Abstract
Background/Objectives
Androgen deprivation therapy (ADT) is commonly used for treatment of prostate cancer, but is associated with side effects such as sarcopenia and insulin resistance. The role of lifestyle factors such as diet and exercise on insulin sensitivity and body composition in testosterone-deficient males is poorly understood. The aim of the present study was to examine the relationships between androgen status, diet, and insulin sensitivity.
Subjects/Methods
Middle-aged (11–12-yo) intact and orchidectomized male rhesus macaques were maintained for two months on a standard chow diet, and then exposed for six months to a Western-style, high-fat/calorie-dense diet (WSD) followed by four months of caloric restriction (CR). Body composition, insulin sensitivity, physical activity, serum cytokine levels, and adipose biopsies were evaluated before and after each dietary intervention.
Results
Both intact and orchidectomized animals gained similar proportions of body fat, developed visceral and subcutaneous adipocyte hypertrophy, and became insulin resistant in response to the WSD. CR reduced body fat in both groups, but reversed insulin resistance only in intact animals. Orchidectomized animals displayed progressive sarcopenia, which persisted after the switch to CR. Androgen deficiency was associated with increased levels of interleukin-6 and macrophage-derived chemokine (CCL22), both of which were elevated during CR. Physical activity levels showed a negative correlation with body fat and insulin sensitivity.
Conclusion
Androgen deficiency exacerbated the negative metabolic side effects of the WSD, such that CR alone was not sufficient to improve altered insulin sensitivity, suggesting that ADT patients will require additional interventions to reverse insulin resistance and sarcopenia.
Keywords: androgen deprivation therapy, hypogonadal, Western-style diet, obesity, sarcopenia
Introduction
Of the approximately two million men annually diagnosed with prostate cancer in the United States, one third receive androgen deprivation therapy (ADT) in combination with other therapies1, 2. Because ADT improves outcomes for high-risk patients treated with radiation therapy for localized disease, and is also a common treatment for patients with increasing prostate-specific antigen levels after local treatment without metastatic disease3, side effects of this therapy constitute a significant social and clinical issue. Adverse effects of ADT include metabolic changes such as obesity4–6, insulin resistance7–9, and diabetes1, 10, all of which constitute independent risk factors for increased mortality rates in men11. Obesity and hyperinsulinemia are also associated with a higher risk of prostate cancer-specific mortality, and may promote the development of a more aggressive form of prostate cancer12. Cardiovascular disease, which is linked to obesity, has been recognized as the second most common cause of mortality in men with prostate cancer13.
These factors contribute to the high risk/benefit ratio of ADT, which makes lifestyle modifications essential for survival of prostate cancer patients. Currently, there is a paucity of studies that have explicitly investigated physiologic outcomes of diet in hypogonadal men. Short-term caloric restriction (CR) in humans has been shown to reduce cardiovascular and metabolic disease risk factors14–17. Because intensive lifestyle changes have been shown to exert positive metabolic effects in men18–20, we hypothesized that dietary restriction could improve metabolic health in prostate cancer patients undergoing ADT, as well as in hypogonadal men with low testosterone levels. To determine whether dietary modifications can alter the side effects of androgen deficiency in the absence of confounding factors introduced by cancer, we developed a non-human primate (NHP) model of hypogonadism using naïve, age-matched male rhesus macaques with no prior medical or drug treatment history and tested the effects of a Western-style, high-fat/calorie-dense diet (WSD) and CR on body composition, metabolic parameters, activity and circulating cytokines.
Materials and Methods
Experimental animals and diets
All procedures described in this study were approved by the ONPRC Institutional Animal Care and Use Committee. Twelve 11 to 12-year-old male rhesus macaques were housed individually, with the cage size adjusted to animal weight according to the USDA Cage Size Guide 8th Edition. Standard chow diet consisted of the two daily meals of Purina Lab Diet fiber-balanced monkey chow (15% calories from fat, 27% from protein, and 59% from carbohydrates; no. 5000; Purina Mills, St. Louis, MO), supplemented with fruits and vegetables. Baseline food intake of individual animals was determined based on the amounts of chow pellets consumed daily as an average over the three-week period. A WSD diet (33% calories from fat, 17% from protein, 51% from carbohydrates; 5A1F, Purina Mills, St. Louis, MO) was given ad libitum.
Activity monitoring
Activity was measured continuously throughout the experiment using Actical omnidirectional accelerometers (Respironics, Phoenix, AZ). Each monkey was fitted with a loose-fitting metal collar (Primate Products, Inc. Immokalee, FL) that housed the accelerometer in a snug, protective stainless steel box. Monitors were programmed to record the total number of activity counts per minute. Activity data were downloaded at least every 45 days while animals were under sedation. Total daily activity level was averaged for a two-month baseline period, over the last week of the six-month WSD period and over the last week of the four-month CR period. All data was checked for normality and homogeneity of variance. If necessary, data was transformed using log or square root transformations to meet criteria for parametric tests. Comparisons between the baseline time period and WSD and CR periods were made using paired Students t tests, with a Bonferroni correction for multiple comparisons. Data are presented as mean ± standard error of the mean (SEM). Alpha values of p< 0.05 were considered statistically significant. All statistical analyses were conducted using the SPSS software package, version 23.0 (SPSS Inc., Chicago, Illinois).
Dual-energy X-ray absorptiometry
Percent body fat was determined using dual-energy X-ray absorptiometry (DEXA) scanning as described21. Monkeys were sedated with ketamine and positioned supine on the bed of a Hologic DEXA scanner (Discovery scanner, Hologic Inc, Bedford, MA).
Glucose tolerance test
Each animal was sedated initially with Telazol (Tiletamine hydrochloride and Zolazepam hydrochloride, Fort Dodge Animal Health, Fort Dodge, IA) and subsequently with ketamine to maintain sedation. The protocol was based on that designed by Bergman et al22. Dextrose (300 mg/kg) was infused intravenously through a catheter and blood samples were taken from 15 minutes before to three hours after the glucose infusion. Tolbutamide (5 mg/kg) was infused intravenously 20 minutes after the dextrose in order to stimulate the pancreas to secrete more insulin. All samples were immediately assayed for glucose using YSI 2300 Stat Plus (YSI Inc., Yellow Springs, OH), and subsequently for insulin by RIA (Linco Human Insulin RIA, Millipore Corporation, Billerica, MA). The sensitivity of the insulin assay was 1 µU/ml and the intra-assay coefficient of variation was 4.9%.
Adipose tissue biopsies
White adipose tissue (WAT) biopsies were performed by expert surgical personnel at ONPRC according to well-accepted veterinary surgical procedures under sterile conditions and appropriate anesthesia with postoperative pain control. Food was withheld for approximately 12 hours prior to the procedure. Animals were sedated with 100 mg Ketamine combined with 0.1 mg glycopyrrolate administered intramuscularly. Once the intravenous catheter was placed, animals received 0.5 mg hydromorphone-HCl intravenously. Animals were endotracheally intubated with an endotracheal tube (size 4.0–6.0) and general anesthesia was induced with 3% Isoflurane for 2–3 minutes. Inhalant anesthesia was maintained at 1–2% isoflurane. Inhalant anesthetics was combined with 100% oxygen administered at a rate of 1–1.5 L/min.
The animal was positioned in dorsal recumbency followed by sterile preparation and draping of the abdomen. A Verres needle was inserted via a 1-cm subumbilical skin incision followed by insufflation to 15 mm Hg pressure with CO2 gas. The Verres was removed and an 11-mm trocar/sheath and 10-mm telescope was inserted by puncture at the same site. A right paralumbar 5-mm accessory port was placed, through which a cutting biopsy grasper was inserted. Pinch biopsy forceps were used to retrieve two fat biopsies from the falciform ligament. Grasping forceps were used to grab a small section of omentum, was pulled through the side port and a 1 × 2 × 1-cm block of omentum was removed via sharp and blunt dissection. A SC-WAT biopsy was retrieved from the site of the scope incision. The incisions was closed with interrupted 4-0 Monocryl in the rectus fascia and skin. Recovery was on the OR table until extubation. Additional heat and oxygen support was provided as needed during the recovery period. Post-operative analgesia was provided for 48–72 hours following the surgical procedure, using hydromorphone HCl (0.05–0.4 mg/kg, administered intramuscularly, three times a day), and buprenorphine (0.01–0.1 mg/kg, administered intramuscularly, once a day). The standard 48 to 72-hr opioid protocol for post-operative analgesia was used. Post-operative monitoring and assessment of pain and distress were accomplished by surgical veterinary staff for a minimum of 7 days.
Bilateral orchiectomy
Positioning was in dorsal recumbency, followed by sterile preparation of the cranial scrotum and caudal abdomen and placement of sterile drapes. A linear 2–3 cm ventral midline skin incision was made cranial to the scrotum, with blunt dissection to reveal the testicular tunic. A 3-cm incision was made through the tunic and the testis will be delivered manually. The spermatic cord (vas deferens, cremaster m. and testicular vasculature) was clamped then double-ligated (one circumferential, one transfixing ligature) with 3-0 Vicryl, transected, and the testis was removed. The contralateral testis was resected in like manner. The subcutis was closed with continuous 4-0 Monocryl, and skin apposition was closed with continuous intradermal 4-0 Monocryl.
Cytokine, chemokine, and growth factor analysis
Plasma samples were thawed and analyzed in duplicates using the Invitrogen Cytokine Monkey Magnetic 29-Plex Panel per the manufacturer’s instructions (Life Technologies, Grand Island, NY). The panel includes monocyte chemoattractant protein 1 (MCP-1; CCL2), fibroblast growth factor basic (FGF-b), IL-1β, granulocyte colony-stimulating factor (G-CSF), IL-10, IL-6, IL-12, RANTES, eotaxin, IL-17, macrophage inflammatory protein 1 alpha (MIP-1α), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein 1 beta (MIP-1β), IL-15, epidermal growth factor (EGF), IL-5, hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), IFN-γ, monocyte-derived chemokine (MDC; CCL22), interferon-inducible T cell alpha chemoattractant (ITAC; CXCL11), migration inhibition factor (MIF), IL-1 receptor agonist (IL-1RA), TNF-α, IL-2, IFN-gamma-inducible protein 10 (IP-10, CXCL10) monokine induced by IFN-gamma (MIG; CXCL9), IL-4, and IL-8. IL-6 and MDC showed the significant differences between intact and orchidectomized groups and were included in the “Results” section.
Results
The effect of ADT on body composition
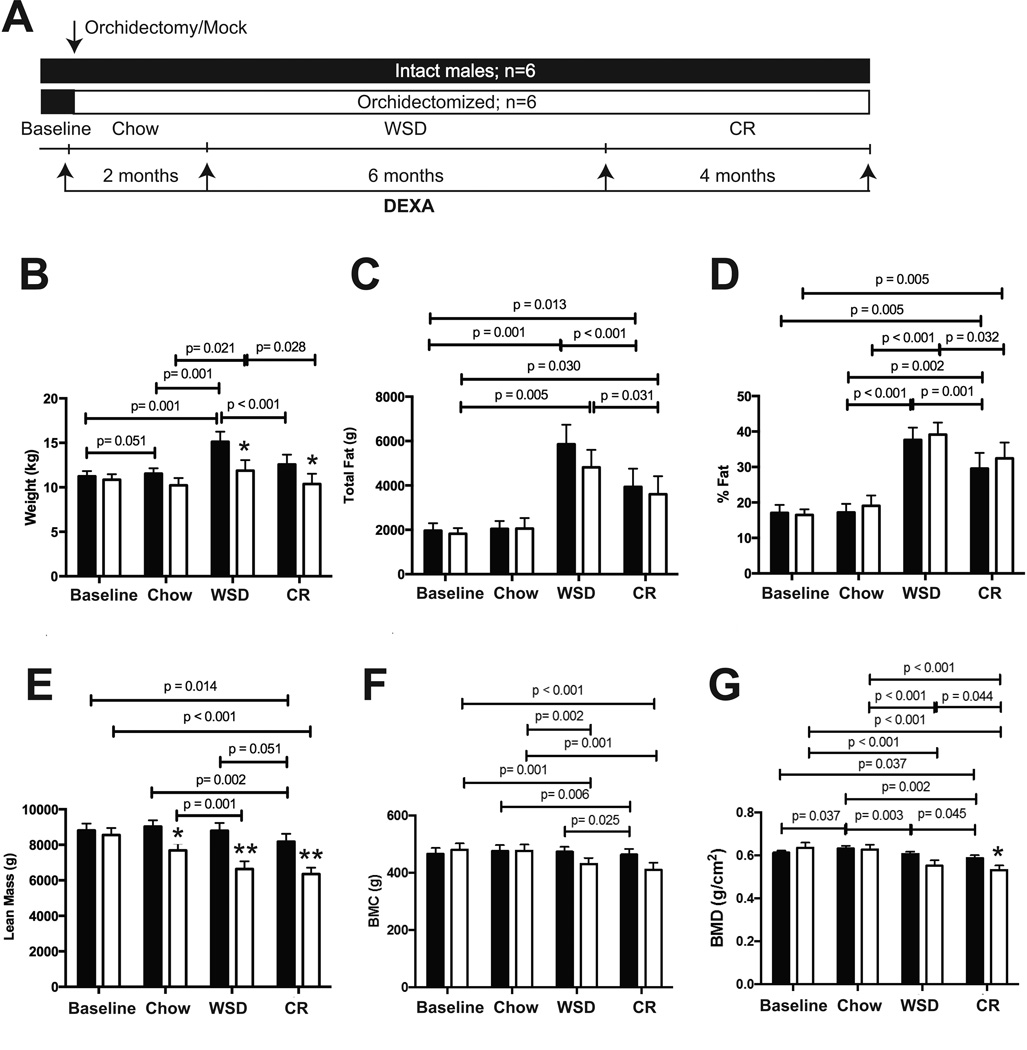
Individually caged middle-aged male rhesus macaques were maintained in a sedentary environment, and then were either orchidectomized or subjected to mock surgery. After the surgery, both groups were exposed to several dietary regimens. For the first two months after surgery they were maintained on a standard chow diet, followed by six months on a WSD, and then calorically-restricted for four months on the chow diet at 70% of baseline caloric intake values (see Figure 1A for experimental outline). Two months after surgery, while still eating the chow diet, orchidectomized but not intact animals showed a decrease in lean mass (Figure 1E). Fat mass and bone mineral content (BMC) remained stable in both experimental groups (Figure 1C, D and F), whereas bone mineral density (BMD) increased significantly in intact but not in orchidectomized animals (Figure 1G).
Figure 1. Changes in body composition following androgen deprivation.
A) Experimental design of NHP studies. Rhesus macaque males were either orchidectomized or had mock surgery. After the surgery, intact (black bars) and orchidectomized (open bars) were maintained for two months on a chow diet (chow), followed by six months on the WSD (WSD), and then calorically-restricted for four months (CR), as indicated in the “Results” section. Body composition were monitored at the end of each dietary period, as indicated with arrows, including the measurements of body weight (A), total body fat (C), % of body fat (D), total lean mass (E), bone mineral content (BMC, F), and bone mineral density (BMD, G). Error bars are means of means ± SEM, n=6. The differences between dietary groups (p-values are indicated) were determined using repeated measures two-way ANOVA followed by t-test. Statistically significant differences between intact and orchidectomized animals (* p<0.05; **p<0.01) were determined by independent samples t-test.
After a subsequent six months on the WSD, both experimental groups gained significant amounts of total fat and the percentage of body fat increased significantly compared to when they were on a chow diet, with no significant differences observed between groups (Figure 1C–D). Lean mass, BMC, and BMD decreased significantly in orchidectomized animals during the WSD period (Figure 1E–G). Following CR, both groups of animals lost a significant amount of fat mass and exhibited a decrease in the percent body fat, with no significant differences seen between groups (Figure 1C–D). Lean mass and BMC showed no significant change compared to the WSD period in orchidectomized animals, but declined significantly in intact males (Figure 1E and F). Following CR, BMD decreased significantly in both groups, with orchidectomized males showing a more dramatic loss of BMD than intact males (Figure 1G). Thus, testosterone deficiency exerted diet-specific effects on lean mass and bone quality, but had no significant effect on fat mass.
The effect of ADT on glucose clearance and insulin sensitivity
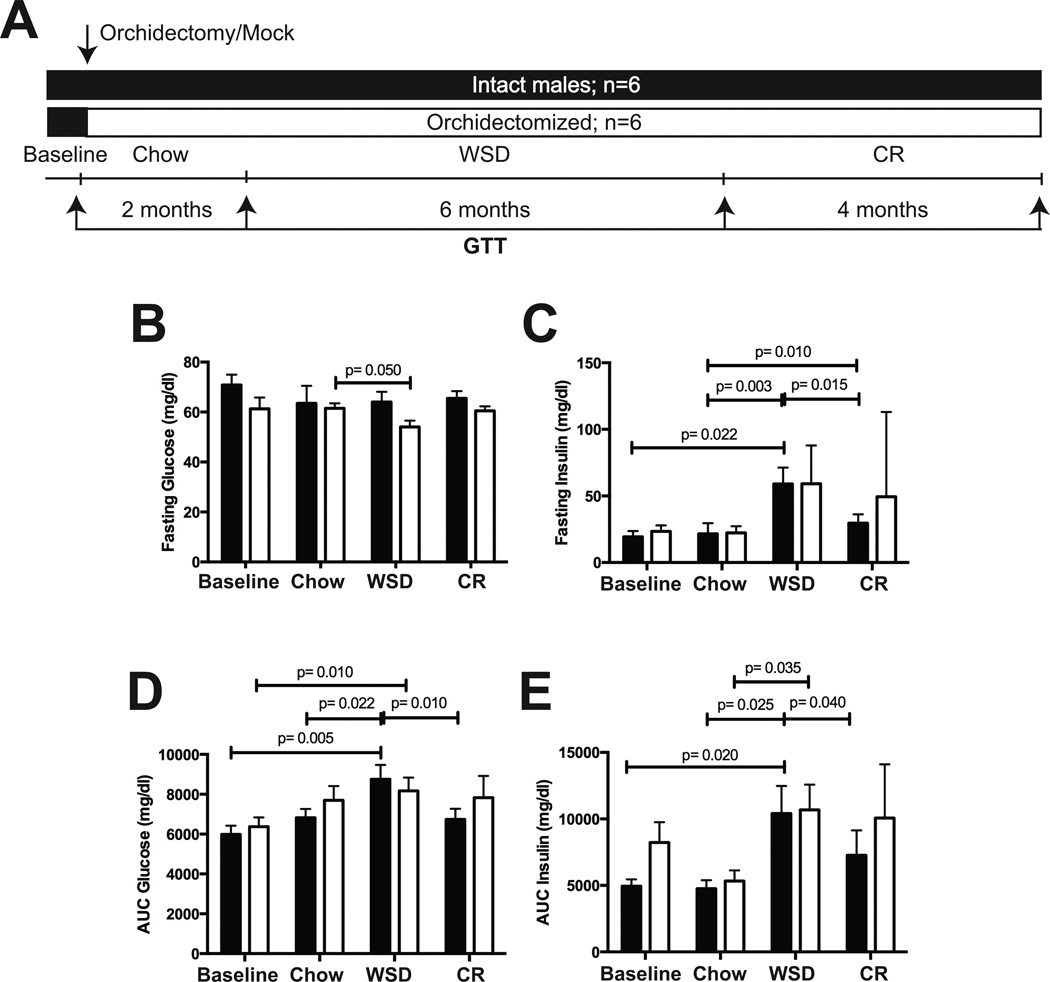
Changes in glucose clearance and insulin sensitivity were assessed by glucose tolerance tests (GTT, Figure 2A). During the low fat diet period, orchidectomy had no significant effect on fasting glucose, insulin, AUC glucose, or AUC insulin values (Figure 2B–E). In contrast, fasting insulin, AUC glucose, and AUC insulin values increased significantly in both groups during the WSD period (Figure 2C–E). These parameters decreased significantly following CR in intact animals, whereas orchidectomized animals showed no significant changes compared to the WSD period (Figure 2C–E). Taken together, diet-induced obesity induced fasting hyperinsulinemia and insulin resistance in both groups of animals. However, CR improved these metabolic parameters only in intact animals, whereas orchidectomized animals remained glucose-intolerant, despite a significant loss in fat mass (Figure 1C–D).
Figure 2. Changes in glucose homeostasis following androgen deprivation.
A) The details of experimental design are described in Figure 1A. A GTT was performed at the end of each dietary period, as indicated by arrows. Intact, black bars; orchidectomized, open bars. B) Fasting glucose, C) fasting insulin, D) AUC glucose, and E) AUC insulin were determined as described in “Materials and Methods.” Error bars are means of means ± SEM, n=6. The differences between dietary groups (p-values are indicated) were determined using repeated measures two-way ANOVA followed by t-test.
The effect of ADT on adipocyte size
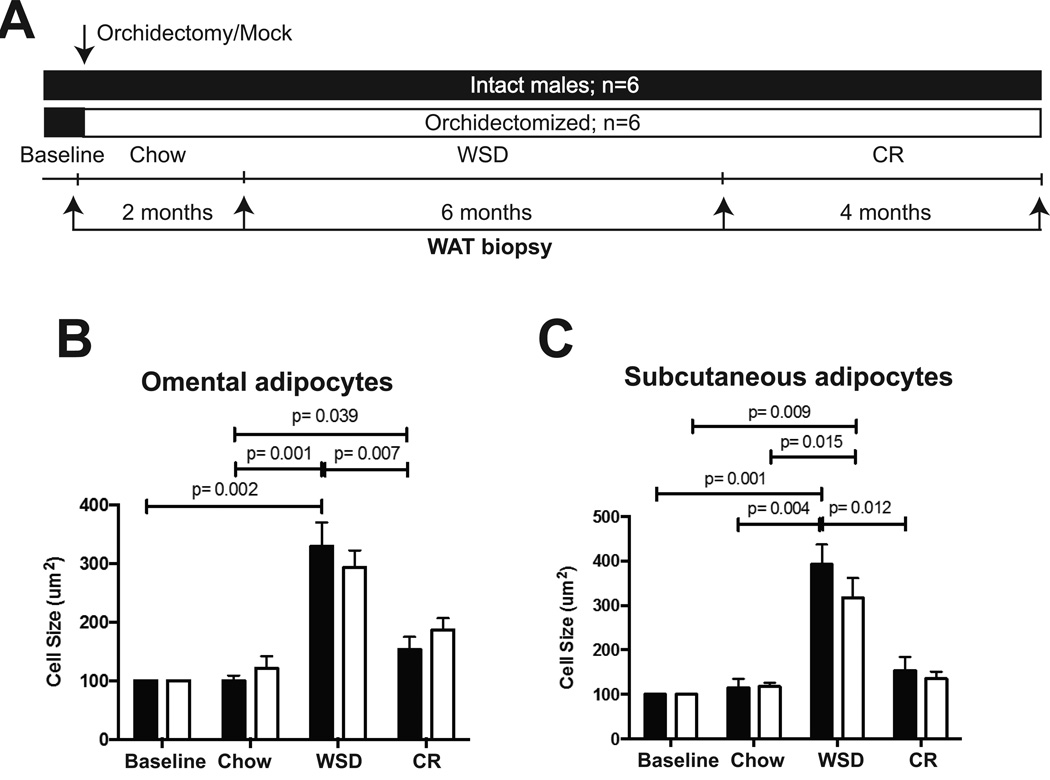
The morphological analysis of subcutaneous (SC) and visceral (collected from omental fat, OM) adipocytes was performed using white adipose tissue (WAT) biopsies collected longitudinally during the study (Figure 3A). OM and SC adipocytes underwent hypertrophy in response to the WSD, with no significant differences observed between groups (Figure 3B–C). CR induced a decrease in OM and SC adipocyte size, although this effect was not statistically significant in orchidectomized animals (Figure 3B–C). The changes in adipocyte size were consistent with the changes in percent body fat seen during the transition from the WSD to CR (Figure 1C–D). The in vitro lipolytic response of OM-WAT and SC-WAT under basal or β-adrenergic agonist-stimulated conditions (10 nM isoproterenol) was not significantly different between orchidectomized and intact animals under any dietary regimen tested in the present study (data not shown).
Figure 3. The effects of androgen deprivation on adipocyte size.
A) The details of experimental design are described in Figure 1A. WAT biopsies were collected at the end of each dietary period, as indicated with arrows. Intact, black bars; orchidectomized, open bars. The area of (B) omental and (C) subcutaneous adipocytes was determined as described in35. Error bars are means of means ± SEM, n=6. The differences between dietary groups (p-values are indicated) were determined using repeated measures two-way ANOVA followed by t-test.
The effect of ADT on food intake and physical activity
Surgery had no significant effect on food intake (intact, 100±6% presurgery food intake; orchidectomized (98±8% presurgery food intake). The WSD stimulated caloric intake in all animals, although no significant differences were found between groups (intact, 215±19%; orchidectomized, 218±26%). Physical activity was monitored continuously using collar-worn accelerometers (Figure 4A). WSD and CR had no significant effect on total daily physical activity levels or changes in daily activity, and there were no significant group differences in these parameters under any of the dietary regimens studied (Figure 4B and C).
Figure 4. Physical activity during androgen deprivation.
A) The details of experimental design are described in Figure 1A. Daily physical activity was monitored continuously, using accelerometers, as described in “Materials and Methods.” Intact, black symbols; orchidectomized, open symbols. B) Daily activity counts; (C) changes in daily activity compared to PreSurgery; (D) correlations between daily activity and the percent of body fat and (E) between daily activity and AUC glucose following the HFD period. Error bars are means of means ± SEM, n=5. Independent samples t-tests were used to analyze differences between the two groups. No significant differences between the two groups was found at any time point in the study. Each group was then analyzed individually using paired t-tests. Correlations were graphed in PRISM. Intact= closed circles (n=6), orchidectomized= open circles (n=4).
Correlation between activity and metabolic responses to orchidectomy and diet during WSD
Correlation analyses were performed to explore the relationship between activity and changes in metabolic parameters that responded to the WSD. Total fat, percent body fat and the ratio of abdominal/gonadal fat were significantly negatively correlated with activity levels, such that the most active animals had the lowest percent body fat at the end of the WSD period (Total fat: r2=0.52, p=0.019; % fat: r2=0.55, p=0.014; Figure 4D). There was a significant negative correlation between activity and AUC glucose at the end of the WSD period, indicating that the most sedentary animals were also the most glucose-intolerant, as shown by the high AUC glucose values for animals with low activity values (r2=0.48, p=0.027; Figure 4E).
The effect of ADT on circulating cytokines
The circulating levels of interleukin-6 (IL-6) increased significantly during CR, and this increase was significantly higher in orchidectomized animals during the WSD period (Figure 5B). In contrast, the levels of macrophage-derived chemokine (MDC, also known as CCL22) elevated during the chow diet period and remained elevated during the WSD and CR periods. During each dietary period, MDC levels were significantly higher in orchidectomized than in intact animals (Figure 5C), suggesting that testosterone deficiency alone is sufficient for triggering the chronic elevation of MDC levels in males.
Figure 5. Changes in circulating cytokines during androgen deprivation.
A) The details of experimental design are described in Figure 1A, except IL-6 (B) and MDC (C) cytokine levels were determines after four months on the WSD. Intact, black symbols; orchidectomized, open symbols. Statistically significant differences between intact and orchidectomized animals (* p<0.05; **p<0.01) were determined by independent t-test.
Discussion
The main metabolic side effects of ADT in prostate cancer patients include the development of obesity4–6, 23 and insulin resistance7–9. Low free testosterone concentrations were also observed in obese diabetic and obese nondiabetic pubertal and post-pubertal males, with the former displaying a significantly higher prevalence of subnormal testosterone levels24, 25. Recent studies demonstrated that males with type 2 diabetes and hypogonadism have additional insulin resistance, while testosterone treatment resulted in its reversal with an improvement in insulin signal transduction26. Additionally, testosterone therapy can help achieve more sustained fat mass loss and improve lean mass and insulin sensitivity in hypogonadal men26, 27, which is consistent with the present report (Figure 1E, 2 and 3). Thus, testosterone may play a protective role in male physiology, while its deficiency may increase the susceptibility of males to metabolic syndrome. Although androgen replacement can improve insulin sensitivity in hypogonadal men, the contribution of environmental factors remains poorly understood28, 29. The benefits of dietary interventions in testosterone-deficient males remain to be determined, and the present study was undertaken to clarify the potential role of WSD and CR in metabolic dysfunction in hypogonadal men.
The present study demonstrates that skeletal muscle loss in testosterone-deficient NHPs correlated with the development of IR and glucose intolerance during the WSD and CR periods. To the best of our knowledge, this is the first study demonstrating that diet-induced insulin resistance persists even after caloric intake and dietary fat content were significantly reduced. Surprisingly, there was no significant effect of testosterone deficiency on diet-induced change in fat mass, including fat gain during the WSD period and fat loss during the CR period, suggesting that insulin resistance in ADT patients is related to the loss of skeletal muscle, which is the primary anatomical site responsible for glucose disposal30. In contrast, ADT patients become insulin-resistant and also gain fat mass following the initiation of therapy6. It is possible that obesity is secondary to changes in lifestyle and diet that may occur during or after initiation of ADT. Because testosterone is the direct precursor of estradiol, some of the observed effects of androgen deficiency in males may be mediated, at least in part, by the lack of estrogen action, which is consistent with the results of clinical studies, suggesting that estrogens are essential for the regulation of body fat in males31. Recently, Dhindsa et al reported that estradiol concentrations are low in type 2 diabetic males with hypogonadotropic hypogonadism and that they increase after treatment with testosterone, suggesting that changes in testosterone and estradiol concentrations are positively related26. Thus, additional studies are needed to compare the effects of aromatizable and non-aromatizable androgens on the regulation of body composition and insulin sensitivity in males.
The second factor that may impact systemic metabolism is WAT dysfunction in response to IL-6. Androgens are involved in various processes in WAT, including adipogenesis32, 33, lipolysis34, 35, adipokine secretion36, and insulin signaling37. New evidence suggests that androgen signaling in WAT protects against high-fat diet-induced obesity, promotes systemic insulin sensitivity, and improves glucose homeostasis36. Thus, ADT may cause dysregulation not only in skeletal muscle, but also in WAT, possibly through altered adipokine secretion36, which may accelerate the development of metabolic syndrome. The lasting increase in the circulating levels of MDC (and IL-6) following androgen deprivation is a new phenomenon, suggesting the possible increase in the levels of alternatively activated macrophages in hypogonadal men38. The present report is consistent with recent human studies that demonstrated the anti-inflammatory effects of testosterone therapy in hypogonadal men26, 27. Specifically, testosterone treatment of type 2 diabetic males with hypogonadotropic hypogonadism suppressed the production of cytokines, including IL-1β and tumor necrosis factor alpha (TNFα)26. IL-1β can induce β-cell death39 while TNFα interferes with insulin signal transduction and induces insulin resistance40. Thus, testosterone treatment will potentially protect patients from the development of diabetes by preventing β-cell loss and potentiating peripheral insulin sensitivity.
Our NHP model recapitulated metabolic and body composition changes that may occur in ADT patients consuming a typical WSD, suggesting that dietary restriction alone is not sufficient for the preservation of lean mass, bone quality, and insulin sensitivity in ADT patients. Dietary restriction may worsen BMD (Figure 1G) associated with ADT5. The observed correlative changes in the bone quality and lean mass are related to the proposed endocrine interactions between bone and skeletal muscle mediated through osteoclast-producing or bone marrow mesenchymal cell-producing factors that have the anabolic effects in muscle41. Similarly, muscle can stimulate bone development through secretion of soluble myokines42. The present NHP study strongly suggests that caloric restriction is not feasible for treatment of osteoporosis in ADT patients43, and other approaches are needed to minimize this and other muscle-related or metabolic adverse effects of ADT. These approaches may include intermittent or local ADT44, 45, resistance training46, 47 and aerobic exercise48, 49, and pharmacological inhibition of myostatin for preserving muscle mass50.
In summary, the present study established, for the first time, a new promising NHP model of ADT and male hypogonadism, allowing us to study diet-specific effects of androgen deprivation and the effects of pharmacological interventions, using highly controlled environment. The use of longitudinal tissue biopsies will allow us to further clarify molecular mechanisms of androgen action in metabolic and musculoskeletal systems.
Acknowledgments
This work was supported by National Institutes of Health Grants R21 AG047543 (to O.V.) and, P51 OD011092 for the operation of the Oregon National Primate Research Center.
We thank Dr. Charles Roberts Jr. from Oregon Health and Science University for critical reading of the manuscript.
Abbreviations
- ADT
androgen-deprivation therapy
- CR
caloric restriction
- NHP
nonhuman primate
- SM
skeletal muscle
- SC
subcutaneous
- VIS
visceral
- WAT
white adipose tissue
- WSD
Western-style diet.
Footnotes
Conflict of interest
Authors report no conflict of interest.
References
- 1.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 2.Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211–223. doi: 10.6004/jnccn.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 4.Basaria S, Lieb J, 2nd, Tang AM, DeWeese T, Carducci M, Eisenberger M, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56(6):779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95(10):2136–2144. doi: 10.1002/cncr.10967. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 7.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86(9):4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 8.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104(2):195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91(4):1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 10.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 11.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30(1):23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 12.Amling CL. Relationship between obesity and prostate cancer. Curr Opin Urol. 2005;15(3):167–171. doi: 10.1097/01.mou.0000165550.94663.fb. [DOI] [PubMed] [Google Scholar]
- 13.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110(7):1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 14.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 18.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85(1):60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Ornish D, Magbanua MJ, Weidner G, Weinberg V, Kemp C, Green C, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008;105(24):8369–8374. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin DW, Neuhouser ML, Schenk JM, Coleman IM, Hawley S, Gifford D, et al. Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2150–2154. doi: 10.1158/1055-9965.EPI-07-0154. [DOI] [PubMed] [Google Scholar]
- 21.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. doi: 10.1093/humrep/der393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104(8):1633–1637. doi: 10.1002/cncr.21381. [DOI] [PubMed] [Google Scholar]
- 24.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186–1192. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol (Oxf) 2013;78(4):593–599. doi: 10.1111/cen.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, et al. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction After Testosterone Replacement in Men With Type 2 Diabetes. Diabetes Care. 2016;39(1):82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond) 2016;40(1):162–170. doi: 10.1038/ijo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dandona P, Dhindsa S, Chandel A, Chaudhuri A. Hypogonadotropic hypogonadism in men with type 2 diabetes. Postgrad Med. 2009;121(3):45–51. doi: 10.3810/pgm.2009.05.2001. [DOI] [PubMed] [Google Scholar]
- 29.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):247–256. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 33.Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 34.Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47(3):420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 35.Varlamov O, Chu MP, McGee WK, Cameron JL, O'Rourke RW, Meyer KA, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154(11):4126–4135. doi: 10.1210/en.2013-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnes KJ, Smith LB, Hunger NI, Saunders PT, Andrew R, Walker BR. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes. 2012;61(5):1072–1081. doi: 10.2337/db11-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153(7):3100–3110. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajaiah R, Perkins DJ, Polumuri SK, Zhao A, Keegan AD, Vogel SN. Dissociation of endotoxin tolerance and differentiation of alternatively activated macrophages. J Immunol. 2013;190(9):4763–4772. doi: 10.4049/jimmunol.1202407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57(11):3034–3044. doi: 10.2337/db07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94(4):1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16(3):272–277. doi: 10.1097/MCO.0b013e32835fe6a5. [DOI] [PubMed] [Google Scholar]
- 42.Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone. 2015;80:109–114. doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassemillante AC, Doi SA, Hooper JD, Prins JB, Wright OR. Prevalence of osteoporosis in prostate cancer survivors: a meta-analysis. Endocrine. 2014;45(3):370–381. doi: 10.1007/s12020-013-0083-z. [DOI] [PubMed] [Google Scholar]
- 44.Hershman DL, Unger JM, Wright JD, Ramsey S, Till C, Tangen CM, et al. Adverse Health Events Following Intermittent and Continuous Androgen Deprivation in Patients With Metastatic Prostate Cancer. JAMA Oncol. 2015:1–9. doi: 10.1001/jamaoncol.2015.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zejnullahu K, Arevalo MG, Ryan CJ, Aggarwal R. Approaches to minimize castration in the treatment of advanced prostate cancer. Urol Oncol. 2016 doi: 10.1016/j.urolonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 47.Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–2052. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 48.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 49.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 50.Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2014;99(10):E1967–E1975. doi: 10.1210/jc.2014-1271. [DOI] [PubMed] [Google Scholar]