Abstract
Sphingosine-1- phosphate (S1P), a simple, bioactive sphingolipid metabolite, plays a key role, both intracellularly and extracellularly, in various cellular processes such as proliferation, survival, migration, inflammation, angiogenesis, and endothelial barrier integrity. The cellular S1P level is low and is tightly regulated by its synthesis and degradation. Sphingosine Kinases (SphKs) 1 and 2, catalyze the ATP-dependent phosphorylation of sphingosine to S1P, while the degradation is mediated by the reversible dephosphorylation catalyzed by the S1P phosphatases and lipid phosphate phosphatases and the irreversible degradation to hexadecenal and ethanolamine phosphate by sphingosine-1-phosphate lyase (S1PL). As a ligand for specific G-protein-coupled receptors, S1P1–5, which are differentially expressed in different cell types, S1P generates downstream signals that play crucial role in developmental and disease related pathologies. In addition to acting extracellularly on receptors located on the plasma membrane, S1P can also act intracellularly, independently of S1P1–5, affecting calcium homeostasis and cell proliferation. The SphKs /S1P /S1PL metabolic pathway is implicated in numerous human pathologies including respiratory disorders, thereby raising the possibility that manipulating intracellular S1P levels could offer therapeutic potential in ameliorating lung diseases. This review focuses on the prospects of targeting S1P signaling and S1P metabolizing enzymes using small molecule inhibitors, receptor agonists, and antagonists in the treatment of lung diseases.
Keywords: Sphingsine-1-Phosphate, Sphingosine Kinase, Sphingosine-1-Phosphate Lyase, Sphingosine-1-phosphate Phosphatase, Sphingomyelinase, Ceramide, S1P receptor, Lung Diseases
1. Introduction
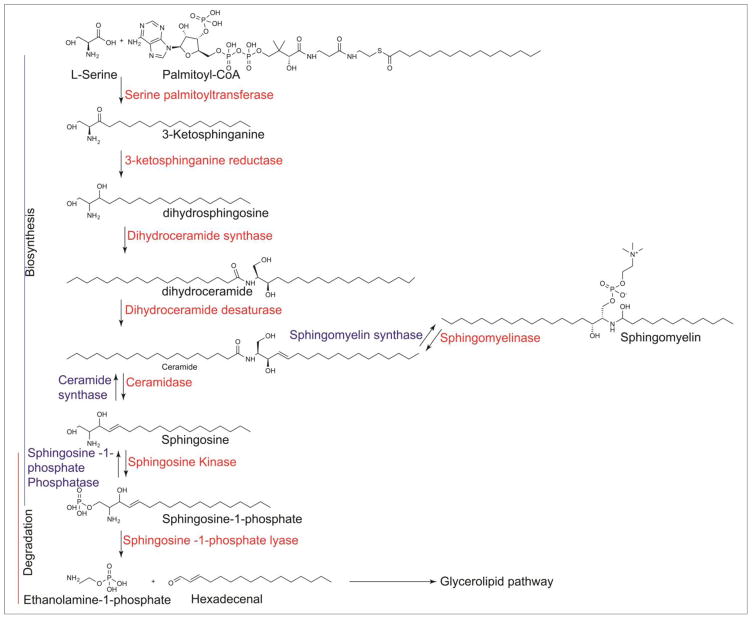
Sphingolipids constitute a class of lipids that contain a sphingoid base such as sphingosine, sphinganine (dihydrosphingosine), or phytosphingosine backbone linked to long-chain fatty acids (ceramides). The resulting ceramides can be linked to hydrophilic head groups such as phosphorylcholine (sphingomyelin) (SM), carbohydrate moieties (glycosphingolipids), or a phosphate group (ceramide-1-phosphate) (Weete, 1974). SM is located on the exoplasmic surface of the eukaryotic plasma membrane where it plays a paramount role in protecting the cell surface from external agents (Simons & Ikonen, 1997), and also functions as a signaling lipid (Maceyka et., 2012). The first step of sphingolipid de novo biosynthesis is the formation of 3-keto-dihydrosphingosine via condensation of L-serine and palmitoyl CoA catalyzed by serine palmitoyltransferase (SPT), the rate limiting enzyme in sphingolipid biosynthesis (Merrill, 2002). 3-Keto-dihydrosphingosine is rapidly reduced to sphinganine (dihydrosphingosine) by ketosphinganine reductase (Stoffel, 1970), followed by ceramide synthase(s) mediated N-acylation to dihydroceramide with different fatty acid chain lengths (Stiban et., 2010). Mammals exhibit six different acyltransferases encoded by lass-genes that show specificities for different fatty acyl CoAs (Futerman & Riezman, 2005). Dihydroceramides can be desaturated to ceramides, which can be channeled to the synthesis of complex sphingolipids such as SM and glycosphingolipids, or phosphorylated by ceramide kinase to ceramide-1-phosphate (Mitsutake et., 2006). Mammalian cells do not convert dihydrosphingosine to sphingosine; however sphingosine can be generated from ceramide by ceramidases (Chalfant & Spiegel, 2005). Also, ceramide can be formed from SM in mammalian cells by sphingomyelinase activation in response to extracellular stimuli such as TNF- α or growth factors (Dbaibo et al., 1993). Sphingosine generated from ceramide is converted to sphingosine-1-phosphate (S1P) by sphingosine kinase (SphK) 1 and/or 2 (Figure 1).
Fig 1. De novo Sphingolipid Metabolism in mammalian cells.
Illustration of the key enzymatic steps in the biosynthesis, degradation and recycling of sphingoid bases.
2. Sphingosine-1-phosphate Metabolism and Signaling
Cellular levels of S1P are tightly regulated by its synthesis from sphingosine through the activation of SphKs and degradation through reversible dephosphorylation of S1P to sphingosine by S1P phosphatases (SPPs), lipid phosphate phosphatases (LPPs), or irreversible degradation by a pyridoxal phosphate-dependent S1P Lyase (S1PL) to Δ2 hexadecenal and ethanolamine phosphate (Saba & Hla, 2004). In unstimulated cells, the balance between S1P production and degradation results in relatively low intracellular levels of S1P. Erythrocytes and platelets have much higher levels of S1P compared to other cells and this is due to lack of S1PL (Ito et al., 2007). S1P is also transported from inside the cell to outside by ABC transporters (Kim et al.,2009; Kobayashi et al., 2009; Mitra et al., 2006; Sato et al., 2007), and the recently identified spinster homolog 2 (Spns2) transporter (Fukuhara et al., 2012; Hisano et al., 2012; Kawahara et al., 2009). In the last two decades, S1P garnered much deserved research attention as it has emerged as a bioactive lipid mediator of diverse cellular processes such as cell growth, and survival (Olivera et al., 1999), motility (Van Brocklyn et al., 2003; Xu et al., 2006; Berdyshev et al., 2011), cytoskeletal organization (Garcia et al., 2001), endothelial permeability (Wang & Dudek, 2009), vascular tone (Levkau, 2008), adherens junctions (Mehta et al., 2005), tight junctions assembly (Lee et al., 1999a; Lee et al., 2006), autophagy (Lavieu et al., 2006; Huang and Natarajan, 2015), immune regulation (Chi, 2011; Spiegel & Milstien, 2011; Walzer, Chiossone, Chaix, & Calver, 2007) and morphogenesis (Lee et al., 1999a). These pleotropic actions are attributed to its unique inside-out (extracellular), and intracellular signaling, highlighting its role as a signaling sphingolipid. Intracellularly, S1P is known to act as a second messenger and plays a role in calcium homeostasis; however very little is known regarding intracellular targets of S1P. Release of S1P in human lung endothelial cells by the photolysis of caged S1P significantly enhanced endothelial cell (EC) barrier function, which was independent of S1P1, but was dependent on Rac1(Usatyuk et al., 2011). Interestingly, S1P generated in the nucleus by the action of SphK2 is shown to directly target HDACs and an integral component of the HDAC repressor complex (Hait et al., 2009; Fu et al., 2014), but the mechanism and its relevance to disease needs further study. Additionally, S1P has been identified as a missing co-factor required for the E3 ligase activity of TNF receptor-associated factor 2 (TRAF2) (Alvarez et al., 2010). Equally important, S1P is a regulator of mitochondrial assembly and function by binding to prohibitin 2 (Strub et al., 2011), and a modulator of BACE1 activity in Alzheimer’s disease (Takasugi et al., 2011). Further, S1P binding to human telomerase reverse trancriptase Tak stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation (Panner Selvam et al., 2015). Taken together, S1P’s actions could be autocrine or paracrine or in situ where it is generated and thus offers immense therapeutic potential for a number of human diseases including respiratory disorders such as sepsis, asthma, fibrosis, pulmonary hypertension, and lung cancer, which is the focus of this review (Table 1).
Table 1.
SphK expression and S1P levels in Lung Diseases
| Lung Disease | Expression of SphK1/SphK2 | S1P Levels | Therapy Option |
|---|---|---|---|
| Sepsis |
Decreased SphK1 Increased S1P Lyase |
Low | Activation of SphK1 |
| BPD | Increased SphK1 | Elevated | Inhibition of SphK1 activity |
| IPF | Increased SphK1 | Elevated | Inhibition of SphK1 activity |
| PH | Increased SphK1 | Elevated | Inhibition of SphK1 activity |
| VILI |
Decreased SphK1 Increased S1P Lyase |
Elevated | Inhibition of SphK1 activity |
| Lung Cancer | Increased SphK1/SphK2 | Elevated | Inhibition of SphK1/SphK2 activity |
| RILI | Increased SphK1/SphK2 | Elevated | Inhibition of SphK1/SphK2 activity |
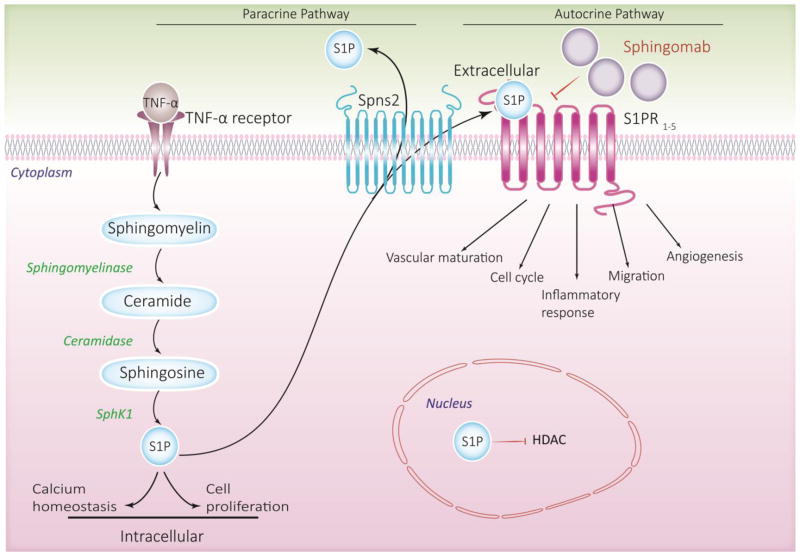
S1P signaling has momentous clinical importance as evidenced by recent research, suggesting its pivotal role in regulating diverse immune regulatory networks that contribute to human health and diseases (Maceyka et al., 2012) including respiratory and lung disorders (Brinkmann & Baumruker, 2006). S1P signaling axis is implicated in regulation of many physiological and pathophysiological processes and diseases (Pyne et al., 2009), and targeting S1P levels in pathologies wherein increase or decrease in circulating or tissue S1P levels has been shown to be detrimental (Pyne & Pyne, 2010; Natarajan et al., 2013; Kunkel et al., 2013) which is of significant interest to scientists and pharmaceutical industry. Of note, anti-S1P monoclonal antibody (sphingomab) (O’Brien et al., 2009), which neutralizes extracellular S1P and inhibits its signaling via its receptors, is currently being investigated in pre-clinical and phase I and II trials of tumor growth suppression and age-related macular degeneration (Sabbadini, 2011) (Figure 2).
Fig 2. S1P signaling Axis.
This scheme depicts the inside-out signaling of S1P. S1P produced by Sphingosine kinase 1 or 2 can act intracellularly on unknown targets in the cytoplasm; S1P generated by sphingosine kinase 2 in the nucleus inhibits HDAC activity. S1P is also exported out of the cell by specific transporter SpnS2 and act extracellularly on S1P receptors in an autocrine or paracrine manner. Extracellular S1P can be targeted by specific monoclonal antibody (sphingomab) to neutralize its effects.
2.1. Sphingosine Kinases as Drug Targets
SphK1 and SphK2 are the two known isoenzymes that have been identified and characterized in mammals (Kohama et al., 1998; Liu et al., 2000a; Nava et al., 2000). Although both share significant sequence homology, they differ significantly in sub-cellular localization, tissue distribution, and function. Albeit ubiquitously expressed in most tissues, SphK1 expression is substantial in the lung and heart, and SphK2 in the liver and spleen (Melendez et al., 2000). Two functional nuclear export signal sequences (NES) direct SphK1 localization to the cytosol (Inagaki et al., 2003). In contrast, SphK2 has both nuclear import and export sequences, and is found predominantly in the nucleus in many cell types (Igarashi et al., 2003). Both SphK1 and SphK2, when activated by growth and survival factors, undergo translocation, post-translational modifications, protein-lipid, and protein-protein interactions that ultimately result in elevated S1P levels in the cell (Alemany et al., 2007), which regulates various biological responses. Cytosolic S1P produced by SphK1 enhances cell growth, whereas Sphk2 generated S1P in ER and/or membranes promote apoptosis, as evident from studies using different model systems (Hait et al., 2006; Liu et al., 2003). Despite their differences, in vivo studies have shown that SphK1 and SphK2 can compensate for each other, as neither SphK1 nor SphK2 knockout mice models reveal any ostensible phenotype in adulthood. The SphK1/2 double knockout mice are embryonically lethal (Mizugishi et al., 2005). Although S1P has barrier protective effects, and proven ameliorative effect against sepsis-induced lung injury in animal models (Peng et al., 2012), there are limitations to S1P therapy in acute lung injury (ALI) (Natarajan et al., 2013; Wang et al., 2014). Infusion of high concentrations of S1P produces pulmonary edema, stimulates contraction of airway and bronchial smooth muscle cells (Rosenfeldt et al., 2003), and increases airway hyper-responsiveness to allergens in mice (Roviezzo et al., 2007). Several small molecule inhibitors of SphK 1 and SphK2 have been reported and some are listed in Table 2.
Table 2.
Sphingosine Kinase Inhibitors
| Inhibitor | Chemical name | Structure | Specificity |
|---|---|---|---|
| SK1-I | (2R,3S,4E)-N-methyl-5-(4′- pentylphenyl)-2-aminopent-4-ene-1,3- diol. HCl |
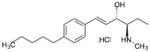
|
SphK1 |
| SKI-I | 5-(2-Naphthalenyl)-1H-pyrazole-3- carboxylic acid 2-[(2-hydroxy-1- naphthalenyl)methylene]hydrazide |
|
SphK1 & SphK2 |
| SKI-II | 2-(p-Hydroxyanilino)-4-(p- chlorophenyl)thiazole |
|
SphK1 |
| Safingol | (2S,3S)-2-amino-1,3-octadecanediol |
|
SphK1 & SphK2 |
| ABC294640 | (1s,3r,5R,7S)-3-(4-chlorophenyl)-N- (pyridin-4-ylmethyl)adamantane-1- carboxamide |
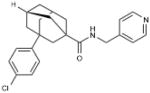
|
SphK2 |
| DMS | (E,2R,3S)-2-(Dimethylamino)-octadec- 4-ene-1,3-diol |
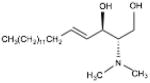
|
SphK1 & SphK2 |
| PF-543 | 1-[[4-[[3-methyl-5- [(phenylsulfonyl)methyl]phenoxy]meth yl]phenyl]methyl]-2R- pyrrolidinemethanol |
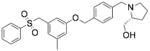
|
SphK1 |
| FTY-720 Methyl ether | 2-amino-2-(methoxymethyl)-4-(4- octylphenyl)butan-1-ol |
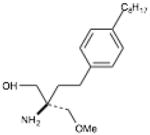
|
SphK2 |
2.2. S1P Lyase as a Drug Target
S1P Lyase (S1PL) is an endoplasmic reticulum-resident, pyridoxal phosphate dependent, type III membrane protein (Ikeda et al., 2004) that is ubiquitously expressed in all tissues (Serra & Saba, 2010). It catalyzes the terminal step in sphingolipid metabolism by irreversibly cleaving the C2-C3 bond of S1P to generate Δ2 hexadecenal and ethanolamine phosphate, thereby controlling the S1P concentrations in the cells. Deletion of both alleles of Sgpl1 gene in mice is lethal and animals do not survive beyond weaning due to congenital anomalies partially attributed to cytotoxic levels of S1P in major organs (Allende et al., 2011); however, partial knock out of Sgpl1 gene results in small increase in S1P with no effect on the survival (Billich et al., 2013). S1PL is known to be up-regulated and activated in response to various stimuli like the bacterial endotoxin lipopolysaccharide (LPS) (Zhao et al., 2011) and radiation exposure of the mouse lung (Huang et al., 2013). S1PL is likely to undergo various post-translational modifications due to many potential phosphorylation and nitrosylation sites; however, they need to be validated experimentally (Huang et al., 2005; Zhan & Desiderio, 2006). Although S1PL is a novel target in immunomodulation, only a few S1PL small molecular inhibitors have been characterized. The vitamin B6 antagonist, 4′-deoxypyridoxine (4-DP) (Bandhuvula et al., 2007) and the food colorant, 2-acetyl-4-tetrahydroxybutylimidazole (THI) (Ohtoyo et al., 2015) have been widely used to block S1PL activity, in vitro and in vivo, respectively, and have limited therapeutic application. 4-DP is a non-specific inhibitor of all pyridoxal phosphate dependent enzymes, and may lead to long-term cytotoxicity. THI modulates S1PL activity in vivo and not in vitro as biotransformation of THI is required for its inhibition of S1PL (Schwab et al., 2005). A recently designed S1PL inhibitor, [(4-benzylpthalazin-1-yl)-2-methylpiperazin-1-yl] nicotinonitrile 5, reduced peripheral T cell numbers after oral administration and conferred protection to experimental autoimmune encephalomyelitis in rats (Weiler et al., 2014). Thus, inhibition of S1PL, which increases intracellular S1P levels, may serve as an alternative pharmacological approach to FTY720 to reduce T cell egress from lymph nodes in treating certain sub-types of multiple sclerosis. Table 3 lists S1PL inhibitors that may have therapeutic potential.
Table 3.
Sphingosine-1-Phosphate Lyase inhibitors
| Inhibitor | Chemical name | Structure | Specificity |
|---|---|---|---|
| LX-2931 | (1R,2S,3R)-1-[(2Z)-2-(1- nitrosoethylidene)-1,3- dihydroimidazol-4-yl]butane-1,2,3,4- tetrol, |
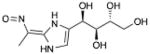
|
S1PL |
| THI | 1-[5-[(1R,2S,3R)-1,2,3,4- tetrahydroxybutyl]-1H-imidazol-2-yl]-ethanone |
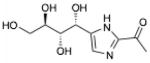
|
S1PL |
| 4-Deoxy pyridoxine | 5-(Hydroxymethyl)-2,4- dimethylpyridin-3-ol |
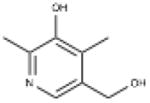
|
S1PL |
| Compound 31 (Novartis) | (4-benzyl-phthalazin-1-yl)-2-methyl- piperazin-1-yl]-nicotinonitrile 5 |
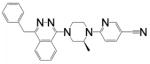
|
S1PL |
2.3. S1P receptors as Drug Targets
S1P is a unique lipid mediator that has both intracellular and extracellular targets. S1P generated in the cell is delivered to the extracellular environment by ABC transporters (Kim et al., 2009; Mitra et al., 2006; Sato et al., 2007; Kobayashi et al., 2009) and the recently identified Spns2 (Fukuhara et al., 2012; Kawahara et al., 2009; Hisano et al., 2012) where it acts in an autocrine or paracrine manner as a ligand for G-protein-coupled receptors on the cell surface. Five such S1P receptors (S1PRs) have been characterized so far that are designated S1P1–5. S1PRs are differentially expressed in cells and tissues, and are coupled to various heterotrimeric G-proteins, thereby accounting for its pleotropic effects (Hla, 2001). The pattern of S1PR activation differentially regulates downstream signaling effector molecules like small GTPases (Kume et al., 2007; H. Zhou & Murthy, 2004), MAP kinase (Guo et al., 1998; Dikic et al., 1996; Sato et al., 1999), Akt (Tanimoto et al.,, 2004; Baudhuin et al., 2004), and phospholipase C/D (Banno et al., 1999; Okamoto et al., 1998), which in turn mediate various cellular processes including migration, immunity, angiogenesis, and vascular development. The differential expression of S1PRs also accounts for the diversity of the activation of down-stream signaling pathways. For example, binding of S1P to S1P1 or S1P3 results in increase migration of endothelial cells, whereas activation of S1P2 has an opposite effect (Lee et al., 2001; Kimura et al., 2000; Ryu et al., 2002). Moreover, the differential regulation of Rho family of small GTPases, especially Rho and Rac, by S1PRs, is critical for barrier maintenance, cytoskeletal rearrangement, and motility of endothelial and epithelial cells (Lee et al., 1999a; Okamoto et al., 2000). S1P activation of S1P1 enhances barrier integrity by cortical actin rearrangement in endothelial cells via Rac activation but S1P3 activation in epithelial cells results in possible activation of Rho, leading to increased vascular permeability (Garcia et al., 2001; Liu et al., 2000b; Natarajan et al., 2013). S1P1 deletion also hinders downstream Rac activation that leads to defects in vascular maturation, as evident from studies done using S1P1 receptor null mice (Liu et al., 2000b). S1P-mediated multiple immune modulatory functions are also attributed to the differential expression of S1PRs. S1P secreted by mast cells was found to enhance mast cell function by binding to its S1PR1&2 in an autocrine manner (Olivera et al., 2010). While activation of S1P1 by S1P in an autocrine manner causes mast cell migration (Olivera et al., 2010), binding to S1P2 inhibits mast cell migration by down-regulation of Rac activity (Olivera & Rivera, 2005; Sanchez & Hla, 2004). Extracellular addition of S1P to rat islet cells has been shown to inhibit apoptosis, which is also mimicked by di-hydro–S1P addition (Laychock et al., 2013). Similarly, blocking S1P1 & S1P3 by antisense nucleotide attenuated S1P mediated effect on cell survival in endothelial cells (Kwon et al., 2001). Although the differential expression and effects of S1PRs underscores the complexity of S1P signaling, it also presents a valuable approach for targeting S1PRs for drug development. Given the limitation of S1P as a therapeutic agent in treating ALI, there has been a growing interest in the development of analogs of sphingosine and S1P for therapy. One such analog is FTY720, a synthetic derivative of the fungal metabolite, myriocin (Troncoso & Kahan, 1998). FTY720 is phosphorylated to FTY720-P by SphKs, especially by SphK2, in vitro and in vivo (Paugh et al., 2003). FTY720-P, similar to S1P, also binds to S1P1 and S1P3 (Gräler & Goetzl, 2004), and modulates endothelial barrier function (Brinkmann et al., 2004). Moreover, FTY720 (Fingolimod) is an anti-inflammatory (Sehrawat & Rouse, 2008) and immune-modulatory agent (Zhou et al., 2009), which has been recently approved by FDA for the treatment of multiple sclerosis (MS) (Chun & Hartung, 2010). Most likely, as in animal models, phosphorylation of FTY720 to FTY720-P by SphK2 is essential for its therapeutic action in MS (Brinkman, 2010). Additionally, FTY720-P enhanced endothelial barrier function via S1P1 and reversed endothelial barrier dysfunction induced by VEGF in endothelial cells (Dudek et al., 2007). In addition to the beneficial effects, both S1P and FTY720-P have therapeutic limitations (Natarajan et al., 2013) as both these mediators induce ubiquitination and proteosomal degradation of S1P1 resulting in increased endothelial permeability after prolonged exposure. However, non-hydrolysable analog of FTY720-P, FTY720-(S)-Phosphonate, which also binds to S1P1 has been shown to exhibit superior efficacy as a barrier protecting agent in cultured ECs and pre-clinical animal models of ALI (Camp et al., 2016; Wang et al., 2014). Although the precise mechanism of action of FTY720-(S)-phosphonate is unclear, its superior efficacy may be attributed to maintenance of S1P1 expression for a prolonged period of time compared to S1P or FTY720-P. Further studies are necessary to delineate the down-stream signaling pathways mediated by S1P, FTY720, and FTY720-P analogs in regulating endothelial barrier function. Several agonists and antagonists that can modulate S1PR activity has been developed as pharmacologic tools to better understand the S1P receptor functions (Table 4 & 5).
Table 4.
S1P receptor Agonists
| Agonist | Chemical name | Structure | Specificity |
|---|---|---|---|
| SEW2871 | 5-[4-phenyl-5-(trifluoromethyl)-2- thienyl]-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole |
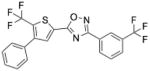
|
S1PR1 |
| Ponesimod | (2Z,5Z)-5-{3-Chloro-4-[(2R)-2,3- dihydroxypropoxy]benzylidene}-3-(2- methylphenyl)-2-(propylimino)-1,3- thiazolidin-4-one |
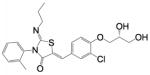
|
S1PR1 |
| FTY 720-P | 2-amino-2[2-(4-octylphenyl)ethyl]-1,3- propanediol, mono dihydrogen phosphate ester |
|
S1PR1, S1PR3, S1PR4, S1PR5 |
| AUY954 | 3-[[2-[4-phenyl-3- (trifluoromethyl)phenyl]-1- benzothiophen-5- yl]methylamino]propanoic acid |
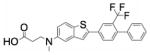
|
S1PR1 |
Table 5.
S1P receptor Antagonists
| Antagonist | Chemical name | Structure | Specificity |
|---|---|---|---|
| VPC 23019 | (R)-2-amino-3-((3-octylphenyl)amino)- 3-oxopropyl dihydrogen phosphate |
|
S1PR1 & 3 |
| VPC 23153 | (R)-phosphoric acid mono-[2-amino-2- (6-octyl-1H-benzoimiazol-2-yl)-ethyl] ester |
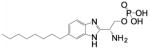
|
S1PR4 |
| SB 649146 | Structure not known | S1PR1 | |
| BML-241 | 2-undecyl-thiazolidine-4-carboxylic acid |
|
S1PR3 |
| JTE 013 | N-(2,6-dichloro-4-pyridinyl)-2-[1,3- dimethyl-4-(1-methylethyl)-1H- pyrazolo[3,4-b]pyridin-6-yl]-hydrazinecarboxamide |
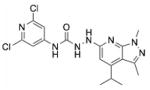
|
S1PR2 |
| W123 | 3-(2-(3-hexylphenylamino)-2- oxoethylamino) propanoic acid |
|
S1PR1 |
| VPC 24191 | (S)-phosphoric acid mono-[2-amino-3- (4-octyl-phenylamino)-propyl] ester |
|
S1PR1 & 3 |
2.4. S1P Phosphatases and Lipid Phosphate Phosphatases as Drug Targets
In addition to S1PL, S1P can also be metabolized by SPPs and LPPs to sphingosine (Le Stunff et al., 2002; Jasinska et al., 1999; Zhao et al., 2007; Tang et al., 2015). However, not much is known about the physiological and pathophysiological roles of SPP 1 and 2 and LPP1–3 in lung diseases. Depletion of SPP1 results in unfolded protein response and endoplasmic stress-induced autophagy (Lépine et al., 2011b) and doxorubicin switches protective autophagy in SPP1-depleted cells to apoptosis by calpain-mediated autophagy-related gene 5 cleavage (Lépine, Allegood, Edmonds, Milstien, & Spiegel, 2011a). Also, there is evidence to suggest a role for SPP1 in cancer as depletion of SPP1 with siRNA confers resistance to chemotherapy (Johnson et al., 2003). In contrast to SPP1, SPP2 seems to have a role in inflammation. SPP2 is induced in human umbilical vein ECs (HUVECs) during inflammatory responses and silencing of SPP2 by siRNA significantly reduced TNF-α mediated IL-1β mRNA and protein expression (Mechtcheriakova et al., 2007). LPPs comprising of three major isoforms, LPP1, LPP2 and LPP3, dephosphorylate several bioactive lipids including S1P, LPA, PA, and ceramide-1-phosphate, and modulate the bioavailability of these lipids for cell signaling (Burnett & Howard, 2003; Tang et al., 2015). Very little is known about the role of LPPs in lung diseases; In human endothelial cells, LPP1 has be shown to modify S1P signaling by decreasing extracellular S1P concentrations, thereby lowering the activation of S1P receptors and downstream signaling. In addition, overexpression of LPP1 increases intracellular S1P signaling by promoting intracellular S1P formation (Zhao et al., 2007). Also, studies done using human bronchial epithelial cells showed a physiological role of LPP1 in attenuating LPA-induced IL-8 secretion (Zhao et al 2005). LPP3 may also have a role in tumor growth. LPP3 has been shown to impact glioblastoma progression by amplifying β-catenin and cyclin-D1 activities (Chatterjee et al., 2011). Several agents such as tetrafluoroaluminate (ALF4), orthovanadate, and N-ethylmaleimide (NEM) are known to inhibit SPPs and LPPs; however, many of these compounds are cytotoxic and have no therapeutic value. Currently, specific small molecule inhibitors to dissect the roles of SPPs and LPPs in human diseases have not been developed.
2.5. Acid Sphingomyelinase as a Drug Target
Acid sphingomyelinase (ASM) belongs to a family of enzymes that catalyze the breakdown of SM to ceramide, a bioactive sphingolipid that has been implicated in the pathogenesis of human diseases including cancer, lung disorders, atherosclerosis, and diabetes (Ogretmen & Hannun, 2004; Brodlie et al., 2012; Petrache et al., 2005; Bismuth et al., 2008; Holland et al., 2007). Ceramide and S1P, two interconvertible lipids, form the ceramide-S1P rheostat that controls the cell fate, mediating opposing responses; ceramides are anti-proliferative whereas S1P stimulates cell survival pathways. In humans, deficiency of ASM results in an inherited lipidosis disorder called type A and B Niemann-Pick disease, characterized by accumulation of SM in neural and other tissues (Brady et al., 1966). Ceramide stimulates apoptosis in cells and increased ceramide levels have been observed in the lungs of patients with emphysema (Petrache et al., 2005); however, contradictory findings have been reported about ceramide levels in cystic fibrosis (CF) patients and in mouse model of CF where reduced ceramide levels correlated with defects in fatty acids (Wojewodka et al., 2010). These contradicting results in CF may be due to the various animal models used and the detection of only selected species of ceramides by mass spectrometry. Several pulmonary disorders result from either elevated ASM activity and/or expression. Pharmacological inhibition or genetic knockdown of ASM has been shown to normalize ceramide levels, decrease inflammation and infection, and prevent pulmonary disorders such as fibrosis (Dhami et al., 2010), cigarette smoke-induced emphysema (Petrache et al., 2005), and CF in mice (Becker et al., 2012). Several small molecule inhibitors of ASM such as amitriptyline, trimipramine, desipramine, chlorprothixene, fluoxetine, amlodipine, sertraline, and bisphosphonates may be useful for the treatment of pulmonary disorders such as CF, fibrosis, or emphysema via inhibition of ASM and modulation of ceramides in the lung (Table 6).
Table 6.
Acid Sphingomyelinase inhibitors
| Inhibitor | Chemical Name | Structure | Specificity |
|---|---|---|---|
| Amytripytline | 3-(10,11-Dihydro-5H-dibenzo [a,d]cycloheptene-5-ylidene)-N,N- dimethylpropan-1-amine |
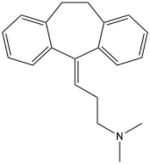
|
Acid Sphingomyelinase |
| Trimipramine | (±) -3-(10,11-dihydro-5H-dibenz o[b,f]azepin-5-yl)-N,N,2- trimethylpropan-1-amine |
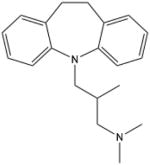
|
Acid Sphingomyelinase |
| Desipramine | 3-(10,11-Dihydro-5H- dibenzo[b,f]azepin-5-yl)-N- methylpropan-1-amine |
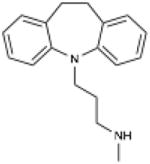
|
Acid Sphingomyelinase |
| Chlorprothixene | (Z)-3-(2-chlorothioxanthen-9- ylidene)-N,N-dimethyl-propan-1- amine |
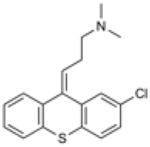
|
Acid Sphingomyelinase |
| Fluoxetine | N-methyl-3-phenyl-3-[4- (trifluoromethyl)phenoxy]propan-1- amine |
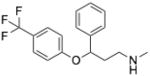
|
Acid Sphingomyelinase |
| Amlodipine | (RS)-3-ethyl 5-methyl 2-[(2- aminoethoxy) methyl]-4-(2- chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate |
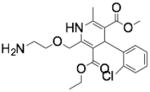
|
Acid Sphingomyelinase |
| Setraline | (1S,4S)-4-(3,4-dichlorophenyl)-N- methyl-1,2,3,4- tetrahydronaphthalen-1-amine |
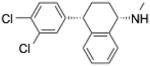
|
Acid Sphingomyelinase |
| Bisphosphanate |
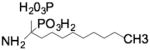
|
Acid Sphingomyelinase |
3. S1P/SphKs/S1PL Signaling Axis in Respiratory and Lung Diseases
Lung diseases are common medical conditions that affect the function of the lungs with symptoms ranging from mild to life threatening. Lung diseases fall into three broad categories: 1. Airway diseases that affect the tracheal and bronchial tubes resulting in structural changes in the airway, 2. Respiratory diseases that affect the lung structure leading to scarring of tissues, and 3. Pulmonary circulation diseases affect the blood vessels causing clotting and inflammation, which can also affect heart function. Irrespective of the nature of the disease, all the lung disorders affect the ability of the lung to exchange gases, which could lead to acute or chronic disease condition. S1P, being a potent signaling molecule, has been implicated in a number of lung disorders and because of its pleotropic nature it provides us with multiple targets, which could be exploited in developing therapeutics (Brinkmann & Baumruker, 2006). Lung disorders that affect the tubes cause constriction of airways, and airway diseases include asthma, chronic obstructive pulmonary disease (COPD), and CF (Barnes, 2008; Lyczak et al., 2002). A number of diseases affecting lung tissues alter the tissue morphology by scarring or inflammation, which affects the lungs ability to expand and exchange gases. Diseases that cause restriction of the lung include pulmonary fibrosis, pneumonia, ARDS, and lung cancer (Chapman, 2004). Lung diseases that cause clotting, scarring, and inflammation of the blood vessels affect the normal functioning of the lungs and the heart; pulmonary hypertension is one such disease (Leeman, 1996).
S1P can signal extracellularly (inside out mechanism) by ligation to G-protein-coupled S1P1–5 receptors or signal inside the cell, independent of the S1PRs. (Chun et al., 2002). However, the possibility of intracellular action of S1P via intracellularly localized S1PRs is understudied and cannot be ruled out. Recent evidences suggest localization and differential expression of S1PRs in normal and malignant human tissues. All the five S1PRs were expressed in both the cytoplasm and nucleus of benign and malignant tissues from multiple human organs as evidenced by Immunohistochemistry and Immunocytochemistry (Wang et al., 2014). Additionally, in estrogen receptor-positive breast cancer, high cytoplasmic S1P1 and nuclear S1P2 and S1P3 expression and association of these receptors with signaling proteins such as ERK1/2, Akt or SphK1 was reported to be associated with survival or recurrence of estrogen receptor-positive breast cancer (Ohotski et al., 2013). A new paradigm of S1P signaling in the nucleus is emerging that may alter gene expression, in which, SphK2 specific generation of S1P in the nucleus can bind to HDACs 1 & 2 and inhibit HDAC activity (Hait et al., 2009; Fu et al., 2014). Similar to S1P, FTY720-P also seems to bind to HDACs 1 and 2 modulating HDAC activity (Hait et al., 2015). Thus, SphK2/S1P nuclear signaling may represent a novel mechanism of epigenetic regulation of gene expression and cell function. This new role of nuclear S1P signaling may have a potential role in the regulation of human lung pathologies such as inflammation, pulmonary hypertension, and pulmonary fibrosis.
Given the pleotropic nature of S1P signaling, it is evident that targeting S1P signaling and S1P metabolizing enzymes holds enormous therapeutic potential for the treatment of lung diseases. So a diverse array of small molecule inhibitors, agonists, and antagonists targeting SphKs, S1PL, and S1PRs have been identified, characterized, and tested in mice models of lung diseases to unravel the distinct roles of the individual players in S1P signaling. Development of new and specific inhibitors will help to target specific components of the S1P signaling in treating the lung diseases.
3.1a. Acute Lung Injury/ Acute Respiratory Distress Syndrome
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are sudden failure of the respiratory system due to accumulation of fluid in the alveoli. Some of the common causes of ALI/ARDS include sepsis, pneumonia, trauma, radiation, multiple blood transfusion, and aspiration of stomach fluids. Inflammation and endothelial dysfunction are hallmark features of ARDS (Leaver & Evans, 2007) and S1P/SphK1/S1PL signaling axis may have an important role in the pathophysiology of the syndrome (Brinkmann & Baumruker, 2006; Natarajan et al., 2013; Proia & Hla, 2015).
S1P’s role as a key regulator of endothelial barrier function is attributed to its signaling through S1P1 & S1P3 that activates downstream Rho GTPases and rearrangement of cytoskeleton (Garcia et al., 2001). Intravenous infusion of S1P has shown to reduce LPS-induced lung vascular permeability and inflammation in murine models (McVerry & Garcia, 2004). The barrier enhancing effects of S1P are generally ascribed to ligation to S1P1, which initiates a series of downstream signaling cascades including Rac activation, cortactin translocation, peripheral myosin light chain phosphorylation, and rearrangement of focal adhesion and adherens junction proteins culminating in increased barrier function in lung endothelium cells in vitro (Sun et al., 2009; Abbasi and Garcia, 2013; Takuma et al., 2012; McVerry and Garcia, 2005; Xiong and Hla. 2014). However, elevated concentrations of S1P (>5–10 μM) produces barrier disruption in vitro and in vivo. Also, Intravenous infusion of S1P at 0.5 mg/kg body wt produces pulmonary edema in mice (Sammani et al., 2010) Ligation of S1P to S1P3 leads to activation of Gi/G/11/G12/13 coupled signaling pathways, and robust Rho/Rho kinase mediated EC contractile apparatus resulting in cell migration and vascular barrier dysfunction (Sun et al., 2012; Singleton et al., 2007; Li et al., 2015; Ni et al., 2014). Consistent with these findings, mice with genetic knock down of SphK1 were much more susceptible to LPS-induced vascular leak (Wadgaonkar et al., 2009). Similarly, SphK1−/− mice showed poor recovery from anaphylaxis and delayed histamine clearance, which was improved after S1P injection (Olivera et al., 2010). S1PL expression is enhanced in LPS-induced lung injury mice models, causing decreased S1P levels thereby increasing inflammation and injury; targeting S1PL ameliorated lung injury as it increased intracellular S1P levels and decreased LPS-induced inflammatory cytokines (Zhao et al., 2011). In addition to ALI, SphK1 may play a role in sub-ALI such as radiation-induced lung injury (RILI). Exposure of mice to thoracic radiation (20–25 Gy) for 6 weeks enhanced SphK1 and SphK2 expression and ceramide to S1P ratio in plasma (Mathew et al., 2011). Genetic deletion of SphK1 potentiated susceptibility to RILI, indicating a protective role for SphK1 against RILI (Mathew et al., 2011). Interestingly, pretreatment with myriocin, an inhibitor of SPT, decreased fibrogenesis and inflammation 18 weeks post-radiation exposure, and inhibiting SPT also modulated SphK1 activity and S1P levels in the lung tissue and plasma (Gorshkova et al., 2012). These results support a role for S1P and S1P metabolizing pathways as potential targets against ALI and RILI.
Given the barrier protective role of S1P in ALI, advances have been made in the development and the use of S1PR agonists. Experiments done in HUVECs indicate that at lower doses (0.1–1.0 μM), FTY720 enhance endothelial barrier function; however at higher doses (10 μM –100 μM), it causes barrier disruption and apoptosis (Müller et al., 2011). Similar observations were also recorded in mechanically ventilated mouse model of lung injury (Müller et al., 2011). This complex action of FTY720 in ECs is due to its phosphorylation to FTY720-P by SphK1 or SphK2 that increases its affinity for S1PRs (Billich et al., 2003; Paugh et al., 2003). Though S1P1 specific agonist SEW2871 increased endothelial barrier function in LPS model of lung injury pretreatment with S1P1 antagonist SB-649146 reduced the effect considerably (Sammani et al., 2012), validating the role of S1P1 in preventing vascular leakage. More importantly, studies carried out in murine model(s) of lung injury have highlighted the possibility of developing S1PL as a potential therapeutic option in ameliorating ALI. Mice orally treated with THI (0.05 mg/ml water) for 2 days, post intratracheal LPS instillation showed elevated S1P levels in the lung tissues and BAL fluids but not in the plasma (Zhao et al., 2011). Moreover, THI treatment also blocked neutrophil infiltration to the alveolar space and attenuated IL-6 secretion, thus clearly offering protection against LPS induced lung injury (Zhao et al., 2011). Targeting S1PL using siRNA in human lung microvascular endothelial cells (HLMVECs) challenged with LPS, paralleled with the murine studies show diminished barrier disruption, IL-6 secretion, and LPS-induced p38 MAPK phosphorylation, emphasizing that targeting S1PL could be a viable therapeutic option (Zhao et al., 2011).
3.1b Pseudomonas aeruginosa mediated lung inflammation and injury
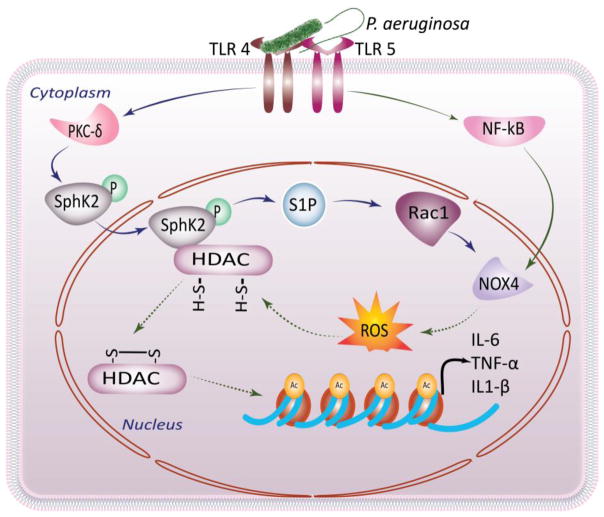
Pulmonary infections remain a significant global health concern, as it is associated with significant morbidity and mortality in neonates and adults. Pseudomonas aeruginosa (PA) is a common, gram negative, environmental, opportunistic pathogen that causes clinical pneumonia in humans. PA mediated pulmonary infections are prevalent in people with CF, COPD, and in the clinical setting of mechanical ventilation. PA infection activates host NADPH oxidase (Nox) proteins (Fu et al., 2013), and acid SM (Managò et al., 2015), leading to the generation of reactive oxygen species (ROS) and ceramides, respectively. Excessive ROS and ceramide production have been implicated in lung inflammation, cell death, and susceptibility to infection in CF (Seitz et al., 2015). However, recent studies also suggest a role for ceramides and sphingosine in mounting lung defense against bacterial pathogens as targeting sphingolipid pathways suppresses the recruitment of neutrophils and other inflammatory immune cells into the lung, thereby reducing lung inflammation (ManagòAntonella et al., 2015). PA infection of mouse lung enhanced global acetylation of histones and genetic deletion of SphK2, but not SphK1, mitigated PA-mediated histone acetylation, and generation of pro-inflammatory cytokines such as IL-6 and TNF-α production both in mouse lungs and mouse lung epithelial cells (Fu et al., 2014). Further, infection of mouse alveolar epithelial cells with heat inactivated PA or flagellin, a principle component of bacterial flagella, induced SphK2 phosphorylation, and its translocation to the nucleus and inhibition of SphK2 with ABC 294760 attenuated histone H3 and H4 acetylation and these data suggest a novel role for SphK2 in regulating HDACs/HATs and expression of pro-inflammatory cytokines in response to PA infection (Fu et al., 2014). The PA mediated H3 and H4 histone acetylation was also attenuated by Nox4 siRNA and SphK2 inhibitor ABC 294760 blocked Nox4 dependent ROS generation (Fu et al., 2014). Thus, PA infection of mouse lungs induces chromatin modification via SphK2/S1P/Nox4 nuclear signaling. The role of nuclear SphK2/S1P signaling mediating chromatin modification and lung inflammation warrants further investigation (Fig. 3).
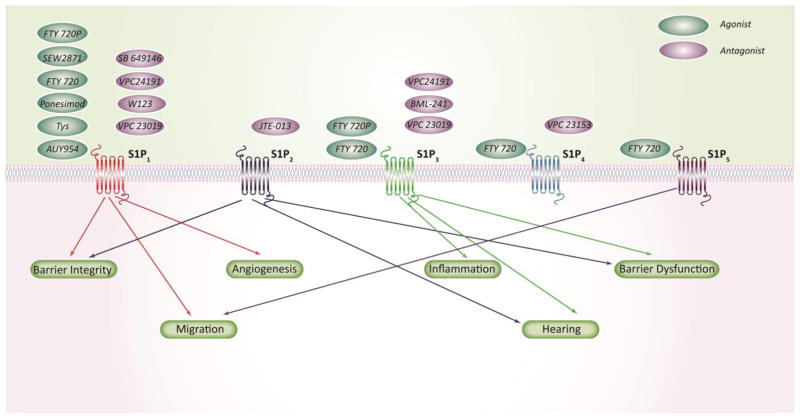
Fig 3. S1P receptors as drug targets.
Extracellular S1P act as a ligand for specific G-protein coupled S1P receptors (S1PR 1–5) and regulate multiple cellular processes such as barrier integrity, migration, angiogenesis and inflammation. S1P receptors expression and activity can be modulated by well characterized S1P receptor agonists or antagonists that offer immense therapeutic potential in the treatment of lung disorders.
3.2 Asthma
Inflamed airways, as a result of exposure to pollutants, allergens, and infection are known to trigger asthmatic symptoms (Nelson et al., 2003); Elias et al., 1999). This causes recurrent wheezing and shortness of breath, accompanied by coughing that could worsen overtime resulting in asthmatic attack. Both in allergic and non-allergic asthma, T-helper cells are recruited to the airways, which secrete cytokines that in turn causes airway inflammation, and promoting leukocytosis (Cohn et al., 2004; Halim et al., 2012). Inflammatory cells such as mast cells, macrophages, and eosinophils that are recruited to the site, exacerbate the inflammation, and work in concert to airway remodeling of the airway. While existing drugs, β2-Adrenergic receptor agonists, leukotriene analogs, and glucocorticoids (Bai, 1992; Chowdhury & Dal Pan, 2010; Hamid et al., 2003; Montuschi & Peters Golden, 2010; Barnes, 2012) can alleviate the clinical manifestations of asthma, there is no permanent cure for this complex airway disorder.
The role of S1P in the development of asthma by regulating inflammatory responses was first established by a study using rat mast cell line that showed Sphk activation and increase in S1P levels in response to FcεRI stimulation (Choi et al., 1996). Regulation of mast cell activation and degranulation has been credited to cytoplasmic S1P-interceded MAPK activation, while sphingosine was shown to have an opposite effect (Prieschl et al., 1999). Moreover, differential roles of SphK1 and SphK2 have been observed in a human mast cell line; SphK1 stimulated TNF-α, IL-6, and inflammatory mediators, and both SphK1 and SphK2 were required for TNF- α secretion (Oskeritzian et al., 2008). Elevated levels of S1P in BAL fluids from allergic asthmatic patients was shown to act on airway smooth cells, prompting airway remodeling in a Rho-Kinase dependent manner (Fuerst et al., 2014). Role of S1P in regulating macrophage function was validated in SphK1 knockdown mouse model of allergic asthma, where reduction in macrophage number and IL-4 and IL-5 secretion was observed (Lai et al., 2008). Evidences additionally point out that eosinophil infiltration into the airway and their adhesion to pulmonary endothelium is mediated by S1P via RhoA/Rho-kinase pathway (Sashio et al., 2012). Elevated levels of S1P in airway smooth muscle cells also increase COX-2 mediated PGE2 production thereby repressing β-2 adrenergic activity (Rumzhum et al., 2016). S1P also induces IL-8 secretion in a dose-dependent manner in BEAS-2B cells, mediated by S1P2, and nuclear factor kB (NF-kB) (O’Sullivan et al., 2014). In rodent asthma models, S1P induced airway hyper responsiveness is mediated by S1P3 as evident from S1P3 agonist based study (Trifilieff & Fozard, 2012). The differential roles of S1P2 and S1P3 in asthma may be due S1P action on lung epithelial S1P2 and pulmonary artery smooth muscle cell S1P3. Given the deleterious effects of SphK1 and SphK2 in aggravating asthmatic conditions, blocking SphK activity and/or S1P2 or S1P3 may be beneficial in ameliorating asthmatic allergic inflammation.
Use of specific SphK1 inhibitor SK1-I in murine allergic asthma models revealed attenuation of inflammation due to the suppression of NF-kB (Price et al., 2013). Oral administration of FTY720-P, a potent agonist of all S1PRs except S1P2, increased airway hyper-reactivity in rodents, whereas AUY954, a highly selective S1P1 agonist did not, suggesting the potential role of S1P3 in S1P induced hyper-reactivity (Trifilieff & Fozard, 2012). However, in repeat allergen exposure models, FTY720 abrogated airway inflammation and hyper -responsiveness by inhibiting Th-2 associated transcription factors (Karmouty-Quintana et al., 2012). In mice subjected to antigen-induced allergic bronchial asthma, pretreatment with SKI-II, a non-selective SphK inhibitor, diminished airway hyper-responsiveness but not inflammation (Chiba et al.,2010b). In antigen-challenged mice, use of W123, JTE-013, and BML-241 – S1PR antagonists selective for S1P1–3 respectively, resulted in down regulation of S1PRs and further substantiated the role of S1P2 involvement in bronchial smooth muscle contractility, a distinctive feature of asthma (Chiba et al., 2010a). Consistent with this study, subcutaneous administration of S1P to Balb/C mice have shown to increase bronchial hyper-responsiveness and lung resistance in a dose-and time-dependent manner (Roviezzo et al., 2010). Further, this study revealed an increase in mast cell number and elevated secretion of IL-4, IL-13, and IL-17 secretion levels, thereby demonstrating the importance of S1P signaling in asthma (Roviezzo et al., 2010). Inhaled delivery of SphK1 inhibitors dimethyl sphingosine and SKI-II also decreased asthmatic symptoms by preventing eosinophil inflammation in OVA administered mice (Nishiuma et al., 2008).
3.3 Bronchopulmonary dysplasia
Bronchopulmonary dysplasia (BPD) is a lung disease of the premature neonates treated for respiratory distress syndrome by mechanical ventilation (Gien & Kinsella, 2011). Babies with BPD have lung tissue scarring and inflammation that leads to interruption of lung development. BPD is characterized by thickened interstitium, alveolar simplification, abnormal pulmonary vasculature, and increased pulmonary resistance and in some cases, pulmonary hypertension (Coalson, 2003). There is no effective treatment for BPD and therapeutic approaches to alleviate symptoms are not efficacious.
A study that characterized sphingolipid profile in newborn mice exposed to hyperoxia showed transient increase in ceramide levels together with alveolar damage and lung function abnormalities. Nonetheless, ceramide levels decreased after treatment with D-sphingosine that improved alveolar histology, suggesting a role for ceramide in BPD (Tibboel et al., 2013). However, recent investigations in the neonatal murine model showed that enhanced S1P generation in mice exposed to hyperoxia is injurious and is associated with BPD (Harijith et al., 2013). Interestingly, in vivo studies using specific knockout mice revealed that SphK1, but not SphK2, appears to have an adversarial role in hyperoxia induced BPD (Harijith et al., 2013). Further, SphK1 deficient mice exposed to hyperoxia revealed significantly less ROS formation and lung injury compared to SphK2 knockout mice under similar conditions of exposure, whereas S1PL heterozygous knockout mice showed increased lung inflammation and injury compared to the wild type neonatal mice exposed to hyperoxia (Harijith et al., 2013). Exogenous addition of S1P to human lung microvascular endothelial cells (HLMVECs) triggered reactive oxygen species (ROS) production whereas knocking down SphK1 using siRNA blocked hyperoxia-induced ROS generation (Harijith et al., 2013). The hyperoxia-induced ROS generation was mediated by Nox2 and involved S1P / SphK1 / Spns2 / S1P1&2 signaling axis in the endothelium.
SKI-II, a small molecule inhibitor of both the isoforms of SphK, attenuated hyperoxia-induced ROS in HLMVECs (Harijith et al., 2013), further confirming that blocking S1P may confer protection in BPD. Mechanism(s) of S1P-mediated activation of ROS generation in BPD is unclear but may involve Nox family members, which require future investigations.
3.4 Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that causes obstructive airflow from the lungs. Cigarette smoke is the leading cause of COPD, followed by irritating gases and pollutants. COPD includes two conditions, emphysema-the damage of the air sacs and chronic bronchitis-the inflammation of air way lining, that obstructs the airflow to lungs causing shortness of breath(Yoshida & Tuder, 2007). One of the noteworthy mechanisms of lung tissue damage in COPD is the disruption of the balance between apoptosis and generation of new structural cells in the lung. The pro-apoptotic effect of ceramide is implicated in alveolar cell apoptosis and oxidative stress in emphysema development, as evident from various in vivo (Petrache et al., 2005) and in vitro (Tepper et al., 1997; Ravid et al., 2003; Sawada et al., 2002) studies carried out in the past decade. Studies aimed to antagonize the effect of ceramides to tilt the balance against apoptosis were effective by increasing intracellular S1P levels, which is anti-apoptotic and pro-proliferative (Cuvillier et al., 1996; Osawa et al., 2005). S1PR profiling in the lungs of COPD patients showed significant down regulation S1P5, which could be a possible target for COPD pharmacotherapy (Cordts et al., 2011). A potential link between S1P5 and S1P metabolizing enzymes and defective alveolar macrophage function in COPD has been reported (Barnawi et al., 2015). A significant increase in mRNA levels of SphK1/2, S1P2, and S1PL in alveolar macrophages from COPD patients compared to control subjects was observed while S1P5 and SGPL1 expression was unaffected by smoking status, suggesting a COPD “disease effect” rather than smoke effect per se (Barnawi et al., 2015). Further, significant associations were noted between S1P5 and both lung function and phagocytosis. Cigarette smoke extract significantly increased mRNA expression of SphK1, SphK2, S1P2, and S1P5 in THP-1 macrophages, confirming the results from patient-derived macrophages, and antagonizing SIP5 significantly improved phagocytosis of the macrophages (Barnawi et al., 2015).
Strategies to mimic S1P signaling are known to be effective in improving COPD. Triggering S1P signaling using FTY720 or S1P receptor agonist SEW2871 increased SphK1 expression and activity, and attenuated lung parenchyma apoptosis in emphysema (Diab et al., 2012). Administration of sphingosine activated pro-survival pathways and increased the S1P to ceramide ratio, thereby restricting alveolar space enlargement. (Ghidoni et al., 2015). Hence, increasing S1P to ceramide ratio and signaling could be a therapeutic option in attenuating lung apoptosis and could offer protection against COPD.
3.5 Cystic Fibrosis
Cystic fibrosis (CF) is a genetic disorder that causes lung damage and affects the cells that secrete mucus, sweat, and digestive juices. Rather than acting as lubricant, the thick and sticky secretions, the products of the defective CFTR gene, clog the airways that interfere with normal function of the lung (Wine, 1999).
The pro-inflammatory cytokine TNF-α-mediated down regulation of CF transmembrane conductance regulator (CFTR) negatively impact S1P signaling in resistance arteries (Meissner et al., 2012); however, a recent investigation suggests a feedback link of S1P, wherein it inhibits CFTR activity through adenosine monophosphate-activated kinase (Malik et al., 2015). Low levels of S1P in BAL fluids detected in CFTR knockout mice partly affect dendritic cell recruitment, which plays a crucial role in chronic infections in CF (Xu et al., 2013).
Altered S1P signaling has been shown to affect dendritic cell function in CF lung. Augmenting cystic fibrotic BALF with S1P recovered the expression of major histocompatibility complex class II molecules (MHCII), CD 40, and CD 86 in both wild type lung and blood dendritic cells (Xu et al., 2013). This finding argues that S1P could restore the innate immune function in CF. It will be interesting to test the effect of FTY720 and its analogs in dendritic cell recruitment, mucus secretion, and lung infections in CFTR knockout mice.
3.6 Pulmonary Hypertension
Pulmonary hypertension is a lung disorder where the blood pressure in the pulmonary arteries rises above normal levels, as a result of constricted arteries that carry blood from the heart to the lungs. This strains the right ventricle of the heart, weakening it to an extent that it loses its ability to pump blood to the lungs (Rubin, 1997).
Influence of S1P and its receptors in vascular remodeling in pulmonary hypertension has been documented through multiple studies. S1P induces vascular inflammation in pulmonary hypertension through S1P1 by activation of p38MAPK and JNK/SAPK pathways mediated by EGFR and PDGFR transactivation (Yogi et al., 2011). S1P2 is identified as the primary cell surface receptor that mediates S1P-induced vasoconstriction of lungs in mice and S1P2 -dependent downstream activation of Rho-Kinase signaling alters pulmonary vascular tone (Szczepaniak et al., 2010). In contrast, S1P4 is implicated in S1P-mediated vasoconstriction in hypertensive rat lungs (Ota et al., 2011). However, in experiments using pulmonary artery smooth muscle cells, SphK1 generated S1P signals through both S1P2 and S1P4, activating downstream Rho kinase causing hypoxic pulmonary vasoconstriction (Tabeling et al., 2015). Recent investigation on the role of S1P signaling in excessive pulmonary vascular remodeling demonstrated that the pro-proliferative effects of SphK1 comes into play to promote pulmonary artery smooth muscle cell (PMSMC) proliferation in hypoxia–dependent pulmonary hypertension mouse model (Chen et al., 2014).. S1P levels were increased in lungs of patients with pulmonary arterial hypertension and pulmonary arteries from rodent models of hypoxia-induced pulmonary hypertension (Chen et al., 2014). Unlike SphK2−/− mice, SphK1−/− mice were protected against hypoxia-induced pulmonary hypertension, whereas Sgpl1 +/− mice were more susceptible (Chen et al., 2014). In hPASMCs, PDGF induces SphK1 expression via Egr-1, a PDGF-associated transcription factor, to promote pulmonary artery SMC proliferation (Sysol etal., 2016). Pharmacologic inhibition of SphK1 prevented the development of hypoxia-induced pulmonary hypertension in rodent models of pulmonary hypertension. Overexpression of SphK1 and stimulation with S1P, potentially via ligation of S1P2, promoted PASMC proliferation in vitro, whereas SphK1 deficiency inhibited PASMC proliferation (Chen et al., 2014).
Use of S1P receptor agonists and antagonists in pulmonary hypertension models uncovered the prominent roles of S1PRs and the downstream signaling pathways. S1P4, initially thought to be restricted in cardiovascular system, was found to mediate S1P-mediated vasoconstriction in normotensive lungs when treated with S1P4 agonists VPC23153 and phytosphingosine-1-phosphate, while dual S1P1 and S1P3 agonist VPC24191 did not elicit such response (Ota et al., 2011). JTE013, a putative inhibitor of S1P2, has been shown to attenuate the S1P induced vasoconstriction in intact mouse lung (Szczepaniak et al., 2010). Further, intraperitoneal treatment of JTE013 prevented hypoxia-mediated pulmonary hypertension in mice, emphasizing a role for S1P2 in pulmonary vascular remodeling (Chen et al., 2014). The collective body of evidence accumulated from the above studies underlines a broader role for S1P signaling in the pathophysiology of pulmonary hypertension and targeting S1P signaling axis could ameliorate hypertension.
3.7 Lung Cancer
Carcinoma of the lung is the uncontrolled growth of lung tissue that is malignant in nature. There are three types of lung cancer: Non-small cell lung cancer (NSCLC), Small cell lung cancer (SCLC) and Lung carcinoid tumor (Cai et al., 2015). Exposure to cigarette smoke is the principal risk factor for lung cancer development (Hecht, 2002). Lung cancer is the leading cause of cancer deaths in US and worldwide (Jemal et al., 2008).
S1P is a potent angiogenic factor (Argraves et al., 2004; Lee et al., 1999b; Liu et al., 2000b), and mitogenic signaling of S1P comes in the forefront of deciding cell survival and proliferation (Augé et al., 1999; Panner Selvam & Ogretmen, 2013). The ceramide/S1P rheostat is a prominent feature of human cancer and perturbed sphingolipid metabolism has been associated with increased risk of lung cancer in a nested-control study. The study also concluded that increased S1P and ceramide levels could be markers for latent lung cancer(Alberg et al., 2013). The reason for increased ceramide levels associated with increased lung cancer risk was attributed to precancerous and cancerous cells producing larger pools of ceramide resulting in the conversion to S1P eventually tilting the balance towards S1P (Alberg et al., 2013). A recent study using non-small cell lung cancer (NSCLC) cells identified intracellular S1P playing a principal role in enhancing cell migration and extracellular S1P in apoptosis (Bradley et al., 2014). These diverse effects of S1P are in part due to the altered expression of Spns2, the S1P transporter and impaired sphingolipid metabolizing enzymes (Bradley et al., 2014). Marked increase of SphK1 expression observed in NSCLC cells activates PI3K/Akt/NF-kB pathways, resulting in tumor progression and poor survival (Fumarola, et al., 2014). In lung adenocarcinoma cells, S1P3 upregulation enhances EGFR expression, increasing proliferation and anchorage independent growth, thereby contributing to tumor progression (Hsu et al., 2012). SphK2 generated S1P is implicated in chemo resistance of A549 lung cancer cell line exposed to hypoxia, where increase in SphK2 protein expression and activity correlated with increased S1P concentration (Schnitzer et al., 2009); however, knockdown of SphK2 using siRNA only resulted in partial protection. The role of S1PRs in tumor growth is not clearly established; S1P1 in ECs promotes angiogenesis and tumor growth, whereas S1P2 in ECs and bone marrow derived cells (BMDC) reveal an opposing effect (Du et al., 2010). Very recently, SphK2 generated S1P was found in association with the catalytic subunit of telomerase in normal fibroblasts and lung cancer cells, promoting cell proliferation and tumor growth (Panneer Selvam et al., 2015).
Targeting S1PRs suggests SphK2 generated S1P is responsible for offering chemo resistance in hypoxic A549 cells (Schnitzer et al., 2009). When A549 cells were incubated with S1P1/S1P3 antagonists, VPC 23019, and JTE013 (specific for S1P2), only VPC 23019 exhibited a protective effect by blocking ERK1/2 signaling (Schnitzer et al., 2009). A combination therapy using SphK2 specific inhibitor ABC294640, and human recombinant TNF-α related apoptosis-induced ligand (TRAIL) heightened the activity of caspase 3/8 and increased the expression levels of death receptors in NSCLC (Yang et al., 2015). A recent study that distinguished the role of S1P in telomerase stabilization in lung cancer cell lines used ABC294640 to delineate the role of SphK2 in hTERT stability in A549 cells. Inhibiting SphK2 with ABC294640 increased the ubiquitination-mediated degradation of hTERT, indicating that SphK2-generated S1P is responsible for the stability of hTERT (Panneer Selvam et al., 2015). Thus, blocking SphK1 and/or SphK2 activity may have beneficial effect in treating lung cancers.
3.8 Mesothelioma
Mesothelioma is a rare yet aggressive cancer that affects the membrane lining the lungs, with asbestos exposure being the principal risk factor (Abakay et al., 2015). Recent evidence points to the pro-apoptotic role of sphingosine in mesothelioma cells, promoting cell cycle arrest at G0/G1 phase by inhibiting PKC δ (Okuwa et al., 2012). The pro-proliferative effects of SphK1, as observed in malignant pleural mesothelioma (MPM) tumor tissues and MPM cell lines, is ascribed to its ability to alter the chromatin landscape by modulating the expression levels of histone acetyl transferases (HATs) and cell cycle checkpoint genes (Kalari et al., 2012). Studies using SKI-II (10μM) to inhibit SphKs in mesothelioma cell lines revealed attenuation of Histone H3 and Histone H4 acetylation and phosphorylation of H3 at serine 10 (Kalari et al., 2012). Also, inhibiting SphKs blocked the phosphorylation of CDK-2 that is vital for G1/S transition, obstructing mesothelioma cell division. Further investigation revealed that in response to SKI-II, there is an increase transcription of CDK-2 inhibitors, P21Cip1, and P27Kip1. Supplementary experiments targeting SphK1/SphK2 using siRNA established SphK1 and not SphK2 as the principal player in the development of malignant mesothelioma (Kalari et al., 2012). These studies suggest that SphK1 could be a therapeutic target for the treatment of mesothelioma; however, the role of SphK2 in malignant mesothelioma cannot be ruled out and warrants further investigation.
3.9 Pulmonary fibrosis
Pulmonary fibrosis is a condition that causes scarring of lung tissues, marked by alveolar epithelial injury, and accumulation of myofibroblasts (Wynn & Ramalingam, 2012); (Selman & Pardo, 2012). The stiffened lung tissue makes breathing difficult and reduces oxygen supply in the blood. A number of factors including chronic inflammation, infections, environmental pollutants, radiation exposure, and autoimmune disease contribute towards pulmonary fibrosis, but if an identifiable cause of the disease cannot be pinpointed then it is termed idiopathic pulmonary fibrosis (IPF) (Bourke, 2006). Lung damage caused by pulmonary fibrosis is permanent and in certain cases a lung transplant, the only viable treatment, is recommended (Mason et al., 2007); (George, Arnaoutakis, & Shah, 2011). Recently, FDA has approved two drugs for the treatment of IPF but it is too early to determine the efficacy of these drugs in ameliorating the disease (Rangarajan et al., 2016; Bonella et al., 2015).
S1P and S1P signaling may contribute to the development and progression of IPF and pulmonary fibrosis in animal models. Increased expression of SphK1/SphK2 correlated negatively with lung function and survival, as analyzed by microarray in peripheral blood mononuclear cells of IPF patients (Huang et al., 2013). Consistent with this observation, SIP levels were found to be elevated in BAL fluids of IPF patients compared to control groups that correlated with poor lung prognosis in IPF patients (Milara et al., 2012). Elevated S1P levels in a murine model of bleomycin-induced pulmonary fibrosis resulted from enhanced SphK1, but not Sphk2 expression (Milara et al., 2012), which exacerbated lung fibrogenesis by increasing expression levels of TGF-β, fibronectin, and lung collagen. Genetic knockdown of SphK1, but not SphK2, offered protection against bleomycin-induced pulmonary fibrosis in mice (Huang et al., 2013). SphK1 levels are found to be elevated in human and mouse lung fibroblast following TGF- β stimulation, causing myofibroblast differentiation by activating S1P2 & 3 (Huang et al., 2013). Similarly, in IPF patients, increased SphK1 levels contributed to epithelial to mesenchymal transition through S1P2 & 3 in alveolar type II cells (Milara et al., 2012). In radiation-induced fibrosis murine model, impairing de novo synthesis of SphK1 delayed the onset of fibrosis and reduced pulmonary inflammation (Gorshkova et al., 2012). Diverse roles of S1P formed after TGF- β stimulation has also been reported in lung fibroblasts, with S1P inhibiting α-smooth muscle actin through S1P1 and stimulating via S1P3 (Sobel et al., 2013). Targeting SphK1 with specific inhibitors decreased pulmonary fibrosis and increased survival in bleomycin-induced lung fibrosis mice models (Huang et al., 2013). Buildup of extracellular matrix proteins (ECM), a characteristic feature of fibrosis, has also been attributed to S1P signaling via S1P2 & 3 agonist-based studies in human lung fibroblasts (Sobel et al., 2013). Consistent with the previous study, S1P3 knockout mice showed decreased inflammation and fibrosis in bleomycin-induced murine model of fibrosis (Murakami et al., 2014). Furthermore, manipulating the intracellular levels of SphK1 by over expressing S1PL attenuated lung fibrosis post bleomycin challenge, suggesting a critical role of S1PL as a natural suppressor of lung fibrosis (Huang & Natarajan, 2015; Huang et al., 2015). These studies suggest a major role for S1P signaling in the development of lung fibrosis, where S1P-S1P1 signaling appears to be protective. Conversely, S1P-S1P2 & 3 signaling seems to promote fibrosis and could be potential therapeutic targets.
Extensive studies carried out using bleomycin and radiation–induced pulmonary fibrosis murine models underscore the therapeutic potential of S1P signaling in IPF. Mice administered with SphK inhibitor SKI-II on day 8-post-bleomycin challenge decreased S1P levels in lung tissue and increased survival (Huang et al., 2013). Use of TGF-β and S1PR agonists S1P, FTY720, Ponesimod, and SEW2871 to investigate the molecular pathway revealed divergent pathways involved in ECM synthesis in normal human lung fibroblasts (Sobel et al., 2013). While TGF- β signals through Smad pathway, S1PR agonists trigger P13K/Akt and ERK 1/2 –dependent pathways to induce ECM (Sobel et al., 2013). Besides, non-specific S1P receptor agonists S1P and FTY720 induced potent ECM synthesis, while highly selective S1P agonists Ponesimod, and SEW 2871 did not, underlining the pro fibrotic role of S1P2/S1P3 (Sobel et al., 2013). However, in a separate bleomycin model study, prolonged exposure to FTY720 and S1P1 specific agonist AUY954 resulted in fibrosis, suggesting S1P-S1P1 pathway could also be a therapeutic target (Shea et al., 2012).
4. Conclusion and Future Directions
Novel therapies are needed for the treatment of lung diseases, as most of them are fatal with no effective cure in sight. S1P, a potent bioactive lipid mediator, regulates plethora of signaling pathways, which have been implicated in the development of a number of lung pathologies. S1P signals intracellularly or extracellularly via G-Protein-coupled receptors and there has been considerable interest and progress made in developing novel and specific antagonist for the S1PRs. Additionally, the recent observations that S1P generated in the nucleus by nuclear SphK2 functions as a epigenetic co-regulator, has opened a new area of nuclear S1P signaling in chromatin modification and epigenetic role of sphingolipids in inflammation and lung injury. Current evidence emerging from studies using small molecule inhibitors in rodent models of lung diseases suggests that SphKs/S1P/S1PR signaling axis could be potential target(s) to ameliorate respiratory ailments. However, decreasing S1P levels in circulation or lung tissue might have some adverse effect in vascular system as it is a potent angiogenic factor. Therefore, localized delivery of SphK or S1PR inhibitors might mitigate any adverse vascular effects of systemic route of altering S1P levels. While SphK1 and to a limited extend SphK2 and S1PRs have been extensively investigated as potential therapeutic targets in a number of lung disorders, targeting S1PL, the enzyme that catalyzes the terminal degradation of S1P in the sphingolipid metabolic pathway, has received less attention. Given its role in regulating intracellular levels of S1P, both blocking and activating S1PL holds immense potential as a therapeutic target in treating lung diseases. Recently, a few specific S1PL inhibitors have been synthesized and their efficacy validated in autoimmune disease models; however, their therapeutic potential in combating lung diseases will require future studies in pre-clinical animal models of lung inflammation. Similarly, blocking ASM with small molecule inhibitor(s) seems to offer some protection against lung inflammation, an area that has potential for drug development. Last but not the least, targeting ceramide synthases with small molecule inhibitors may have beneficial outcome in COPD/emphysema and development of novel inhibitors specific for the six isoforms of the ceramide synthase is essential for in vivo studies in rodent models. Despite lack of Phase I or Phase II clinical trials with many of the sphingolipid inhibitors, there is tremendous potential to develop and test new and novel inhibitors of sphingolipid metabolizing enzymes and S1PRs to ameliorate respiratory disorders.
Fig 4. Nuclear S1P signaling mediating chromatin modification in Pseudomonas aeruginosa-induced Lung inflammation and injury.
Nuclear S1P generated by activated Sphk2 in response to P. aeruginosa infection activates Nox 4 and generates nuclear ROS. SphK2/S1P signaling also induce histone acetylation and block HDAC activity in P. aeruginosa-induced lung inflammation.
Acknowledgments
The authors thank Dr. Prasad Kanteti for valuable suggestions, and editing the manuscript. The technical support of Ms. Alison W. Ha is greatly appreciated. This work was supported by NIH grant P01 HL98050 to VN.
Abbreviations
- 4-DP
4′-deoxypridoxine
- ABC transporter
ATP-binding cassette transporter
- Akt
Protein kinase B
- ALF4
Tetrafluoroaluminate
- ALI
Acute Lung Injury
- ARDS
Acute Respiratory Distress Syndrome
- ASM
Acid Sphingomyelinase
- BACE1
beta-site APP-cleaving enzyme
- BAL
Bronchoalveolar Lavage
- BMDC
Bone marrow derived cells
- BPD
Broncho Pulmonary Dysplasia
- CDK-2
Cyclin Dependent Kinase
- CF
Cystic Fibrosis
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- CoA
Co Enzyme A
- COPD
Chronic Obstructive Pulmonary Disease
- COX-2
Cyclooxygenase-2
- EC
Endothelial Cell
- ECM
Extracellular Matrix
- EGFR
Epidermal Growth Factor Receptor
- ERK
Extracellular Signal-Regulated Kinase
- FcεRI
High-Affinity Receptor for IgG
- HAT
Histone Acetyltransferase
- HDAC
Histone Deacetylase
- HLMVEC
Human Lung Microvascular Endothelial Cells
- hTERT
Human Telomerase Reverse Transcriptase
- HUVEC
Human Umbilical Vein Endothelial Cell
- IL-1β
Interleukin-1-beta
- IL-4
Interleukin-4
- IL-5
Interleukin-5
- IL-6
Interleukin-6
- IPF
Idiopathic Pulmonary Fibrosis
- JNK
c-Jun N-terminal kinase
- LPA
Lysophosphatidic Acid
- LPP
Lipid Phosphate Phosphatase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-Activated Protein Kinase
- MPM
Malignant Pleural Mesothelioma
- mRNA
Messenger RNA
- NEM
N-ethylmaleimide
- NES
Nuclear Export Sequences
- NOX
NADPH-oxidase
- NSCLC
Non-Small Cell Lung Cancer
- P21Cip1
Cyclin-Dependent Kinase Inhibitor 1A
- P27Kip1
Cyclin-Dependent Kinase Inhibitor 1B
- PASMC
Pulmonary Artery Smooth Muscle Cell
- PDGFR
Platelet-Derived Growth Factor Receptor
- PGE2
Prostaglandin E2
- PI3K
Phosphoinositide-3-Kinase
- PKCδ
Protein Kinase C-delta
- ROS
Reactive Oxygen Species
- S1P
Sphingosine-1-Phosphate
- S1PL
Sphingosine-1-Phosphate Lyase
- S1PR
Sphingosine-1-phosphate receptor
- SAPK
Stress-Activated Protein Kinase
- SCLC
Small Cell Lung Cancer
- siRNA
Short Interfering RNA
- SM
Sphingomyelin
- SphK1
Sphingosine Kinase 1
- SphK2
Sphingosine Kinase 2
- SPT
Serine Palmitoyltransferase
- Spns2
Spinster Homolog 2
- SPP
Sphingosine-1-phosphate Phosphatase
- TGF- β
Transforming Growth Factor- beta
- Th-2
T helper 2, THI, 2-acetyl-4-tetrahydroxybutylimidazole
- TNF-α
Tumor Necrosis factor- alpha
- TRAF-2
TNF Receptor-Associated Factor 2
Footnotes
Disclosures
The authors have no conflict of interest, and nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abakay A, Tanrikulu AC, Ayhan M, Imamoglu MS, Taylan M, Kaplan MA, Abakay O. High-risk mesothelioma relation to meteorological and geological condition and distance from naturally occurring asbestos. Environmental Health and Preventive Medicine. 2015:1–9. doi: 10.1007/s12199-015-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi T, Garcia JG. Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handbook of Experimental Pharmacology. 2013;(216):201–26. doi: 10.1007/978-3-7091-1511-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberg AJ, Armeson K, Pierce JS, Bielawski J, Bielawska A, Visvanathan K, et al. Plasma sphingolipids and lung cancer: a population-based, nested case-control study. Cancer Epidemiology Biomarkers & Prevention. 2013;22(8):1374–1382. doi: 10.1158/1055-9965.EPI-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R, van Koppen CJ, Danneberg K, Braak Ter M, Meyer zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2007;374(5–6):413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, et al. Sphingosine-1-phosphate Lyase Deficiency Produces a Pro-inflammatory Response While Impairing Neutrophil Trafficking. The Journal of Biological Chemistry. 2011;286(9):7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves KM, Wilkerson BA, Argraves WS, Fleming PA, Obeid LM, Drake CJ. Sphingosine-1-phosphate Signaling Promotes Critical Migratory Events in Vasculogenesis. The Journal of Biological Chemistry. 2004;279(48):50580–50590. doi: 10.1074/jbc.M404432200. [DOI] [PubMed] [Google Scholar]
- Augé N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Nègre-Salvayre A, Salvayre R, et al. Role of Sphingosine 1-Phosphate in the Mitogenesis Induced by Oxidized Low Density Lipoprotein in Smooth Muscle Cells via Activation of Sphingomyelinase, Ceramidase, and Sphingosine Kinase. The Journal of Biological Chemistry. 1999;274(31):21533–21538. doi: 10.1074/jbc.274.31.21533. [DOI] [PubMed] [Google Scholar]
- Bai TR. Beta2 adrenergic receptors in asthma: A current perspective. Lung. 1992;170(3):125–141. doi: 10.1007/BF00174316. [DOI] [PubMed] [Google Scholar]
- Bandhuvula P, Fyrst H, Saba JD. A rapid fluorescence assay for sphingosine-1-phosphate lyase enzyme activity. The Journal of Lipid Research. 2007;48(12):2769–2778. doi: 10.1194/jlr.D700010-JLR200. [DOI] [PubMed] [Google Scholar]
- Banno Y, Fujita H, Ono Y, Nakashima S, Ito Y, Kuzumaki N, Nozawa Y. Differential Phospholipase D Activation by Bradykinin and Sphingosine 1-Phosphate in NIH 3T3 Fibroblasts Overexpressing Gelsolin. The Journal of Biological Chemistry. 1999;274(39):27385–27391. doi: 10.1074/jbc.274.39.27385. [DOI] [PubMed] [Google Scholar]
- Barnawi J, Tran H, Jersmann H, Pitson S, Roscioli E, Hodge G, et al. Potential Link between the Sphingosine-1-Phosphate (S1P) System and Defective Alveolar Macrophage Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD) PLoS ONE. 2015;10(10):e0122771. doi: 10.1371/journal.pone.0122771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nature Reviews Immunology. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mechanisms of Action of Glucocorticoids in Asthma. American Journal of Respiratory and Critical Care Medicine. 1996;154(2):521–527. doi: 10.1164/ajrccm/154.2_Pt_2.S21. [DOI] [PubMed] [Google Scholar]
- Baudhuin LM, Jiang Y, Zaslavsky A, Ishii I, Chun J, Xu Y. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR) FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(2):341–343. doi: 10.1096/fj.03-0302fje. [DOI] [PubMed] [Google Scholar]
- Becker KA, Riethmüller J, Lüth A, Döring G, Kleuser B, Gulbins E. Acid Sphingomyelinase Inhibitors Normalize Pulmonary Ceramide and Inflammation in Cystic Fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2010;42(6):716–724. doi: 10.1165/rcmb.2009-0174OC. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, et al. Intracellular S1P Generation Is Essential for S1P-Induced Motility of Human Lung Endothelial Cells: Role of Sphingsoine Kinase 1 and S1P Lyase. PloS ONE. 2011;6(1):e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Baumruker T, Beerli C, Bigaud M, Bruns C, Calzascia T, et al. Partial Deficiency of Sphingosine-1-Phosphate Lyase Confers Protection in Experimental Autoimmune Encephalomyelitis. PLoS ONE. 2013;8(3):e59630. doi: 10.1371/journal.pone.0059630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the Immunomodulatory Drug FTY720 by Sphingosine Kinases. The Journal of Biological Chemistry. 2003;278(48):47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196(2):497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonella F, Stowasser S, Wollin L. Idiopathic pulmonary fibrosis: current treatment options and critical appraisal of nintedanib. Drug Design, Development and Therapy. 2015;9:6407–6419. doi: 10.2147/DDDT.S76648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke SJ. Interstitial lung disease: progress and problems. Postgraduate Medical Journal. 2006;82(970):494–499. doi: 10.1136/pgmj.2006.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Dasgupta S, Jiang X, Zhao X, Zhu G, He Q, et al. Critical Role of Spns2, a Sphingosine-1-Phosphate Transporter, in Lung Cancer Cell Survival and Migration. PLoS ONE. 2014;9(10):e110119. doi: 10.1371/journal.pone.0110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proceedings of the National Academy of Sciences of the United States of America. 1966;55(2):366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, BAUMRUKER T. Pulmonary and vascular pharmacology of sphingosine 1-phosphate. Current Opinion in Pharmacology. 2006;6(3):244–250. doi: 10.1016/j.coph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Reviews Drug Discovery. 2010;9(11):883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. FTY720: Sphingosine 1-Phosphate Receptor-1 in the Control of Lymphocyte Egress and Endothelial Barrier Function. American Journal of Transplantation. 2004;4(7):1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Brodlie M, McKean MC, Johnson GE, Gray J, Fisher AJ, Corris PA, et al. Ceramide Is Increased in the Lower Airway Epithelium of People with Advanced Cystic Fibrosis Lung Disease. American Journal of Respiratory and Critical Care Medicine. 2012;182(3):369–375. doi: 10.1164/rccm.200905-0799OC. [DOI] [PubMed] [Google Scholar]
- Burnett C, Howard K. Fly and mammalian lipid phosphate phosphatase isoforms differ in activity both in vitro and in vivo. EMBO Reports. 2003;4(8):793–799. doi: 10.1038/sj.embor.embor900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Xu D, Zhang Q, Zhang J, Ngai SM, Shao J. Classification of lung cancer using ensemble-based feature selection and machine learning methods. Molecular BioSystems. 2015;11(3):791–800. doi: 10.1039/c4mb00659c. [DOI] [PubMed] [Google Scholar]
- Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, et al. Pulmonary Endothelial Cell Barrier Enhancement by Novel FTY720 Analogs: Methoxy-FTY720, Fluoro-FTY720, and β-Glucuronide-FTY720. Chemistry and Physics of Lipids. 2016;194:85–93. doi: 10.1016/j.chemphyslip.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. Journal of Cell Science. 2005;118(20):4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- Chapman HA. Disorders of lung matrix remodeling. Journal of Clinical Investigation. 2004;113(2):148–157. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee I, Humtsoe JO, Kohler EE, Sorio C, Wary KK. Lipid phosphate phosphatase-3 regulates tumor growth via β-catenin and CYCLIN-D1 signaling. Molecular Cancer. 2011;10(1):51. doi: 10.1186/1476-4598-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2014;190(9):1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Suzuki K, Uechi M, Kurihara E, Goto K, Sakai H, Misawa M. Downregulation of sphingosine-1-phosphate receptors in bronchial smooth muscle of mouse experimental asthma. Pharmacological Research. 2010a;62(4):357–363. doi: 10.1016/j.phrs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Takeuchi H, Sakai H, Misawa M. SKI-II, an inhibitor of sphingosine kinase, ameliorates antigen-induced bronchial smooth muscle hyperresponsiveness, but not airway inflammation, in mice. Journal of Pharmacological Sciences. 2010b;114(3):304–310. doi: 10.1254/jphs.10202fp. [DOI] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380(6575):634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Chowdhury BA, Dal Pan G. The FDA and Safe Use of Long-Acting Beta-Agonists in the Treatment of Asthma. New England Journal of Medicine. 2010;362(13):1169–1171. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid Receptor Nomenclature. Pharmacological Reviews. 2002;54(2):265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Coalson JJ. Pathology of new bronchopulmonary dysplasia. Seminars in Neonatology. 2003;8(1):73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Cohn L, Elias JA, Chupp GL. Asthma: Mechanisms of Disease Persistence and Progression. Annual Review of Immunology. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- Cordts F, Pitson S, Tabeling C, Gibbins I, Moffat DF, Jersmann H, et al. Expression profile of the sphingosine kinase signalling system in the lung of patients with chronic obstructive pulmonary disease. Life Sciences. 2011;89(21–22):806–811. doi: 10.1016/j.lfs.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Obeid LM, Hannun YA. Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. The Journal of Biological Chemistry. 1993;268(24):17762–17766. [PubMed] [Google Scholar]
- Dhami R, He X, Schuchman EH. Acid Sphingomyelinase Deficiency Attenuates Bleomycin-Induced Lung Inflammation and Fibrosis in Mice. Cellular Physiology and Biochemistry. 2010;26(4–5):749–760. doi: 10.1159/000322342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of Sphingosine 1-Phosphate Signaling as an Alveolar Cell Survival Strategy in Emphysema. American Journal of Respiratory and Critical Care Medicine. 2012;181(4):344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383(6600):547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, et al. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Research. 2010;70(2):772–781. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- Dudek S, Camp S, Chiang E, Singleton P, Usatyuk P, Zhao Y, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cellular Signalling. 2007;19(8):1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. Journal of Clinical Investigation. 1999;104(8):1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Ebenezer D, Huang L, Usatuk P, Harijith A, Berdyshev E, et al. Epigenetic Regulation of P. Aeruginosa Mediated Acute Lung Injury by Sphingosine Kinase 2. American Journal of Respiratory Critical Care Medicine. 2014:A1061. [Google Scholar]
- Fu P, Mohan V, Mansoor S, Tiruppathi C, Sadikot RT, Natarajan V. Role of Nicotinamide Adenine Dinucleotide Phosphate–Reduced Oxidase Proteins in Pseudomonas aeruginosa–Induced Lung Inflammation and Permeability. American Journal of Respiratory Cell and Molecular Biology. 2013;48(4):477–488. doi: 10.1165/rcmb.2012-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst E, Foster HR, Ward JPT, Corrigan CJ, Cousins DJ, Woszczek G. Sphingosine-1-phosphate induces pro-remodelling response in airway smooth muscle cells. Allergy. 2014;69(11):1531–1539. doi: 10.1111/all.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. Journal of Clinical Investigation. 2012;122(4):1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochemical Pharmacology. 2014;90(3):197–207. doi: 10.1016/j.bcp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends in Cell Biology. 2005;15(6):312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. Journal of Clinical Investigation. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TJ, Arnaoutakis GJ, Shah AS. Lung Transplant in Idiopathic Pulmonary Fibrosis. Archives of Surgery. 2011;146(10):1204–1209. doi: 10.1001/archsurg.2011.239. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Caretti A, Signorelli P. Role of Sphingolipids in the Pathobiology of Lung Inflammation. Mediators of Inflammation. 2015;2015:487508. doi: 10.1155/2015/487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Current Opinion in Pediatrics. 2011;23(3):305–313. doi: 10.1097/MOP.0b013e328346577f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. The Journal of Lipid Research. 2012;53(8):1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(3):551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Guo C, Zheng C, Martin-Padura I, Bian ZC, Guan JL. Differential stimulation of proline-rich tyrosine kinase 2 and mitogen-activated protein kinase by sphingosine 1-phosphate. European Journal of Biochemistry / FEBS. 1998;257(2):403–408. doi: 10.1046/j.1432-1327.1998.2570403.x. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science Signaling. 2009;325(5945):1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ER|[alpha]| expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4(6):e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochimica Et Biophysica Acta-Biomembranes. 2006;1758(12):2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Halim TYF, Krauß RH, Sun AC, Takei F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity. 2012;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Tulic MK, Liu MC, Moqbel R. Inflammatory cells in asthma: Mechanisms and implications for therapy. Journal of Allergy and Clinical Immunology. 2003;111(1):S5–S17. doi: 10.1067/mai.2003.22. [DOI] [PubMed] [Google Scholar]
- Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, et al. Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. The American Journal of Pathology. 2013;183(4):1169–1182. doi: 10.1016/j.ajpath.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. The Lancet Oncology. 2002;3(8):461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7(6):e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T. Sphingosine 1-phosphate receptors. Prostaglandins & Other Lipid Mediators. 2001;64(1–4):135–142. doi: 10.1016/s0090-6980(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Hsu A, Zhang W, Lee JF, An J, Ekambaram P, Liu J, et al. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. International Journal of Oncology. 2012;40(5):1619–1626. doi: 10.3892/ijo.2012.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HD, Lee TY, Tzeng SW, Horng JT. KinasePhos: a web tool for identifying protein kinase-specific phosphorylation sites. Nucleic Acids Research. 2005;33(Web Server issue):W226–229. doi: 10.1093/nar/gki471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Natarajan V. Sphingolipids in pulmonary fibrosis. Advances in Biological Regulation. 2015;57:55–63. doi: 10.1016/j.jbior.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Berdyshev E, Tran JT, Xie L, Chen J, Ebenezer DL, et al. Sphingosine-1-phosphate lyase is an endogenous suppressor of pulmonary fibrosis: role of S1P signalling and autophagy. Thorax. 2015;70(12):1138–1148. doi: 10.1136/thoraxjnl-2014-206684. [DOI] [PubMed] [Google Scholar]
- Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2013;27(4):1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura SI. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of Biological Chemistry. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochemical and Biophysical Research Communications. 2004;325(1):338–343. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Li PY, Wada A, Mitsutake S, Igarashi Y. Identification of functional nuclear export sequences in human sphingosine kinase 1. Biochemical and Biophysical Research Communications. 2003;311(1):168–173. doi: 10.1016/j.bbrc.2003.09.194. [DOI] [PubMed] [Google Scholar]
- Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochemical and Biophysical Research Communications. 2007;357(1):212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- Jasinska R, Zhang QX, Pilquil C, Singh I, Xu J, Dewald J, et al. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochemical Journal. 1999;340(Pt 3):677–686. [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA: a Cancer Journal for Clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. The Journal of Biological Chemistry. 2003;278(36):34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- Kalari S, Moolky N, Pendyala S, Berdyshev EV, Rolle C, Kanteti R, et al. Sphingosine Kinase 1 Is Required for Mesothelioma Cell Proliferation: Role of Histone Acetylation. PLoS ONE. 2012;7(9):e45330. doi: 10.1371/journal.pone.0045330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Siddiqui S, Hassan M, Tsuchiya K, Risse PA, Xicota-Vila L, et al. Treatment with a sphingosine-1-phosphate analog inhibits airway remodeling following repeated allergen exposure. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2012;302(8):L736–L745. doi: 10.1152/ajplung.00050.2011. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323(5913):524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochimica Et Biophysica Acta. 2009;1791(7):692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochemical Journal. 2000;348(Pt 1):71–76. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Naoki, Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. The Journal of Biological Chemistry. 2009;284(32):21192–21200. doi: 10.1074/jbc.M109.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. The Journal of Biological Chemistry. 1998;273(37):23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Kume H, Takeda N, Oguma T, Ito S, Kondo M, Ito Y, Shimokata K. Sphingosine 1-phosphate causes airway hyper-reactivity by rho-mediated myosin phosphatase inactivation. Journal of Pharmacology and Experimental Therapeutics. 2007;320(2):766–773. doi: 10.1124/jpet.106.110718. [DOI] [PubMed] [Google Scholar]
- Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation, and beyond. Nature Reviews. Drug Discovery. 2013;12(9):688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-Phosphate Protects Human Umbilical Vein Endothelial Cells from Serum-deprived Apoptosis by Nitric Oxide Production. The Journal of Biological Chemistry. 2001;276(14):10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- Lai WQ, Goh HH, Bao Z, Wong WSF, Melendez AJ, Leung BP. The Role of Sphingosine Kinase in a Murine Model of Allergic Asthma. Journal of Immunology (Baltimore, Md: 1950) 2008;180(6):4323–4329. doi: 10.4049/jimmunol.180.6.4323. [DOI] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, et al. Regulation of Autophagy by Sphingosine Kinase 1 and Its Role in Cell Survival during Nutrient Starvation. The Journal of Biological Chemistry. 2006;281(13):8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD. Sphingosine 1-Phosphate Affects Cytokine-Induced Apoptosis in Rat Pancreatic Islet β-Cells. Endocrinology. 2013;147(10):4705–4712. doi: 10.1210/en.2006-0456. [DOI] [PubMed] [Google Scholar]
- Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. The Journal of Cell Biology. 2002;158(6):1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ (Clinical Research Ed) 2007;335(7616):389–394. doi: 10.1136/bmj.39293.624699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual Roles of Tight Junction-associated Protein, Zonula Occludens-1, in Sphingosine 1-Phosphate-mediated Endothelial Chemotaxis and Barrier Integrity. The Journal of Biological Chemistry. 2006;281(39):29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular Endothelial Cell Adherens Junction Assembly and Morphogenesis Induced by Sphingosine-1-Phosphate. Cell. 1999a;99(3):301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al. Akt-Mediated Phosphorylation of the G Protein-Coupled Receptor EDG-1 Is Required for Endothelial Cell Chemotaxis. Molecular Cell. 2001;8(3):693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, et al. Sphingosine 1-Phosphate Induces Angiogenesis: Its Angiogenic Action and Signaling Mechanism in Human Umbilical Vein Endothelial Cells. Biochemical and Biophysical Research Communications. 1999b;264(3):743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- Leeman M. The pulmonary circulation in lung diseases. Baillieres Clinical Anaesthesiology. 1996;10(1):215–230. [Google Scholar]
- Lépine S, Allegood JC, Edmonds Y, Milstien S, Spiegel S. Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. Journal of Biological Chemistry. 2011a;286(52):44380–44390. doi: 10.1074/jbc.M111.257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépine S, Allegood JC, Park M, Dent P, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death & Differentiation. 2011b;18(2):350–361. doi: 10.1038/cdd.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen B, Zeng C, Fan A, Yuan Y, Guo X, Huang X, Huang Q. Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Experimental Physiology. 2015;100(1):95–107. doi: 10.1113/expphysiol.2014.082149. [DOI] [PubMed] [Google Scholar]
- Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular Cloning and Functional Characterization of a Novel Mammalian Sphingosine Kinase Type 2 Isoform. Journal of Biological Chemistry. 2000a;275(26):19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. The Journal of Biological Chemistry. 2003;278(41):40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. Journal of Clinical Investigation. 2000b;106(8):951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung Infections Associated with Cystic Fibrosis. Clinical Microbiology Reviews. 2002;15(2):194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends in Cell Biology. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik FA, Meissner A, Semenkov I, Molinski S, Pasyk S, Ahmadi S, et al. Sphingosine-1-Phosphate Is a Novel Regulator of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Activity. PLoS ONE. 2015;10(6):e0130313. doi: 10.1371/journal.pone.0130313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managò A, Becker KA, Carpinteiro A, Wilker B, Soddemann M, Seitz AP, et al. Pseudomonas aeruginosa Pyocyanin Induces Neutrophil Death via Mitochondrial Reactive Oxygen Species and Mitochondrial Acid Sphingomyelinase. Antioxidants & Redox Signaling. 2015;22(13):1097–1110. doi: 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, et al. Lung Transplantation for Idiopathic Pulmonary Fibrosis. The Annals of Thoracic Surgery. 2007;84(4):1121–1128. doi: 10.1016/j.athoracsur.2007.04.096. [DOI] [PubMed] [Google Scholar]
- Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, et al. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2011;25(10):3388–3400. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JGN. Endothelial cell barrier regulation by sphingosine 1-phosphate. Journal of Cellular Biochemistry. 2004;92(6):1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cellular Signaling. 2005;17(2):131–9. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakova D, Wlachos A, Sobanov J, Kopp T, Reuschel R, Bornancin F, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cellular Signalling. 2007;19(4):748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-Phosphate-induced Mobilization of Intracellular Ca2+ Mediates Rac Activation and Adherens Junction Assembly in Endothelial Cells. The Journal of Biological Chemistry. 2005;280(17):17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- Meissner A, Yang J, Kroetsch JT, Sauvé M, Dax H, Momen A, et al. TNFα-Mediated Down-Regulation of CFTR Drives Pathological S1P Signaling in a Mouse Model of Heart Failure. Circulation. 2012;125(22):2739–2750. doi: 10.1161/CIRCULATIONAHA.111.047316. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251(1):19–26. doi: 10.1016/s0378-1119(00)00205-5. [DOI] [PubMed] [Google Scholar]
- Merrill AH., Jr De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway. The Journal of Biological Chemistry. 2002;277(29):25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- Milara J, Navarro R, Juan G, Peiró T, Serrano A, Ramón M, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67(2):147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsutake S, Kim T-J, Igarashi Y. Sphingolipid Biology. Springer; Japan: 2006. Ceramide 1-Phosphate; pp. 207–218. [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential Role for Sphingosine Kinases in Neural and Vascular Development. Molecular and Cellular Biology. 2005;25(24):11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Peters Golden ML. Leukotriene modifiers for asthma treatment. Clinical & Experimental Allergy. 2010;40(12):1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kohno M, Kadoya M, Nagahara H, Fujii W, Seno T, et al. Knock Out of S1P3 Receptor Signaling Attenuates Inflammation and Fibrosis in Bleomycin-Induced Lung Injury Mice Model. PLoS ONE. 2014;9(9):e106792. doi: 10.1371/journal.pone.0106792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, Schönrock SM, et al. The Sphingosine-1 Phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulmonary Pharmacology & Therapeutics. 2011;24(4):377–385. doi: 10.1016/j.pupt.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, et al. Sphingosine-1–Phosphate, FTY720, and Sphingosine-1–Phosphate Receptors in the Pathobiology of Acute Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2013;49(1):6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava VE, Lacana E, Poulton S, Liu H, Sugiura M, Kono K, et al. Functional characterization of human sphingosine kinase-1. FEBS Letters. 2000;473(1):81–84. doi: 10.1016/s0014-5793(00)01510-6. [DOI] [PubMed] [Google Scholar]
- Nelson HS, Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: New insights. Journal of Allergy and Clinical Immunology. 2003;111(2):215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- Ni X, Epshtein Y, Chen W, Zhou T, Xie L, Garcia JG, Jacobson JR. Interaction of integrin β4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement. Journal of cellular biochemistry. 2014;115(6):1187–95. doi: 10.1002/jcb.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura SI. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2008;294(6):L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- O’Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. The Journal of Lipid Research. 2009;50(11):2245–2257. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan MJ, Hirota N, Martin JG. Sphingosine 1-phosphate (S1P) induced interleukin-8 (IL-8) release is mediated by S1P receptor 2 and nuclear factor κB in BEAS-2B cells. PLoS ONE. 2014;9(4):e95566. doi: 10.1371/journal.pone.0095566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Reviews Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Ohotski J, Edwards J, Elsberger B, Watson C, Orange C, Mallon E, Pyne S, Pyne NJ. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1-phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor-positive breast cancer. International Journal of Cancer. 2013;132(3):605–16. doi: 10.1002/ijc.27692. [DOI] [PubMed] [Google Scholar]
- Ohtoyo M, Tamura M, Machinaga N, Muro F, Hashimoto R. Sphingosine 1-phosphate lyase inhibition by 2-acetyl-4-(tetrahydroxybutyl)imidazole (THI) under conditions of vitamin B6 deficiency. Molecular and Cellular Biochemistry. 2015;400(1–2):125–133. doi: 10.1007/s11010-014-2268-z. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. The Journal of Biological Chemistry. 1998;273(42):27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Molecular and Cellular Biology. 2000;20(24):9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuwa H, Kanno T, Fujita Y, Gotoh A, Tabata C, Fukuoka K, et al. Sphingosine Suppresses Mesothelioma Cell Proliferation by Inhibiting PKC-δ and Inducing Cell Cycle Arrest at the G0/G1 Phase. Cellular Physiology and Biochemistry. 2012;30(4):995–1004. doi: 10.1159/000341476. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rivera J. Sphingolipids and the Balancing of Immune Cell Function: Lessons from the Mast Cell. Journal of Immunology (Baltimore, Md: 1950) 2005;174(3):1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. Journal of Clinical Investigation. 2010;120(5):1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine Kinase Expression Increases Intracellular Sphingosine-1-Phosphate and Promotes Cell Growth and Survival. The Journal of Cell Biology. 1999;147(3):545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C16-ceramide and Sphingosine 1-Phosphate in Regulating Hepatocyte Apoptosis in Response to Tumor Necrosis Factor-α. The Journal of Biological Chemistry. 2005;280(30):27879–27887. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111(8):4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF. S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulmonary Circulation. 2011;1(3):399–404. doi: 10.4103/2045-8932.87309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneer Selvam S, De Palma RM, Oaks JJ, Oleinik N, Peterson YK, Stahelin RV, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Science Signaling. 2015;8(381):ra58–ra58. doi: 10.1126/scisignal.aaa4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Letters. 2003;554(1–2):189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, et al. Protective Effects of Sphingosine 1-Phosphate in Murine Endotoxin-induced Inflammatory Lung Injury. American Journal of Respiratory and Critical Care Medicine. 2012;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature Medicine. 2005;11(5):491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. The Journal of Allergy and Clinical Immunology. 2013;131(2):501–11. e1. doi: 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc epsilon receptor I triggering. The Journal of Experimental Medicine. 1999;190(1):1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. The Journal of Clinical Investigation. 2015;125(4):1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Reviews. Cancer. 2010;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signaling. 2009;21(1):14–21. doi: 10.1016/j.cellsig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Locy ML, Luckhardt TR, Thannickal VJ. Targeted Therapy for Idiopathic Pulmonary Fibrosis: Where To Now? Drugs. 2016:1–10. doi: 10.1007/s40265-015-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2003;284(6):L1082–L1092. doi: 10.1152/ajplung.00172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2003;17(13):1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- Roviezzo F, D’Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, et al. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. American Journal of Respiratory Cell and Molecular Biology. 2010;42(5):572–577. doi: 10.1165/rcmb.2009-0108OC. [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, et al. Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. American Journal of Respiratory Cell and Molecular Biology. 2007;36(6):757–762. doi: 10.1165/rcmb.2006-0383OC. [DOI] [PubMed] [Google Scholar]
- Rubin LJ. Primary Pulmonary Hypertension. New England Journal of Medicine. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ. Effect of Sphingosine 1-Phosphate on Cyclo-Oxygenase-2 Expression, Prostaglandin E2 Secretion, and β2-Adrenergic Receptor Desensitization. American Journal of Respiratory Cell and Molecular Biology. 2016;54(1):128–135. doi: 10.1165/rcmb.2014-0443OC. [DOI] [PubMed] [Google Scholar]
- Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circulation Research. 2002;90(3):325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circulation Research. 2004;94(6):724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. British Journal of Pharmacology. 2011;162(6):1225–1238. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, et al. Differential Effects of Sphingosine 1–Phosphate Receptors on Airway and Vascular Barrier Function in the Murine Lung. American Journal of Respiratory Cell and Molecular Biology. 2010;43(4):394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. Journal of Cellular Biochemistry. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Sashio T, Hiroaki K, Takeda N, Asano T, Tsuji S, Kondo M, et al. Possible Involvement of Sphingosine-1-Phosphate/Gi/RhoA Pathways in Adherence of Eosinophils to Pulmonary Endothelium. Allergology International. 2012;61(2):283–293. doi: 10.2332/allergolint.10-OA-0299. [DOI] [PubMed] [Google Scholar]
- Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, et al. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. Journal of Neurochemistry. 2007;103(6):2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Tomura H, Igarashi Y, Ui M, Okajima F. Possible involvement of cell surface receptors in sphingosine 1-phosphate-induced activation of extracellular signal-regulated kinase in C6 glioma cells. Molecular Pharmacology. 1999;55(1):126–133. doi: 10.1124/mol.55.1.126. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Kiyono T, Yamada J, Hara S, Nakagawa M, et al. Acid Sphingomyelinase Activation Requires Caspase-8 but Not P53 nor Reactive Oxygen Species during Fas-Induced Apoptosis in Human Glioma Cells. Experimental Cell Research. 2002;273(2):157–168. doi: 10.1006/excr.2001.5437. [DOI] [PubMed] [Google Scholar]
- Schnitzer SE, Weigert A, Zhou J, Brüne B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Molecular Cancer Research. 2009;7(3):393–401. doi: 10.1158/1541-7786.MCR-08-0156. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Sehrawat S, Rouse BT. Anti-Inflammatory Effects of FTY720 against Viral-Induced Immunopathology: Role of Drug-Induced Conversion of T Cells to Become Foxp3+ Regulators. Journal of Immunology (Baltimore, Md: 1950) 2008;180(11):7636–7647. doi: 10.4049/jimmunol.180.11.7636. [DOI] [PubMed] [Google Scholar]
- Seitz AP, Grassmé H, Edwards MJ, Pewzner-Jung Y, Gulbins E. Ceramide and sphingosine in pulmonary infections. Biological Chemistry. 2015;396(6–7):611–620. doi: 10.1515/hsz-2014-0285. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. Role of Epithelial Cells in Idiopathic Pulmonary Fibrosis: From Innocent Targets to Serial Killers. Proceedings of the American Thoracic Society. 2012;3(4):364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- Selvam SP, Ogretmen B. Sphingosine Kinase/Sphingosine 1-Phosphate Signaling in Cancer Therapeutics and Drug Resistance. Handbook of Experimental Pharmacology. 2013;216:3–27. doi: 10.1007/978-3-7091-1511-4_1. [DOI] [PubMed] [Google Scholar]
- Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Advances in Enzyme Regulation. 2010;50(1):349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged Exposure to Sphingosine 1–Phosphate Receptor-1 Agonists Exacerbates Vascular Leak, Fibrosis, and Mortality after Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2012;43(6):663–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnatrexone: role of mOP-R and S1P3 transactivation. American Journal of Respiratory Cell and Molecular Biology. 2007;37(2):222–31. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- Sobel K, Menyhart K, Killer N, Renault B, Bauer Y, Studer R, et al. Sphingosine 1-Phosphate (S1P) Receptor Agonists Mediate Pro-fibrotic Responses in Normal Human Lung Fibroblasts via S1P2 and S1P3 Receptors and Smad-independent Signaling. The Journal of Biological Chemistry. 2013;288(21):14839–14851. doi: 10.1074/jbc.M112.426726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiban J, Tidhar R, Futerman AH. Ceramide Synthases: Roles in Cell Physiology and Signaling. Advances in Experimental Medicine and Biology. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- Stoffel W. Studies on the biosynthesis and degradation of sphingosine bases. Chemistry and Physics of Lipids. 1970;5(1):139–158. doi: 10.1016/0009-3084(70)90014-9. [DOI] [PubMed] [Google Scholar]
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2011;25(2):600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, Dudek SM, Garcia JG. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvascular Research. 2009;77(3):304–13. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, Chiang ET, Moreno-Vinasco L, Wade MS, Zhou T, Liu B, Parastatidis I, Thomson L, Ischiropoulos H, Natarajan V, Jacobson JR, Machado RF, Dudek SM, Garcia JG. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. American Journal of Respiratory Cell and Molecular Biology. 2012;47(5):628–36. doi: 10.1165/rcmb.2012-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysol JR, Natarajan V, Machado RF. PDGF Induces SphK1 Expression via Egr-1 to Promote Pulmonary Artery Smooth Muscle Cell Proliferation. American Journal of Physiology - Cell Physiology. 2016;310(11):C983–92. doi: 10.1152/ajpcell.00059.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2010;299(1):L137–L145. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D, et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proceedings of the National Academy of Sciences. 2015;112(13):E1614–23. doi: 10.1073/pnas.1421190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Suzuki K, Osawa S, Isshiki H, Hori Y, et al. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(18):6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors. 2012;38(5):329–37. doi: 10.1002/biof.1030. [DOI] [PubMed] [Google Scholar]
- Tang X, Benesch MG, Brindley DN. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. Journal of Lipid Research. 2015;56(11):2048–2060. doi: 10.1194/jlr.R058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto T, Lungu AO, Berk BC. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circulation Research. 2004;94(8):1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- Tepper AD, Cock JGRBD, de Vries E, Borst J, van Blitterswijk WJ. CD95/Fas-induced Ceramide Formation Proceeds with Slow Kinetics and Is Not Blocked by Caspase-3/CPP32 Inhibition. The Journal of Biological Chemistry. 1997;272(39):24308–24312. doi: 10.1074/jbc.272.39.24308. [DOI] [PubMed] [Google Scholar]
- Tibboel J, Joza S, Reiss I, de Jongste JC, Post M. Amelioration of hyperoxia-induced lung injury using a sphingolipid-based intervention. European Respiratory Journal. 2013;42(3):776–784. doi: 10.1183/09031936.00092212. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, Fozard JR. Sphingosine-1-Phosphate-Induced Airway Hyper-Reactivity in Rodents Is Mediated by the Sphingosine-1-Phosphate Type 3 Receptor. Journal of Pharmacology and Experimental Therapeutics. 2012;342(2):399–406. doi: 10.1124/jpet.112.191585. [DOI] [PubMed] [Google Scholar]
- Troncoso P, Kahan BD. Preclinical evaluation of a new immunosuppressive agent, FTY720. Clinical Biochemistry. 1998;31(5):369–373. doi: 10.1016/s0009-9120(98)00043-5. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JGN, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2011;300(6):L840–L850. doi: 10.1152/ajplung.00404.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Letters. 2003;199(1):53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, et al. Differential regulation of sphingosine kinases 1 and 2 in lung injury. American Journal of Physiology Lung Cellular and Molecular Physiology. 2009;296(4):L603–613. doi: 10.1152/ajplung.90357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvascular Research. 2009;77(1):39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sammani S, Moreno-Vinasco L, Letsiou E, Wang T, Camp SM, et al. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Critical Care Medicine. 2014;42(3):e189–99. doi: 10.1097/CCM.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Mao J, Redfield S, Mo Y, Lage JM, Zhou X. Systemic distribution, subcellular localization and differential expression of sphingosine-1-phosphate receptors in benign and malignant human tissues. Experimental Molecular Pathology. 2014;97(2):259–65. doi: 10.1016/j.yexmp.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weete JD. Fungal Lipid Biochemistry. Boston, MA: Springer US; 1974. Sphingolipids; pp. 267–286. [Google Scholar]
- Weiler S, Braendlin N, Beerli C, Bergsdorf C, Schubart A, Srinivas H, Oberhauser B, Billich A. Orally active 7-substituted (4-Benzylphthalazin-1yl)-2-methylpiperazin-1-yl]nicotinonitriles as active-site inhibitors of sphingosine 1-phosphate lyase for the treatment of multiple sclerosis. J Med Chem. 2014;57:5074–5084. doi: 10.1021/jm500338n. [DOI] [PubMed] [Google Scholar]
- Wine JJ. The genesis of cystic fibrosis lung disease. Journal of Clinical Investigation. 1999;103(3):309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojewodka G, De Sanctis JB, Radzioch D. Ceramide in Cystic Fibrosis: A Potential New Target for Therapeutic Intervention. Journal of Lipids. 2010:674968. doi: 10.1155/2011/674968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Hla T. S1P control of endothelial integrity. Current topics in microbiology and immunology. 2014;378:85–105. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A Sphingosine-1–Phosphate-Activated Calcium Channel Controlling Vascular Smooth Muscle Cell Motility. Circulation Research. 2006;98(11):1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Krause A, Limberis M, Worgall TS, Worgall S. Low Sphingosine-1–Phosphate Impairs Lung Dendritic Cells in Cystic Fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2013;48(2):250–257. doi: 10.1165/rcmb.2012-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biology & Therapy. 2015;16(8):1194–1204. doi: 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Aranha AB, Antunes TT, Graham D, McBride M, et al. Sphingosine-1-phosphate-induced inflammation involves receptor tyrosine kinase transactivation in vascular cells: upregulation in hypertension. Hypertension. 2011;57(4):809–818. doi: 10.1161/HYPERTENSIONAHA.110.162719. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiological Reviews. 2007;87(3):1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Analytical Biochemistry. 2006;354(2):279–289. doi: 10.1016/j.ab.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Usatyuk PV, Cummings R, Saaatian B, He D, Watkins T, et al. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF- κ B activation and interleukin-8 secretion in human bronchial epithelial cells. Biochemical Journal. 2005;385(Pt 2):493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. The Journal of Biological Chemistry. 2007;282(19):14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, et al. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. American Journal of Respiratory Cell and Molecular Biology. 2011;45(2):426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. American Journal of Physiology. Cell Physiology. 2004;286(5):C1130–1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- Zhou PJ, Wang H, Shi GH, Wang XH, Shen ZJ, Xu D. Immunomodulatory drug FTY720 induces regulatory CD4+CD25+ T cells in vitro. Clinical & Experimental Immunology. 2009;157(1):40–47. doi: 10.1111/j.1365-2249.2009.03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]