus, there was evidence for a crossover effect in maternal smoking rates. Only one pattern of desistance was identified in the White mothers: mothers who were decreasingly likely to smoke over time (10%). African-American mothers who smoked were split into two patterns of desistance: desisting in the postpartum versus desisting later in the time frame. Women from all three of these desistance groups were likely to smoke during the third trimester of pregnancy, placing their children at risk of multiple negative outcomes (Bernstein et al., 2005; Cornelius & Day, 2009; El Marroun et al., 2014; England et al., 2001; Lindell et al., 2012; Lux et al., 2000). Hostility was associated with membership in a smoking group in the African-American mothers, consistent with previous research demonstrating a link between smoking and hostility in women (Eiden et al., 2011; Whiteman et al., 1997). It is not clear why hostility did not predict smoking in White mothers. Lower levels of maternal education predicted smoking trajectories only for White mothers, which may partly be a function of greater variability in education among the White mothers. Economic hardship was a significant predictor of smoking among only for African-American mothers.
These results should be interpreted considering several limitations. The analyses did not consider smoking in partners, even though there is evidence that partners’ smoking, alcohol, and marijuana use in the prenatal period influences maternal pre- and postpartum smoking in women (Desrosiers et al., 2015). The sample was limited to urban, lower income African-American and White mothers and thus may not generalize to other populations. The rich longitudinal datasets had excellent retention rates over the 16-year period. However, attrition was higher in the White mothers and among mothers who smoked during their first trimester of pregnancy, which may limit the generalizability of our findings. Additionally, it is possible that there may not have been enough power to detect smaller effect sizes in the analyses on the different racial groups due to small cell sizes. Finally, our study relied on maternal self-report of smoking. However, biological validation of self-reported smoking indicates that self-report is reliable in both pregnant (Pickett et al., 2009) and non-pregnant women (Vartiainen et al., 2002).
This study addresses several gaps in the literature on maternal smoking, including the impact of maternal age at first birth and race on smoking trajectories over a 17-year span in White and African-American mothers. Future directions for research include studies of alternate tobacco products that mothers may use before, during, and after pregnancy (such as electronic cigarettes, hookah/water pipe, cigarillos), polydrug use, and concurrent use (blunts, or marijuana rolled into a tobacco leaf). It is likely that younger mothers are more likely to use these other tobacco products than older mothers, given the increase in use of these products in youth compared to a relative decline in the use of combustible cigarettes (Arrazola et al., 2015; Johnston et al., 2016). Other areas that warrant future research are prospective studies that examine long-term trajectories of smoking in women with specific patterns during pregnancy, trajectory analyses that consider both maternal and partners’ smoking patterns, and trajectory analyses on smoking in mothers from samples that include important subpopulations such as Latina and Asian American mothers.
Clinical Implications
The results of this study have several implications for clinical practice. We identified two groups of mothers who were increasingly likely to smoke across pregnancy, with higher rates reported for the third trimester. These two maternal smoking trajectories were also associated with post-natal cigarette use, suggesting that it is crucial to provide these prenatal smokers with additional resources to prevent both prenatal and postnatal tobacco exposure in offspring. These findings also identify targets for cessation efforts that differ by maternal age and race. While prenatal smoking interventions are appropriate with disadvantaged women from both groups, there is a much greater risk of smoking for White women during the prenatal period and there was no evidence of a postpartum desistance pattern among White mothers. There was an increasing likelihood of smoking among the younger mothers overall, and in African-American mothers in the postpartum. Prenatal alcohol use was a risk factor for chronic smoking in both groups, whereas prenatal marijuana use predicted chronic cigarette use in African-American mothers. This information may benefit health care providers to effectively target smoking cessation efforts to the most vulnerable mothers.
Highlights.
This is the first study of maternal smoking spanning 17 years.
Growth mixture modeling revealed 5 distinct maternal smoker groups.
Young mothers were more likely to desist later or increasingly likely to smoke.
Different trajectories were identified for the African-American and White mothers.
Acknowledgments
This research was funded by the National Institutes of Health. Data collection was funded by the National Institute on Alcohol Abuse and Alcoholism (AA06390: PI N. Day; AA08284: PI M Cornelius) and the National Institute on Drug Abuse (DA03874: PI N. Day; DA09275: PI M. Cornelius). The analyses were funded by the National Institute on Drug Abuse (DA037209: PI N. De Genna).
Role of Funding Sources Funding for this study was provided by the National Institute of National Institute on Alcohol Abuse and Alcoholism (AA06390: PI N. Day; AA08284: PI M Cornelius) and the National Institute on Drug Abuse (DA03874: PI N. Day; DA09275: PI M. Cornelius; DA037209: PI De Genna). NIAAA and NIDA had no role in the study design, analysis or interpretation of the data, manuscript preparation, or the decision to submit the paper for publication.
Footnotes
Contributors Drs Day and Cornelius designed the original birth cohort studies. Dr De Genna designed and wrote the protocol for the secondary data analysis. Dr Goldschmidt conducted the statistical analysis. Dr De Genna wrote the first draft of the manuscript, and all authors contributed to and have approved the final manuscript.
Conflict of Interest All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lidush Goldschmidt, Email: lidush@pitt.edu.
Nancy L. Day, Email: nday@pitt.edu.
Marie D. Cornelius, Email: mdc1@pitt.edu.
References
- Allen AM, Oncken C, Hatsukami D. Women and smoking: the effect of gender on the epidemiology, health effects, and cessation of smoking. Current Addiction Reports. 2014;1(1):53–60. doi: 10.1007/s40429-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS. Tobacco Use Among Middle and High School Students—United States, 2011–2014. Morbidity and Mortality Weekly Report (MMWR) 2015 Apr 17;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birth weight. Obstetrics & Gynecology. 2005;106:986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang C, Whiteman M, Cohen P, Finch SJ. Developmental trajectories of cigarette smoking from adolescence to the early thirties: personality and behavioral risk factors. Nic Tob Res. 2008;10:1283–1291. doi: 10.1080/14622200802238993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a Midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19:223–231. [PubMed] [Google Scholar]
- Cornelius M, Day N. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22:121–125. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Geva D, Day NL, Cornelius JR, Taylor PM. Patterns and covariates of tobacco use in a recent sample of pregnant teenagers. J Adolesc Health. 1994;15:528–35. doi: 10.1016/1054-139x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nic Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol Teratol. 2005;27:667–676. doi: 10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Hussong AM. Integrative data analysis: The simultaneous analysis of multiple data sets. Psychol Methods. 2009;14:81–100. doi: 10.1037/a0015914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Genna NM, Cornelius MD, Donovan JE. Risk factors for young adult substance use among women who were teenage mothers. Addict Behav. 2009;34:463–470. doi: 10.1016/j.addbeh.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Genna NM, Cornelius MD, Goldschmidt L, Day NL. Maternal age and trajectories of cannabis use. Drug Alcohol Dependence. 2015;156:199–206. doi: 10.1016/j.drugalcdep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Genna NM, Goldschmidt L, Day N, Cornelius MD. Prenatal and postnatal maternal trajectories of cigarette use predict adolescent cigarette use. Nicotine Tob Res. 2016;18:988–92. doi: 10.1093/ntr/ntv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers A, Thompson A, Divney A, Magriples U, Kershaw T. Romantic partner influences on prenatal and postnatal substance use in young couples. J Public Health. 2015 doi: 10.1093/pubmed/fdv039. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Kessler LG. Rates of symptoms of depression in a national sample. Am J Epidem. 1981;114:528–538. doi: 10.1093/oxfordjournals.aje.a113218. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Leonard KE, Colder CR, Homish GG, Schuetze P, Gray TR, Huestis MA. Anger, hostility, and aggression as predictors of persistent smoking during pregnancy. J Stud Alcohol Drugs. 2011;72:926–932. doi: 10.15288/jsad.2011.72.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH., Jr Age differentiation and the life course. Ann Rev Sociol. 1975;1:165–190. [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ. High-risk behaviors associated with early smoking: results from a 5-year follow-up. J Adolesc Health. 2001;28:465–473. doi: 10.1016/s1054-139x(00)00202-0. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Schmidt MN, Franken IH, Jaddoe VW, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White T. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharm. 2014;39(4):792–800. doi: 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Polce RJ, Vasilenko SA, Lanza ST. Changes in gender and racial/ethnic disparities in rates of cigarette use, regular heavy episodic drinking, and marijuana use: ages 14 to 32. Addict Behav. 2015;41:218–222. doi: 10.1016/j.addbeh.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J, Epidemiol. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Neidert LJ, Bound J. Age patterns of smoking in US black and white women of childbearing age. Am J Pub Health. 1993;83:1258–1264. doi: 10.2105/ajph.83.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nic Tob Res. 2012;14(6):694–702. doi: 10.1093/ntr/ntr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H, Hawkins SS, Law C. Lifecourse influences on women's smoking before, during and after pregnancy. Soc Sci Med. 2010;70:582–587. doi: 10.1016/j.socscimed.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Hardaway CR, Cornelius MD. Economic hardship and adolescent problem drinking: family processes as mediating influences. J Youth Adolesc. 2014;43:1191–1202. doi: 10.1007/s10964-013-0063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Miech RA. Monitoring the Future national survey results on drug use, 1975–2015: Volume II, college students and adults ages 19–55. Ann Arbor: Institute for Social Research, The University of Michigan; 2016. p. 427. [Google Scholar]
- Kandel D, Schaffran C, Hu M, Thomas Y. Age-related differences in cigarette smoking among whites and African-Americans: evidence for the crossover hypothesis. Drug Alcohol Depend. 2011;118:280–287. doi: 10.1016/j.drugalcdep.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Vo T, Wall MM, Caetano R, Suglia SF, Martins SS, Galea S, Hasin D. Racial/ethnic differences in use of alcohol, tobacco, and marijuana: is there a cross-over from adolescence to adulthood? Soc Sci Med. 2015;124:132–141. doi: 10.1016/j.socscimed.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EM, Pampel FC, Mollborn S. Life course transitions and racial and ethnic differences in smoking prevalence. Adv Life Course Res. 2014;22:27–40. doi: 10.1016/j.alcr.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Lindell G, Maršál K, Källén K. Impact of maternal characteristics on fetal growth in the third trimester: a population-based study. Ultrasound Obstet Gynecol. 2012;40:680–687. doi: 10.1002/uog.11125. [DOI] [PubMed] [Google Scholar]
- Liu W, Mumford EA, Petras H. Maternal Alcohol Consumption During the Perinatal and Early Parenting Period: A Longitudinal Analysis. Matern child health. 2015:1–10. doi: 10.1007/s10995-015-1836-5. [DOI] [PubMed] [Google Scholar]
- Lux AL, Henderson AJ, Pocock SJ. Wheeze associated with prenatal tobacco smoke exposure: a prospective, longitudinal study. Arch DIS child. 2000;83(4):307–12. doi: 10.1136/adc.83.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy JT, Li J, Xun P, Presson CC, Chassin L. Dual trajectories of cigarette smoking and smokeless tobacco use from adolescence to midlife among males in a midwestern US community sample. Nic Tob Res. 2015 doi: 10.1093/ntr/ntv070. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott L, Dobson A, Russell A. Changes in smoking behaviour among young women over life stage transitions. Aust NZ J Pub Health. 2004;28:330–335. doi: 10.1111/j.1467-842x.2004.tb00439.x. [DOI] [PubMed] [Google Scholar]
- McDermott L, Dobson A, Owen N. Determinants of continuity and change over 10 years in young women's smoking. Addiction. 2009;104:478–487. doi: 10.1111/j.1360-0443.2008.02452.x. [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S. Predictors of persistent smoking and quitting among women smokers. Addict Behav. 2006;31:1711–1715. doi: 10.1016/j.addbeh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Melchior M, Chastang JF, Mackinnon D, Galéra C, Fombonne E. The intergenerational transmission of tobacco smoking--the role of parents' long-term smoking trajectories. Drug Alc Depend. 2010;107:257–260. doi: 10.1016/j.drugalcdep.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Mumford EA, Hair EC, Yu TC, Liu W. Women’s longitudinal smoking patterns from preconception through child’s kindergarten entry: profiles of biological mothers of a 2001 U.S. birth cohort. Mat Child Health. 2014;18:810–820. doi: 10.1007/s10995-013-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford EA, Liu W. Growth models of maternal smoking behavior: individual and contextual factors. Subst Use Misuse. 2015 doi: 10.3109/10826084.2014.998234. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM. Symptom scales and diagnostic schedules in adult psychiatry. In: Tsuang MT, Tohen M, editors. Textbook in Psychiatric Epidemiology. Wiley-Liss; New York: 2002. pp. 273–332. [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Eq Model. 2007;14:535–569. [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nic Tob Res. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psyc Meas. 1977;1:385–401. [Google Scholar]
- Radloff LS, Locke BZ. The community mental health assessment survey and the CES-D Scale. In: Weissman MM, Myers JK, Ross CE, editors. Community surveys of psychiatric disorders. Rutgers University Press; New Brunswick, N.J: 1986. pp. 177–189. [Google Scholar]
- Rostron B. Smoking-attributable mortality by cause in the United States: revising the CDC’s data and estimates. Nic Tob Res. 2013;15(1):238–46. doi: 10.1093/ntr/nts120. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Tucker JS, Ellickson PL, Orlando M, Klein DJ. Cigarette smoking from adolescence to young adulthood: women's developmental trajectories and associates outcomes. Womens Health. 2006;16:30–37. doi: 10.1016/j.whi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Vartiainen E, Seppälä T, Lillsunde P, Puska P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Comm Health. 2002;56:167–170. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Nagin D, Replogle E, Stouthamer-Loeber M. Racial differences in trajectories of cigarette use. Drug Alc Depend. 2004;76:219–227. doi: 10.1016/j.drugalcdep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- White HR, Pandina RJ, Chen PH. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug Alc Depend. 2002;65:167–178. doi: 10.1016/s0376-8716(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Whiteman MC, Fowkes FG, Deary IJ, Lee AJ. Hostility, cigarette smoking and alcohol consumption in the general population. Soc Sci Med. 1997;44:1089–1096. doi: 10.1016/s0277-9536(96)00236-5. [DOI] [PubMed] [Google Scholar]
- Yuan ASV. Black-White differences in aging out of substance use and abuse. Sociol Spectr. 2011;31:3–31. [Google Scholar]