Abstract
Background & Aims
Although it is well established that fatty acids (FA) are indispensable for the proliferation and survival of cancer cells in hepatocellular carcinoma (HCC), inhibition of Fatty Acid Synthase (FASN) cannot completely repress HCC cell growth in culture. Thus, we hypothesized that uptake of exogenous FA by cancer cells might play an important role in the development and progression of HCC. Lipoprotein lipase (LPL) is the enzyme that catalyzes the hydrolysis of triglycerides into free fatty acids (FFA) and increases the cellular uptake of FA.
Methods
We used immunohistochemistry and quantitative reverse transcription real-time polymerase chain reaction to evaluate LPL expression in human and mouse HCC samples. Using lipoprotein-deficient medium as well as siRNAs against LPL and/or FASN, we investigated whether human HCC cells depend on both endogenous and exogenous fatty acids for survival in vitro.
Results
We found that LPL is upregulated in mouse and human HCC samples. High expression of LPL in human HCC samples is associated with poor prognosis. In HCC cell lines, silencing of FASN or LPL or culturing the cells in lipoprotein-deficient medium significantly decreased cell proliferation. Importantly, when FASN suppression was coupled to concomitant LPL depletion, the growth restraint of cell lines was further augmented.
Conclusions
The present study strongly suggests that both de novo synthetized and exogenous FA play a major role along hepatocarcinogenesis. Thus, combined suppression of LPL and FASN inhibitors might be highly beneficial for the treatment of human HCC.
Keywords: hepatocellular carcinoma, lipogenesis, fatty acid synthase, lipoprotein lipase
Introduction
Human hepatocellular carcinoma (HCC), the most frequent primary liver cancer, is one of the most lethal tumors worldwide [1, 2]. Few patients are amenable to surgery because of late HCC diagnosis, and alternative treatments do not substantially improve the patients’ prognosis when HCC is unresectable [3-5]. Sorafenib, an oral multi-kinase inhibitor that suppresses tumor cell proliferation and angiogenesis as well as the only targeted therapy available for the treatment of advanced HCC, has limited efficacy in improving survival of HCC patients [6, 7]. Thus, the investigation of the molecular mechanisms leading to HCC development and progression is mandatory to identify new targets for its early diagnosis, prevention and treatment.
Occurrence of HCC is frequently associated with fibrotic or cirrhotic chronic liver disease (CLD), whose main etiology is either viral infection by hepatitis B (HBV) or hepatitis C (HCV), or alcohol abuse [8]. In recent years, an emerging role in the development of HCC has been recognized for Non Alcoholic Fatty Liver Disease (NAFLD) and Non Alcoholic Steatohepatitis (NASH) [9, 10]. NAFLD encompasses a large spectrum of features, ranging from simple reversible intracellular accumulation of lipids in hepatocytes to the presence of inflammation and/or fibrosis, which can ultimately progress to cirrhosis and HCC [11, 12]. In support of a pro-tumorigenic role of NAFLD in the liver, it has been found that diabetes and obesity, two conditions characterized by a pro-lipogenic state, are predisposing conditions to develop HCC [3, 13]. In particular, epidemiologic evidence indicates that obesity almost doubles the risk of HCC [14]. As a consequence, there is an urgent need for a better understanding of the relationship between aberrant lipogenesis and hepatocarcinogenesis in order to develop novel therapeutic strategies against HCC.
In proliferating tumor cells, fatty acids (FA) are required for energy supply, membrane formation, and cell signaling [15, 16]. Based on the current knowledge, FA are predominantly synthetized through de novo lipogenesis in tumor cells [17, 18]. Fatty acid synthase (FASN), the enzyme responsible for the production of long-chain FA from acetyl-coA and malonyl-CoA, is the most investigated lipogenic protein in cancer [19]. Recently, we discovered that ablation of FASN both suppresses AKT-driven hepatocarcinogenesis in mice and restrains the growth of human HCC cell lines in vitro [20]. In spite of the essential role of FA in the proliferation and survival of cancer cells, however, we found that inhibition of FASN is not able to suppress completely HCC cell growth in culture [20]. The latter experimental evidence suggests that HCC cells might compensate the blockade of endogenous FA biosynthesis by the uptake of exogenous FA present in the medium. Thus, it is conceivable that uptake of exogenous FA by cancer cells might play an important role in hepatocarcinogenesis.
It has been hypothesized that triglycerides in circulating lipoprotein particles provide an additional, exogenous source of FA for tumor cells. This event would require triglyceride-rich chylomicrons or very low density lipoproteins (VLDL) as substrates, extracellular lipoprotein lipase (LPL) for hydrolysis, and FA transporters (CD36 and SLC27A family members) for cellular uptake of free FA [21]. In particular, LPL, commonly expressed in adipocytes and muscle cells, plays a central role in lipid metabolism. LPL catalyzes the hydrolysis of triglycerides into free fatty acids (FFA) and increases the cellular uptake of lipoproteins in a non-catalytic manner [22]. As LPL is a secreted enzyme that is bound to the luminal surface of capillary endothelial cells, it could potentially be supplied by tumor cells or by nonmalignant cells from the tumor microenvironment [23].
While the functional significance of endogenous de novo lipogenesis along HCC development and progression has been extensively investigated, whether exogenous FA also contribute to hepatocarcinogenesis remains unknown. In the present study, we evaluated LPL expression in human and mouse HCC samples. In addition, we subjected HCC cell lines to lipoprotein-deficient medium (LDM) and LPL inhibition to investigate the requirement of exogenous FA for HCC cell growth.
Materials and Methods
Human hepatocellular carcinoma samples
A collection of 70 formalin-fixed, paraffin-embedded HCC samples was used in the present study. Forty-eight frozen HCC and corresponding non-tumorous surrounding livers from the same collection were also utilized. Tumors were divided in HCC with poorer (HCCP; n=32) and better (HCCB; n=30) outcome, characterized by <3 and >3 years survival following partial liver resection, respectively. The clinicopathological features of liver cancer patients are summarized in Supplementary Table 1. HCC specimens were kindly provided by Dr. Snorri S. Thorgeirsson (National Cancer Institute, Bethesda, MD, USA) or collected at the Institute of Pathology of the University of Greifswald (Greifswald, Germany) and the Department of Surgery of the University of Sassari (Sassari, Italy). Institutional Review Board approval was obtained at the National Institutes of Health and the local Ethics Review Board of the Universities of Greifswald and Sassari. Informed consent was obtained from all subjects.
Histopathologic Analysis
Liver histopathologic analysis on mouse lesions was performed by two experienced liver pathologists (SR and FD) on tissue slides stained with H&E and the PAS reaction in accordance with the criteria by Frith et al. [24].
HCC cell lines
The following human HCC cell lines were used in this study: SK-Hep1, MHCC97H, SNU475, SNU449, PLC, HLE, Huh7, and HEP40. In addition, the mouse AKT/Ras [25] cells were used. All cells were grown in a 5% CO2 atmosphere, at 37°C, in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and penicillin/streptomycin (Gibco). Lipoprotein-deficient FBS was purchased from Thermo Fisher Scientific, Inc (Catalog number: NC9283729). Lipoproteins were depleted from FBS using ultracentrifugation. Lipoprotein-deficient FBS was added to DMEM to generate 10% Lipoprotein-deficient FBS containing DMEM (LDM). Cell growth was analyzed using crystal violet cell viability assay and/or BrdU Cell Proliferation Assay Kit (Cell Signaling Technology, Danvers, MA). Cell Apoptosis was assessed with the Cell Death Detection Elisa Plus Kit (Roche Molecular Biochemicals, Indianapolis, IN).
Knockdown of FASN and LPL
For knockdown studies, cells were transfected with 30 pmol/L siRNA targeting FASN (ID # s5032; Life Technologies, Grand Island, NY) and/or LPL (siRNA A: 50-GGUAGAUAUUGGAGAACUA; Dharmacon Inc., Lafayette, CO) and with the use of Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer's recommendations. A scramble small interfering RNA (siRNA; ID # 4390846; Life Technologies) was used as negative control RNA and GAPDH siRNA (siRNA; ID # AM4605; Life Technologies) as a positive control. RNA was extracted 48 hours after transfection. Experiments were repeated at least three times in triplicate.
Statistical analysis
Unless otherwise noted, for all experiments that contained single comparisons, 1-way ANOVA with Newman–Keuls post-hoc tests were used. For grouped samples, 2-way ANOVA with Bonferroni post-hoc tests were performed with the GraphPad Prism software (San Diego, CA). All graphs are the mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Kaplan–Meier survival analysis was performed using Statistical Package for Social Science software (SPSS, version 16.0, Chicago, IL, USA).
Detailed description of Materials and methods is provided as Supplementary material.
Results
LPL mRNA is upregulated in human and mouse HCC
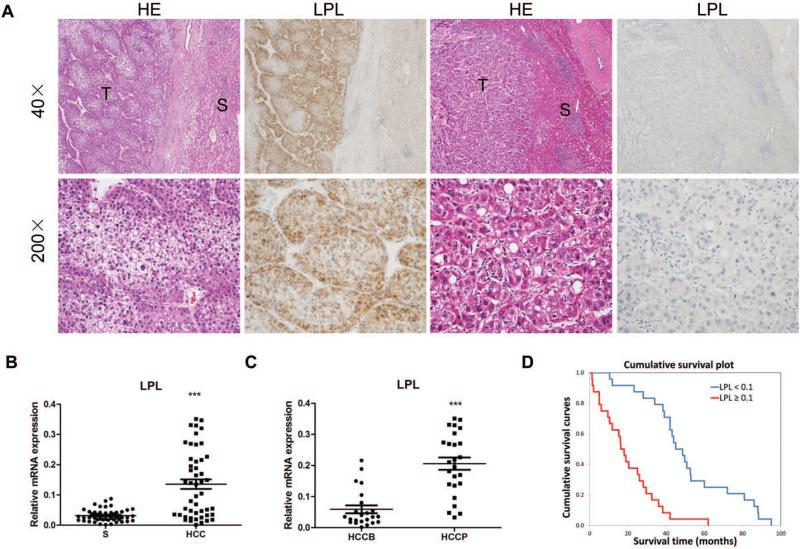
First, we determined the levels of LPL in human HCC samples. For this purpose, we examined the protein expression patterns of LPL in collection of human HCC specimens by immunohistochemistry. We found that LPL levels are upregulated, when compared with non-tumorous surrounding counterparts, in 43 of 70 (61.4%) HCC specimens (Fig.1A, left panels). Equivalent levels of LPL immunoreactivity in HCC and corresponding non-neoplastic livers were detected in the remaining samples (Fig. 1A, right panels). Of note, 28 of 43 (65.1%) tumors showing increased LPL immunolabeling in the tumors when compared with the non-neoplastic counterpart were associated with a poor patient outcome (HCCP), thus suggesting that induction of LPL is associated with a dismal prognosis in HCC.
Figure 1.
Expression patterns of LPL in human HCC. (A) Immunohistochemical patterns of LPL. Left panel, HCC (T) showing upregulation of LPL when compared to the non-neoplastic counterpart (S). Right panel, equivalent immunoreactivity for LPL protein in an HCC and corresponding surrounding non-tumorous liver. Original magnification: 40X in upper panel; 200X in lower panel. Abbreviation: HE, hematoxylin and eosin staining. (B) qRT-PCR analysis of LPL mRNA expression in 48 paired human HCC and non-tumor liver tissues (S). (C) LPL mRNA levels in HCCP (n=25) and HCCB (n=23). The ΔCt value of each sample was calculated by subtracting the average Ct value of the LPL gene from the average Ct value of the β- Actin or RNR-18 gene. (D) Kaplan–Meier survival analysis of HCC patients with high and low expression of LPL. Tukey-Kramer's test: ***P < 0.001.
To further validate the latter findings, LPL mRNA levels were analyzed in 48 paired human non-tumor liver tissue and HCC specimens from the same collection using real-time quantitative RT-PCR. In accordance with the immunohistochemical data, we found that LPL mRNA expression is significantly upregulated in HCC samples compared to respective non-tumor liver samples (P<0.0001; Fig. 1B). In particular, higher levels of LPL were detected in 32 of 48 HCC specimens (64.6%) when compared with corresponding non-tumorous surrounding livers. Once again, 22 of 32 (68.7%) HCCs showing upregulation of LPL in the tumor part belonged to the HCC subset with poorer outcome (HCCP). In addition, LPL expression was significantly higher in HCCP than in HCC with better outcome (HCCB) (P<0.0001; Fig. 1C). Importantly, analysis of LPL expression with HCC survival rate demonstrated that high expression of LPL correlates with poor patient outcome (Fig. 1D). This association is still significant after multivariate Cox regression analysis (p<0.001; Table 1), supporting LPL expression as an independent prognostic factor for HCC. Subsequent analysis of an additional collection of human HCC specimens (n = 28) whose survival data were missing, showed LPL upregulation in the tumor part in 18 of 28 samples (64.3%; data not shown). Furthermore, equivalent results were found in a dataset from The Cancer Genome Atlas (374 surgically resected HCCs and 59 surrounding non-tumor liver tissues) and Fudan database (247 HCC tissues and 239 surrounding non-tumor liver tissues) using microarray analysis (p<0.0001; Supplemental Fig. 1).
Table 1.
Multivariate Cox regression analysis demonstrating that LPL expression is an independent predictor of survival in HCC patients.
| Covariates | Each covariate separately (HR and 95% CI) | Full model (HR and 95% CI) | Stepwise backward (HR and 95% CI) |
|---|---|---|---|
| Age | 1.005 (0.970–1.041) | 0.776 (0.414–1.456) | – |
| Male sex | 1.017 (0.515–2.011) | 1.199 (0.501–2.867) | – |
| Cirrhosis (y/n) | 0.858 (0.438–1.682) | 0.864 (0.411–1.813) | 0.429 (0.198–0.932)* |
| Etiology | |||
| HCV | Reference | Reference | Reference |
| HBV | 0.816 (0.429–1.553) | 1.095 (0.493–2.431) | 1.253 (0.630–2.492) |
| Ethanol | 0.648 (0.245–1.714) | 1.153 (0.384–3.462) | 1.297 (0.443–3.798) |
| Wilson's disease | 0.409 (0.054–3.122) | 0.017 (0.001–0.256)* | 0.035 (0.004–0.346)* |
| Diameter > 3 cm | 1.446 (0.729–2.869) | 1.239 (0.600–2.561) | – |
| AFP > 300 ng/ml | 1.514 (0.813–2.820) | 1.193 (0.570–2.498) | – |
| Edm-Ste | – | ||
| II | Reference | ||
| III | 1.053 (0.513–2.159) | ||
| IV | 2.084 (0.929–4.675) | ||
| LPL > 0.10566 | 4.853 (2.491–9.453)** | 5.901 (2.668–13.050)** | 12.301 (4.868–31.084)** |
p<0.05
p<0.0001
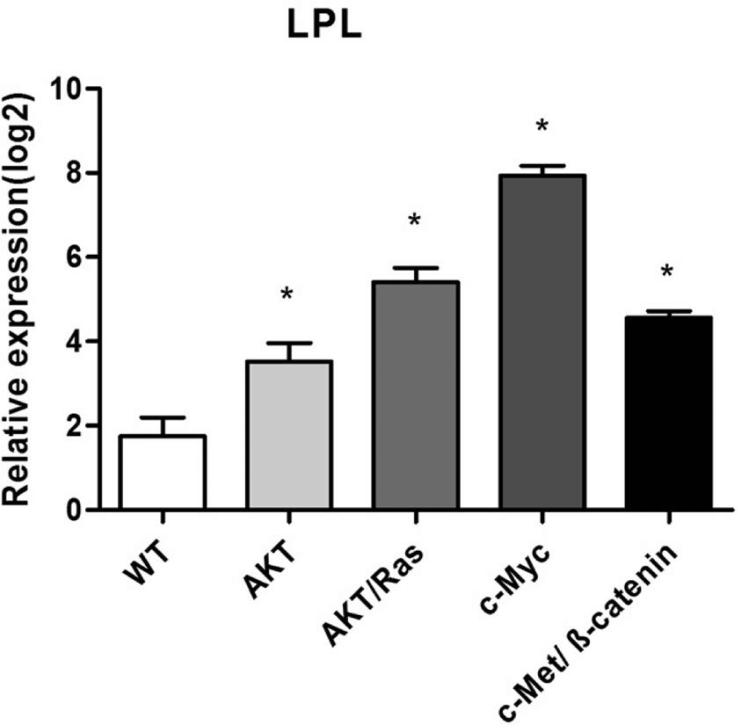
Previously, we have shown that hydrodynamic transfection of activated forms of AKT [15], AKT/Ras [26], c-Myc[27] and c-Met/β-catenin [28] led to HCC development in mice. Thus, we sought to determine the mRNA levels of LPL in liver lesions from these mouse models. Consistent with human HCC data, we found that LPL was expressed at a much higher level in tumor lesions from the various mouse models when compared with wild-type (WT) liver tissues (P<0.05; Fig. 2).
Figure 2.
Expression levels of LPL in wild-type (WT) and tumor samples from different HCC mouse models (overexpressing AKT/Ras, c-Myc, or c-Met/β-catenin). Data were obtained by qRT-PCR and normalized to 18S rRNA gene (n=5 in each group). The ΔCt value of each sample was calculated by subtracting the average Ct value of the target gene from the average Ct value of the 18S rRNA gene. Tukey-Kramer's test: * P < 0.05.
Finally, we determined LPL expression in the collection of human HCC cell lines. We found that LPL mRNA could be detected from all human HCC cell lines tested, although the expression levels varied significantly among these cells (Supplementary Fig. 2A). Furthermore, LPL protein could also be detected using anti-LPL antibody; and the protein levels were consistent with mRNA expression levels (Supplementary Fig. 2B).
Altogether, the present findings indicate that LPL expression is upregulated in both human and mouse HCC samples. In the latter, overexpression of LPL is associated with aggressive tumor growth and poor patient survival.
Depletion of lipoprotein in the serum inhibits HCC cell growth in culture
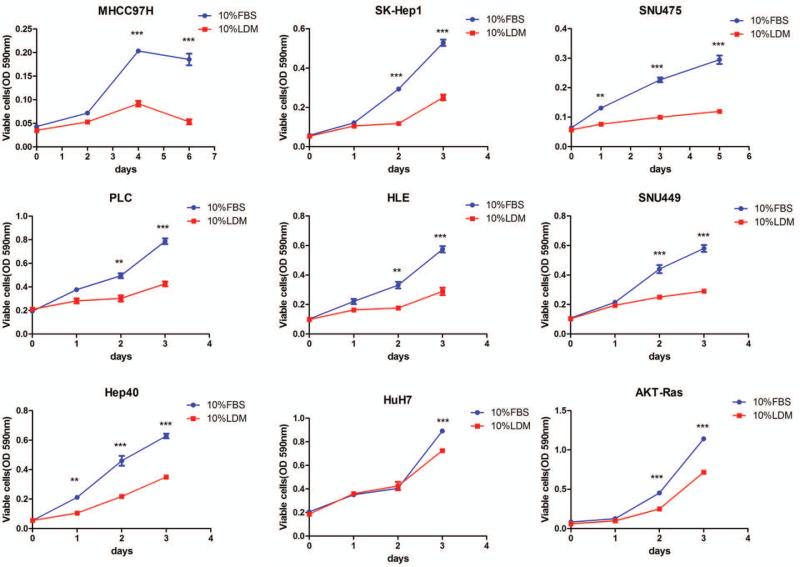
As the first step to investigate whether exogenous FA are required for HCC cell growth, HCC cell lines were cultured either in 10% Fetal Bovine Serum (FBS) medium or 10% lipoprotein depleted FBS medium (LDM). Eight human HCC cell lines (SK-HEP1, MHCC97H, SNU475, SNU449, PLC, HLE, Huh7, and HEP40) and the AKT/Ras mouse HCC cell line [25, 29] were used. Their ability to grow in FBS or LDM was assayed using crystal violet cell viability assay. Strikingly, we found that all the HCC cell lines grew significantly more slowly in 10% LDM compared with 10% Fetal Bovin Serum (FBS) medium (Fig. 3), although the degree of growth inhibition activity of LDM varied among HCC cell lines.
Figure 3.
Effect of lipoprotein deficient medium (LDM) on the growth of human and mouse HCC cell lines in culture. HCC cell lines were incubated either in 10% LDM or 10% FBS; and cell viability was analyzed after 3-6 day of incubation. Tukey-Kramer's test: *P < 0.05; and **P < 0.01.
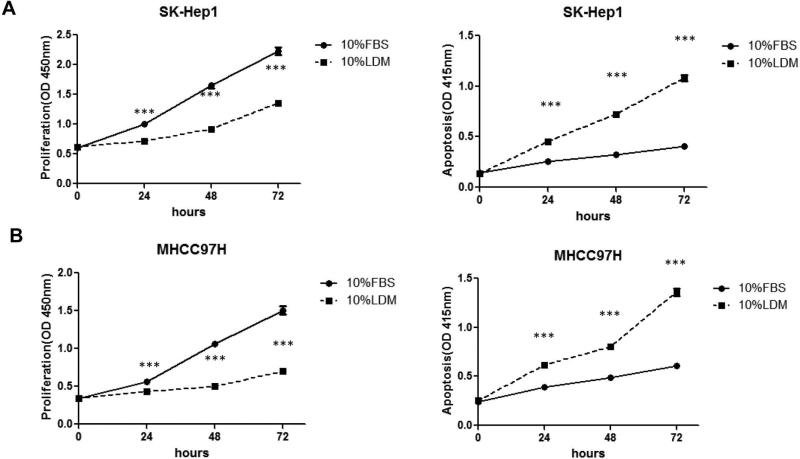
Next, we analyzed the cell proliferation and apoptosis rates in two of these cell lines, namely SK-Hep1 and MHCC97H cells, growing in FBS or LDM using BrdU labeling assay and cell death assay, respectively. Importantly, we found that growing cells in LDM resulted in significantly decreased cell proliferation and increased cell apoptosis (Fig. 4).
Figure 4.
Culture of human HCC cells in LDM led to decreased cell proliferation (left panel) and increased apoptosis (right panel). (A) SK-Hep1 cells and (B) MHCC97H cells. Tukey-Kramer's test: *P < 0.05; **P < 0.01 and ***P<0.0001.
Altogether, our data show that depletion of lipoproteins results in decreased cell proliferation and increased apoptosis, indicating that exogenous FA contribute to HCC cell growth.
Human HCC cells depend on both endogenous and exogenous fatty acids for survival
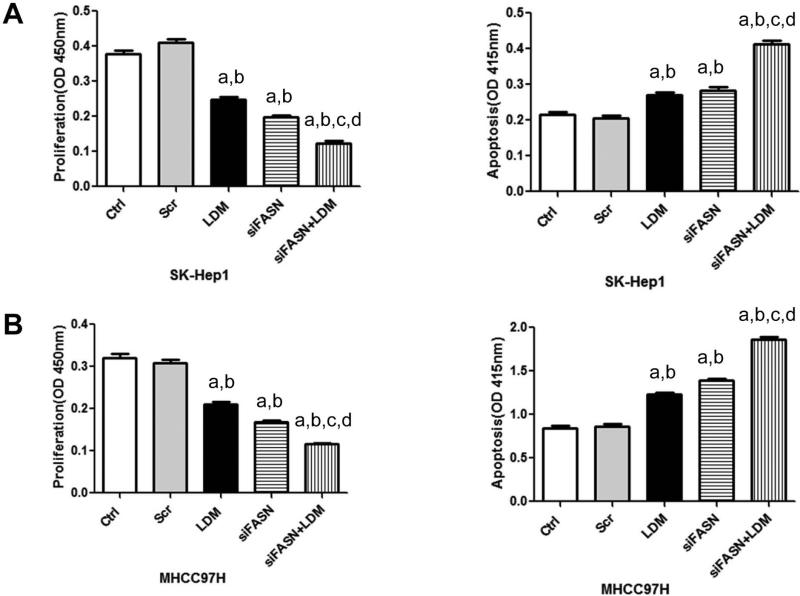
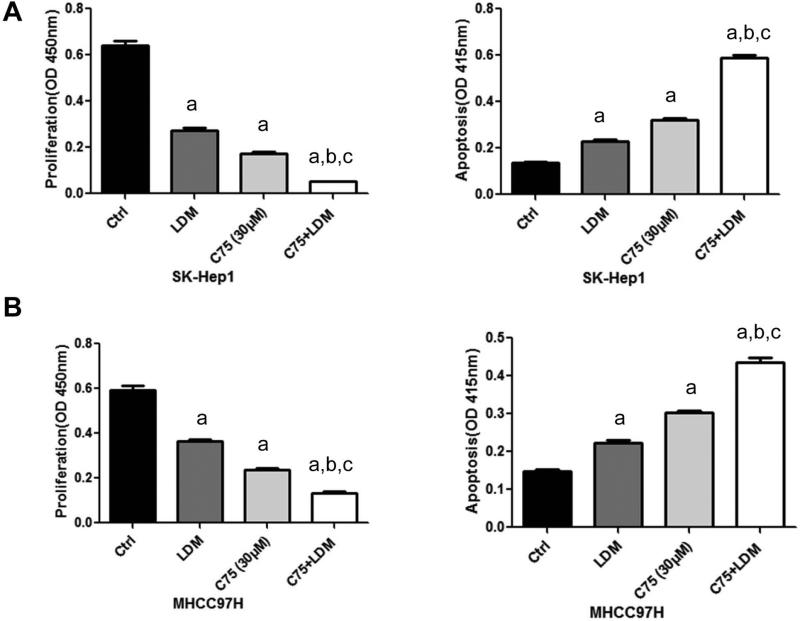
It has been previously shown that FASN blockade triggers tumor growth restraint and massive apoptosis in numerous in vitro and in vivo models [25, 29]. To evaluate whether human HCC cells depend on both endogenous and exogenous FA for survival, the SK-Hep1 and MHCC97H cell lines, which exhibit high LPL mRNA and protein levels (Supplementary Fig. 2), were grown in LDM medium in the presence of FASN small interfering RNA (siRNA; Fig. 5 and Supplementary Fig. 3); or the FASN inhibitor C75 (Fig. 6). Similar to that described in other HCC cell lines [25, 29], FASN silencing via specific siRNA resulted in a significant reduction of cell proliferation in SK-Hep1 and MHCC97H cells. Of note, a significantly more pronounced growth restraint was achieved and increased apoptosis was noted in these cell lines when FASN depletion by siRNA was associated with culture in LDM medium (P<0.05; Fig. 5). Similarly, a pronounced decrease in cell proliferation and a rise in apoptosis were obtained when SK-Hep1 and MHCC97H cell lines were subjected to administration of the FASN chemical inhibitor, C75, while being cultured in LDM medium (P<0.05; Fig. 6).
Figure 5.
Effect of FASN silencing via siRNA and growth in 10% LDM on cell proliferation (left panel) and apoptosis (right panel) of SK-Hep1 (A) and MHCC97H (B) human HCC cell lines. Cells were treated with transfection reagent only (control), scramble siRNA, FASN siRNA, LDM or FASN siRNA+LDM and collected for analysis after 48 hours of treatment. Tukey–Kramer test: P < 0.05 a, vs control; b, vs scramble siRNA; c, vs LDM; d, vs FASN siRNA.
Figure 6.
Effect of C75 (FASN inhibitor; 30ug/l) and LDM on cell proliferation (left panel) and apoptosis (right panel) of SK-Hep1 (A) and MHCC97H (B) human HCC cell lines. Each bar represents mean ± SEM. Tukey–Kramer test: P < 0.05 a, vs control; b, vs LDM; c, vs C75.
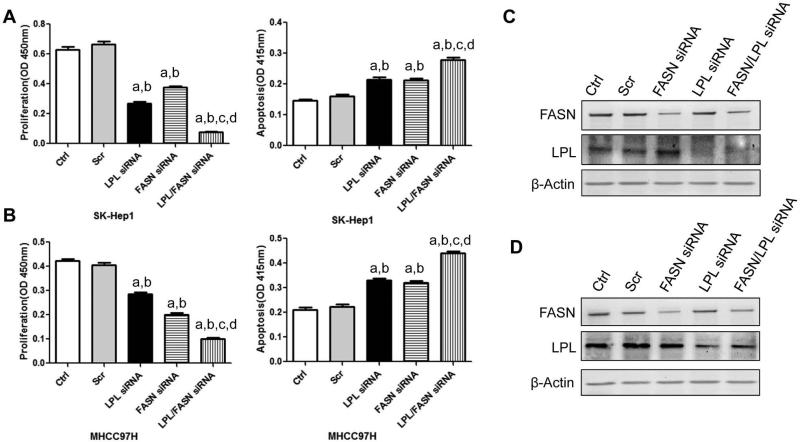
Next, we assessed the importance of concomitant silencing of FASN and LPL on cell proliferation and apoptosis in SK-Hep1 and MHCC97H cells (Supplementary Fig. 4). Suppression of FASN or LPL via specific siRNA significantly decreased cell proliferation and increased apoptosis in both cell lines. Importantly, when FASN suppression was coupled to concomitant LPL depletion, the growth restraint and increased cell apoptosis of both cell lines was further augmented (P<0.05; Fig. 7).
Figure 7.
Effect of LPL and FASN knockdown on cell proliferation (left panel) and apoptosis (middle panel) of SK-Hep1 (A) and MHCC97H (B) human HCC cell lines. Cells were treated with transfection reagent only (control), scramble siRNA, FASN siRNA, siLPL or siFASN+siLPL and collected for analysis after 48 hours treatment. Each bar represents mean ± SEM. Tukey–Kramer test: P < 0.05 a, vs control; b, vs scramble siRNA; c, vs LPL siRNA; d, vs FASN si RNA. (C) and (D): Western blotting analysis showing the decreased expression of FASN and/or LPL protein expression after siRNA treatment in SK-Hep1 (C) and MHCC97H (D) human HCC cell lines.
Altogether, the present findings indicate that human HCC cells depend on both endogenous and exogenous FA for survival.
Discussion
In this study, we investigated the functional contribution of de novo synthetized and exogenous FA during hepatocarcinogenesis. To the best of our knowledge, this is the first study to demonstrate that both endogenous and exogenous FAs contribute to HCC development. Specifically, we hypothesized that, in addition to de novo biosynthesis, liver cancer cells may obtain preformed, diet-derived FA by uptake from the bloodstream. This activity would require hydrolytic release of FA from triglyceride in circulating lipoprotein particles by the secreted enzyme LPL for cellular FA uptake. Noticeably, it has been previously reported that LPL activity, the crucial enzyme for intravascular catabolism of triglyceride-rich lipoproteins, is increased in non-small cell lung cancer (NSCLC), chronic lymphocytic leukemia (CLL), breast and prostate cancer as well as in liposarcoma [22, 23, 30, 31]. However, whether LPL plays a role in HCC is largely unknown. Here, we show that overexpression of LPL is a common feature of most HCC tissues. Indeed, LPL mRNA expression was found to be significantly upregulated in both human and mouse HCC samples compared to corresponding non-tumorous liver counterparts. Importantly, our analysis shows that high levels of LPL correlate with aggressive tumor phenotype and short patient survival, indicating that LPL represents a negative prognostic factor in human HCC. The latter data are in agreement with previous findings in non-small cell lung cancer [30] and chronic lymphocytic leukemia [22], where LPL levels inversely correlate with the patients’ outcome.
The role of LPL in HCC was further substantiated in our study by a body of evidence obtained in in vitro cultured HCC cell lines. Firstly, we found the growth of all HCC cell lines was significantly slower in 10% LDM compared with 10% FBS medium, although the degree of growth inhibition activity of LDM varied among HCC cell lines. This observation clearly indicates that exogenous FA contribute to HCC cell growth and proliferation. Secondly, significantly stronger growth restraint and enhanced cell apoptosis were achieved in HCC cell lines expressing elevated levels of LPL when FASN depletion by siRNA or FASN inhibitor was associated with culture in LDM medium. Similar results with decreased cell proliferation and increased apoptosis were obtained when FASN suppression was coupled to concomitant LPL depletion. Altogether, the present data indicate that human HCC cells depend on both endogenous and exogenous FA for survival.
It is interesting to note that, although the PLC HCC cell line expresses LPL at relatively low levels (Supplementary Fig. 2), the latter cell line shows a reduction in cell viability in response to LDM that is similar to other HCC cell lines displaying high LPL expression (Fig. 3). It is possible that lipolysis by PLC cells depends on a lipase other than LPL. A candidate lipase is hepatic lipase (HL). HL is expressed in normal hepatocytes and shares several functional domains with LPL [32]. However, analysis of expression data using TCGA and Fudan microarray dataset shows that HL is expressed at high levels in normal liver, yet its expression is significantly downregulated in human HCC samples (data not shown). This indicates that HL may not be the major lipase for uptaking FA in the majority of human HCC cases. However, in a small subset of human HCC samples, HL expression remains high (data not shown). Thus, it is possible that, in these HCCs, HL but not LPL is the key lipase responsible for exogenous FA uptake. It would be therefore interesting to assess HL expression in PLC cells, and use functional assays to determine the relative contribution of LPL and HL in regulating PLC cell growth in culture.
The present findings provide a possible mechanism whereby obesity and its related metabolism syndrome, including NAFLD, contribute to the initiation and progression of HCC. Indeed, increased uptake of exogenous FA may be a major route by which HCC cells obtain FA, as FA are important building blocks required for tumor cell growth. In addition, the data shown in the present study might have therapeutic implications. Molecules involved in exogenous FA uptake, including LPL and FA uptake transporters, might represent excellent targets for innovative strategies for the treatment of liver cancer. Inhibitors able to suppress FA uptake might be extremely useful as chemopreventive agents in patients who are at high risk to develop HCC. Also, these inhibitors should be used in association with drugs that suppress the endogenous FA pathway, such as ACAC or FASN inhibitors, for the efficient treatment of HCC.
As concerns the development of LPL inhibitors, a possible molecular approach to suppress LPL expression could be achieved via the upregulation miR-29, as LPL is a specific target of miR-29 in the liver [33]. In a recent study, we found that miR-29 administration is able to inhibit both AKT/Ras and c-Myc driven HCC development in mice [34]. Consistently, we discovered that LPL expression is significantly downregulated in miR-29 overexpressing AKT/Ras and c-Myc liver tumor lesions (Supplemental Fig. 5). As it has been shown that microRNAs can be efficiently delivered into hepatocytes as well as HCC cells [35], in vivo delivery of miR-29 mimic oligonucleotide might represent a possible and effective strategy to inhibit LPL function for chemoprevention and treatment of human HCC.
Supplementary Material
Key Points.
LPL expression is upregulated in mouse and human HCC samples.
High expression of LPL in human HCC samples is associated with poor prognosis.
In HCC cell lines, silencing of FASN or LPL or culturing the cells in lipoprotein-deficient medium significantly decreases cell proliferation.
When FASN suppression is coupled to concomitant LPL depletion, the growth restraint of HCC cell lines is further augmented.
In addition to de novo biosynthesis of fatty acids, uptake of fatty acids from the bloodstream might represent an important source of lipids for HCC cells.
Acknowledgments
Financial support: This work was supported by NIH R01CA136606 and R21CA187306 to XC; P30DK026743 to UCSF Liver Center; National Natural Science Foundation of China (Grant No. 81201553) to Lei Li; and grant from the Italian Association Against Cancer (AIRC; grant number IG 12139) to DFC.
Abbreviations
- LPL
lipoprotein lipase
- FA
fatty acids
- FASN
fatty acid synthase
- HCC
hepatocellular carcinoma
- LDM
lipoprotein deficient medium
- NAFLD
Non Alcoholic Fatty Liver Disease
- NASH
Non Alcoholic Steatohepatitis
- siRNA
small interfering RNA
Footnotes
Conflict of interests: The authors declare there is no conflict of interests.
References
- 1.EL-SERAG HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–8. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.JEMAL A, BRAY F, CENTER MM, FERLAY J, WARD E, FORMAN D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.EL-SERAG HB, KANWAL F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–75. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BRUIX J, BOIX L, SALA M, LLOVET JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5(3):215–9. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 5.LLOVET JM, BRUIX J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 6.CHENG AL, KANG YK, CHEN Z, TSAO CJ, QIN S, KIM JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.KANE RC, FARRELL AT, MADABUSHI R, BOOTH B, CHATTOPADHYAY S, SRIDHARA R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14(1):95–100. doi: 10.1634/theoncologist.2008-0185. [DOI] [PubMed] [Google Scholar]
- 8.PISCAGLIA F, SVEGLIATI-BARONI G, BARCHETTI A, PECORELLI A, MARINELLI S, TIRIBELLI C, et al. Clinical patterns of hepatocellular carcinoma (hcc) in non alcoholic fatty liver disease (NAFLD): A multicenter prospective study. Hepatology. 2015 doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 9.MARCHESINI G, BUGIANESI E, FORLANI G, CERRELLI F, LENZI M, MANINI R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 10.TAKAMATSU S, NOGUCHI N, KUDOH A, NAKAMURA N, KAWAMURA T, TERAMOTO K, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepato-gastroenterology. 2008;55(82-83):609–14. [PubMed] [Google Scholar]
- 11.WHITE DL, KANWAL F, EL-SERAG HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(12):1342–59. e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.YOUNOSSI ZM. Review article: current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Alimentary pharmacology & therapeutics. 2008;28(1):2–12. doi: 10.1111/j.1365-2036.2008.03710.x. [DOI] [PubMed] [Google Scholar]
- 13.ASCHA MS, HANOUNEH IA, LOPEZ R, TAMIMI TA, FELDSTEIN AF, ZEIN NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–8. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 14.MANCEBO A, GONZALEZ-DIEGUEZ ML, CADAHIA V, VARELA M, PEREZ R, NAVASCUES CA, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(1):95–101. doi: 10.1016/j.cgh.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 15.CALVISI DF, WANG C, HO C, LADU S, LEE SA, MATTU S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140(3):1071–83. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SANTOS CR, SCHULZE A. Lipid metabolism in cancer. Febs J. 2012;279(15):2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 17.MENENDEZ JA, LUPU R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 18.SWINNEN JV, BRUSSELMANS K, VERHOEVEN G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9(4):358–65. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 19.FLAVIN R, PELUSO S, NGUYEN PL, LODA M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6(4):551–62. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LI L, PILO GM, LI X, CIGLIANO A, LATTE G, CHE L, et al. Inactivation of fatty acid synthase impairs hepatocarcinogenesis driven by AKT in mice and humans. Journal of hepatology. 2015 doi: 10.1016/j.jhep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GOLDBERG IJ, ECKEL RH, ABUMRAD NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. Journal of lipid research. 2009;50(Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ROZOVSKI U, GRGUREVIC S, BUESO-RAMOS C, HARRIS DM, LI P, LIU Z, et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Molecular cancer research : MCR. 2015;13(5):944–53. doi: 10.1158/1541-7786.MCR-14-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KUEMMERLE NB, RYSMAN E, LOMBARDO PS, FLANAGAN AJ, LIPE BC, WELLS WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Molecular cancer therapeutics. 2011;10(3):427–36. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DOEGE H, GRIMM D, FALCON A, TSANG B, STORM TA, XU H, et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283(32):22186–92. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.HO C, WANG C, MATTU S, DESTEFANIS G, LADU S, DELOGU S, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55(3):833–45. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HO C, WANG CM, MATTU S, DESTEFANIS G, LADU S, DELOGU S, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55(3):833–45. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CHOW EK, FAN LL, CHEN X, BISHOP JM. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56(4):1331–41. doi: 10.1002/hep.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.TWARD AD, JONES KD, YANT S, CHEUNG ST, FAN ST, CHEN X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14771–6. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CHEN X, CALVISI DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. The American journal of pathology. 2014;184(4):912–23. doi: 10.1016/j.ajpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CERNE D, ZITNIK IP, SOK M. Increased fatty acid synthase activity in non-small cell lung cancer tissue is a weaker predictor of shorter patient survival than increased lipoprotein lipase activity. Archives of medical research. 2010;41(6):405–9. doi: 10.1016/j.arcmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 31.KRISTENSEN L, KRISTENSEN T, ABILDGAARD N, ROYO C, FREDERIKSEN M, MOURITS-ANDERSEN T, et al. LPL gene expression is associated with poor prognosis in CLL and closely related to NOTCH1 mutations. European journal of haematology. 2015 doi: 10.1111/ejh.12700. [DOI] [PubMed] [Google Scholar]
- 32.PERRET B, MABILE L, MARTINEZ L, TERCE F, BARBARAS R, COLLET X. Hepatic lipase: structure/function relationship, synthesis, and regulation. Journal of lipid research. 2002;43(8):1163–9. [PubMed] [Google Scholar]
- 33.MATTIS AN, SONG G, HITCHNER K, KIM RY, LEE AY, SHARMA AD, et al. A screen in mice uncovers repression of lipoprotein lipase by microRNA-29a as a mechanism for lipid distribution away from the liver. Hepatology. 2015;61(1):141–52. doi: 10.1002/hep.27379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.TAO J, JI J, LI X, DING N, WU H, LIU Y, et al. Distinct anti-oncogenic effect of various microRNAs in different mouse models of liver cancer. Oncotarget. 2015;6(9):6977–88. doi: 10.18632/oncotarget.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CALLEGARI E, GRAMANTIERI L, DOMENICALI M, D'ABUNDO L, SABBIONI S, NEGRINI M. MicroRNAs in liver cancer: a model for investigating pathogenesis and novel therapeutic approaches. Cell death and differentiation. 2015;22(1):46–57. doi: 10.1038/cdd.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.