Abstract
Atrial fibrillation (AF), the most common cardiac arrhythmia, is associated with increased risk of cerebrovascular stroke, and with several other pathologies, including heart failure. Current therapies for AF are targeted at reducing risk of stroke (anticoagulation) and tachycardia-induced cardiomyopathy (rate or rhythm control). Rate control, typically achieved by atrioventricular nodal blocking drugs, is often insufficient to alleviate symptoms. Rhythm control approaches include antiarrhythmic drugs, electrical cardioversion, and ablation strategies. Here, we offer several examples of how computational modeling can provide a quantitative framework for integrating multi scale data to: (a) gain insight into multi-scale mechanisms of AF; (b) identify and test pharmacological and electrical therapy and interventions; and (c) support clinical decisions. We review how modeling approaches have evolved and contributed to the research pipeline and preclinical development and discuss future directions and challenges in the field.
Keywords: Antiarrhythmic drugs, atrial selectivity, ablation, cardioversion, systems pharmacology, simulation
1. Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, occurring in 1-2% of the general population - projected to increase to 4% by 2050 (Andrade et al. 2014). Because age is the most powerful predictor of AF risk, the impact of AF is furthermore estimated to increase dramatically in coming decades, as Westernized populations continue to age. AF markedly increases risk for cerebrovascular stroke and thrombo-embolic events, impairs quality of life and exercise capacity, and often coexists with other pathologies, such as heart failure (HF) and left ventricular dysfunction, causing increased morbidity and mortality. AF has significant economic impact: at least 1% of the healthcare budget of the United States and European countries is currently spent on AF management: annual related costs are estimated at $6.65 billion in 2001 and €13.5 billion respectively (Coyne et al. 2006; Fuster et al. 2006). These are largely attributed to hospitalization and acute care, whereas outpatient treatment and pharmacotherapy account for about one-third of the economic burden (Coyne et al. 2006).
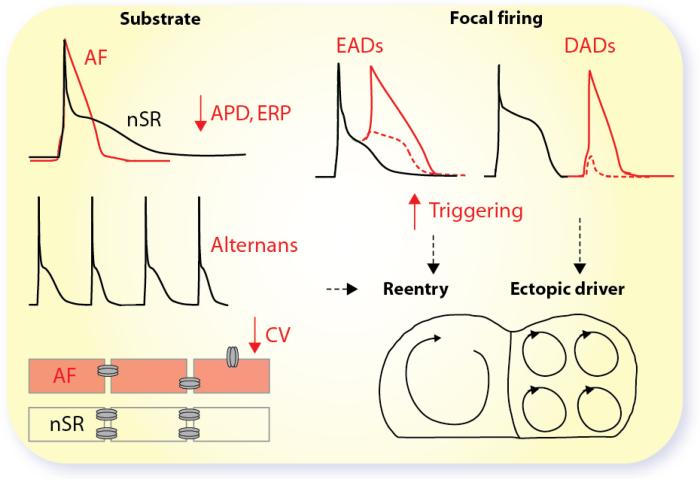
In the majority of patients, there appears to be an inexorable progression from sporadic paroxysmal AF (pAF) to persistent or chronic (cAF) forms, associated with arrhythmia-induced changes of atrial electrophysiological properties, mechanical function, and atrial ultrastructure that may perpetuate the arrhythmia. For AF to be initiated and maintained, both triggers for its onset and a substrate for its maintenance are required. We refer the readers to recent thorough reviews for detailed descriptions of the mechanisms underlying AF (Guillem et al. 2016; Heijman et al. 2014; Wakili et al. 2011). These involve both increased ectopic firing of atrial cells and impulse reentry through atrial tissue (Fig. 1). At the cellular level, focal ectopic/triggered activity is likely caused by early and delayed afterdepolarizations (EADs and DADs) or enhanced automaticity. At the tissue level, afterdepolarization-prone regions have to overcome the surrounding stable tissue to produce ectopic activity. Focal ectopic activity can maintain AF as a driver or act on vulnerable reentrant substrates. Reentry is promoted by shorter action potential (AP) duration (APD) and effective refractory period (ERP), and APD alternans. At the tissue level, it is favored by slow conduction velocity (CV) and heterogeneous conduction, spatially heterogeneous APD, and abbreviated refractoriness. Fibrotic regions may additionally serve as architectural anchors, which stabilize AF reentry rotors. Virtually all of these mechanisms are modulated by the autonomic nervous system, which has a profound influence on the occurrence of AF (Bettoni & Zimmermann 2002; Dimmer et al. 1998). Indeed, simultaneous sympathovagal discharges commonly precede arrhythmias, and both sympathetic and vagal activation have been shown capable of producing proarrhythmic atrial myocyte APD changes and predispose to Ca-dependent triggered activity (Fig. 1).
Figure 1. Arrhythmia Mechanisms in AF.
AF remodeling causes abbreviated APD and ERP, alternans, decreased CV (e.g., due to connexin downregulation or lateralization, and/or fibrosis), all contributing to a reentrant substrate. Focal firing via enhanced automaticity or triggered activity due to early or delayed afterdepolarizations causes local ectopic activity, which can act as an AF-maintaining ectopic driver, or can trigger AF-maintaining re-entry in a vulnerable substrate.
Current therapies for AF are targeted at treating the faulty heartbeat and reducing risk of tachycardia-induced cardiomyopathy (rate or rhythm control) and/or stroke (anticoagulation). Rhythm control is the preferred treatment approach for the majority of patients with pAF or cAF (Ball et al. 2013; Camm et al. 2012; January et al. 2014). Rate control, typically achieved by atrioventricular nodal blocking drugs, such as β-blockers, L-type calcium channel blockers, or digoxin, is often insufficient to alleviate symptoms. Rhythm control is attempted with antiarrhythmic drugs, electrical cardioversion, and ablation strategies. Drug treatment of AF is limited by low efficacy and the side effects of currently available antiarrhythmic agents, which often increase the propensity to life-threatening ventricular arrhythmias. Section 2 documents the ongoing search for new agents against AF with a more favorable benefit-to-harm relation, which has led to the development of atrial-selective antiarrhythmic drugs. In Sections 3, we focus on non-pharmacological interventions to limit AF, e.g., ablation techniques, which have been substantially developed in the past decade and proven successful to reduce the symptomatic burden associated with the arrhythmia. Throughout the article, we discuss how atrial modeling and simulation have enhanced our mechanistic understanding of AF, and contributed to the development of therapeutic strategies.
Many commonly used human atrial myocyte models (Courtemanche et al. 1998; Grandi et al. 2011; Koivumaki et al. 2011; Maleckar et al. 2009; Nygren et al. 1998), their main properties and their evolution, have been recently reviewed and compared elsewhere (Heijman et al. 2016b). These models show important differences in AP morphology and rate dependence, which affect reentrant behaviors in multicellular simulations (Cherry et al. 2008; Wilhelms et al. 2012). Notably, a recent study adopting population-based simulations to study inter-subject variability has shown remarkable similarities in the mechanisms identified using three widely used different human atrial AP models (Sanchez et al. 2014), and also provided a new approach to analyzing differences among various models and identifying which model might be best suited for certain questions.
More recent models, such as the Grandi and Koivumaki models, have focused more in depth on the simulation of atrial Ca handling, and emphasized the importance of Ca and Na homeostasis for atrial electrophysiological properties (Grandi et al. 2011; Koivumaki et al. 2011). The Koivumäki model also provided a spatial representation of Ca handling, with centripetal Ca diffusion between transverse cytosolic and SR compartments (Koivumaki et al. 2011). Heijman and colleagues recently developed the first human atrial cardiomyocyte model with both transverse and longitudinal Ca compartmentation (Voigt et al. 2014), coupled with the Grandi et al. electrophysiological model (Grandi et al. 2011). The latter allows simulating the consequences of sympathetic and vagal (as in the Maleckar model (Maleckar et al. 2009; Maleckar et al. 2008)) stimulation, and has been further updated with incorporation of Na-dependent regulation of IK1 and IK,ACh (Voigt et al. 2013), two-pore K current and its regulation in AF (Schmidt et al. 2015), and a Markov-type Na channel model accounting for non-equilibrium gating (Morotti et al. 2015).
When applied in concert with major new drug developments such as novel antithrombotic agents, the emerging safer antiarrhythmic drugs and therapeutic strategies, aided by computational modeling approaches as outlined below, should help to improve outcomes in AF patients.
2. Ionic anti-AF targets
2.1 K channels
A large variety of K channels are expressed in atria (Fig. 2) (Nerbonne 2000). These include the voltage-gated K channels, i.e., the slow, rapid, and ultrarapid delayed rectifier (IKs, IKr and IKur) and the transient-outward (Ito); the inward-rectifier K currents, including the basal IK1, the acetylcholine-dependent (IK,ACh), and the ATP-sensitive K current (IKATP); the small-conductance Ca-activated K (SK) current (IK,Ca), and the hyperpolarization-activated cyclic nucleotide-gated current (‘funny’ current; If). Similarly, several members of the large family of two-pore-domain K (K2P) channels, including TREK1 (K2P2.1) and TASK1 (K2P3.1) are expressed in the heart and contribute to the background K currents.
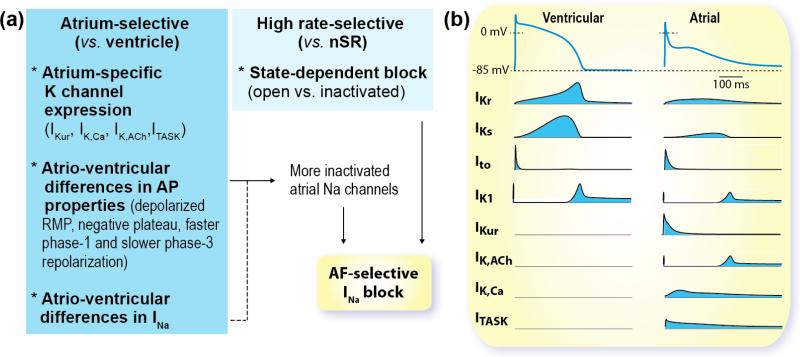
Figure 2. AF-selective Pharmacology and Atrial-specific K currents.
(a) To maximize efficacy and safety an AF-selective drug should exhibit potent effects in atrial tissue at high rates (during fibrillation) without impacting ventricular AP and nSR. To achieve this, drugs could target channels that are predominantly expressed in atria vs. ventricles, shown in (b), or take advantage of atrioventricular differences in membrane potential and/or ion channel gating, as discussed here for Na channel blockers. (b) Ventricular (left) and atrial (right) APs and K currents underlying repolarization.
Targeting selectively atrial myocytes to reduce off-target side effects is a major goal in AF therapeutics (Fig. 2). To maximize efficacy and minimize proarrhythmic risk, an AF-selective drug should exert potent effects on fibrillating atria without significantly impacting ventricular tissue during normal heart rates. A potential strategy to achieve this is targeting channels that are predominantly expressed in atria vs. ventricles, such as those carrying IKur, IK,ACh, IK2P, and IK,Ca. Inhibition of these channels may selectively affect atrial electrophysiology, while causing little or no effect in the ventricles.
IKur
IKur (and its alpha subunit Kv1.5) is detected in atrial myocytes (and in ventricular myocytes in certain species), it activates more rapidly than IKs and IKr, and inactivates slowly (in seconds). Human cAF has been associated with strong reduction of IKur density (Brandt et al. 2000; Caballero et al. 2010; Christ et al. 2008; Dobrev & Ravens 2003; Van Wagoner et al. 1997), paralleled by diminished expression of Kv1.5 (Brundel et al. 2001a; Brundel et al. 2001b; Van Wagoner et al. 1997). However, others have reported no changes in IKur density (Bosch et al. 1999; Grammer et al. 2000; Workman et al. 2001). Inconsistent results regarding IKur function have been attributed to different strategies for identification of IKur (e.g., pharmacological or with Ito-inactivating prepulse), and to a fraction of IKur that is not accounted for by Kv1.5 (Christ et al. 2008). The reduction in Ito and IKur explains the slight prolongation in earlier phases of the AP of cAF patients (Grandi et al. 2011; Van Wagoner & Nerbonne 2000).
Experimental evidence suggests that block of IKur enhances force of contraction of isolated human atrial trabeculae both in patients in normal sinus rhythm (nSR) and AF (Schotten et al. 2007; Shibata et al. 1989; Wettwer et al. 2004). Our human atrial myocytes model predicted that block of IKur results in prolongation and elevation of the AP plateau, which augments the Ca transient amplitude that would elicit a positive inotropic effect (Grandi et al. 2011). Taken together, these studies suggest that IKur might be a useful atrial-specific target to potentially counteract hypocontractility associated with cAF. A slight AP prolongation associated to IKur blockade may also be beneficial, although our modeling results also predicted that IKur block may result in proarrhythmic EADs during simulated sympathetic stimulation (Grandi et al. 2011). Interestingly, APD and ERP were prolonged in AF but shortened in nSR preparations, depending on the overall repolarization profile and relative strengths of ICaL and IKr (Wettwer et al. 2004). Indeed, our sensitivity analysis of a human atrial myocytes model reveals that APD changes negatively or positively correlate with IKur block depending on the degree of remodeling e.g., in other K currents such as IK,Ca (Morotti et al. 2016). Population-based approaches applied to atrial models (Sanchez et al. 2014) support IKur importance in modulating inter-subject variability in APD50 and APD20, with smaller importance in modulating APD90. Thus the effects of changes in IKur on Ca influx and APD are potentially complex and modeling may help identifying the net impact of opposite mechanisms in various remodeling conditions. Population-based studies may be used to identify the implication of variability (e.g., the extent of remodeling) in modulating the response to pharmacological block of IKur.
While numerous Kv1.5 selective compounds have been screened, characterized, and tested in various animal models of AF, proof-of-concept of antiarrhythmic efficacy in human is still lacking (Ravens et al. 2013). The novel IKur inhibitor MK-0448 did not appreciably alter atrial refractoriness in patients with history of AF (Pavri et al. 2012), though only relatively slow pacing rates were tested (150 bpm). Notably, the highly selective Kv1.5 inhibitor XEN-D0103 was recently shown to prolong APD and ERP preferentially at fast stimulation rates in human nSR and pAF preparations (Ford et al. 2016). The authors concluded that this frequency-dependence could be useful, as APD prolongation leading to failure of excitation response at high stimulation rates is expected to reduce AF burden. A computational study of the frequency-dependent effects of various IKr blockers on the human atrial APD had indeed suggested that the voltage- and time-dependent nature of IKr blockade by drugs may be critical for the drug effect on atrial AP (Tsujimae et al. 2007). However, this concept needs to be confirmed in clinical trials.
IK1
The inward rectifying K current IK1 is increased in cAF vs. nSR human atrial myocytes (Dobrev et al. 2001). Many studies have highlighted the importance of IK1 in human atrial electrophysiology, pointing to IK1 as one of the most relevant currents in the perpetuation of reentrant mechanisms both in experiments (Filgueiras-Rama et al. 2012; Gomez et al. 2014) and simulation (Koivumaki et al. 2014; Pandit et al. 2005; Sanchez et al. 2012). Thus IK1 is considered a potential antiarrhythmic target, based on the effect of IK1 blockade: to lengthen APD and ERP and therefore reduce the dominant frequency (DF).
IK,ACh
IK,ACh is composed of two homologous GIRK channel subunits, GIRK1 and GIRK4 (encoded by kir3.1 and kir3.4), prominently expressed in atrial and nodal cells, and activated by muscarinic agonists such as acetylcholine. Patients with cAF exhibit agonist-independent constitutive IK,ACh activity that contributes to the enhanced basal inward rectifier current and may result from abnormal channel phosphorylation by protein kinase C (PKC) (Dobrev et al. 2005; Dobrev et al. 2001; Voigt et al. 2010). Activation of IK,ACh hyperpolarizes atrial resting membrane potential (RMP), and shortens APD and ERP (Maleckar et al. 2008; Zaza et al. 1995). Constitutively active IK,ACh is considered to support the maintenance of AF, together with increased IK1, by stabilizing reentrant activity sustained by rotors (faster activation, less meander) (Pandit et al. 2005). In a vagally induced canine model, AF is terminated by intravenous application of the IK,ACh inhibitor Tertiapin-Q (Hashimoto et al. 2006), but the Ravens group reported Tertiapin-Q prolonged refractoriness in isolated cardiomyocytes, but had little effect in multicellular preparations (Ravens et al. 2013). The recently reported IK,ACh blocker NTC-801/BMS 914392 was suggested to exert antifibrillatory action by atrial-selective ERP prolongation (Machida et al.), but failed to meet its primary endpoint in a phase II study of AF burden in pAF patients (Podd et al. 2015). The kir3.x inhibitor XEN-R0706 potently inhibits the kir3.4/3.4 homotetramer and prolongs APD and ERP in human atrial tissue from AF patients, suggesting a possible role for kir3.4/3.4 homotetramer in the remodeled atria (Milnes et al. 2014).
IK,Ca
SK channels are also a putative atrial-selective target for antiarrhythmic drug therapy, given their higher abundance in atrial vs. ventricular tissue, and the fact that IK,Ca was found not to contribute substantially to the human ventricular AP (Skibsbye et al. 2014). SK activation is Ca-dependent (mediated by calmodulin tethered to the channel), and can be due to either Ca entered through the L-type Ca channels, or Ca released from the sarcoplasmic reticulum (SR). Recent data revealed that SK trafficking also increases in a Ca-dependent manner (Rafizadeh et al. 2014). These properties make IK,Ca of particular interest during fast pacing or tachyarrhythmias (e.g. AF), when Ca accumulation is likely to up-regulate the current. Both SK hyperactivity and suppression have been implicated in AF, likely from different mechanisms depending on species and heart rates. Several studies reported prolongation of ERP and/or AF termination resulting from SK channel inhibition (Diness et al. 2011; Diness et al. 2010; Skibsbye et al. 2011). In contrast, APD prolongation upon SK ablation has been shown to increase the occurrence of EADs (Li et al. 2009), and to increase APD heterogeneity leading to alternans and wave breaks in isolated canine left atrium (LA) (Hsueh et al. 2013).
The role of IK,Ca in human atrial physiology and AF is not well understood (it is found reduced in cAF patient tissue (Skibsbye et al. 2014), but data from the Dobrev group suggest otherwise (Zhou et al. 2012)). Our preliminary simulation studies (Morotti et al. 2016) suggest that IK,Ca is protective against triggered events (EADs, DADs, triggered activity), but contributes to a reentrant substrate (favoring ERP shortening and alternans). Defining the conditions that determine a trigger in the setting of a dynamic substrate is quite challenging. In such complex scenarios, computational approaches can be most useful to determine whether a therapeutic window for IK,Ca modulation exists, as the very large number of combinations of variables may be prohibitive to test in wet lab experiments.
K2P channels
Recent studies show predominant atrial expression of mRNA in humans for other K channels (TWIK1 and TASK1) that function similarly to the voltage-independent inward rectifying K channels responsible for IK1 (Gaborit et al. 2007; Limberg et al. 2011; Schmitt et al. 2014). TWIK1 and TASK1 belong to a family of K2P channel proteins that are responsible for background K currents and can be regulated by pH, oxygen, stretch, temperature, drugs, lipids, and second messengers (Gurney & Manoury 2009; Limberg et al. 2011; Schmitt et al. 2014). Inhibition of K2P channels in human atria is expected to prolong atrial APD and increase the effective refractory period, which suggests the possibility of targeting these channels in atrial-selective anti-arrhythmic drugs. Indeed, some atrial-predominant K2P channels (e.g., K2P3.1) are upregulated in cAF, and computational modeling has shown that upregulation of IK2P contributes to APD shortening in AF (Schmidt et al. 2015).
Na/K pump current
The electrogenic Na/K ATPase does not seem to be involved in AF-induced electrophysiological remodeling in patients (Workman et al. 2003). Nevertheless, modeling studies have highlighted the importance of Na/K pump current in modulating atrial (and ventricular) repolarization (Grandi et al. 2011; Koivumaki et al. 2011; Sanchez et al. 2012; Xie et al. 2015) and its importance in inter-subject variability (Sanchez et al. 2014), affecting APD, APD restitution, ERP, and reentrant DF. This is often overlooked in discussions regarding treatment, despite the fact that the Na/K ATPase has also been implicated in the effects of amiodarone, and cardiac glycosides (e.g., digoxin) are commonly used for rate control (though the inhibitory effects of cardiac glycosides on atrio-ventricular conduction are largely due to their parasympathetic effects (Gheorghiade et al. 2004)).
2.2 Na channels
The cardiac Na current (INa, mainly carried by the cardiac specific alpha subunit NaV1.5) peaks at the onset of the AP (fast INa) and continues throughout systole, with a so-called late component (INaL), which decays gradually. Specific Na channel block has been demonstrated to effectively terminate AF both experimentally and in mathematical models by increasing rotor core size and decreasing reentry frequency while decreasing generation of secondary wavelets by wavebreak (Kneller et al. 2005). Remarkably, although the role of INa in AF remodeling is unclear (from no changes (Bosch et al. 1999; Brundel et al. 2001a) to slight decrease in cAF patients’ samples (Sossalla et al. 2010b)), AF-selective Na channel blockers are emerging as a promising AF-suppressing strategy. Indeed, atrioventricular differences in membrane potential and/or ion channel gating can confer atrial selectivity to certain Na blockers, depending on the drugs’ interaction with complex kinetics, conformational state specificity, and intrinsic voltage dependence. Na channel blockers with Em- and frequency-dependent action preferentially suppress AF because of the high excitation rate and less negative atrial vs. ventricular RMP, which promote drug binding in atria (Fig. 2). Flecainide, recommended as one of the first-line treatment options for restoring and maintaining nSR in patients with AF under current treatment guidelines, exhibits very slow unbinding kinetics from the Na channels during diastole, thus prolonging post-repolarization refractoriness, decreasing excitability and slowing intracardiac conduction (though in all cardiac tissues). Vernakalant and ranolazine, which mainly block atrial Na channels, are clinically effective (Ravens et al. 2013). The former is in clinical use for cardioversion of AF in Europe, the latter has efficacy for AF and is being tested in prospective clinical trials. These drugs are far from pure Na channel blockers, in that they have multi-target effects, and are discussed in the “polytherapy” section below.
Computational models have proven useful in understanding and developing novel antiarrhythmic drug therapy for AF. Modeling has been used to investigate the electrophysiological effects and mechanisms of AF termination by available drugs (Comtois et al. 2008). Recently, this approach has been taken a step forward to determine the relationship between drug dynamic properties and atrial-selectivity (vs. ventricles) (Morotti et al. 2015), AF-selectivity (vs. nSR), and AF-termination effectiveness (Aguilar et al. 2015; Aguilar-Shardonofsky et al. 2012). The anti-anginal drug ranolazine selectively blocks atrial peak INa, given the negatively shifted steady-state inactivation of atrial vs. ventricular INa and the more depolarized atrial RMP (Antzelevitch & Burashnikov 2010; Burashnikov et al. 2008), and has proven successful to suppress AF in certain species (Burashnikov et al. 2014). On one hand, ranolazine prolongs atrial ERP and slows CV without affecting ventricular parameters, on the other hand it may prevent INa-mediated phase-3 EADs, as we have recently shown (Morotti et al. 2015). These examples demonstrate the potential for modeling and simulation approaches to aid development of therapeutic agents by predicting optimized pharmacodynamic properties for AF treatment (Aguilar & Nattel 2015).
Sossalla et al. have recently shown that INaL is significantly increased in cAF patients (Sossalla et al. 2010b), possibly due to an increase in neuronal Na channel isoforms (Nav1.1 expression is increased), or mediated by the Ca/Calmodulin dependent protein kinase II (CaMKII), which is increased in AF (Neef et al. 2010; Tessier et al. 1999) and known to regulate INaL (Wagner et al. 2006), or caused by oxidative stress (Mihm et al. 2001; Wagner et al. 2011). Independent of the mechanism, our simulations suggested that an increased INaL does not contribute significantly to repolarization duration in cAF, where the overall APD was still shorter than that in normal healthy cells (Grandi et al. 2011). On the other hand, an increase in INaL could potentially cause cellular Na and Ca overload and lead to contractile dysfunction and electrical instability (via reverse-mode NCX) (Bers 2001). A modeling and simulation framework would provide a useful tool to test this hypothesis quantitatively.
2.3 Na and K channel “polytherapy”
Amiodarone and dofetilide are the only available drugs in the Unites States for treatment of AF in the setting of HF, but they often have cardiac (dofetilide: long QT and torsades de pointes) and extra-cardiac (amiodarone: toxicity) adverse effects. While being categorized as a “Class III” antiarrhythmic drug because it primarily blocks voltage-gated K channels, amiodarone exerts actions typical of all antiarrhythmic classes (on β-adrenergic receptors as well as on Na and Ca channels) and reduces arrhythmia triggers through normalization of restitution and Na and Ca homeostasis. Thus there is growing interest in multi-ion channel blockade or “polytherapy”, which allows lower drug doses and thus less adverse/non-specific effects. Vernakalant is another complex electrophysiological agent, which shows broad K channel inhibition and rate-dependent Na channel inhibition. Vernakalant has shown efficacy in nSR conversion and maintenance in phase I-III clinical trials, and is in clinical use for cardioversion of AF in Europe. Ranolazine reduces peak INa in nSR but not in cAF patients, where it blocks late INa more strongly (Poulet et al. 2015; Sossalla et al. 2010a), and also blocks IKr. Promising results from the phase II clinical trial RAFFAELLO (NCT01534962) showed that 500- and 750-mg ranolazine reduced AF recurrences (De Ferrari et al. 2015). Recently, the Antzelevitch group has reported greater antiarrhythmic efficacy of the combination of ranolazine (Class I effect) and amiodarone or dronedarone (Class III effect) compared with either drug alone (Burashnikov et al. 2010; Sicouri et al. 2010). This effect was confirmed by the recently reported HARMONY study (Reiffel et al. 2015), which showed synergistic AF burden reduction by moderate dose of ranolazine plus reduced dose of dronedarone.
Using (atrial and ventricular) cardiac electrophysiological models, Dr. Nattel's group has made significant headway in the direction of defining pharmacodynamic properties that, on one hand, maximize therapeutic effects on the fibrillating atrium and on the other hand minimize ventricular proarrhythmic actions at the much slower rate of nSR (Aguilar & Nattel 2015; Aguilar-Shardonofsky et al. 2012). Aguilar et al. recently employed a combined experimental and computational approach to demonstrate that K channel block potentiates the anti-AF properties of Na channel blockers (Aguilar et al. 2015). Their results suggested that IKr or IKur block act on prolonging the AP plateau, thus reducing INa availability in a rate-dependent manner, and on lengthening the APD, thus depolarizing the AP takeoff potential at fast rates (fewer available Na channels) and decreasing the diastolic interval, i.e., the time available for drug unbinding (Aguilar et al. 2015). These effects led to a synergistic reduction of Na-dependent parameters (peak INa, upstroke velocity, and CV) and were associated with a more rapid termination of AF, and reduced AF inducibility (Aguilar et al. 2015). However, blocking IKr (though at low doses) might not be a safe choice, especially in patients with prolonged QT interval, due to the increased risk of torsade des pointes. As discussed earlier, the IKur role in AF is additionally controversial. We contend that IK,Ca could be a new atrial-selective target and aid to optimize Na channel block. Indeed, a low dose of the IK,Ca blocker ICAGEN in guinea pig atria has been shown to enhance the anti-arrhythmic properties of the INa inhibitors flecainide and ranolazine (Kirchhoff et al. 2015), likely by further increasing the atrioventricular differences that support atrial selectivity.
2.4 Ca channels
L-type calcium current (ICaL) reduction in human cAF is well established (e.g., (Grandi et al. 2011; Voigt et al. 2012)), and has been demonstrated to play a relevant role in reentrant behavior (Samie et al. 2000), being associated with the progressive DF increase during the transition from pAF to persistent AF (Martins et al. 2014). On the other hand, ICaL blockers resulted in a reduction of fibrillation complexity and a reduction in the DF (Climent et al. 2015). ICaL blocking agents, such as verapamil and diltiazem, reduce the likelihood of early recurrence of AF after cardioversion by attenuating changes in atrial ERP (Daoud et al. 1997), but they do not affect AF in other settings (aside from their role as ventricular rate control agents, (Dobrev 2007)).
2.5 RyR channels
Studies in animal models of AF indicate a role for Ca-handling abnormalities leading to ectopic (triggered) activity via DADs, whose role in AF pathophysiology is somewhat controversial (see discussion in (Schotten et al. 2016)). Spontaneous Ca release events and Ca waves through leaky ryanodine receptor (RyR) channels have been reported in myocytes from cAF patients (Chelu et al. 2009; Neef et al. 2010; Vest et al. 2005; Voigt et al. 2012) despite unaltered SR Ca content. The synthetic agent JTV-519 (K201) suppresses spontaneous Ca release and RyR2 activity, and has been shown to reduce AF in dogs and mice (Kumagai et al. 2003a), but has documented off-target effects on the Ca channel and SR Ca pump (in the micromolar range of concentrations). The drug has completed phase II clinical trials, but no data have yet been posted.
One potential contributor to RyR hyperactivity may be oxidative stress, which is known to play a critical role in AF pathophysiology (Mihm et al. 2001) and increases RyR open probability. Neef et al. suggested that the CaMKII-dependent increase in SR Ca leak caused by RyR hyperphosphorylation in AF is a potential arrhythmogenic mechanism (Neef et al. 2010), because elimination of Ca via inward Na-Ca exchange current could lead to cell depolarization and cause DADs. Voigt et al. directly measured single RyRs isolated from cAF patients and demonstrated a higher channel open probability in cAF that responded to CaMKII inhibition (Voigt et al. 2012). Thus, while results in human samples seem to rule out a role for CaMKII in pAF (Voigt et al. 2014), CaMKII inhibition may reduce the propensity for atrial arrhythmias in cAF patients. Indeed, crosstalk and/or synergy between CaMKII signaling and Ca handling deserve further investigation and would be important extensions for future modeling studies, as done in ventricular models (Morotti et al. 2014).
2.6 Gap junction channels
AF-related remodeling involves reduction in gap junctions via decreased connexin expression and distribution (lateralization, from cell-end gap junctions to lateral margins) in both humans and animal models. Abnormal intercellular communication caused by connexin dysfunction may also be involved in AF, though this remains unclear. Small-molecule drugs enhancing gap junction conductance, such as rotigaptide, have been developed as potential treatments for AF, and lead to improvement in some models (ischemia and mitral valve disease–related AF). However, little or no change is reported in other clinically relevant models (Guerra et al. 2006; Laurent et al. 2009; Shiroshita-Takeshita et al. 2007). Gene transfer of connexin 43 suppressed AF in a rapid atrial pacing porcine model (Bikou et al. 2011). Thus, the role of connexin abnormalities in AF and the potential value of modulating connexin function to treat AF remain unclear. Data from phase II clinical data on ZP123 and phase I on GAP-134 might help to shed light into the potential clinical benefit of targeting gap junction channels.
2.7 Summary and recommendations
The table summarizes novel antiarrhythmic targets and the current status of each with regard to clinical development. Many newly designed selective blockers (e.g., IKur and IK,ACh blockers) have proven effective in animal models and human preparations. However, limited data are available in humans for these drugs, and their clinical benefit in nSR conversion and maintenance, or reduction of AF burden have yet to be demonstrated. Indeed, there are a number of factors still precluding translation of basic research findings to the clinic, as recently reviewed by (Heijman et al. 2016a). These challenges include the lack of animal models with spontaneous AF initiation, wherein pacing is generally used to induce AF; the slow time course of AF progression; and the limited information about the evolving pAF substrate. Limitations in human preparations include procurement issues, restricted access to tissues other than right atrial appendage, and lack of control over confounding clinical variables, including comorbidities and pharmacological therapies. Patient-specific induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are a relatively novel option providing potentially unlimited supplies of human cardiomyocytes, and might become a useful resource for preclinical studies. Unfortunately, the phenotype of hiPSC-CMs remains generally immature as well as variable with source laboratory.
Table.
Novel antiarrhythmic agents (ClinicalTrials.gov or EU Clinical Trials Register identifiers).
| Target | Agent | Status |
|---|---|---|
| NaV and KV | Vernakalant | Promising phase I-III data both in terms of conversion and maintenance of nSR (see review: Vasc Health Risk Manag. 2013; 9: 165–175.) Phase IV trial ongoing |
| IK,ACh | NTC-801 | Phase II terminated; failed to meet primary end point to reduce AF burden or secondary end point to reduce AF duration or the number of AF episode |
| IKur | MK-0448 | Failed to alter ARP in humans |
| BMS-919373 | Ongoing clinical studies assessing AF burden in pAF patients (NCT02156076) and effect in ERP (NCT02153437) in subjects with dual chamber pacemaker | |
| F373280 | Currently being evaluated for its ability to maintain nSR after electrical cardioversion in pAF patients with HF (NCT01831856) | |
| XEND0103/ S66913 | Currently being evaluated in phase II clinical studies to assess efficacy in reducing AF burden in pAF patients (2014-002333-63) and pAF patients with implanted pacemaker (2013-004456-38) | |
| IK,ACh or dual IK,ACh / IKur inhibitor | OPC- 108459 | Ongoing phase I studies to assess safety (NCT02069119) and efficacy to cardiovert AF in paroxysmal and persistent AF (NCT01483183) |
| RyR | S107 | No human data |
| RyR | JTV-519 (K201) | Phase II terminated (NCT00626652), data not available |
| Gap Junction | ZP123 | Phase II terminated (NCT00901563), no data available |
| Gap Junction | GAP-134 | Phase I completed (NCT00510029, NCT00543946, NCT00783341) |
Computational modeling can provide an integrative framework to overcome some of the challenges. We have offered several examples of how cardiac electrophysiological models and computational pharmacology can be powerful tools to aid rational drug design and cardiac safety. We contend that mechanistic insight into AF triggers and substrates/maintenance is paramount for predicting the conditions of AF termination by antiarrhythmic drugs. This includes a multiscale understanding of how ionic, structural, and neurohormonal remodeling conspire to promote and sustain AF. Furthermore, we have shown that modeling and simulation provide a quantitative understanding of atrio-ventricular differences and a framework to understand how pharmacokinetic properties interact with a dynamically changing substrate. Many recent computational studies have highlighted the importance of accurately depicting channel interaction with drugs, including state-dependent binding, instead of relying on steady-state concentration response curves or EC50 values (Aguilar et al. 2015; Aguilar-Shardonofsky et al. 2012; Lee et al. 2016; Moreno et al. 2011; Morotti et al. 2015). These aspects are crucial for safer and more effective pharmacological anti-AF options.
Molecular modeling of ion channel structures and drug components has proven useful to predict the behavior of novel compounds (Gomez et al. 2014), and can potentially be exploited for drug design. Nevertheless, several challenges need to be addressed before we can leverage information from molecular modeling of cardiac ion channels and drug interaction for developing better therapeutic interventions – the most obvious being the lack of molecular structures for most ion channels. Progress in structural modeling of ion channels and molecular dynamics simulation, including membrane simulations with realistic lipid compositions and models of protein-protein interaction, will allow prediction of drug affinities and rate constants of drug bindings to various channel conformational states, which could then be used in higher scale simulations.
Population-based computational approaches have been recently developed (Britton et al. 2013; Sarkar & Sobie 2010; Sobie 2009) and proven valuable for understanding variability in cardiotoxicity (Sarkar & Sobie 2010) as well as identifying drug targets with favorable anti-arrhythmic properties (Cummins et al. 2014). Similar approaches could be applied to identify drug targets with AF-selective properties. Furthermore, computation permits one to build “populations of drugs” and use parameter sensitivity analyses to define optimal theoretical channel blockers (those with safer profile and broader therapeutic window). This will help to delineate the ideal properties for AF-specific modulation of APD, ERP, and arrhythmia (e.g., for INa: optimal ratio of late vs. peak INa block, state-dependence of block, drug binding kinetics). Definition of such ideal drug characteristics might generate predictions for novel/previously unappreciated effects of known drugs, which could be mapped on the simulated population. Known drugs with certain characteristics could then be screened experimentally to see if they have expected effects on atrial electrophysiological parameters (Lee et al. 2016). Mathematical modeling and simulation thus offer promising techniques for both top-down as well as bottom-up approaches to new drugs for AF rhythm control (Tveita & Lines 2016). Indeed, it is not surprising that the novel safety screening FDA proposal, Comprehensive in vitro Proarrhythmia Assay (CiPA), aims at moving from a predominantly traditional pharmacodynamics approach to an in silico and in vitro drug toxicity assessment, and to replace lone hERG testing with assessment of overall proarrhythmic risk, integrating electrophysiological effects of drugs on cardiac currents in heterologous expression systems in cellular computer models.
3. Non-pharmacological rhythm-control therapies
Rhythm control is currently the first line of action against AF. Rhythm control did not show improved survival over rate-control in AF patients (AFFIRM study), but long-term nSR control and anticoagulation therapy were associated with a lower risk of death (Corley et al. 2004). In most patients, at least one attempt at cardioversion (either electrical or chemical) to nSR will improve symptom status and minimize risk for lifelong AF, particularly in younger patients (January et al. 2014). For first episodes, electrical cardioversion is often the preferred strategy for rhythm control. In some patients, antiarrhythmic drugs are administered prior to cardioversion to increase the chance of successful reversion and to prevent early, intermediate, and late recurrence (Walkey et al. 2014). However, for long-term rhythm control, strategies include antiarrhythmic drug therapies (as addressed in sections 2-3), as well as catheter ablation, which uses either radiofrequency ablation, laser, or cryotherapy. Guidelines make a strong recommendation for catheter ablation for patients with symptomatic pAF who have failed treatment with at least one antiarrhythmic drug and a weak recommendation for catheter ablation as first-line therapy (Calkins et al. 2012).
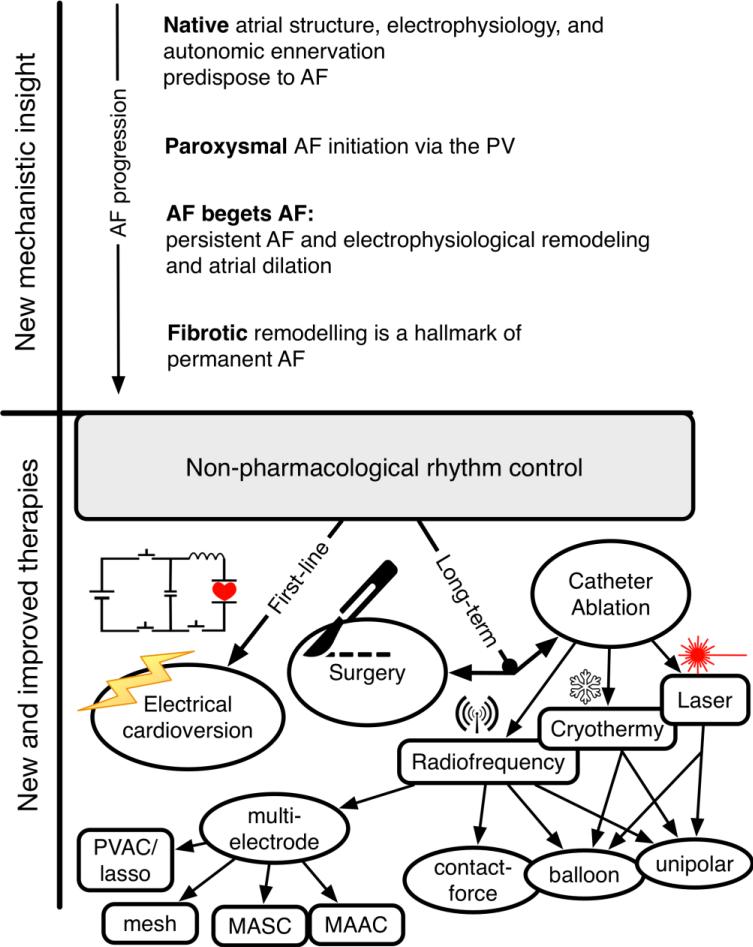
Biophysical modeling and simulation strategies have long been used to understand electrical cardioversion and have been reviewed elsewhere (Trayanova et al. 2011; Trayanova & Rantner 2014), and in more recent years used to evaluate ablative therapies (Guillem et al. 2016; Jacquemet 2016). Here, we offer an overview of how computational modeling has contributed to the research pipeline and new mechanistic insight, as well as novel and improved diagnostics and therapeutics (Fig. 3).
Figure 3. Biophysical simulation has provided both new mechanistic insight and contributed to improved therapeutics in AF.
Computation has provided fundamental understanding of AF mechanisms in terms of the contributions of native atrial heterogeneities, and stage-specific mechanisms as AF progressed from paroxysmal to persistent to permanent (top panel). These insights have enabled improvements to strategies for non-pharmacological rhythm control (summarized in the bottom panel). Notably, biophysical modeling and simulation is a viable strategy for therapy planning in the context of catheter ablation, as discussed in section 3.2.
3.1 The research pipeline and preclinical development
Defining mechanisms for AF in humans has been challenging because fibrillation exhibits complex, chaotic activations, and electrical mapping in patients is practically constrained to lower temporal resolutions and smaller spatial fields. Computational modeling can provide a quantitative framework for integrating multi scale data and understanding complex spatio-temporal dynamics that emerge at the level of the atria.
Basic science, theoretical, and methodological contributions
Early studies using computer simulations employed simple low dimensional models to tease out complex mechanisms underlying AF origins. Ashihara and colleagues investigated mechanisms underlying the longer duration of parasympathetic vs. sympathetically-mediated pAF, and found that dispersion of refractoriness in vagally-stimulated AF was a critical factor in maintenance of the arrhythmia (Ashihara et al. 2002). Other early studies used very simple geometries and rough anatomical approximations to demonstrate how coupling heterogeneities could result in AF-like behavior as opposed to stable atrial flutter, and confirmed an indicated role for drivers in AF (Ellis et al. 2000). Harrild and Henriquez used the finite-volume method to develop the first model of 3D conduction in a realistic atrial geometry (Harrild & Henriquez 2000). Other work employed simulation to test novel techniques for automatic identification of phase singularities incorporating mathematical convolution and image analysis. Notably, these simulations suggested that the established understanding that tissue size was a primary determinant of AF may have been overstated, as they employed an atrial grid size over an 11.1-fold range (Zou et al. 2002). Models have additionally been used to examine the concept of critical cardiac wavelength in the initiation and maintenance of AF, and concluded that a priori information about underlying wavelet dynamics is needed for a correct interpretation of the cardiac wavelength as used in clinical practice (Jacquemet et al. 2005). More recently, simpler computational models neglecting the influence of ionic dynamics have also been used to simulate AF. The essentially two-dimensional approach for thin-walled geometries of Herlin and Jacquemet employs the Eikonal-diffusion equation rather than a full reaction-diffusion system incorporating detailed cell-level electrophysiology. Their results demonstrate that these simpler models could indeed be used to realize similar dynamics as more complex ones, including variance of the level of complexity (i.e. the number of phase singularities) with substantial speedup (Herlin & Jacquemet 2011).
Computational tools have been developed to facilitate the design of in silico AF experiments. Patient-specific biophysical models of the human atria have been constructed from medical imaging of AF patients, often based on diffusion tensor magnetic resonance imaging (MRI) reconstructions of tissue geometry, including fiber orientation and detailed structure for the sino-atrial node. These are often combined with heterogeneous AP models, and used to simulate atrial electrophysiology in nSR as well as arrhythmia. Simulations recapitulate diverse forms of AF dynamics: stable rotors, wavelets broken by repolarization heterogeneities, and multiple unstable meandering wavelets. The use of multiple simulated substrates in concert with different initial conditions could prove a useful testing ground for the clinical utility of potential interventions, in order to identify substrate-specific optimal therapies for AF (Aslanidi et al. 2011; Colman et al. 2013; Krueger et al. 2014; Matene & Jacquemet 2012; McDowell et al. 2012; Schotten et al. 2012; Zhao et al. 2013). The 3D atria can also be incorporated into a torso model to simulate body-surface electrocardiogram (ECG) patterns: virtual atria models such as this can be used as a platform to provide in-depth insights into AF mechanisms beyond current technical capabilities of experiments or the clinic (Aslanidi et al. 2011).
Translational medicine – mechanistic insight
While the most significant mechanistic insights made by atrial simulation have been reviewed elsewhere (Trayanova 2014), the below will review newer work which has moved definitively into employing multiple modalities at the intersection of medicine, biology, and engineering to offer detailed mechanistic insight into AF origins.
Investigating the roles of structural and electrical heterogeneity and AF-associated remodeling
AF is underpinned by and potentiates both electrical and fibrotic remodeling (see section 4.2), further complicating the complex anatomical and electrical substrate of the human atria. Several groups have developed biophysically detailed image-based models of human AF including regional fibrosis to examine the role of structural and electrical heterogeneity in AF. These studies have found that including fibrosis reproduces activation as seen in patient data more precisely, and that fibrosis-associated conduction slowing (via gap junction remodeling) is necessary for reentry (Krueger et al. 2014; McDowell et al. 2012). Haissaguerre and colleagues furthermore recently showed that AF in persistent AF patients is driven by meandering, transitory reentries attached to fibrosis borders, which may be driven via a percolation mechanism (Haissaguerre et al. 2016).
Specific effects of AF-induced structural dissociation between the epicardial layer and the endocardial bundle network have additionally been investigated via a dual-layer computer model. Fibrosis-induced dissociation leads to more complex patterns of AF: as epi-endocardial dissociation allows fibrillation waves to propagate between epicardium and endocardium, and become visible as ‘breakthrough waves’ that increase AF complexity. Models support that this behavior can also increase AF stability (Gharaviri et al. 2012; Gharaviri et al. 2016; Verheule et al. 2014).
Computations have also elucidated the effects of electrophysiological heterogeneity and AF remodeling. (Aslanidi et al. 2009) found that shorter effective refractory periods in the LA translated into a shorter period of spiral rotation, such that reentry in the LA drove the overall excitation patterns in both atria. More complexly heterogeneous electrophysiological modeling in 2- and pseudo 3D models showed that IK1 heterogeneity was dominant compared to other currents in determining rotor drift direction in pAF, through its impact on the gradient of excitability (Calvo et al. 2014). Results from biophysically detailed human AF models have shown that tissue electrophysiological heterogeneity caused breakdown of normal wavefronts at rapid rates (Aslanidi et al. 2011). Electrical remodeling both stabilized and accelerated re-entrant excitation waves in a cAF model; results indicated that electrical remodeling produced regionally heterogeneous and shortened APD; these respectively facilitated initiation and maintenance of re-entrant waves (Colman et al. 2013). The role of non-myocyte cell types in creating a heterogeneous electrical substrate in AF was investigated by (McDowell et al. 2013), who showed that myofibroblast proliferation and subsequent local electrophysiological changes were the sufficient condition for formation of reentrant circuits following ectopic stimuli. While functional coupling between fibroblasts and cardiac myocytes has been demonstrated in animal models and cell cultures (Camelliti et al. 2004), it has yet to be shown in human tissue, and its physiological relevance has yet to be demonstrated in patients.
Remodeling of both structure and electrophysiology in the setting of AF has been the focus of a number of studies. Investigation into the mechanisms of pulmonary vein (PV) arrhythmogenecity via computational modeling offers a convenient illustration. One study concluded that PV reentrant activity depended critically on vein size and coupling properties; reentry occurred readily in PVs with realistic properties in the context of specific connection heterogeneities and could certainly contribute significantly to PV arrhythmogenesis (Cherry et al. 2007). Others have used multiscale 3D modeling to investigate the role of the PVs as a substrate for AF, resulting in novel insight. The authors created a detailed model of the cellular electrophysiology of the LA and PVs with tissue geometry and fiber orientation via high-resolution μCT data. The combination of electrical heterogeneity and conduction anisotropy between the PVs and LA tissues led to the generation of a high-frequency re-entrant source near the PV sleeves (Aslanidi et al. 2013). Progress in this vein has also employed novel techniques designed to speed-up computation times to enable clinically relevant time course for obtaining results. For instance, a rule-based approach was used to simulate AF initiation via PV activity. The model's purpose was to provide a platform wherein realistic anatomical structures, heterogeneous electrophysiology, and arrhythmogenic activity can be evaluated, and the authors suggested that such models could potentially be used in therapy planning in the future (Reumann et al. 2006).
Uncovering mechanisms underlying AF organization and termination
Biophysical modeling and simulation has permitted detailed investigation of several alternate hypotheses regarding AF organization and mechanisms of its termination. Haissaguerre et al. found that AF cycle length was inversely associated with the number of sources and that it was an important predictor of baseline duration of AF, its type, and ease of termination via catheter ablation (Haissaguerre et al. 2007), Vidmar et al. recently used activation times recorded during human AF to compute phase synchrony between tissue regions: domains controlled by spiral waves exhibited regions of high phase synchrony. When in silico analysis was applied to clinical data, it was shown that pharmaceutical intervention with ibutilide organizes activation by increasing the size of the synchronized region. A distribution of synchrony found in patients is inconsistent with distributions obtained from simulations that mimic multiwavelet reentry, but is consistent with a spatially conserved spiral wave surrounded by tissue with disorganized activation (Vidmar et al. 2015). Consistent with the concept of an organized rotor driving AF, it was shown that local catheter ablation may indeed terminate AF by several mechanisms relating to local tissue properties which are increasingly detectable in patients (Rappel et al. 2015). Biophysical modeling has also been used to investigate the spontaneous termination of AF, wherein results suggested that termination mechanisms were dependent on the underlying complexity of the arrhythmia (Uldry et al. 2012).
Simulations have similarly been used to investigate the mechanisms of observed fractionated electrograms forming in the posterior LA during pAF, wherein computer simulations of rotors helped to interpret results from patient recordings. Systolic interval shortening after either drift or acceleration of a source results in intermittent fibrillatory conduction and formation of fractionated electrograms at the posterior LA wall (Atienza et al. 2011). Tobon et al. used a realistic 3D model including fiber orientations, anisotropic conductivity and electrophysiological heterogeneity to correlate both simple and fractionated electrograms to atrial tachycardic/flutter/simple ectopy and fibrillatory activity, respectively; in the latter, it was possible to correlate morphology of electrograms to complex activation dynamics in AF tissue (Tobon et al. 2013).
The mechanisms promoting AF are varied and complex; computation continues to be an avenue to mechanistic insight when clinical data is difficult or impossible to obtain. To illustrate, parasympathetic and sympathetic nerve activity has been known to play a crucial role in AF. Recent studies have aimed to shed light into the role of local vagal stimulation and AF promotion. Hwang et al. employed an “octopus hypothesis”-based model of the ganglionated plexus and vagal nerve to show higher AF inducibility with higher acetylcholine concentrations (Hwang et al. 2016). Similarly, newer work employing a 3D electromechanical model of the human atria has probed the effects of AF electrical remodeling on atrial mechanics, which also presents challenges for in-vitro and in-vivo studies, illustrating that AF-induced electrical remodeling impaired atrial contraction via reduced intracellular Ca transients (Adeniran et al. 2015).
3.2 Computational cardiology
Within the last 10 years, work has also moved towards employing clinical measurements and computational simulations in concert. These clinically impactful studies have often employed relatively simple computational approaches. For instance, automated classification of spiral waves based on catheter recordings for qualitative comparison of arrhythmias has large clinical utility for therapy planning. A recent phenomenological model for cardiac electrical propagation simulated spiral waves on a 2D grid, and labeled these as stable, meandering, or breakup. The authors mimicked commonly used catheter types, star-shaped and circular, both of which record local readings from the atrial wall. Results suggest that qualitative wavefront activation patterns could be determined during AF without highly invasive mapping techniques (Alagoz et al. 2015). Notably, Rappel and Narayan theoretically and computationally addressed the minimum spatial and temporal required resolutions to determine stationary spiral waves and focal sources, and then applied these results to clinical data acquired during human AF via focal impulse and rotor mapping, which is both 2D and coarse. Their results showed theoretical and clinical evidence that the approach was able to reliably identify rotors and focal sources in human AF (Rappel & Narayan 2013).
Electrical cardioversion
Although the use of computational models to terminate fibrillation has been reviewed elsewhere (Schotten et al. 2012; Triedman et al. 2008), computations can continue to add value to clinical practice of electrical cardioversion in AF. One recent study employed an anatomically realistic computer model of human atria and torso to investigate the relationship between esophageal electric fields and atrial defibrillation thresholds, and found that starting with a clinically established electrode placement protocol and making small translational shifts in position has the potential to increase the probability of successful cardioversion on the first shock (Fitch & de Jongh Curry 2015).
Electrical mapping and frequency analysis
Modeling and simulation can also be employed to encourage incremental improvement to existing clinical practice. Catheter ablation relies absolutely on contemporary electrical mapping methods, which, in turn, are highly dependent on the accuracy of anatomic localization of rotor sources within the atria. DF is a key parameter for AF analysis from intracardiac recordings. Simulations have been used to understand and improve dominant DF analysis at high noise levels (Ciaccio et al. 2009) and to test measures of signal amplitude aimed at differentiation of the rotor core and assist in clinical rotor mapping (Ganesan et al. 2013a). Others have addressed the classical preprocessing approach for mapping signals (Botteron & Smith 1995, 1996) via both simulations and real electrogram recordings and showed that this classical approach presents some weaknesses, and were able to move towards recommendations for improvements (Castells et al. 2014). Recently, simulated atrial intracardiac electrograms were employed to test a computationally-efficient method for localization of the spiral wave core and showed that the method could efficiently localize the spiral wavefront with either chirality (Shariat et al. 2015).
Furthermore, combination of 3D atrial and torso models may provide an additional avenue to assimilate clinical observation. In a recent study on non-invasive cardiac mapping, the authors used computer simulations to find that the combination of body surface potential mapping with dominant frequency analysis enable the noninvasive localization of atrial reentries during AF and may promote a rationale for personalized diagnosis and treatment of patients with AF (Atienza et al. 2015). A recently developed 3D human atrial and torso model was also used to develop and test an algorithm to find the location of a focal stimulus from a 64-lead ECG system (Alday et al. 2015).
A number of studies have employed computational modeling to evaluate the accuracy of noninvasive estimation of DF measurements via inverse modeling of echocardiography. Clinical and computational data are combined in studies that use realistic models of the atria and torso anatomy. The inverse problem solution was able to reconstruct the frequency spectrum and the DF maps with relative errors much smaller as compared to reconstruction of the electrograms or the instantaneous phase, revealing that noninvasive reconstruction of atrial frequency maps can be achieved by solving the inverse problem of electrocardiography with a higher accuracy than temporal distribution patterns (Hocini et al. 2015; Pedron-Torrecilla et al. 2016).
Ablation modeling
A comprehensive review of simulations of catheter ablation in computer models of the atria has recently been described elsewhere (Jacquemet 2016). Jacquemet notes that detailed biophysical models can form a platform for integration of pathophysiological data, which can then be used to coalesce understanding of mechanisms and provide theoretical basis for substrate-specific ablation strategies. Here, we offer a brief overview of the most impactful studies and a take on recent work in ablative virtual electrophysiology wherein assumed substrates of AF can be incorporated into computational models, and modeling studies can be used to test ablation strategies and clinical take-aways.
Earlier work used a biophysical 3D model of the human atria to evaluate several different ablation patterns, and compared results to both Cox's MAZE III procedure as reference and clinical data. Studies were able to recapitulate findings made in vivo, demonstrating the ability of biophysical modeling as a useful tool in evaluating current clinical practice and making recommendations as to the gold standard procedures, and noting that the models could be parameterized to individual patients to yield an effective tool for future investigation of tailored ablation strategies (Dang et al. 2005) (Reumann et al. 2008; Ruchat et al. 2007).
Recent proof-of-concept virtual electrophysiology studies investigated the role of fibrosis in ablation failure using detailed patient-specific MRI-based models; as for earlier work, results showed that patient-specific distribution of fibrosis was a critical factor in AF initiation and maintenance. Modeling ablation lesions in restricted regions of persistent rotor core meander rendered the atria uninducible (McDowell et al. 2015). In a prediction of optimal ablation targets using a novel approach based on flow network theory, in silico ablation was applied to a ‘minimum cut’ – the smallest amount of tissue separating the flow. Resultant lesions were similar in length and location to clinical ablation lesions for these patients (Zahid et al. 2016). These studies demonstrate that a patient-specific modeling approach to non-invasively identify AF ablation targets prior to the clinical procedure may present a powerful tool for optimizing ablation procedures.
3.3 Summary and recommendations
We have offered several examples wherein modeling and simulation have served as useful tools to both contribute fundamental understanding of AF as well as more detailed mechanistic insight via biophysically-accurate modeling frameworks. Biophysical-based computational modeling may offer mechanistic insight when experimental or clinical data are difficult or impossible to obtain. Mathematical models have proven their value for basic cardiac research, not just confirming experimental findings, but refining our understanding of biological systems and facilitating new mechanistic predictions, which have been subsequently tested by further experimentation. We have provided several examples of these. Now, computational models are poised to deliver breakthroughs at the bedside. One case in point is the use of clinically-derived computer models to optimize catheter ablation. In a recent retrospective study, Hwang et al. validated the outcomes of a series of patient-specific computer models with subsequent clinical ablations, demonstrating the usefulness of computer models in clinical practice for planning personalized pre-intervention strategies (Hwang et al. 2014). A prospective clinical trial is currently recruiting participants (NCT02171364) to compare virtual ablation based on 3D CT images of AF patients with conventional ablation procedure in the clinical setting.
Thus detailed biophysical models may form a platform for integration of pathophysiological data which can both used to coalesce mechanistic insight and provide the basis for substrate- and patient-specific ablation strategies. Nevertheless, it is important to remark that lack of sufficient clinical data to inform personalized models (including, e.g., fiber orientation, fibrosis, limited spatial and temporal resolution of available data, etc.) is a clear hurdle to model validation and clinical inroads. In addition, there are noted discrepancies between the stable AF often represented in models and the observed variability in clinical recordings from patients. Future lines of improvement of extant organ-level mathematical models may address standard inclusion of natural variability in cell electrophysiology (i.e. a population of models approach), inclusion of patient-specific electrophysiology via inverse modeling of the ECG, consistent representation of fibrosis from validated image-based methodologies, and representation of atrial innervation; work in these directions has already begun. Furthermore, as detailed and 3D patient-specific biophysical models of AF employed in translational and clinical medicine to date may be limited by time-consuming image-processing and high computational cost compared to clinical time scales, focus areas of research for the next years should continue to address model tractability via automatization, parameter reduction, and sensitivity analyses.
4. Upstream therapy approaches
Upstream therapy broadly refers to agents that target molecules that are upstream (or independent) of ion channels, and prevent or delay myocardial remodeling associated with hypertension, HF, or inflammation (e.g., post-operative). By limiting the AF substrate, these agents may oppose the development of new AF (primary prevention) or its recurrence or progression to permanent AF (secondary prevention). Upstream therapies may be more effective and safer than ion channel blockade, particularly for patients with structural heart disease, at increased risk of torsade de pointes with antiarrhythmic drugs.
4.1 Renin-Angiotensin-Aldosterone System (RAAS)
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ARBs) inhibit the arrhythmogenic effects of Angiotensin II, which is involved in myocardial fibrosis, inflammation, oxidative stress, electrical abnormalities, and impaired Ca handling in AF and other cardiovascular morbidities. There is evidence of antifibrotic and antifibrillatory effects of angiotensin-converting enzyme inhibitors and ARBs in various experimental models (Goette et al. 2000a; Goette et al. 2000b; Kumagai et al. 2003b; Li et al. 1999). Retrospective studies indicate that RAAS inhibition may reduce the new onset of AF in patients with left ventricular hypertrophy or HF, but not in post-myocardial infarction patients, whereas it remains uncertain whether RAAS inhibition is effective in the secondary prevention of AF (post-cardioversion) (Healey et al. 2005; Schneider et al. 2010). The Angiotensin II-Antagonist in pAF (ANTIPAF) trial (NCT00098137) showed that the ARB olmesartan does not reduce the AF burden compared with placebo during 1-year follow-up in patients with pAF without structural heart disease (Goette et al. 2012). The progression from pAF to persistent AF in these patients was not altered by ARB therapy, suggesting that ARBs do not have an antiarrhythmic effect and/or do not affect the progression of the arrhythmic substrate per se. Aldosterone antagonists reduce fibrosis in animal models (Shroff et al. 2006; Zhao et al. 2010), but did not appear to prevent onset of AF in a retrospective study of human HF (Khatib et al. 2013). It remains to be investigated whether aldosterone may be more helpful in preventing the progression rather than the onset of AF. Ongoing clinical trials are designed to evaluate the efficacy of aldosterone antagonists in AF and other cardiovascular disease such as HF (Desai et al. 2011; Rossi et al. 2013).
4.2 Oxidative Stress and Inflammation
There is evidence of enhanced oxidative stress, i.e., elevated level of reactive oxygen species (ROS), in atrial samples from patients with AF (Mihm et al. 2001), and there is correlation between oxidative stress and ERP shortening in animal models (Carnes et al. 2001). Despite an expanding amount of evidence implicating oxidative stress to AF, there are surprisingly little mechanistic data linking changes in redox homeostasis to the associated cellular dysfunction, including the source of increased ROS and their direct effects on myocardial electrical targets and properties. An altered redox environment may also promote adverse structural remodeling, providing a substrate for AF sustenance. In particular, inflammation and fibrosis have been linked to changes in the redox system (Ishii et al. 2005; Rudolph et al. 2010), and atrial strain may also promote ROS release (Hoit et al. 1995). Thus, blunting pathological oxidative stress may be an effective strategy for AF therapy. Indeed, treatment with anti-oxidant/anti-inflammatory agents has been shown to reduce atrial electrical remodeling, fibrosis, and AF in animal models (Carnes et al. 2001; Shiroshita-Takeshita et al. 2004), though the clinical outcome of many of these agents is not well established. For example, antioxidant vitamins have not been shown to exert a marked antiarrhythmic effect in clinical trials (Rasoli et al. 2011), and the clinical utility of n-3 polyunsaturated fatty acids in the management of AF is controversial (Christou et al. 2015). A recently concluded clinical trial (PAFRIOSIES, pAF: Role of Inflammation, Oxidative Stress Injury and Effect of Statins, NCT00321802), designed to determine the effect of statin (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) therapy for prevention of AF in pacemaker and non-pacemaker patients with pAF, might confirm promising results (Fauchier et al. 2013) on the effect of statins on clinical outcome in AF.
ROS and CaMKII inhibitors
A recent review suggested future antioxidant drug design would benefit from focusing more on specific antioxidants that either target the sources of ROS/reactive nitrogen species production directly (e.g. nitric oxide synthase re-couplers, or inhibitors of xanthine oxidoreductase), or enhance the expression of endogenous antioxidant defenses (Simon et al. 2016). There may also be therapeutic potential for agents that act as a concentrated ROS scavenger in a very specific location (e.g., mitochondrial ROS scavengers), or which pharmacologically modify downstream effectors of oxidant signaling (e.g., CaMKII inhibitors).
Oxidation of CaMKII is increased in AF patients (Purohit et al. 2013). CaMKII expression and activity are increased in animal models of AF (Greiser et al. 2009; Wakili et al. 2010) as well as in cAF patients (Neef et al. 2010; Tessier et al. 1999; Voigt et al. 2012). While high atrial rates and neuronal autonomic imbalance (e.g., increase in sympathetic activity) may contribute to CaMKII activation, oxidative stress may be an important player in promoting CaMKII hyperactivation.
It has also been suggested that the CaMKII activation mediated by the guanine nucleotide exchange factor Epac involves phosphorylation of CaMKII-Thr287 by PKC type-ε (PKCε) (Oestreich et al. 2009), and upregulation of PKCε in cAF patients (Voigt et al. 2007) might contribute to increased CaMKII activity (besides regulating IK,ACh). Since PKCε translocation to the membrane is increased in atrial myocytes following in vitro tachypacing (Makary et al. 2011), this might promote local atrial tachycardia-dependent CaMKII stimulation, although this remains to be proven in future studies. Unfortunately, there are currently no agents that allow testing the effectiveness of inhibiting these targets in clinical studies.
4.3 Summary and recommendations
While treatment with anti-oxidant/anti-inflammatory agents has shown promise in animal models to reduce AF-associated structural remodeling and improve function, the clinical outcome for primary or secondary prevention of many of these agents is not well established, and possibly dependent on the etiology of AF and associated comorbidities.
Many of these proposed “upstream” interventions may influence not only organ structural remodeling (see section 3.1 for modeling of structural alterations), but also electrical remodeling and intracellular signaling (e.g., CaMKII). Indeed, most described AF changes relate to altered cellular signal transduction. Stimulation of various signaling pathways, altered atrial metabolism, and increased oxidative stress (Ghezelbash et al. 2015; Goette et al. 2002) and their complex interactions may exert acute electrophysiological abnormalities, and accumulation of these changes (e.g., during subsequent paroxysms of AF) may cause prolonged alterations in atrial signal transduction and gene expression. The multiplicity of targets and the complex crosstalk between cellular signaling pathways create a complex and non-linear feedback system that can benefit from quantitative computational approaches. As a case in point, crosstalk and/or synergy between β-adrenergic and parasympathetic or CaMKII pathways, and the effects of oxidative stress (and oxidation vs. nitrosylation) deserve further investigation and would be important extensions for future atrial modeling studies, as done in ventricular models (Heijman et al. 2011; Morotti et al. 2014; Soltis & Saucerman 2010). In addition, the resulting altered Ca signaling may be involved in AF-associated remodeling of ion-channel expression and/or function (Makary et al. 2011; Qi et al. 2008). The incorporation of such processes would be a challenging next step in modeling AF. Recently, atrial-specific upregulation of miR-31 in human AF has been identified as a key mechanism causing atrial dystrophin and nNOS depletion, which in turns contributes to proarrhythmic atrial electrical remodeling (Reilly et al. 2016). Indeed, the downstream effects of nNOS inhibition were incorporated in an experimentally calibrated population of human atrial cells model, which supported experimental findings on the role of nNOS depletion in AF (Reilly et al. 2016).
Models of mitochondrial energetics have also been developed and coupled with electrophysiological models to investigate the key role of energetics in modulating ventricular myocyte mechanical activity and ion concentration gradients (e.g., (Cortassa et al. 2006)), especially during impaired metabolic states in pathological conditions, such as ischemia and HF. These models could provide useful frameworks in the context of AF modeling, e.g., to understand how changes in the electrical (and contractile) activity of atrial myocytes influence and are influenced by mitochondrial energetics through the ATP, Ca, and Na concentrations in the myoplasmic and mitochondrial matrix compartments: acting on controlling cytosolic Ca and Na levels can indirectly target the mitochondrial source of ROS.
Development of urgently needed new strategies for AF treatment hinges upon improved understanding of how the changes in structural pathogenic alterations synergize with ionic and Ca handling remodeling to trigger and sustain arrhythmia in the atria. We contend that the use of computational models as outlined above may shed mechanistic insight into AF management.
5. Research Horizons
5.1 Exploring multi-physics simulation in AF
Notwithstanding the preponderance of work presented here on multiscale human atrial electrophysiology, detailed biophysical simulations focused on both atrial mechanics and hemodynamics may hold the key to further mechanistic insight and innovative therapeutics. Studies combining the effects of stretch activated-channels in 3D atria predicted that heterogeneity in these current contributed to filament stabilization (Yamazaki et al. 2012). Others have used biophysical modeling and simulation to investigate how hemodynamic perturbations affect the potential embolization of blood clots that ultimately cause stroke. Results suggest that these reduce cardiac output and cycle length induced by AF can significant increase incidence of embolism (Choi et al. 2013). The same group recently extended their work to simulate a stroke-prevention device designed to deflect a blood clot. The results suggest that the deflector stent in the aortic branch could possibly function as an effective stroke-prevention device and motivate pre-clinical animal studies (Choi et al. 2015). Other studies have employed circulatory models to investigate the implications of rate-control approaches to AF management and suggest that lower heart rates during permanent AF relate to improved hemodynamic parameters, cardiac efficiency, and lower oxygen consumption. A future combination of models of rate and rhythm control may offer an interesting perspective in patients receiving both therapeutic regimes (Anselmino et al. 2015). We contend that a multi-physics approach, integrating human atrial electrophysiology, mechanics, and hemodynamics could potentially yield novel insight and therapy regimes for both the arrhythmia and its consequences.
5.2 Clinical decision support and integration with other modalities
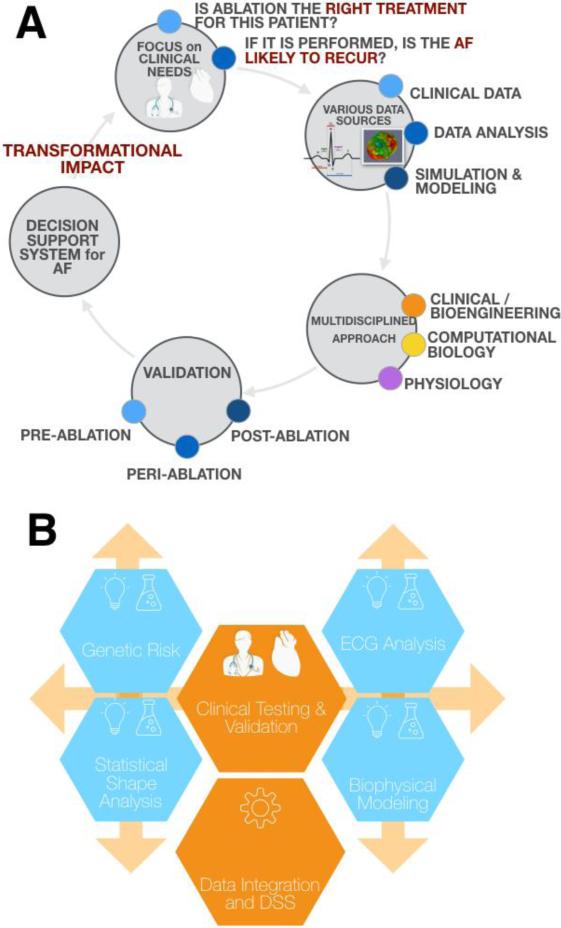
Despite advances in addressing a variety of AF targets (e.g. rotors, fractionated electrograms, ganglionated plexi, PV isolation, and ectopic foci), early recurrence of AF occurs in ~40% of patients (Oral et al. 2002), and late recurrence in as much as 50% for a single ablation procedure (Ganesan et al. 2013b). Further complicating treatment strategy is that in patients that may benefit from cardioversion, efficacy is significantly increased if the procedure is performed or regime is started as early as possible. An overall ambition is to integrate existing tools and related datasets presently at our disposal (Schotten et al. 2012) to build and validate a clinical decision support system for AF therapy planning (Fig. 4). Specifically, the system could provide support for clinicians to determine which is the right treatment for the patient in question, and, when ablation is performed or drug therapy started, if the AF is likely to recur.
Figure 4. A new multidisciplinary approach to decision support employing computational models.
One direction for cutting-edge research may be integration of biophysical modeling and simulation with other research and risk-assessment modalities to serve clinical needs for decision support (A). Such an approach would likely be iterative and require strongly multidisciplinary teams as well as ongoing validation (B).
While biophysical modeling of the atria and AF has been highlighted in this review, it may be that combination of computational strategies with diverse modalities will yield the greatest insight for decision-making and therapy planning. Genetic profiling for patient stratification is one such area. Common clinical risk factors for AF were identified and combined into the Framingham risk score (Schnabel et al. 2009). Addition of a genetic risk score based on common genetic variants at 12 markers identified in genome wide association studies moderately improved the classification performance (Tada et al. 2014). Recent studies have shown an association between genetic variants and response to AF ablation (Shoemaker et al. 2015) but the relative importance of genetic and clinical risk factors is unknown thus far.
Similarly, there has been very active research in computational anatomy over the last decade in order to provide tools for the statistical analysis of 3D shapes in a patient population. Applying such methods to a database of AF patients would make it possible to extract emergent characteristics of the patient cohort (McLeod et al. 2015). Statistical shape analysis has shown its potential to assess the severity of a disease and predict the shape evolution due to remodeling, which would be a key strength in AF. Since the direct biophysical modeling of biological phenomena involved in long-term cardiac remodeling is very difficult, recent advances in deformation-based shape modeling could be used to assess the population variability of model variables and to model the evolution of the heart over time. Although shape modeling has been used in cardiac applications to a high degree, it has been underused in delineating functional changes in the context of AF thus far.
Inverse modeling related to ECG measurements and rotor/source localization was highlighted in section 3.2. More generally, several techniques for the analysis of electrophysiological properties of the atria and the complexity of AF from the surface ECG have been suggested (Lankveld et al. 2016; Schotten et al. 2012). Investigations have been limited in size (up to 50 patients) and heterogeneous in terms of predicted outcome. However, Schotten et al. have developed a unique software package for advanced analysis of AF ECGs, which could be employed to perform quantitative analysis of atrial frequency and complexity of AF conduction patterns (Schotten et al. 2012). Additionally, inverse problems have benefited from the boom of new mathematical solution strategies as well as more detailed information available through additional clinical modalities, e.g. from MRI for mechanical activity, which is expected to significantly increase the quality of estimated electrical and mechanical activity. Combining these state-of-the-art approaches from inverse modeling with data assimilation techniques for more precise estimation of the electro-mechanical activity of the human heart is on the horizon.
Potentially combining patient-specific genetic information with personalized computational models of cardiac cells, tissues, structure and function as well as population-based shape models is unprecedented in AF research and cardiology in general. The independence of information arising from these sources is a major point. By spanning the scale from associative genetic data to biophysically based patient-specific simulation to data-driven population-based modeling, new approaches in this direction may capture information from the most basic biological level up to integrated functional outcomes.
Lastly, it may be crucial to follow and integrate trends in new technologies to reap maximal benefit from biophysical modeling and simulation techniques. A recent study used non-biophysically based simulation strategies to evaluate whether short-term daily, temporally-optimized ECG monitoring would permit a high percentage of episode detection compared to 1-day Holter monitoring, to great effect (Censi et al. 2013). This implies certain future potential for combination with patient-specific computational models for out-of-hospital detection. Furthermore, and in keeping with the decision support system framework suggested above, novel work suggests that a visual aid be used by clinicians to support the process of informing patients about a critical decision. To illustrate the method, a decision tree for thromboembolic risk prevention in patients with non-valvular AF is described producing new graphical outputs representing the distribution of outcomes over the lifetime for different decision options. Integration of such tools with patient-specific simulation could revolutionize patient care in years to come (Rubrichi et al. 2015). Finally, technologies in wearables and mobile technologies are diffusing into medical practice swiftly; hand-held devices, such as smartphones, can record short-duration (e.g., 1-minute) ECGs. Effectiveness in identifying patients with pAF was recently evaluated: daily monitoring detected half of patients as compared to implantable devices (Yano et al. 2016), a score that could arguably be improved via integration with other modalities and the insight offered by computational simulation.
5.3 Final Remarks
In this review, we have examined first-line anti-AF rhythm-control therapies, and how their present and future have been supported by biophysical computational modeling. First, we have presented ionic anti-AF targets (Section 2), including K, Na, Ca, RyR channels, as well as polytherapy, and highlighted how molecular modeling, kinetic models of drug-channel interaction, cardiac electrophysiological models and new computational approaches can aid rational drug design to enhance drug efficacy and cardiac safety. Indeed, implementation of these strategies is in line with current regulatory efforts aiming at using in silico platform for assessing drug toxicity and proarrhythmic risk.
We have additionally reviewed non-pharmacological rhythm control therapies, electrical cardioversion and ablation (Section 3), which have been substantially developed in the past decade and proven successful to reduce the symptomatic burden associated with AF. We have highlighted areas, namely clinically-derived computer models to optimize catheter ablation, in which computational models are poised to deliver breakthroughs at the bedside, and identified roadblocks and future lines of improvements, such as the lack of sufficient clinical data to inform personalized models and issues of computational tractability for clinically-relevant timescales.
In Section 4, we have illustrated upstream therapy approaches, which target molecules that are upstream (or independent) of ion channels, and prevent or delay myocardial remodeling associated with hypertension, HF, or inflammation. The clinical outcome of many of these agents is not well established, and possibly dependent on the etiology of AF and associated comorbidities. While not much has yet been done in the modeling arena, we have proposed potentially important extensions for future atrial modeling studies, including cellular signaling and energetics.
Finally, we have presented the next generation of studies and translational therapies (Section 5), which could be advanced by multi-physics approaches, integrating human atrial electrophysiology, mechanics, and hemodynamics. Furthermore, we suggest modeling and computational strategies could be integrated with novel screening tools, such as genetic screening, to aid patient-specific therapeutics. Work in many of these research frontiers has already begun, and holds great promise for revolutionizing future approaches to anti-AF therapy.
Acknowledgements
This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (R01HL131517 to EG), the American Heart Association (15SDG24910015 to EG), and via the Research Council of Norway through its support of the Center for Biomedical Computing and Center for Cardiological Innovation at Simula Research Laboratory (M.M.M.).
Abbreviations
- AF
atrial fibrillation
- AP
action potential
- APD
action potential duration
- ARB
angiotensin receptor blocker
- cAF
chronic AF
- CaMKII
Ca/calmodulin dependent protein kinase II
- CV
conduction velocity
- DAD
delayed afterdepolarization
- DF
dominant frequency
- EAD
early afterdepolarization
- ECG
electrocardiogram
- ERP
effective refractory period
- If
hyperpolarization-activated cyclic nucleotide-gated ‘funny’ current
- IK1
inward-rectifier K current
- IK,Ach
acetylcholine-dependent K current
- IK,Ca
small-conductance Ca-activated K current
- IKATP
ATP-sensitive K current
- IKr
rapidly activating delayed rectifier K current
- IKs
slowly activating delayed rectifier K current
- IKur
ultra-rapid delayed rectifier K current
- INa
Na current
- INaL
late Na current
- Ito
transient outward K current
- K2P
2-pore domain K (channel)
- LA
left atrium
- MRI
magnetic resonance imaging
- nSR
normal sinus rhythm
- pAF
paroxysmal AF
- PKC
protein kinase C
- PKCε
PKC type-ε
- PV
pulmonary vein
- RAAS
Renin-Angiotensin-Aldosterone System
- RMP
resting membrane potential
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SK
small-conductance calcium-activated K (channel)
- SR
sarcoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Reference List
- Adeniran I, MacIver DH, Garratt CJ, Ye J, Hancox JC, Zhang H. Effects of Persistent Atrial Fibrillation-Induced Electrical Remodeling on Atrial Electro-Mechanics - Insights from a 3D Model of the Human Atria. PloS one. 2015;10:e0142397. doi: 10.1371/journal.pone.0142397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar M, Nattel S. The Past, Present, and Potential Future of Sodium Channel Block as an Atrial Fibrillation Suppressing Strategy. Journal of cardiovascular pharmacology. 2015;66:432–440. doi: 10.1097/FJC.0000000000000271. [DOI] [PubMed] [Google Scholar]
- Aguilar M, Xiong F, Qi XY, Comtois P, Nattel S. Potassium Channel Blockade Enhances Atrial Fibrillation-Selective Antiarrhythmic Effects of Optimized State-Dependent Sodium Channel Blockade. Circulation. 2015;132:2203–2211. doi: 10.1161/CIRCULATIONAHA.115.018016. [DOI] [PubMed] [Google Scholar]
- Aguilar-Shardonofsky M, Vigmond EJ, Nattel S, Comtois P. In silico optimization of atrial fibrillation-selective sodium channel blocker pharmacodynamics. Biophys J. 2012;102:951–960. doi: 10.1016/j.bpj.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagoz C, Guez A, Cohen A, Bullinga JR. Spiral wave classification using normalized compression distance: Towards atrial tissue spatiotemporal electrophysiological behavior characterization.. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2015. pp. 4503–4506. [DOI] [PubMed] [Google Scholar]
- Alday EA, Colman MA, Langley P, Butters TD, Higham J, Workman AJ, Hancox JC, Zhang H. A new algorithm to diagnose atrial ectopic origin from multi lead ECG systems--insights from 3D virtual human atria and torso. PLoS computational biology. 2015;11:e1004026. doi: 10.1371/journal.pcbi.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circulation research. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- Anselmino M, Scarsoglio S, Camporeale C, Saglietto A, Gaita F, Ridolfi L. Rate control management of atrial fibrillation: may a mathematical model suggest an ideal heart rate? PloS one. 2015;10:e0119868. doi: 10.1371/journal.pone.0119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. Annals of the New York Academy of Sciences. 2010;1188:78–86. doi: 10.1111/j.1749-6632.2009.05086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashihara T, Yao T, Namba T, Kawase A, Ikeda T, Nakazawa K, Ito M. Differences in sympathetic and vagal effects on paroxysmal atrial fibrillation: a simulation study. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56(Suppl 2):359s–363s. doi: 10.1016/s0753-3322(02)00317-7. [DOI] [PubMed] [Google Scholar]
- Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV, Zhang H. 3D virtual human atria: A computational platform for studying clinical atrial fibrillation. Progress in biophysics and molecular biology. 2011;107:156–168. doi: 10.1016/j.pbiomolbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidi OV, Colman MA, Varela M, Zhao J, Smaill BH, Hancox JC, Boyett MR, Zhang H. Heterogeneous and anisotropic integrative model of pulmonary veins: computational study of arrhythmogenic substrate for atrial fibrillation. Interface focus. 2013;3:20120069. doi: 10.1098/rsfs.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidi OV, Robinson R, Cheverton D, Boyett MR, Zhang H. Electrophysiological substrate for a dominant reentrant source during atrial fibrillation.. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2009. pp. 2819–2822. [DOI] [PubMed] [Google Scholar]
- Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martinez-Alzamora N, Gonzalez-Torrecilla E, Arenal A, Fernandez-Aviles F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza F, Climent AM, Guillem MS, Berenfeld O. Frontiers in Non-invasive Cardiac Mapping: Rotors in Atrial Fibrillation-Body Surface Frequency-Phase Mapping. Cardiac electrophysiology clinics. 2015;7:59–69. doi: 10.1016/j.ccep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. International journal of cardiology. 2013;167:1807–1824. doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd ed. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. [Google Scholar]
- Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–225. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- Botteron GW, Smith JM. A technique for measurement of the extent of spatial organization of atrial activation during atrial fibrillation in the intact human heart. IEEE transactions on bio-medical engineering. 1995;42:579–586. doi: 10.1109/10.387197. [DOI] [PubMed] [Google Scholar]
- Botteron GW, Smith JM. Quantitative assessment of the spatial organization of atrial fibrillation in the intact human heart. Circulation. 1996;93:513–518. doi: 10.1161/01.cir.93.3.513. [DOI] [PubMed] [Google Scholar]
- Brandt MC, Priebe L, Bohle T, Sudkamp M, Beuckelmann DJ. The ultrarapid and the transient outward K+ current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–1896. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- Britton OJ, Bueno-Orovio A, Van Ammel K, Lu HR, Towart R, Gallacher DJ, Rodriguez B. Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2098–2105. doi: 10.1073/pnas.1304382110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001a;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001b;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Barajas-Martinez H, Hu D, Zygmunt AC, Cordeiro JM, Moise NS, Kornreich BG, Belardinelli L, Antzelevitch C. Ranolazine Effectively Suppresses Atrial Fibrillation in the Setting of Heart Failure. Circulation. Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block as a strategy for suppression of atrial fibrillation. Annals of the New York Academy of Sciences. 2008;1123:105–112. doi: 10.1196/annals.1420.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of ranolazine and dronedarone to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero R, de la Fuente MG, Gómez R, Barana A, Amorós I, Dolz-Gaitón P, Osuna L, Almendral J, Atienza F, Fernández-Avilés F, Pita A, Rodríguez-Roda J, Pinto Á, Tamargo J, Delpón E. In Humans, Chronic Atrial Fibrillation Decreases the Transient Outward Current and Ultrarapid Component of the Delayed Rectifier Current Differentially on Each Atria and Increases the Slow Component of the Delayed Rectifier Current in Both. Journal of the American College of Cardiology. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Heart Rhythm Society Task Force on, C., & Surgical Ablation of Atrial, F. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:632–696. e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Calvo CJ, Deo M, Zlochiver S, Millet J, Berenfeld O. Attraction of rotors to the pulmonary veins in paroxysmal atrial fibrillation: a modeling study. Biophys J. 2014;106:1811–1821. doi: 10.1016/j.bpj.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circulation research. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European heart journal. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circulation research. 2001;89:E32–38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- Castells F, Cervigon R, Millet J. On the preprocessing of atrial electrograms in atrial fibrillation: understanding Botteron's approach. Pacing and clinical electrophysiology : PACE. 2014;37:133–143. doi: 10.1111/pace.12288. [DOI] [PubMed] [Google Scholar]
- Censi F, Calcagnini G, Mattei E, Gargaro A, Biancalana G, Capucci A. Simulation of monitoring strategies for atrial arrhythmia detection. Annali dell'Istituto superiore di sanita. 2013;49:176–182. doi: 10.4415/ANN_13_02_09. [DOI] [PubMed] [Google Scholar]
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry EM, Ehrlich JR, Nattel S, Fenton FH. Pulmonary vein reentry--properties and size matter: insights from a computational analysis. Heart rhythm : the official journal of the Heart Rhythm Society. 2007;4:1553–1562. doi: 10.1016/j.hrthm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Cherry EM, Hastings HM, Evans SJ. Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells. Progress in biophysics and molecular biology. 2008;98:24–37. doi: 10.1016/j.pbiomolbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Navia JA, Kassab GS. Stroke propensity is increased under atrial fibrillation hemodynamics: a simulation study. PloS one. 2013;8:e73485. doi: 10.1371/journal.pone.0073485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Navia JA, Kassab GS. Thrombus deflector stent for stroke prevention: A simulation study. Journal of biomechanics. 2015;48:1789–1795. doi: 10.1016/j.jbiomech.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, Varro A, Dobrev D, Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou GA, Christou KA, Korantzopoulos P, Rizos EC, Nikas DN, Goudevenos JA. The Current Role of Omega-3 Fatty Acids in the Management of Atrial Fibrillation. International journal of molecular sciences. 2015;16:22870–22887. doi: 10.3390/ijms160922870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio EJ, Biviano AB, Whang W, Wit AL, Garan H, Coromilas J. New methods for estimating local electrical activation rate during atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6:21–32. doi: 10.1016/j.hrthm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Climent AM, Guillem MS, Fuentes L, Lee P, Bollensdorff C, Fernandez-Santos ME, Suarez-Sancho S, Sanz-Ruiz R, Sanchez PL, Atienza F, Fernandez-Aviles F. Role of atrial tissue remodeling on rotor dynamics: an in vitro study. Am J Physiol Heart Circ Physiol. 2015;309:H1964–1973. doi: 10.1152/ajpheart.00055.2015. [DOI] [PubMed] [Google Scholar]
- Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt C, Hancox JC, Zhang H. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria. J Physiol. 2013;591:4249–4272. doi: 10.1113/jphysiol.2013.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295:H1489–1504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, O'Rourke B, Jacques R, Tseng HJ, Marban E, Winslow RL. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J. 2006;91:1564–1589. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Cummins MA, Dalal PJ, Bugana M, Severi S, Sobie EA. Comprehensive analyses of ventricular myocyte models identify targets exhibiting favorable rate dependence. PLoS computational biology. 2014;10:e1003543. doi: 10.1371/journal.pcbi.1003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Virag N, Ihara Z, Jacquemet V, Vesin JM, Schlaepfer J, Ruchat P, Kappenberger L. Evaluation of ablation patterns using a biophysical model of atrial fibrillation. Annals of biomedical engineering. 2005;33:465–474. doi: 10.1007/s10439-005-2502-7. [DOI] [PubMed] [Google Scholar]
- Daoud EG, Knight BP, Weiss R, Bahu M, Paladino W, Goyal R, Man KC, Strickberger SA, Morady F. Effect of verapamil and procainamide on atrial fibrillation-induced electrical remodeling in humans. Circulation. 1997;96:1542–1550. doi: 10.1161/01.cir.96.5.1542. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Maier LS, Mont L, Schwartz PJ, Simonis G, Leschke M, Gronda E, Boriani G, Darius H, Guillamon Toran L, Savelieva I, Dusi V, Marchionni N, Quintana Rendon M, Schumacher K, Tonini G, Melani L, Giannelli S, Alberto Maggi C, Camm AJ. Ranolazine in the treatment of atrial fibrillation: Results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion) study. Heart rhythm : the official journal of the Heart Rhythm Society. 2015;12:872–878. doi: 10.1016/j.hrthm.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. American heart journal. 2011;162:966–972. e910. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 1998;82:22–25. doi: 10.1016/s0002-9149(98)00231-8. [DOI] [PubMed] [Google Scholar]
- Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, Grunnet M. Effects on atrial fibrillation in aged hypertensive rats by Ca(2+)-activated K(+) channel inhibition. Hypertension. 2011;57:1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613. [DOI] [PubMed] [Google Scholar]
- Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- Dobrev D. Cardiomyocyte Ca2+ overload in atrial tachycardia: is blockade of L-type Ca2+ channels a promising approach to prevent electrical remodeling and arrhythmogenesis? Naunyn-Schmiedeberg's archives of pharmacology. 2007;376:227–230. doi: 10.1007/s00210-007-0199-x. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S, Ravens U. Molecular Basis of Downregulation of G-Protein-Coupled Inward Rectifying K+ Current (IK,ACh) in Chronic Human Atrial Fibrillation: Decrease in GIRK4 mRNA Correlates With Reduced IK,ACh and Muscarinic Receptor-Mediated Shortening of Action Potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol. 2003;98:137–148. doi: 10.1007/s00395-003-0409-8. [DOI] [PubMed] [Google Scholar]
- Ellis WS, SippensGroenewegen A, Auslander DM, Lesh MD. The role of the crista terminalis in atrial flutter and fibrillation: a computer modeling study. Annals of biomedical engineering. 2000;28:742–754. doi: 10.1114/1.1289456. [DOI] [PubMed] [Google Scholar]
- Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Current opinion in cardiology. 2013;28:7–18. doi: 10.1097/HCO.0b013e32835b0956. [DOI] [PubMed] [Google Scholar]
- Filgueiras-Rama D, Martins RP, Mironov S, Yamazaki M, Calvo CJ, Ennis SR, Bandaru K, Noujaim SF, Kalifa J, Berenfeld O, Jalife J. Chloroquine terminates stretch-induced atrial fibrillation more effectively than flecainide in the sheep heart. Circulation. Arrhythmia and electrophysiology. 2012;5:561–570. doi: 10.1161/CIRCEP.111.966820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch DA, de Jongh Curry AL. Esophageal electric fields are predictive of atrial cardioversion success-a finite element analysis. Annals of translational medicine. 2015;3:196. doi: 10.3978/j.issn.2305-5839.2015.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J, Milnes J, El Haou S, Wettwer E, Loose S, Matschke K, Tyl B, Round P, Ravens U. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2016;13:555–564. doi: 10.1016/j.hrthm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan AN, Kuklik P, Lau DH, Brooks AG, Baumert M, Lim WW, Thanigaimani S, Nayyar S, Mahajan R, Kalman JM, Roberts-Thomson KC, Sanders P. Bipolar electrogram shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2013a;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Journal of the American Heart Association. 2013b;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaviri A, Verheule S, Eckstein J, Potse M, Kuijpers NH, Schotten U. A computer model of endo-epicardial electrical dissociation and transmural conduction during atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14(Suppl 5):v10–v16. doi: 10.1093/europace/eus270. [DOI] [PubMed] [Google Scholar]
- Gharaviri A, Verheule S, Eckstein J, Potse M, Kuklik P, Kuijpers NH, Schotten U. How disruption of endo-epicardial electrical connections enhances endo-epicardial conduction during atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 doi: 10.1093/europace/euv445. [DOI] [PubMed] [Google Scholar]
- Gheorghiade M, Adams KF, Jr., Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–2964. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- Ghezelbash S, Molina CE, Dobrev D. Altered atrial metabolism: an underappreciated contributor to the initiation and progression of atrial fibrillation. Journal of the American Heart Association. 2015;4:e001808. doi: 10.1161/JAHA.115.001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette A, Arndt M, Rocken C, Spiess A, Staack T, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000a;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- Goette A, Lendeckel U, Klein HU. Signal transduction systems and atrial fibrillation. Cardiovasc Res. 2002;54:247–258. doi: 10.1016/s0008-6363(01)00521-1. [DOI] [PubMed] [Google Scholar]
- Goette A, Schon N, Kirchhof P, Breithardt G, Fetsch T, Hausler KG, Klein HU, Steinbeck G, Wegscheider K, Meinertz T. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circulation. Arrhythmia and electrophysiology. 2012;5:43–51. doi: 10.1161/CIRCEP.111.965178. [DOI] [PubMed] [Google Scholar]
- Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000b;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- Gomez R, Caballero R, Barana A, Amoros I, De Palm SH, Matamoros M, Nunez M, Perez-Hernandez M, Iriepa I, Tamargo J, Delpon E. Structural basis of drugs that increase cardiac inward rectifier Kir2.1 currents. Cardiovasc Res. 2014;104:337–346. doi: 10.1093/cvr/cvu203. [DOI] [PubMed] [Google Scholar]
- Grammer JB, Bosch RF, Kuhlkamp V, Seipel L. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:626–633. doi: 10.1111/j.1540-8167.2000.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circulation research. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Guerra JM, Everett T. H. t., Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- Guillem MS, Climent AM, Rodrigo M, Fernandez-Aviles F, Atienza F, Berenfeld O. Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications. Cardiovasc Res. 2016;109:480–492. doi: 10.1093/cvr/cvw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. European biophysics journal : EBJ. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Lim KT, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P, Virag N. Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2007;9(Suppl 6):vi64–70. doi: 10.1093/europace/eum208. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Shah AJ, Cochet H, Hocini M, Dubois R, Efimov I, Vigmond E, Bernus O, Trayanova N. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiologic mechanism of human persistent atrial fibrillation. J Physiol. 2016 doi: 10.1113/JP270617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrild D, Henriquez C. A computer model of normal conduction in the human atria. Circulation research. 2000;87:E25–36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Yamashita T, Tsuruzoe N. Tertiapin, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacological research. 2006;54:136–141. doi: 10.1016/j.phrs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res. 2016a;109:467–479. doi: 10.1093/cvr/cvv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D. Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation. J Physiol. 2016b;594:537–553. doi: 10.1113/JP271404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation research. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- Heijman J, Volders PG, Westra RL, Rudy Y. Local control of beta-adrenergic stimulation: Effects on ventricular myocyte electrophysiology and Ca(2+)-transient. J Mol Cell Cardiol. 2011;50:863–871. doi: 10.1016/j.yjmcc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlin A, Jacquemet V. Eikonal-based initiation of fibrillatory activity in thin-walled cardiac propagation models. Chaos (Woodbury, N.Y.) 2011;21:043136. doi: 10.1063/1.3670060. [DOI] [PubMed] [Google Scholar]
- Hocini M, Shah AJ, Neumann T, Kuniss M, Erkapic D, Chaumeil A, Copley SJ, Lim PB, Kanagaratnam P, Denis A, Derval N, Dubois R, Cochet H, Jais P, Haissaguerre M. Focal Arrhythmia Ablation Determined by High-Resolution Noninvasive Maps: Multicenter Feasibility Study. J Cardiovasc Electrophysiol. 2015;26:754–760. doi: 10.1111/jce.12700. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Shao Y, Gabel M, Walsh RA. Left atrial mechanical and biochemical adaptation to pacing induced heart failure. Cardiovasc Res. 1995;29:469–474. [PubMed] [Google Scholar]
- Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M, Kwon SS, Wi J, Park M, Lee HS, Park JS, Lee YS, Shim EB, Pak HN. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: comparison with clinical catheter ablation. Progress in biophysics and molecular biology. 2014;116:40–47. doi: 10.1016/j.pbiomolbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Hwang M, Lee HS, Pak HN, Shim EB. Inducibility of human atrial fibrillation in an in silico model reflecting local acetylcholine distribution and concentration. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2016;20:111–117. doi: 10.4196/kjpp.2016.20.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, Damiano RJ., Jr. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- Jacquemet V. Lessons from computer simulations of ablation of atrial fibrillation. J Physiol. 2016 doi: 10.1113/JP271660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet V, Virag N, Kappenberger L. Wavelength and vulnerability to atrial fibrillation: Insights from a computer model of human atria. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2005;7(Suppl 2):83–92. doi: 10.1016/j.eupc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice, G. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. International journal of cardiology. 2013;165:17–24. doi: 10.1016/j.ijcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kirchhoff JE, Diness JG, Sheykhzade M, Grunnet M, Jespersen T. Synergistic antiarrhythmic effect of combining inhibition of Ca(2)(+)-activated K(+) (SK) channels and voltage-gated Na(+) channels in an isolated heart model of atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2015;12:409–418. doi: 10.1016/j.hrthm.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EJ, Leon LJ, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circulation research. 2005;96:e35–47. doi: 10.1161/01.RES.0000160709.49633.2b. [DOI] [PubMed] [Google Scholar]
- Koivumaki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS computational biology. 2011;7:e1001067. doi: 10.1371/journal.pcbi.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivumaki JT, Seemann G, Maleckar MM, Tavi P. In silico screening of the key cellular remodeling targets in chronic atrial fibrillation. PLoS computational biology. 2014;10:e1003620. doi: 10.1371/journal.pcbi.1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger MW, Rhode KS, O'Neill MD, Rinaldi CA, Gill J, Razavi R, Seemann G, Doessel O. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. Journal of electrocardiology. 2014;47:324–328. doi: 10.1016/j.jelectrocard.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003a;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003b;41:2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- Lankveld T, Zeemering S, Scherr D, Kuklik P, Hoffmann BA, Willems S, Pieske B, Haissaguerre M, Jais P, Crijns HJ, Schotten U. Atrial Fibrillation Complexity Parameters Derived From Surface ECGs Predict Procedural Outcome and Long-Term Follow-Up of Stepwise Catheter Ablation for Atrial Fibrillation. Circulation. Arrhythmia and electrophysiology. 2016;9:e003354. doi: 10.1161/CIRCEP.115.003354. [DOI] [PubMed] [Google Scholar]
- Laurent G, Leong-Poi H, Mangat I, Moe GW, Hu X, So PP, Tarulli E, Ramadeen A, Rossman EI, Hennan JK, Dorian P. Effects of chronic gap junction conduction-enhancing antiarrhythmic peptide GAP-134 administration on experimental atrial fibrillation in dogs. Circulation. Arrhythmia and electrophysiology. 2009;2:171–178. doi: 10.1161/CIRCEP.108.790212. [DOI] [PubMed] [Google Scholar]
- Lee W, Mann SA, Windley MJ, Imtiaz MS, Vandenberg JI, Hill AP. In silico assessment of kinetics and state dependent binding properties of drugs causing acquired LQTS. Progress in biophysics and molecular biology. 2016;120:89–99. doi: 10.1016/j.pbiomolbio.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J, Chiamvimonvat N. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg SH, Netter MF, Rolfes C, Rinne S, Schlichthorl G, Zuzarte M, Vassiliou T, Moosdorf R, Wulf H, Daut J, Sachse FB, Decher N. TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:613–624. doi: 10.1159/000335757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida T, Hashimoto N, Kuwahara I, Ogino Y, Matsuura J, Yamamoto W, Itano Y, Zamma A, Matsumoto R, Kamon J, Kobayashi T, Ishiwata N, Yamashita T, Ogura T, Nakaya H. Effects of a highly selective acetylcholine-activated K+ channel blocker on experimental atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2011;4:94–102. doi: 10.1161/CIRCEP.110.951608. [DOI] [PubMed] [Google Scholar]
- Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circulation research. 2011;109:1031–1043. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol. 2009;297:H1398–1410. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleckar MM, Greenstein JL, Trayanova NA, Giles WR. Mathematical simulations of ligand-gated and cell-type specific effects on the action potential of human atrium. Progress in biophysics and molecular biology. 2008;98:161–170. doi: 10.1016/j.pbiomolbio.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–1482. doi: 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matene E, Jacquemet V. Fully automated initiation of simulated episodes of atrial arrhythmias. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14(Suppl 5):v17–v24. doi: 10.1093/europace/eus271. [DOI] [PubMed] [Google Scholar]
- McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, MacLeod RS, Trayanova NA. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. Journal of electrocardiology. 2012;45:640–645. doi: 10.1016/j.jelectrocard.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, Macleod RS, Trayanova NA. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys J. 2013;104:2764–2773. doi: 10.1016/j.bpj.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Zahid S, Vadakkumpadan F, Blauer J, MacLeod RS, Trayanova NA. Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PloS one. 2015;10:e0117110. doi: 10.1371/journal.pone.0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod K, Saberniak J, Haugaa K. Statistical analysis of ventricular shape of ARVC patients and correlation with clinical diagnostic indices. Journal of Cardiovascular Magnetic Resonance. 2015;17:1–2. [Google Scholar]
- Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- Milnes J, El-Haou S, Loose S, Jackson C, Tang R, Muirhead I, Ford J, Ravens U. Abstract 20199: In the Absence of Muscarinic-Activation, Inhibition of Kir3.1/3.4 and Kir3.4/3.4, but Not Kir3.1/3.4-Alone Prolongs Repolarisation of Atrial Tissue From Patients With Atrial Fibrillation. Circulation. 2014;130:A20199. [Google Scholar]
- Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, Wang L, Bayer JD, Christini DJ, Trayanova NA, Ripplinger CM, Kass RS, Clancy CE. A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Science translational medicine. 2011;3:98ra83. doi: 10.1126/scitranslmed.3002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotti S, Edwards AG, McCulloch AD, Bers DM, Grandi E. A novel computational model of mouse myocyte electrophysiology to assess the synergy between Na+ loading and CaMKII. J Physiol. 2014;592:1181–1197. doi: 10.1113/jphysiol.2013.266676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotti S, Koivumäki JT, Maleckar MM, Chiamvimonvat N, Grandi E. Small-Conductance Ca2+-Activated K+ Current in Atrial Fibrillation: Both Friend and Foe. Biophysical Journal. 2016;110:274a. [Google Scholar]
- Morotti S, McCulloch AD, Bers DM, Edwards AG, Grandi E. Atrial-selective targeting of arrhythmogenic phase-3 early afterdepolarizations in human myocytes. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circulation research. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525(Pt 2):285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circulation research. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. The Journal of biological chemistry. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr., Strickberger SA, Morady F. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002;40:100–104. doi: 10.1016/s0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri BB, Greenberg HE, Kraft WK, Lazarus N, Lynch JJ, Salata JJ, Bilodeau MT, Regan CP, Stump G, Fan L, Mehta A, Wagner JA, Gutstein DE, Bloomfield D. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circulation. Arrhythmia and electrophysiology. 2012;5:1193–1201. doi: 10.1161/CIRCEP.111.969782. [DOI] [PubMed] [Google Scholar]
- Pedron-Torrecilla J, Rodrigo M, Climent AM, Liberos A, Perez-David E, Bermejo J, Arenal A, Millet J, Fernandez-Aviles F, Berenfeld O, Atienza F, Guillem MS. Noninvasive Estimation of Epicardial Dominant High-Frequency Regions During Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:435–442. doi: 10.1111/jce.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podd SJ, Freemantle N, Furniss SS, Sulke N. First clinical trial of specific IKACh blocker shows no reduction in atrial fibrillation burden in patients with paroxysmal atrial fibrillation: pacemaker assessment of BMS 914392 in patients with paroxysmal atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015 doi: 10.1093/europace/euv263. [DOI] [PubMed] [Google Scholar]
- Poulet C, Wettwer E, Grunnet M, Jespersen T, Fabritz L, Matschke K, Knaut M, Ravens U. Late Sodium Current in Human Atrial Cardiomyocytes from Patients in Sinus Rhythm and Atrial Fibrillation. PloS one. 2015;10:e0131432. doi: 10.1371/journal.pone.0131432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circulation research. 2008;103:845–854. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- Rafizadeh S, Zhang Z, Woltz RL, Kim HJ, Myers RE, Lu L, Tuteja D, Singapuri A, Bigdeli AA, Harchache SB, Knowlton AA, Yarov-Yarovoy V, Yamoah EN, Chiamvimonvat N. Functional interaction with filamin A and intracellular Ca2+ enhance the surface membrane expression of a small-conductance Ca2+-activated K+ (SK2) channel. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9989–9994. doi: 10.1073/pnas.1323541111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappel WJ, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos (Woodbury, N.Y.) 2013;23:023113. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappel WJ, Zaman JA, Narayan SM. Mechanisms for the Termination of Atrial Fibrillation by Localized Ablation: Computational and Clinical Studies. Circulation. Arrhythmia and electrophysiology. 2015;8:1325–1333. doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasoli S, Kakouros N, Harling L, Gukop P, Soni M, Athanasiou T, Kourliouros A. Antioxidant vitamins in the prevention of atrial fibrillation: what is the evidence? Cardiology research and practice. 2011;2011:164078. doi: 10.4061/2011/164078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens U, Poulet C, Wettwer E, Knaut M. Atrial selectivity of antiarrhythmic drugs. J Physiol. 2013;591:4087–4097. doi: 10.1113/jphysiol.2013.256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffel JA, Camm AJ, Belardinelli L, Zeng D, Karwatowska-Prokopczuk E, Olmsted A, Zareba W, Rosero S, Kowey P. The HARMONY Trial: Combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: Mechanistic and Therapeutic Synergism. Circulation. Arrhythmia and electrophysiology. 2015;8:1048–1056. doi: 10.1161/CIRCEP.115.002856. [DOI] [PubMed] [Google Scholar]
- Reilly SN, Liu X, Carnicer R, Recalde A, Muszkiewicz A, Jayaram R, Carena MC, Wijesurendra R, Stefanini M, Surdo NC, Lomas O, Ratnatunga C, Sayeed R, Krasopoulos G, Rajakumar T, Bueno-Orovio A, Verheule S, Fulga TA, Rodriguez B, Schotten U, Casadei B. Up-regulation of miR-31 in human atrial fibrillation begets the arrhythmia by depleting dystrophin and neuronal nitric oxide synthase. Science translational medicine. 2016;8:340ra374. doi: 10.1126/scitranslmed.aac4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann M, Bohnert J, Doessel O. Simulating pulmonary vein activity leading to atrial fibrillation using a rule-based approach on realistic anatomical data.. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2006. pp. 3943–3946. [DOI] [PubMed] [Google Scholar]
- Reumann M, Bohnert J, Seemann G, Osswald B, Dossel O. Preventive ablation strategies in a biophysical model of atrial fibrillation based on realistic anatomical data. IEEE transactions on bio-medical engineering. 2008;55:399–406. doi: 10.1109/TBME.2007.912672. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Seccia TM, Gallina V, Muiesan ML, Leoni L, Pengo M, Ragazzo F, Caielli P, Belfiore A, Bernini G, Cipollone F, Cottone S, Ferri C, Giacchetti G, Grassi G, Letizia C, Maccario M, Olivieri O, Palumbo G, Rizzoni D, Rossi E, Sechi L, Volpe M, Mantero F, Morganti A, Pessina AC. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. Journal of human hypertension. 2013;27:158–163. doi: 10.1038/jhh.2012.21. [DOI] [PubMed] [Google Scholar]
- Rubrichi S, Rognoni C, Sacchi L, Parimbelli E, Napolitano C, Mazzanti A, Quaglini S. Graphical representation of life paths to better convey results of decision models to patients. Medical decision making : an international journal of the Society for Medical Decision Making. 2015;35:398–402. doi: 10.1177/0272989X14565822. [DOI] [PubMed] [Google Scholar]
- Ruchat P, Dang L, Virag N, Schlaepfer J, von Segesser LK, Kappenberger L. A biophysical model of atrial fibrillation to define the appropriate ablation pattern in modified maze. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007;31:65–69. doi: 10.1016/j.ejcts.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K, Didie M, Seniuk A, von Leitner EC, Szoecs K, Schrickel JW, Treede H, Wenzel U, Lewalter T, Nickenig G, Zimmermann WH, Meinertz T, Boger RH, Reichenspurner H, Freeman BA, Eschenhagen T, Ehmke H, Hazen SL, Willems S, Baldus S. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nature medicine. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samie FH, Mandapati R, Gray RA, Watanabe Y, Zuur C, Beaumont J, Jalife J. A mechanism of transition from ventricular fibrillation to tachycardia : effect of calcium channel blockade on the dynamics of rotating waves. Circulation research. 2000;86:684–691. doi: 10.1161/01.res.86.6.684. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Bueno-Orovio A, Wettwer E, Loose S, Simon J, Ravens U, Pueyo E, Rodriguez B. Inter-subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation. PloS one. 2014;9:e105897. doi: 10.1371/journal.pone.0105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Corrias A, Bueno-Orovio A, Davies M, Swinton J, Jacobson I, Laguna P, Pueyo E, Rodriguez B. The Na+/K+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. Am J Physiol Heart Circ Physiol. 2012;302:H1146–1159. doi: 10.1152/ajpheart.00668.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AX, Sobie EA. Regression analysis for constraining free parameters in electrophysiological models of cardiac cells. PLoS computational biology. 2010;6:e1000914. doi: 10.1371/journal.pcbi.1000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabo G, Kallenbach K, Karck M, Borggrefe M, Biliczki P, Ehrlich JR, Baczko I, Lugenbiel P, Schweizer PA, Donner BC, Katus HA, Dobrev D, Thomas D. Upregulation of K(2P)3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation. 2015;132:82–92. doi: 10.1161/CIRCULATIONAHA.114.012657. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiological reviews. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr., Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Schotten U, de Haan S, Verheule S, Harks EG, Frechen D, Bodewig E, Greiser M, Ram R, Maessen J, Kelm M, Allessie M, Van Wagoner DR. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007;73:37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Schotten U, Dobrev D, Platonov PG, Kottkamp H, Hindricks G. Current controversies in determining the main mechanisms of atrial fibrillation. Journal of internal medicine. 2016 doi: 10.1111/joim.12492. [DOI] [PubMed] [Google Scholar]
- Schotten U, Maesen B, Zeemering S. The need for standardization of time- and frequency-domain analysis of body surface electrocardiograms for assessment of the atrial fibrillation substrate. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14:1072–1075. doi: 10.1093/europace/eus056. [DOI] [PubMed] [Google Scholar]
- Shariat MH, Gazor S, Redfearn D. Computationally efficient method for localizing the spiral rotor source using synthetic intracardiac electrograms during atrial fibrillation.. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2015. pp. 4483–4486. [DOI] [PubMed] [Google Scholar]
- Shibata EF, Drury T, Refsum H, Aldrete V, Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989;257:H1773–1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–2319. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J, Edwards T, Yoneda Z, Sinner MF, Arya A, Sommer P, Delaney J, Goyal SK, Saavedra P, Kanagasundram A, Whalen SP, Roden DM, Hindricks G, Ellis CR, Ellinor PT, Darbar D, Husser D. Common genetic variants and response to atrial fibrillation ablation. Circulation. Arrhythmia and electrophysiology. 2015;8:296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff SC, Ryu K, Martovitz NL, Hoit BD, Stambler BS. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17:534–541. doi: 10.1111/j.1540-8167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circulation. Arrhythmia and electrophysiology. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JN, Ziberna K, Casadei B. Compromised redox homeostasis, altered nitroso-redox balance, and therapeutic possibilities in atrial fibrillation. Cardiovasc Res. 2016;109:510–518. doi: 10.1093/cvr/cvw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibsbye L, Diness JG, Sorensen US, Hansen RS, Grunnet M. The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca(2+)-activated K(+) channels. Journal of cardiovascular pharmacology. 2011;57:672–681. doi: 10.1097/FJC.0b013e318217943d. [DOI] [PubMed] [Google Scholar]
- Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, Matschke K, Wettwer E, Ravens U, Grunnet M, Christ T, Jespersen T. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res. 2014;103:156–167. doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- Sobie EA. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J. 2009;96:1264–1274. doi: 10.1016/j.bpj.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and beta-adrenergic signaling in regulation of cardiac myocyte Ca(2+) handling. Biophys J. 2010;99:2038–2047. doi: 10.1016/j.bpj.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. Journal of the American College of Cardiology. 2010a;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010b;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Tada H, Shiffman D, Smith JG, Sjogren M, Lubitz SA, Ellinor PT, Louie JZ, Catanese JJ, Engstrom G, Devlin JJ, Kathiresan S, Melander O. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke; a journal of cerebral circulation. 2014;45:2856–2862. doi: 10.1161/STROKEAHA.114.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circulation research. 1999;85:810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- Tobon C, Ruiz-Villa CA, Heidenreich E, Romero L, Hornero F, Saiz J. A three-dimensional human atrial model with fiber orientation. Electrograms and arrhythmic activation patterns relationship. PloS one. 2013;8:e50883. doi: 10.1371/journal.pone.0050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova N, Constantino J, Ashihara T, Plank G. Modeling defibrillation of the heart: approaches and insights. IEEE reviews in biomedical engineering. 2011;4:89–102. doi: 10.1109/RBME.2011.2173761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova NA. Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circulation research. 2014;114:1516–1531. doi: 10.1161/CIRCRESAHA.114.302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova NA, Rantner LJ. New insights into defibrillation of the heart from realistic simulation studies. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014;16:705–713. doi: 10.1093/europace/eut330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triedman JK, Jolley M, Stinstra J, Brooks DH, MacLeod R. Predictive modeling of defibrillation using hexahedral and tetrahedral finite element models: recent advances. Journal of electrocardiology. 2008;41:483–486. doi: 10.1016/j.jelectrocard.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimae K, Suzuki S, Murakami S, Kurachi Y. Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H660–H669. doi: 10.1152/ajpheart.01083.2006. [DOI] [PubMed] [Google Scholar]
- Tveita AA, Lines GT. S. I. Publishing, editor. Computing Characterizations of Drugs for Ion Channels and Receptors Using Markov Models. Lecture Notes in Computational Science and Engineering. 2016;111 [Google Scholar]
- Uldry L, Jacquemet V, Virag N, Kappenberger L, Vesin JM. Estimating the time scale and anatomical location of atrial fibrillation spontaneous termination in a biophysical model. Medical & biological engineering & computing. 2012;50:155–163. doi: 10.1007/s11517-011-0859-3. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circulation research. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, Schotten U. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Progress in biophysics and molecular biology. 2014;115:173–185. doi: 10.1016/j.pbiomolbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Vidmar D, Narayan SM, Rappel WJ. Phase synchrony reveals organization in human atrial fibrillation. Am J Physiol Heart Circ Physiol. 2015;309:H2118–2126. doi: 10.1152/ajpheart.00407.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, Strasser RH, Ravens U, Dobrev D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Voigt N, Heijman J, Trausch A, Mintert-Jancke E, Pott L, Ravens U, Dobrev D. Impaired Na(+)-dependent regulation of acetylcholine-activated inward-rectifier K(+) current modulates action potential rate dependence in patients with chronic atrial fibrillation. J Mol Cell Cardiol. 2013;61:142–152. doi: 10.1016/j.yjmcc.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive Oxygen Species-Activated Ca/Calmodulin Kinase II{delta} Is Required for Late INa Augmentation Leading to Cellular Na and Ca Overload. Circulation research. 2011 doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–2968. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakili R, Yeh YH, Yan Qi X, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve LR, Boknik P, Voigt N, Krysiak J, Kaab S, Ravens U, Linke WA, Stienen GJ, Shi Y, Tardif JC, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circulation. Arrhythmia and electrophysiology. 2010;3:530–541. doi: 10.1161/CIRCEP.109.933036. [DOI] [PubMed] [Google Scholar]
- Walkey AJ, Benjamin EJ, Lubitz SA. New-onset atrial fibrillation during hospitalization. J Am Coll Cardiol. 2014;64:2432–2433. doi: 10.1016/j.jacc.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, Varro A, Ravens U. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- Wilhelms M, Hettmann H, Maleckar MM, Koivumaki JT, Dossel O, Seemann G. Benchmarking electrophysiological models of human atrial myocytes. Frontiers in physiology. 2012;3:487. doi: 10.3389/fphys.2012.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovascular Research. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- Workman AJ, Kane KA, Rankin AC. Characterisation of the Na, K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc Res. 2003;59:593–602. doi: 10.1016/s0008-6363(03)00466-8. [DOI] [PubMed] [Google Scholar]
- Xie Y, Liao Z, Grandi E, Shiferaw Y, Bers DM. Slow [Na]i Changes and Positive Feedback Between Membrane Potential and [Ca]i Underlie Intermittent Early Afterdepolarizations and Arrhythmias. Circulation. Arrhythmia and electrophysiology. 2015;8:1472–1480. doi: 10.1161/CIRCEP.115.003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Mironov S, Taravant C, Brec J, Vaquero LM, Bandaru K, Avula UM, Honjo H, Kodama I, Berenfeld O, Kalifa J. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res. 2012;94:48–57. doi: 10.1093/cvr/cvr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano Y, Greenland P, Lloyd-Jones DM, Daoud EG, Koehler JL, Ziegler PD. Simulation of Daily Snapshot Rhythm Monitoring to Identify Atrial Fibrillation in Continuously Monitored Patients with Stroke Risk Factors. PloS one. 2016;11:e0148914. doi: 10.1371/journal.pone.0148914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid S, Whyte KN, Schwarz EL, Blake RC, 3rd, Boyle PM, Chrispin J, Prakosa A, Ipek EG, Pashakhanloo F, Halperin HA, Calkins H, Berger RD, Nazarian S, Trayanova NA. Feasibility of Using Patient-Specific Models and the “Minimum Cut” Algorithm to Predict Optimal Ablation Targets for Left Atrial Flutter. Heart rhythm : the official journal of the Heart Rhythm Society. 2016 doi: 10.1016/j.hrthm.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaza A, Malfatto G, Schwartz PJ. Effects on atrial repolarization of the interaction between K+ channel blockers and muscarinic receptor stimulation. The Journal of pharmacology and experimental therapeutics. 1995;273:1095–1104. [PubMed] [Google Scholar]
- Zhao J, Butters TD, Zhang H, LeGrice IJ, Sands GB, Smaill BH. Image-based model of atrial anatomy and electrical activation: a computational platform for investigating atrial arrhythmia. IEEE transactions on medical imaging. 2013;32:18–27. doi: 10.1109/TMI.2012.2227776. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li J, Li W, Li Y, Shan H, Gong Y, Yang B. Effects of spironolactone on atrial structural remodelling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Br J Pharmacol. 2010;159:1584–1594. doi: 10.1111/j.1476-5381.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XB, Voigt N, Wieland T, Dobrev D. Enhanced Frequency-Dependent Retrograde Trafficking Of Small-Conductance Ca2-activated K+ Channels May Contribute To Electrical Remodeling In Human Atrial Fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:S319. [Google Scholar]
- Zou R, Kneller J, Leon LJ, Nattel S. Development of a computer algorithm for the detection of phase singularities and initial application to analyze simulations of atrial fibrillation. Chaos (Woodbury, N.Y.) 2002;12:764–778. doi: 10.1063/1.1497505. [DOI] [PubMed] [Google Scholar]