Abstract
Background
Few studies have examined multidrug-resistant (MDR) tuberculosis (TB) treatment outcomes among HIV-infected persons after widespread expansion of antiretroviral therapy (ART). We describe MDR-TB treatment outcomes among HIV-infected and HIV-uninfected patients in Botswana after ART expansion.
Methods
We retrospectively reviewed data from patients who started MDR-TB therapy in Botswana during 2006-2013. Multivariable regression models were used to compare treatment outcomes between HIV-infected and HIV-uninfected patients.
Results
We included 588 MDR-TB patients in the analysis, of whom, 47 (8.0%) and 9 (1.5%) were diagnosed with pre-extensively drug-resistant (XDR)-TB and XDR-TB, respectively. Of the 408 (69.4%) HIV-infected patients, 352 (86.0%) were on ART or started ART during treatment, and median baseline CD4+ T cell count was 234 cells/mm3. Treatment success rates were 79.4% and 73.0% among HIV-uninfected and HIV-infected patients, respectively (P = 0.121). HIV-infected patients with CD4+ T cell count <100 cells/mm3 were more likely to die during treatment compared to HIV-uninfected patients (adjusted risk ratio=1.890; 95% CI=1.098-3.254).
Conclusions
High rates of treatment success were achieved with programmatic management of MDR-TB and HIV in Botswana after widespread expansion of ART. However, a two-fold increase in mortality was observed among HIV-infected persons with baseline CD4 <100 cells/mm3 compared with HIV-uninfected persons.
Keywords: tuberculosis, treatment outcomes, HIV/AIDS, immune suppression, antiretroviral therapy
Introduction
Multidrug-resistant (MDR) tuberculosis (TB) is caused by Mycobacterium tuberculosis that is resistant to at least isoniazid (INH) and rifampicin (RIF), the two most important anti-TB drugs and, therefore, is more difficult and expensive to treat than drug-susceptible TB. While initiation of antiretroviral therapy (ART) during treatment for drug-susceptible TB significantly improves survival, few studies have described treatment outcomes among HIV-infected patients in MDR-TB treatment programs after widespread expansion of ART.1–3 A recent systematic review found an overall 56.9% treatment success and 38% mortality among HIV-infected adults with MDR-TB.4 However, most of the studies included in the review were conducted prior to widespread access to ART.4
The objective of the present study is to describe treatment outcomes among HIV-infected and HIV-uninfected MDR-TB patients after the expansion of the ART program in Botswana, using routinely collected MDR-TB program data. We hypothesized that high rates of treatment success can be achieved among HIV-infected MDR-TB patients, particularly among those that do not have advanced immune suppression at initial diagnosis.
Methods
Study population and program description
The study population was previously described in a paper based on an earlier version of the study database.5 All patients enrolled in the national MDR-TB program were registered into a patient cohort database starting in 2006. For this study, we included data from adult and adolescent patients (age ≥15 years) diagnosed with confirmed or presumed MDR-TB. Patients with unknown HIV status, those who never started treatment, and those still on treatment at the time of the analysis were excluded.
During the study period (2006 – 2013), patients diagnosed with MDR-TB in Botswana were referred to one of five specialized MDR-TB clinics across the country.5 MDR-TB clinics were staffed by physicians, nurses, and nursing assistants trained and supervised by one of the co-authors (Dr. Modongo). At each clinic, a designated TB focal person provided comprehensive counseling to patients regarding the MDR-TB treatment and its adverse effects, infection control, and the importance of treatment adherence. The clinic physician was responsible for initiating the MDR-TB treatment, monthly monitoring of adverse effects, and monitoring of sputum results. The MDR-TB nurse provided individual follow-up of each patient, including phone calls to patients who missed their monthly consultation. Patients were initiated on a standardized regimen of amikacin, levofloxacin, ethionamide, cycloserine, and pyrazinamide, for a minimum of 18 months after culture conversion.5 The treatment was ambulatory with the exception of the extensively drug-resistant (XDR)-TB cases, patients living with children less than five years old, and those sharing a room with other household members. These patients were treated as inpatients in the MDR-TB wards until they had culture converted. The treatment was administered daily under directly observed therapy (DOT) at the nearest clinic to the patient's residence. Audiologists and social workers also worked closely with the physician and MDR-TB nurse to provide psychosocial support and hearing aids, if needed. While no monetary incentives were provided, treatment support was provided through monthly food distribution and transportation to the MDR-TB clinic. At completion of treatment, patients continued to be followed up at the MDR-TB clinic to assess for relapse every quarter for two years.
Laboratory procedures
Drug susceptibility testing (DST) for first-line anti-TB drugs (INH, RIF, ethambutol [ETH], and streptomycin [STR]) was performed using the proportional agar method on Lowenstein-Jensen medium or the Mycobacteria Grow Indicator Tube (MGIT) 960 (Becton Dickinson Microbiology Systems, Sparks, MD).6 DST for second-line drugs (fluoroquinolones, ethionaminde, kanamycin, amikacin, and capreomycin) was performed for patients who remained culture positive after six months of treatment.7 HIV testing was done at the clinics on an opt-out basis using rapid HIV diagnostic tests.8
Measures
Treatment outcome was defined based on the 2008 World Health Organization (WHO) guidelines.9 Patients who were cured or completed treatment were categorized as having achieved treatment success. Treatment failure was defined as having two or more positive cultures during the final 12 months of treatment, having a positive culture on any one of the final three cultures, or clinical decision to stop treatment for any reason. Any patient whose treatment was interrupted for two or more consecutive months was considered lost to follow-up. Death was defined as having died during treatment due to any cause.
We extracted HIV infection status and CD4+ T cell values closest to MDR-TB treatment initiation from the cohort database. CD4+ T cell counts were categorized as <100, 100 – 199, 200 – 349, and ≥350 cells/mm3. Patients with second-line drug resistance patterns available at baseline were categorized as MDR-TB only, pre-XDR-TB (MDR-TB plus resistance to ≥1 second-line injectable drug [amikacin, capreomycin or kanamycin] or ≥1 fluoroquinolone), and XDR-TB (MDR-TB plus resistance to ≥1 second-line injectable drug and ≥1 fluoroquinolone).
Statistical analysis
Bivariate comparisons were performed using the χ2 test or the Fisher's exact test, and continuous variables were compared using the Wilcoxon Rank Sum test. Two variables for treatment outcome were examined: 1) treatment success, defined as cure or complete (vs. death, failure, or lost to follow-up); and 2) death (vs. all other outcomes). Probabilities of treatment success and death were estimated using frequencies, and the 95% confidence intervals (95% CI) were estimated using the Score method.10 Dose-response relationship between CD4+ T cell count categories and treatment success or death was assessed using the χ2 test for trend.10
We constructed multivariable Poisson regression models with robust variance to estimate the association between HIV-related immunosuppression and treatment outcomes while controlling for confounding variables.11–14 This method allowed us to estimate risk ratios, instead of logistic regression models, which generate odds ratio estimates.11–14 Separate models were constructed for treatment success and death as dependent variables. The primary independent variable was HIV-associated immune suppression, which was created by combining HIV infection status with CD4+ T cell count categories. We used this combined variable to compare the probability of outcome in each CD4+ T cell count category to that among HIV-uninfected patients. Patients with unknown CD4+ T cell counts were excluded from the bivariate and multivariable analysis. We included the following covariates into the model as potential confounders based on a pre-specified conceptual framework of the causal relationship between HIV-associated immune suppression and TB treatment outcomes: age, sex, and prior history of TB. Factors that could have been influenced by HIV associated immune suppression, including weight and other comorbidities, were not considered as confounders, because controlling for such factors could introduce bias in effect estimates.15,16
Ethical considerations
This study was approved by the Botswana Ministry of Health Human Research Development Committee, Centers for Disease Control and Prevention Institutional Review Board (IRB), and the University of Pennsylvania IRB. Informed consent was not obtained from participants as this study involved analysis of routine program data.
Results
Patient characteristics
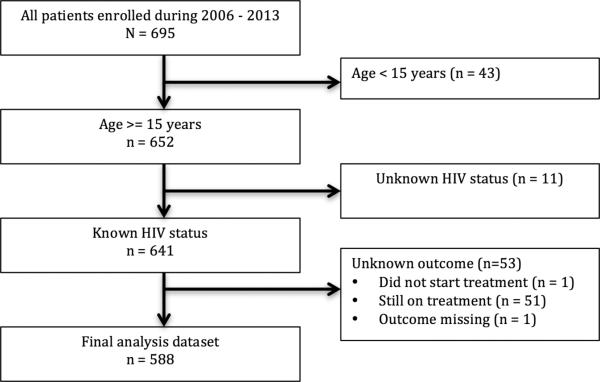
During 2006 – 2013, 695 patients were diagnosed with MDR-TB and entered into the MDR-TB registry. Twelve patients had multiple episodes, resulting in 708 MDRTB cases during the period. We excluded data for 43 patients aged < 15 years, 11 patients with unknown HIV status, and 53 patients with unknown outcome status (51 patients were still on treatment, one patient did not start treatment, and the outcome for one patient was missing) (Figure 1). Of the remaining 588 patients included in this analysis, 322 (54.9%) were male and the median age was 36 years (interquartile range [IQR], 29 – 47 years) (Table 1). Most of the patients had bacteriologically confirmed pulmonary MDR-TB and a prior history of TB treatment. Among 194 patients who had available second-line DST results, 47 (24.2%) were diagnosed with pre-XDR-TB and 9 (4.6%) were diagnosed with XDR-TB.
Figure 1.
Study flowchart of multidrug-resistant tuberculosis patients in Botswana, 2006 – 2013.
Table 1.
Characteristics of multidrug-resistant tuberculosis patients in Botswana by HIV status, 2006 – 2013.
| Characteristic | All patients N = 588 | HIV-uninfected N = 180 | HIV-infected N = 408 | P value** | |
|---|---|---|---|---|---|
| Sex* | Female | 265 (45.1%) | 84 (46.7%) | 181 (44.4%) | 0.687 |
| Male | 322 (54.9%) | 96 (53.3%) | 226 (55.5%) | ||
| Age in years, median (IQR) | 36 (29-47) | 32 (24-51) | 37 (31-45) | 0.019 | |
| Year of treatment initiation | 2006-2007 | 89 (15.1%) | 26 (14.4%) | 63 (15.4%) | 0.625 |
| 2008-2009 | 228 (38.8%) | 75 (41.7%) | 153 (37.5%) | ||
| 2010-2011 | 166 (28.2%) | 45 (25%) | 121 (29.7%) | ||
| 2012-2013 | 105 (17.9%) | 34 (18.9%) | 71 (17.4%) | ||
| Prior TB | No | 25 (4.3%) | 10 (5.6%) | 15 (3.7%) | 0.039 |
| Yes | 492 (83.7%) | 157 (87.2%) | 335 (82.1%) | ||
| Unknown | 71 (12.1%) | 13 (7.2%) | 58 (14.2%) | ||
| Site of Infection | Pulmonary | 388 (66%) | 122 (67.8%) | 266 (65.2%) | 0.697 |
| Extrapulmonary | 42 (7.1%) | 15 (8.3%) | 27 (6.6%) | ||
| Unknown | 158 (26.9%) | 43 (23.9%) | 115 (28.2%) | ||
| Baseline weight in kg, median (IQR) | 49.3 (42-56) | 49 (42-56) | 50 (42.7-57) | 0.709 | |
| Comorbidities | Yes | 71 (12.1%) | 22 (12.2%) | 49 (12.0%) | 1.000 |
| No | 350 (59.5%) | 111 (61.7%) | 239 (58.6%) | ||
| Unknown | 167 (28.4%) | 47 (26.1%) | 120 (29.4%) | ||
| Drug Resistance | Presumed MDR | 109 (18.5%) | 32 (17.8%) | 77 (18.9%) | 0.658 |
| MDR (no 2nd line DST done) | 285 (48.5%) | 93 (51.7%) | 192 (47.1%) | ||
| Confirmed MDR only | 138 (23.5%) | 40 (22.2%) | 98 (24.0%) | ||
| Pre-XDR | 47 (8.0%) | 14 (7.8%) | 33 (8.1%) | ||
| XDR | 9 (1.5%) | 1 (0.6%) | 8 (2.0%) | ||
| Anti-TB Drug Regimen | am-lfx-eto-cs-z¶ only | 217 (36.9%) | 67 (37.2%) | 150 (36.8%) | 0.090 |
| am-cfx-eto-cs-z‡ + other | 83 (14.1%) | 30 (16.7%) | 53 (13%) | ||
| am-lfx-eto-cs-z¥ + other | 65 (11.1%) | 25 (13.9%) | 40 (9.8%) | ||
| Other | 48 (8.2%) | 8 (4.4%) | 40 (9.8%) | ||
| Unknown | 175 (29.8%) | 50 (27.8%) | 125 (30.6%) | ||
| Outcome | Completed | 264 (44.9%) | 90 (50%) | 174 (42.6%) | 0.318 |
| Cured | 177 (30.1%) | 53 (29.4%) | 124 (30.4%) | ||
| Deceased | 118 (20.1%) | 28 (15.6%) | 90 (22.1%) | ||
| Failure | 5 (0.9%) | 2 (1.1%) | 3 (0.7%) | ||
| Loss to follow-up | 24 (4.1%) | 7 (3.9%) | 17 (4.2%) | ||
| ART administered | Yes | 351 (86.0%) | |||
| No | 24 (5.9%) | ||||
| Unknown | 33 (8.1%) | ||||
| On ART before TB treatment initiation | Yes | 208 (51.0%) | |||
| No | 79 (19.4%) | ||||
| Unknown | 121 (29.7%) | ||||
| Baseline CD4+ T cell count, cells/mm3, median (IQR)1 | 234 (124-379) | ||||
| Baseline CD4+ T cell categories, cells/mm3 | <100 | 48 (11.8%) | |||
| 100 - 199 | 64 (15.7%) | ||||
| 200 - 349 | 77 (18.9%) | ||||
| ≥350 | 78 (19.1%) | ||||
| Unknown | 141 (34.6%) | ||||
| ART regimen*** | First line | 236 (57.8%) | |||
| Second line | 24 (5.9%) | ||||
| Not on ART | 24 (5.9%) | ||||
| Unknown | 124 (30.4%) |
Sex unknown for one patient
Based on the X2 –test or Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables
CD4+ T cell counts were unknown for 141 patients
Amikacin-Levofloxacin-ethionamide-cycloserine-pyrazinamide only
Amikacin-Ciprofloxacin-ethionamide-cycloserine-pyrazinamide + other second-line drugs
Amikacin-Levofloxacin-ethionamide-cycloserine-pyrazinamide + other second-line drugs
First line ART regimens were primarily: Tenofovir-Emtricitabine-Nevirapine, Zidovudine-Lamivudine-Efaviranz, Tenofovir-Emtricitabine-Efaviranz, or Zidovudine-Lamivudine-Nevirapine
IQR = interquartile range; ART = antiretroviral therapy; TB = tuberculosis
Overall, 440 (74.8%) patients achieved treatment success and 118 (20.1%) died. Treatment outcomes for 9 patients who had initially failed MDR-TB treatment were categorized as completed (n = 1), cured (n = 5), or death (n = 4) based on their subsequent treatment outcome. Of the patients diagnosed with pre-XDR-TB, 32 (68.1%) achieved treatment success and 13 (27.7%) died. Of the patients diagnosed with XDR-TB, 5 (55.6%) achieved treatment success and 3 (33.3%) died.
Our study population included 408 (69.4%) HIV-infected patients. Of these, 208 (51.0%) patients were on ART prior to starting MDR-TB treatment and 351 (86.0%) received ART during MDR-TB treatment (Table 1). Of the 260 patients with known ART regimen, 236 (90.8%) were on first line ART regimens. The median CD4+ T cell count at baseline was 234 cells/mm3 (IQR, 124 – 379 cells/mm3). Among HIV-infected patients with known CD4+ T cell counts, 17.9%, 24.0%, 28.8%, and 29.2% had CD4+ T cell counts <100, 100 – 199, 200 – 349, and ≥350 cells/mm3, respectively. HIV-infected MDR-TB patients were older (median age of 37 years vs. 32 years for HIV-uninfected patients; P = 0.019) and less likely to have previous history of TB treatment (P = 0.039). None of the other characteristics we examined differed between HIV-infected and HIV-uninfected MDR-TB patients.
HIV-associated immune suppression and treatment success
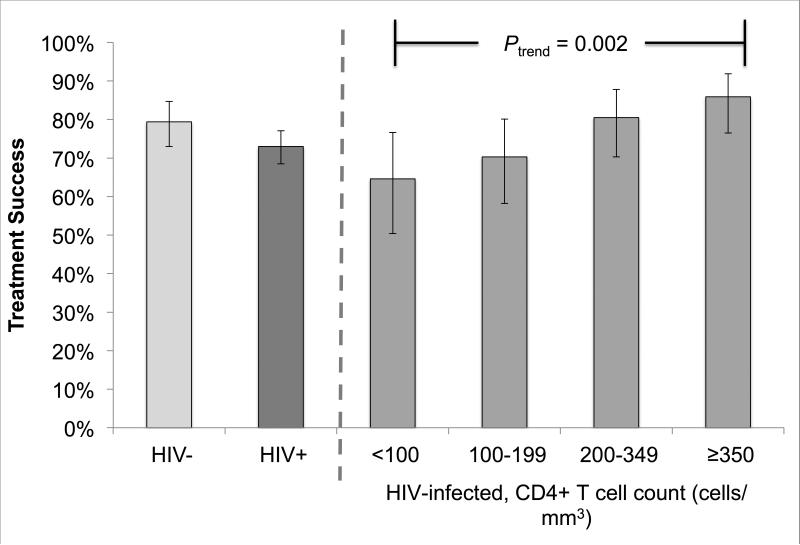
In bivariate analysis, the observed probability of treatment success was higher among HIV-uninfected patients compared to HIV-infected patients, but this difference was not statistically significant (79.4% vs. 73.0%; P = 0.121) (Figure 2a). Likewise, no statistically significant differences were found when treatment success rates were compared between HIV-infected patients in the CD4+ T cell categories (<100, 100-199, 200-349, ≥350 cells/mm3) and HIV-uninfected patients (Table 2). However, we found evidence of an increasing trend in the probability of treatment success with increasing CD4+ T cell count categories (from 64.6% among <100 cells/mm3 to 85.9% among ≥350 cells/mm3; Ptrend = 0.002) (Table 2; Figure 2a). In addition, we observed lower probability of treatment success among patients who were male (70.8% vs. 80.0% among female patients; RR = 0.885; 95% CI = 0.807 – 0.971), aged 45 – 54 years (69.0% vs. 83.3% among patients aged 15 - 24 years; RR = 0.828; 95% CI = 0.709 – 0.966), and patients with previous history of TB treatment (74.2% vs. 92.0% among patients with no previous history of TB treatment; RR = 0.807; 95% CI = 0.712 – 0.915) (Table 2).
Figure 2.
Treatment success (A) and death (B) by HIV-infection status and baseline CD4+ T cell counts among multidrug-resistant tuberculosis patients in Botswana, 2006 – 2013.
Table 2.
Bivariate and multivariable analysis of the association between HIV-associated immune suppression and treatment success among multidrug-resistant tuberculosis patients in Botswana, 2006 – 2013.
| Variable | Percent with treatment success (n/N) | Bivariate analysis Risk ratio (95% confidence intervals) | Multivariable model Risk ratio (95% confidence intervals) |
|---|---|---|---|
| Sex | |||
| Female | 80.0% (212/265) | 1.00 | 1.00 |
| Male | 70.8% (228/322) | 0.885 (0.807-0.971) | 0.934 (0.847 - 1.031) |
| Age in years | |||
| 15 - 24 | 83.3% (70/84) | 1.00 | |
| 25 - 34 | 77.1% (121/157) | 0.925 (0.814-1.051) | |
| 35 – 44 | 76.0% (127/167) | 0.913 (0.803-1.037) | |
| 45 – 54 | 69.0% (80/116) | 0.828 (0.709-0.966) | |
| ≥55 | 67.9% (36/53) | 0.815 (0.662-1.004) | |
| Age (+10 year) | 0.945 (0.907 - 0.985) | ||
| Prior TB | |||
| No | 92.0% (23/25) | 1.00 | 1.00 |
| Yes | 74.2% (418/563) | 0.807 (0.712-0.915) | 0.895 (0.772 - 1.037) |
| CD4+ T cell count, cells/mm3* | |||
| HIV-uninfected | 79.4% (143/180) | 1.00 | 1.00 |
| <100 | 64.6% (31/48) | 0.813 (0.651-1.015) | 0.835 (0.667 - 1.046) |
| 100 – 199 | 70.3% (45/64) | 0.885 (0.742-1.055) | 0.958 (0.810 - 1.133) |
| 200 – 349 | 80.5% (62/77) | 1.014 (0.888-1.157) | 1.021 (0.896 - 1.164) |
| ≥350 | 85.9% (67/78) | 1.081 (0.962-1.215) | 1.077 (0.963 - 1.205) |
Patients with unknown CD4+ T cell counts (n = 141) were excluded from the analysis.
In the multivariable model, no statistically significant differences were found in treatment success probabilities among HIV-infected patients in the CD4+ T cell count categories compared to HIV-uninfected patients after controlling for sex, age, and prior history of TB treatment (Table 2). Age was the only variable that remained independently associated with treatment success in the model (+10 years; risk ratio [RR] = 0.945; 95% CI = 0.907 – 0.985).
HIV-associated immune suppression and death
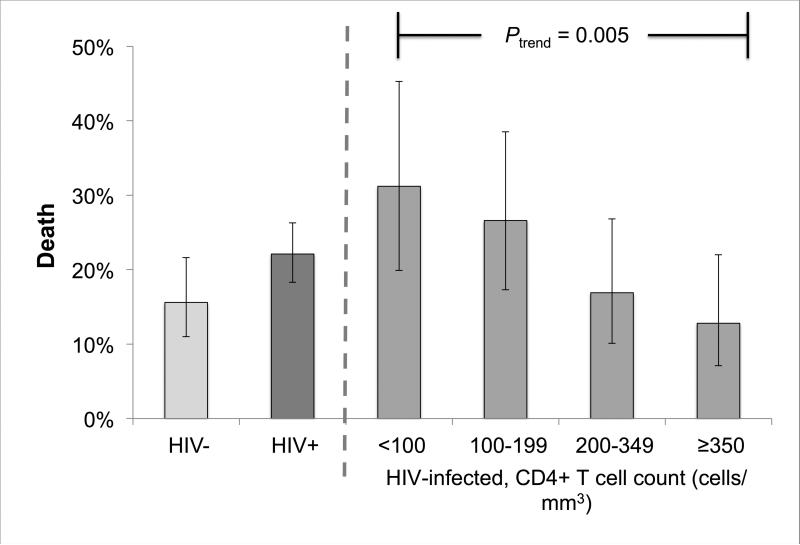
In bivariate analysis, the observed probability of death was higher among HIV-infected patients compared to HIV-uninfected patients, but this difference was not statistically significant (22.1% vs. 15.6%; P = 0.089) (Figure 2b). HIV-infected patients with CD4+ T cell counts <100 (RR = 2.009; 95% CI = 1.170 - 3.448) and 100 - 199 cells/mm3 (RR = 1.708; 95% CI = 1.004 – 2.904) were more likely to die during treatment compared to HIV-uninfected patients (Table 3). We found evidence of a decreasing trend in the probability of death with increasing CD4+ T cell count categories (from 31.2% among <100 cells/mm3 to 12.8% among ≥ 350 cells/mm3; Ptrend = 0.005) (Table 3; Figure 2b). In addition, we observed higher risk of death among patients who were male (24.5% vs. 14.7% among female patients; RR = 1.667; 95% CI = 1.178 – 2.360) and aged 45 – 54 years (29.3% vs. 14.3% among patients aged 15 - 24 years; RR = 2.052; 95% CI = 1.131 – 3.721) (Table 3).
Table 3.
Bivariate and multivariable analysis of the association between HIV-associated immune suppression and death among multidrug-resistant tuberculosis patients in Botswana, 2006 – 2013.
| Variable | Percent of patients who died (n/N) | Bivariate analysis Risk ratio (95% confidence intervals) | Multivariable model Risk ratio (95% confidence intervals) |
|---|---|---|---|
| Sex | |||
| Female | 14.7% (39/265) | 1.00 | 1.00 |
| Male | 24.5% (79/322) | 1.667 (1.178-2.360) | 1.630 (1.041 - 2.553) |
| Age in years | |||
| 15 - 24 | 14.3% (12/84) | 1.00 | |
| 25 - 34 | 15.9% (25/157) | 1.115 (0.591-2.104) | |
| 35 - 44 | 17.4% (29/167) | 1.216 (0.654-2.259) | |
| 45 - 54 | 29.3% (34/116) | 2.052 (1.131-3.721) | |
| ≥55 | 26.4% (14/53) | 1.849 (0.927-3.687) | |
| Age (+10 year) | 1.228 (1.072 - 1.406) | ||
| Prior TB | |||
| No | 4% (1/25) | 1.00 | 1.00 |
| Yes | 20.8% (117/563) | 5.195 (0.756-35.691) | 3.558 (0.493 - 25.664) |
| CD4+ T cell count* | |||
| HIV-uninfected | 15.6% (28/180) | 1.00 | 1.00 |
| <100 | 31.2% (15/48) | 2.009 (1.170-3.448) | 1.890 (1.098 - 3.254) |
| 100 - 199 | 26.6% (17/64) | 1.708 (1.004-2.904) | 1.338 (0.756 - 2.370) |
| 200 - 349 | 16.9% (13/77) | 1.085 (0.595-1.98) | 1.137 (0.634 - 2.036) |
| ≥350 | 12.8% (10/78) | 0.824 (0.421-1.613) | 0.886 (0.441 - 1.781) |
Patients with unknown CD4+ T cell counts (n = 141) were excluded from the analysis.
In the multivariable model, HIV-infected patients with CD4+ T cell counts <100 cells/mm3 had higher risk of death compared to HIV-uninfected patients (RR = 1.890; 95% CI = 1.098 – 3.254), after controlling for sex, age, and prior history of TB treatment (Table 3). No statistically significant differences were found in risk of death between HIV-uninfected patients and HIV-infected patients in CD4+ T cell categories ≥ 100 cells/mm3 (Table 3). Male sex (vs. female; RR = 1.630; 95% CI = 1.041 – 2.553) and older age (+10 years; RR = 1.228; 95% CI = 1.072 – 1.406) were also independently associated with death.
Discussion
We found high treatment success rates among patients managed by the national MDR-TB program in Botswana. Aspects of this program that may have contributed to high success rates include pre-initiation counseling, strict DOT, food and transport incentives, and follow-up of patients who miss their monthly consultations. In this setting, we found similar probabilities of treatment success and death between HIV-uninfected MDR-TB patients and HIV-infected MDR-TB patients in CD4+ T cell categories ≥100 cells/mm3. Notably, the WHO goal of 75% treatment success for MDRTB was reached for HIV-uninfected patients and HIV-infected patients with CD4+ T cell count ≥200 cells/mm3 (Table 2; Figure 2a). However, HIV-infected MDR-TB patients with advanced immune suppression (CD4+ T cell count < 100 cells/mm3) were more likely to die during treatment compared to HIV-uninfected patients. Thus, our data suggest that, in the ART era, high rates of treatment success can be achieved for HIV-infected MDR-TB patients who have not progressed to an advanced stage of immune suppression.
Our findings are consistent with a recent systematic review, which suggests that similar levels of treatment success can be achieved between HIV-infected and HIV-uninfected MDR-TB patients.4 However, the 73.0% treatment success among HIV-infected MDR-TB patients in our study is substantially higher than the 56.9% reported in that systematic review.4 The difference in treatment success might be largely due to inclusion in the systematic review of studies done prior to ART access.4 Our findings are similar to a recent study in Haiti that reported a 78% treatment success among HIV-infected MDR-TB patients on ART.17 That study was limited by a small sample size (N = 110), including only 27 HIV-infected MDR-TB patients.17 Using information from a large national cohort of MDR-TB patients, we confirm and extend the findings from the Haiti study by showing that similar levels of treatment success can be achieved among HIV-uninfected MDR-TB patients and HIV-infected MDR-TB patients with CD4+ T cell count ≥200 cells/mm3, in the context of concomitant ART availability in a well-managed MDR-TB program.
Treatment success among HIV-uninfected patients in our study was lower than the 84% – 89% treatment success reported for the short-course MDR-TB regimen recently endorsed by the WHO.18–21 Additional research is needed to determine the effectiveness of the WHO-endorsed short-course therapy in Botswana, particularly for HIV-infected patients who comprise nearly 70% of the diagnosed MDR-TB patients.
Although Botswana's MDR-TB treatment program achieved successful outcomes and very low rates of treatment failure and loss to follow-up, mortality remained unacceptably high among HIV-infected patients with baseline CD4+ T cell counts <100 cells/mm3. Among HIV-infected patients with known CD4+ T cell counts, nearly 20% had <100 cells/mm3 at the time of MDR-TB treatment initiation. These findings highlight the importance of reaching all HIV-infected persons with ART as part of comprehensive HIV care to prevent advanced immunosuppression and reduce mortality from MDR-TB and other causes. While intensified public health efforts have significantly increased ART coverage in Botswana, our study suggests that gaps in coverage continue to adversely affect HIV-related mortality.22,23 In June 2016, Botswana launched a “test and treat” program, designed to administer ART to all patients diagnosed with HIV. Based on our findings, this new program could improve treatment outcomes for HIV-infected patients who develop MDR-TB. Additional research is needed to improve the clinical management of MDR-TB patients with advanced immunosuppression.24
Strengths of our study include the use of programmatic data that reflect “real-life” experience of the MDR-TB program and its patients. In addition, our sample size of nearly 600 patients, including 408 HIV-infected patients allowed us to make comparisons between HIV-uninfected patients and HIV-infected patients stratified by CD4+ T cell counts.
Our study should be interpreted with consideration of the following limitations. First, we did not collect information on the cause of death. HIV-infected persons with CD4 T cell counts <100 cells/mm3 are at high risk of death caused by numerous etiologies other than TB, including cryptococcal meningitis and other opportunistic infections.25 However, ascertaining the cause of death for patients with multiple comorbidities is challenging, and we used the WHO definition of defining death as death due to any cause during TB treatment.9 Of note, we classified outcomes based on the patient's latest MDR-TB treatment episode, which partially explains the low failure rate in our study. It is also possible that more patients who died were patients who initially failed treatment. However, given the program's strong emphasis on individual patient follow-up, such “missed” failure events are likely to be minimal. Another limitation is that the program database did not include detailed HIV-related treatment variables, including ART failure and interruption, which could have affected MDR-TB treatment outcomes.
DST for second-line drugs was not done for all MDR-TB patients. Therefore, our study could have underestimated the proportion of patients identified as having pre-XDR and XDR-TB. Similarly, we did not perform DST for pyrazinamide resistance and, as the latest national drug resistance survey did not systematically test for pyrazinamide resistance, we do not know its background prevalence.26 During the study period, molecular diagnostics, such as Xpert MTB/RIF and line probe assays, were not routinely used. Recently, the Botswana Ministry of Health recommended replacing smear microscopy with Xpert MTB/RIF as the primary TB diagnostic tool. This policy change could lead to earlier diagnosis and more complete ascertainment of MDR-TB cases in Botswana.
Conclusions
We report treatment outcomes from a large national cohort of MDR-TB patients with access to ART in Botswana. High rates of treatment success are possible for HIV-infected MDR-TB patients with moderate to high immune function. Based on our findings, we recommend improved efforts to prevent advanced immunosuppression among HIV-infected persons and additional research to reduce mortality among MDR-TB patients with advanced HIV-associated immunosuppression.
Acknowledgments
We would like to thank the patients and staff at the MDR-TB clinics in Botswana who made this study possible.
Source of Funding
This work was supported in part by National Institutes of Health grants R01AI097045, 1R21AI120838, K01AI118559, MH58107 (UCLA Center for HIV Identification, Prevention, and Treatment), 5P30AI028697 (UCLA Center for AIDS Research), and UL1TR000124 (National Center for Advancing Translational Sciences). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest
All authors declare that there are no conflicts of interests.
Meetings where the material has been presented: This paper was presented in part at the Conference on Retroviruses and Opportunistic Infections, Feb 22-25, 2016.
References
- 1.Blanc F-X, Sok T, Laureillard D, et al. Earlier versus Later Start of Antiretroviral Therapy in HIV-Infected Adults with Tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. doi:10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of Initiation of Antiretroviral Drugs during Tuberculosis Therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. doi:10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells CD, Cegielski JP, Nelson LJ, et al. HIV Infection and Multidrug-Resistant Tuberculosis—The Perfect Storm. J Infect Dis. 2007;196(Supplement 1):S86–S107. doi: 10.1086/518665. doi:10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 4.Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2015;19(8):969–978. doi: 10.5588/ijtld.15.0123. doi:10.5588/ijtld.15.0123. [DOI] [PubMed] [Google Scholar]
- 5.Modongo C, Sobota RS, Kesenogile B, et al. Successful MDR-TB treatment regimens including Amikacin are associated with high rates of hearing loss. BMC Infect Dis. 2014;14(1):542. doi: 10.1186/1471-2334-14-542. doi:10.1186/1471-2334-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bemer P, Palicova F, Rüsch-Gerdes S, Drugeon HB, Pfyffer GE. Multicenter Evaluation of Fully Automated BACTEC Mycobacteria Growth Indicator Tube 960 System for Susceptibility Testing of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40(1):150–154. doi: 10.1128/JCM.40.1.150-154.2002. doi:10.1128/JCM.40.1.150-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Republic of Botswana Ministry of Health [February 15, 2016];National Tuberculosis Programme Manual. http://www.who.int/hiv/pub/guidelines/botswana_tb.pdf. Published 2007.
- 8.Botswana Ministry of Health [February 15, 2016];Botswana National HIV/AIDS Treatment Guidelines. 2008 http://www.hsph.harvard.edu/population/aids/botswana.aids.08.pdf.
- 9.World Health Organization E. Emergency update. World Health Organization, Stop TB Department; Geneva: 2008. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: [Emergency Update 2008]. [Google Scholar]
- 10.Agresti A. Categorical Data Analysis. 3 edition. Wiley; Hoboken, NJ: 2012. [Google Scholar]
- 11.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. doi:10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 12.Donoghoe MW, Marschner IC. Flexible Regression Models for Rate Differences, Risk Differences and Relative Risks. Int J Biostat. 2015;11(1):91–108. doi: 10.1515/ijb-2014-0044. doi:10.1515/ijb-2014-0044. [DOI] [PubMed] [Google Scholar]
- 13.Knol MJ, Cessie SL, Algra A, Vandenbroucke JP, Groenwold RHH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Can Med Assoc J. 2012;184(8):895–899. doi: 10.1503/cmaj.101715. doi:10.1503/cmaj.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marschner IC, Gillett AC. Relative risk regression: reliable and flexible methods for log-binomial models. Biostatistics. 2012;13(1):179–192. doi: 10.1093/biostatistics/kxr030. doi:10.1093/biostatistics/kxr030. [DOI] [PubMed] [Google Scholar]
- 15.Schisterman EF, Cole SR, Platt RW. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. doi:10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanderWeele TJ. On the Relative Nature of Overadjustment and Unnecessary Adjustment. Epidemiology. 2009;20(4):496–499. doi: 10.1097/EDE.0b013e3181a82f12. doi:10.1097/EDE.0b013e3181a82f12. [DOI] [PubMed] [Google Scholar]
- 17.Charles M, Vilbrun SC, Koenig SP, et al. Treatment outcomes for patients with multidrug-resistant tuberculosis in post-earthquake Port-au-Prince, Haiti. Am J Trop Med Hyg. 2014;91(4):715–721. doi: 10.4269/ajtmh.14-0161. doi:10.4269/ajtmh.14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piubello A, Harouna SH, Souleymane MB, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis. 2014;18(10):1188–1194. doi: 10.5588/ijtld.13.0075. doi:10.5588/ijtld.13.0075. [DOI] [PubMed] [Google Scholar]
- 19.Sotgiu G, Tiberi S, D'Ambrosio L, Centis R, Zumla A, Migliori GB. WHO recommendations on shorter treatment of multidrug-resistant tuberculosis. The Lancet. 2016;387(10037):2486–2487. doi: 10.1016/S0140-6736(16)30729-2. doi:10.1016/S0140-6736(16)30729-2. [DOI] [PubMed] [Google Scholar]
- 20.Aung KJM, Van Deun A, Declercq E, et al. Successful “9-month Bangladesh regimen” for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18(10):1180–1187. doi: 10.5588/ijtld.14.0100. doi:10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 21.Van Deun A, Maug AKJ, Salim MAH, et al. Short, Highly Effective, and Inexpensive Standardized Treatment of Multidrug-resistant Tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–692. doi: 10.1164/rccm.201001-0077OC. doi:10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 22.Gaolathe T, Wirth K, Holme MP, et al. Botswana is Close to Meeting UNAIDS 2020 Goals of 90-90-90 Coverage. Boston, MA: 2016. [Google Scholar]
- 23.Okatch H, Bellamy SL, Han X, et al. CD4 Cell Counts at Antiretroviral Therapy Initiation in Botswana Have Been Increasing. Clin Infect Dis. 2016;62(5):669–670. doi: 10.1093/cid/civ965. doi:10.1093/cid/civ965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisson GP, Zetola N, Collman RG. Persistent High Mortality in Advanced HIV/TB Despite Appropriate Antiretroviral and Antitubercular Therapy: an Emerging Challenge. Curr HIV/AIDS Rep. 2015;12(1):107–116. doi: 10.1007/s11904-015-0256-x. doi:10.1007/s11904-015-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longley N, Jarvis JN, Meintjes G, et al. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin Infect Dis. 2016;62(5):581–587. doi: 10.1093/cid/civ936. doi:10.1093/cid/civ936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies HJ, Moalosi G, Anisimova V, et al. Increase in anti-tuberculosis drug resistance in Botswana: results from the fourth National Drug Resistance Survey. Int J Tuberc Lung Dis. 2014;18(9):1026–1033. doi: 10.5588/ijtld.13.0749. doi:10.5588/ijtld.13.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]