Abstract
Purpose
We present three dimensional ultrashort echo time Cones (3D UTE Cones) techniques for quantification of total water T1s (T1TW), bound water T1s (T1BW) and pore water T1s (T1PW) in vitro and in vivo using a 3T scanner.
Methods
T1TW, T1BW and T1PW were measured with 3D Cones and adiabatic inversion recovery Cones (IR-Cones) sequences. 2D non-selective UTE techniques, including saturation recovery, variable TRs, and IR preparation approaches were compared with 3D-Cones techniques on bovine cortical bone samples (n=8). The 3D Cones sequences were used to measure T1TW, T1BW and T1PW in the tibial midshaft of healthy volunteers (n=8).
Results
Comparable T1s were achieved for cortical bone between 3D Cones and 2D UTE techniques as well as those published in the literature. The 3D Cones sequences showed a mean T1TW of 208±22 ms, a mean T1PW of 545±28 ms and a mean T1BW of 131±12 ms for bovine cortical bone, and a mean T1TW of 246±32 ms, a mean T1PW of 524±46 ms and a mean T1BW of 134±11 ms for the tibial midshaft of healthy volunteers.
Conclusions
The 3D Cones sequences can be used for fast volumetric assessment of bound and pore water T1s in vitro and in vivo.
Keywords: cortical bone, UTE, Cones, bound water, pore water, T1 measurement
Introduction
Osteoporosis is defined by decreased bone strength [1] and is characterized by thinning and increased porosity of cortical bone as well as architectural deterioration of trabecular bone [2–5]. Cortical bone is particularly important since it composes approximately 80% of the skeleton and approximately 80% of all fractures associated with advanced age arise at sites that are mainly composed of cortical bone [6]. Cortical bone has a hierarchical physical structure [7] and consists of mineral (~43% by volume), organic matrix (~35%) and water (~22%) [8, 9]. The water exists in various locations and in different states including water bound to the organic matrix (bound water BW) and water residing in Haversian canals and in lacunae-canalicular systems (pore water PW) [9–14]. Bound and pore water pools show opposite correlations with biomechanical measures of bone competence [15, 16]. Therefore, it is of critical importance to develop techniques to noninvasively evaluate properties of cortical bone, including bound and pore water components.
Magnetic resonance (MR) imaging is uniquely suited for imaging of water. However, cortical bone water has a short T2* which can barely be detected by conventional clinical MR sequences [17]. Ultrashort echo time (UTE) techniques have been used to acquire signal from cortical bone water before it decays to zero or near zero levels. Using UTE-based techniques, total bone water (including both bound water and pore water) T1 (T1TW) and concentration can be measured using clinical MR scanners [18–20]. Furthermore, bound and pore water T2*s and relative fractions can be accessed using bi-exponential fitting of UTE signal decay [19–23]. Bound water T2*s can be selectively measured with adiabatic inversion recovery prepared UTE (IR-UTE) techniques in which pore water with a longer T2* can be selectively suppressed [24]. However, T1s of bound water (T1BW) and pore water (T1PW) have not been well investigated using clinical MR scanners, although T1BW and T1PW have been reported using high performance nuclear magnetic resonance (NMR) spectrometers [22,25]. Accurate measurement of bound and pore water concentrations require compensation of T1 and T2* effects [25–27]. Reliability and fast measurements of T1BW and T1PW would also be necessary to be performed in vivo in the clinical setting.
The 3D Cones UTE sequence employs a short radio frequency (RF) rectangular pulse for signal excitation, followed by 3D spiral trajectories sampled on the Cones [28,29]. The Cones sequence provides 3D volumetric UTE imaging in a time efficient way with greatly reduced eddy current artifacts compared with the regular 2D slice-selective UTE sequence. Our prior studies have shown that the 2D spiral UTE sequence has improved signal-to-noise (SNR) efficiency than the 2D radial UTE sequence [30]. The 3D Cones sequence is expected to have further improved SNR efficiency compared with the 2D spiral UTE sequence as well as the 2D or 3D radial UTE sequences [30,31]. The purpose of this study was to utilize the efficient, 3D-Cones sequences for fast volumetric measurement of T1BW and T1PW of cortical bone in vitro and in vivo. Comparison studies between 2D UTE and 3D Cones sequences were performed on a rubber phantom and ex vivo bovine bone samples. Finally the 3D Cones techniques were applied to healthy volunteers on a 3T scanner to demonstrate the feasibility of fast volumetric assessment of T1BW and T1PW in vivo.
Methods
Sample Preparation
Eight bovine cortical bone samples were harvested from mature bovine femoral midshafts obtained from a local slaughterhouse, and were cleared of external muscle and soft tissue. Bone marrow was removed with a scalpel. Cross-sectional cortical bone segments with an approximate thickness of 60 mm were sectioned using a low-speed diamond saw (Isomet 1000, Buehler) with constant saline irrigation, and stored in phosphate buffered saline (PBS) solution for 24 hours prior to use. A piece of rubber (Pink Eraser, Paper Mate Products Inc) was used as a reference phantom for T1 quantification (details shown below).
Pulse Sequences
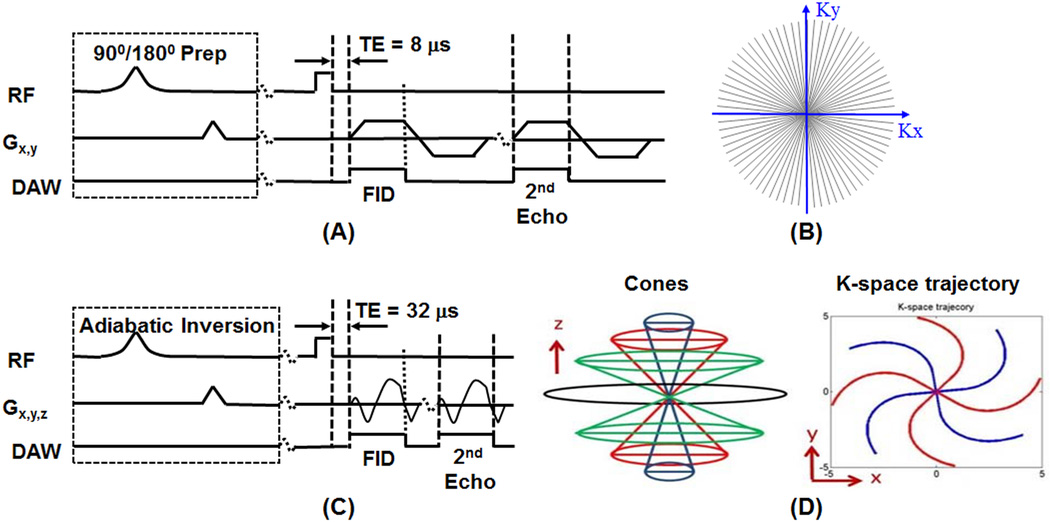
Figure 1 shows the 3D Cones UTE sequences as well as previously reported 2D radial UTE sequences implemented on a 3T Signa TwinSpeed scanner (GE Healthcare Technologies, Milwaukee, WI). The basic 3D Cones sequence (Figure 1C) employed a short radio frequency (RF) rectangular pulse (duration = 26–52 µs) for signal excitation, followed by 3D spiral trajectories sampled on the Cones (Figure 1D) [29]. The Cones sequence provides 3D volumetric UTE imaging in a time efficient way with eddy currents artifacts greatly reduced over the regular 2D UTE sequence, which employs half-pulses for selective excitation and thus are sensitive to eddy currents and gradient errors [32]. To minimize sensitivity to eddy currents, we used 2D non-slice selective UTE rather than slice-selective UTE sequences (Figure 1A) in which the slice selective half-pulse excitation was replaced with a short rectangular pulse (duration = 26–52 µs). This eliminates the eddy currents while offering much improved SNR due to projection imaging in the slice dimension. The non-selective UTE imaging also speeds up data acquisition since only one excitation is needed (2D slice-selective imaging requires two excitations). The 2D non-selective UTE sequences are used to compare with the 3D Cones UTE sequences in T1 analysis of bound and pore water components in bovine cortical bone samples.
Figure 1.
The 2D non-slice selective UTE (A) and 3D UTE Cones (C) sequences, as well as sampling trajectories for 2D UTE (B) and 3D Cones (D). Both 2D UTE and 3D Cones sequences employ a short rectangular pulse (duration = 26–52 µs) for signal excitation followed by single or dual-echo radial ramp sampling. Magnetization preparation including short 90° saturation pulse (duration = 248 µs) and long adiabatic inversion pulse (duration = 8.64 ms) can be applied before UTE data acquisitions.
Both bound and pore water are detectable with the basic 2D radial UTE and 3D Cones UTE sequences. The UTE sequences can also be combined with an adiabatic inversion recovery preparation pulse (Silver-Hoult pulse with duration of 8.64 ms, spectral bandwidth of 1.5 kHz) for IR-Cones or IR-UTE imaging of bound water [29]. The purpose of the adiabatic IR pulse is to invert the longitudinal magnetization of the long T2 signal components, including those in muscle and fat as well as pore water [25,33]. The magnetization of collagen-bound water which has a very short T2* is not inverted but is largely saturated by the adiabatic IR pulse [25]. After an inversion time (TI) delay during which the inverted pore water magnetization approaches the null point, the Cones acquisition is initiated to selectively detect signal from collagen-bound water. To speed up data acquisition, one IR preparation is followed by five Cones sampling [29].
T1TW and T1PW Measurement with Saturation Recovery UTE (SR-UTE)
Saturation recovery UTE (SR-UTE) has been employed for T1 measurement of cortical bone [33]. In this technique, a 90° rectangular pulse (duration = 232 s) was devised in conjunction with large dephasing gradients to suppress signals from both long and short T2 species. UTE acquisition started at a series of saturation recovery time (TSR) to detect the signal recovery from bone. Only the 2D non-selective UTE sequence was combined with the short 90° rectangular pulse for SR-UTE imaging. The 3D Cones sequence was not combined with the SR approach due to excessively lengthy scan times. The single exponential signal recovery model shown below was used to fit T1 [18,33]:
| (1) |
where S(TSR,TE=8µs) is the UTE-TSR signal intensity, S0 is the steady state UTE signal intensity, k accounts for the residual fraction of the longitudinal magnetization of cortical bone after a nominal 90 pulse, T1TW is the effective T1 of bone water with signal contribution from both bound and pore water in cortical bone.
Bound water signal has an extremely short T2* of around ~300 µs, while pore water has a much longer T2* of several milliseconds. Therefore, a longer TE (e.g., TE = 2.5 ms) can be used to selectively detect signal from pore water with near zero signal contribution from bound water. In this case, SR-UTE can be used to measure T1 of pore water (T1PW) based on the following equation:
| (2) |
Each bovine cortical bone sample was placed in Fomblin solution which helped maintain the hydration of cortical bone and minimize susceptibility effects at tissue-air interfaces. A wrist coil (BC-10, Medspira, Minneapolis, MN) was used for signal excitation and reception. The 2D dual-echo SR-UTE sequence employed the following imaging parameters for T1 quantification: field of view (FOV) = 15 cm, sampling bandwidth (BW) = 125 kHz, flip angle = 20, TE = 8 µs and 2.5 ms, TR = 1000 ms, 8 SR-UTE acquisitions (TSRs = 7, 25, 50, 100, 200, 400, 600, 800ms), reconstruction matrix size = 256×256, in-plane pixel size = 0.31×0.31 mm2, scan time = 28 min.
T1TW and T1PW Measurement with UTE Variable TR (UTE-VTR) Approach
The steady-state UTE signal SUTE can be written as [18]:
| (3) |
where fxy and fz describe the behavior of the transverse magnetization and longitudinal magnetization, respectively, as a function of the pulse B1(t) as well as the T2 and T1 of the ultrashort T2* components, S0UTE is the UTE signal with full longitudinal recovery. For both the 2D non-selective UTE and 3D Cones UTE sequences, the duration of the excitation pulse (i.e., 52 µs, 20° rectangular pulse) is significantly shorter than both T2 and T1 of bone water, therefore relaxation effects during RF excitation could be ignored as a first order approximation. Therefore, the above equation can be simplified as below:
| (4) |
Where T2*TW is the effective T2* of bound and pore water in cortical bone.
When a longer TE is used, bound water signal decays to near zero and only pore water is detected. As a result, T1PW can be selectively measured with UTE-VTR acquisitions with a longer TE (e.g., 2.5 ms). Pore water T1 can then be measured with the following equation [34]:
| (5) |
Since only pore water is detected with a TE of 2.5 ms, T2*PW should be used in the above equation.
A bi-component model shown below was used to quantify the T2*s and relative fractions of bound and pore water components in cortical bone [13]:
| (6) |
Where SBW and SPW are the corresponding signal intensities of bound and pore water components at TE of 0.
The experimental setup was similar to that used in the SR-UTE approach. The dual-echo 2D non-selective UTE-VTR technique employed similar imaging parameters except eight TRs of 14, 25, 50, 100, 200, 400, 600 and 800 ms, and a total scan time 12 min. The dual-echo 3D Cones-VTR technique employed similar imaging parameters except 10 slices, a slice thickness of 7 mm, nine TRs of 6, 10, 15, 20, 25, 30, 50, 100 and 200 ms, 280 sampling points per Cones trajectory (sampling window = 1120 µs, spiral trajectories = 3728), and a total scan time of 28 min. T2* was measured with single-echo 2D UTE and 3D Cones with 15 TEs (TEs = 8 or 32 µs, 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1, 1.5, 2, 3, 4, 6, 8 and 10 ms), and a constant TR of 100 ms for 2D UTE and 20 ms for 3D Cones. The total scan time for T2* quantification was 6 min for 2D UTE and 19 min for 3D Cones.
T1BW Measurement with IR-UTE Variable TR/TI Approach
In IR-UTE the long T2 signal from pore water is inverted and nulled while the short T2 signal from bound water is saturated and recovered during the inversion time of TI, and subsequently detected by UTE data acquisitions. The steady state IR-UTE signal following an adiabatic IR pulse can be calculated as follows [35]:
| (7) |
Where S0IR-UTE is the steady state IR-UTE signal of cortical bone, and Q is the fraction of longitudinal magnetization of bound water following the adiabatic IR pulse. Our previous studies suggest that bound water T2* is around ~0.3 ms, yielding a Q value of less than 0.05 following Bloch equation simulation. This near complete saturation of bound water component is consistent with results reported by other groups [25–27, 36]. As a result, Eq. 7 can be simplified as follows [35]:
| (8) |
Eq. 8 suggests that T1 of bound water can be reliably measured via exponential fitting of IR-UTE images acquired with different combinations of TR and TI, on the condition that all these TR/TI combinations satisfy the nulling of pore water in cortical bone. Although TR is not shown explicitly in Eq. 8, varying TI is associated with varying TR since TI depends on TR in the nulling condition [35].
The experimental setup was similar to those used in the SR-UTE and UTE-VTR approaches. The 2D non-selective IR-UTE sequence employed similar imaging parameters except reduced reconstruction matrix size of 128×128, five TR/TI combinations (representative TR/TI values = 50/24; 100/48; 200/90; 300/130 and 400/160 ms, where TI was adjusted based on the measured T1pw and was further verified by measuring the decay of IR-UTE signals, and a total scan time of 6 min. A single-component T2* signal decay would suggest the nulling of pore water and selective detection of bound water [24]). The 3D IR-Cones UTE sequence employed similar imaging parameters except reconstruction matrix size = 128×128×10, a slice thickness of 7 mm, the same five TR/TI combinations, and a total scan time of 10 min. T2* was measured with 2D IR-UTE and 3D IR-Cones sequences, respectively, with 15 TEs (TEs = 8 or 32 µs, 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1, 1.5, 2, 3, 4, 6, 8 and 10 ms), and a total scan time of 90 min for 2D IR-UTE and 150 min for 3D IR-Cones imaging, respectively.
T1 Measurements In Vivo
The 3D Cones and IR-Cones sequences were applied to the tibial midshaft of eight healthy volunteers (all males, 27–42 years old, mean/standard deviation = 32 ± 5) for bound and pore water T1 measurements in vivo. Written informed consent approved by our Institutional Review Board was obtained prior to their participation in this study. An 8-channel knee coil was used for signal excitation and reception.
To measure T1TW and T1PW, the following dual-echo 3D Cones-VTR imaging parameters were used for in vivo studies: FOV = 15 cm, BW = 250 kHz, flip angle = 18, TE = 32 µs and 2.5 ms, 10 slices, slice thickness = 7 mm, five TRs of 7.8, 11, 15, 20 and 30 ms, reconstruction matrix size = 192×192, scan time = 14 min. To measure T1BW, the following 3D IR-Cones imaging parameters were used for in vivo studies: FOV = 15 cm, BW = 250 kHz, flip angle = 18, TE = 32 µs, 10 slices, slice thickness = 7 mm, five TR/TI combinations (representative TR/TI values = 50/24; 100/48; 200/90; 300/130; 400/160 ms. TI was adjusted based on the measured T1pw), reconstruction matrix size = 128×128×10, scan time = 11 min.
Data Analysis
The analysis algorithm was written in Matlab (The MathWorks Inc., Natick, MA, USA) and was executed offline on the DICOM images obtained by the protocols described above. The program allowed placement of ROIs on the first UTE image of the series, which was then copied onto each of the subsequent images. The mean intensity within each of the ROIs (approximately 50 pixels) was used for subsequent curve fitting. T1TW was estimated using Eq. 1 for the SR-UTE approach and Eq. 4 for the UTE acquisitions with variable TR approach. T1PW was estimated using Eq. 2 for the SR-UTE approach and Eq. 5 for the UTE acquisitions with variable TR approach. T1BW was estimated using Eq. 8 for the IR-UTE approach (TI was calculated for each TR based on the measured T1PW). Bi-component T2* analysis was performed on 2D UTE and 3D Cones as well as 2D IR-UTE and 3D IR-Cones images using Eq. 6. The estimated results of T1TW, T1PW and T1BW were compared between non-selective 2D UTE and 3D Cones sequences in the bovine bone study. Then the 3D Cones and IR-Cones techniques were applied to healthy volunteers with mean and standard deviation of T1TW, T1PW and T1BW calculated. Signal to noise ratio (SNR) was introduced to evaluate the efficiency of 3D Cones and IR-Cones UTE imaging of cortical bone in vivo. SNR was calculated by dividing the mean signal intensity measured in cortical bone by the noise measured in air.
Results
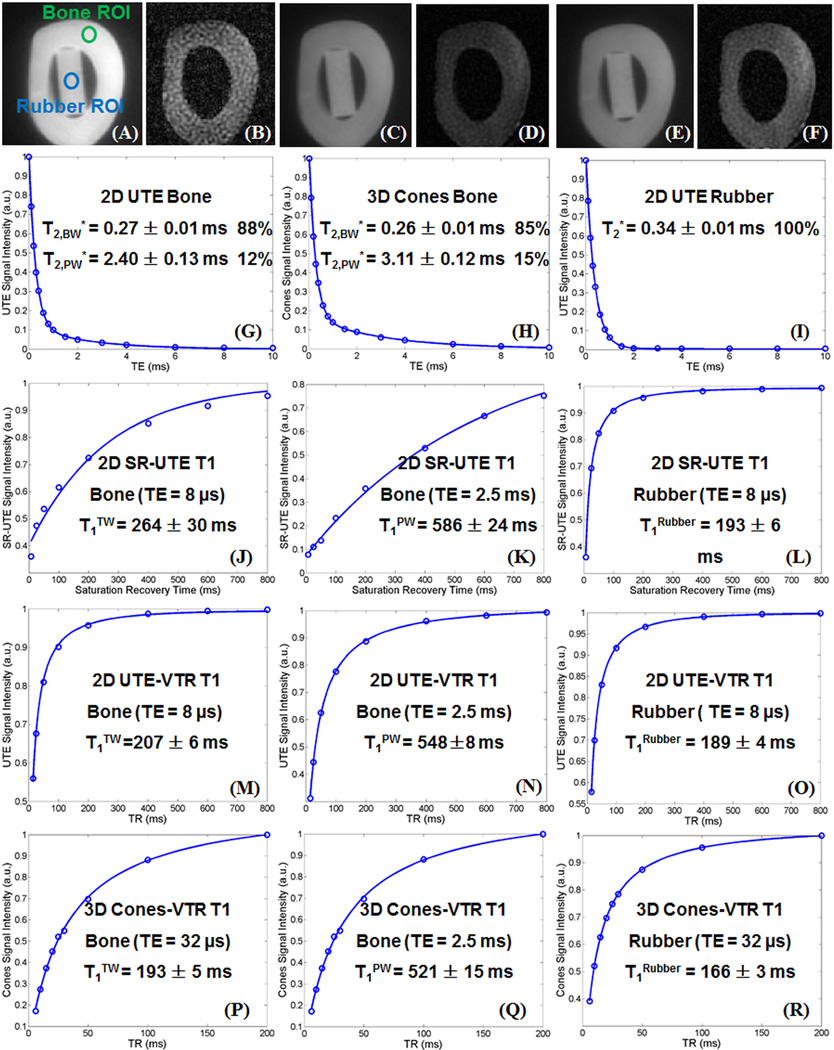
Figure 2 shows representative dual echo 2D SR-UTE, 2D UTE-VTR and 3D Cones-VTR images of a bovine cortical bone sample, as well as bi-component fitting of T2* data, and single-component fitting of T1 data from a representative ROI drawn in cortical bone and rubber phantom, respectively. Two distinct T2* components were observed in cortical bone while a single component was observed in the rubber eraser. T1TW, T1PW and T1BW values derived from all three techniques were largely consistent for both the cortical bone and the rubber eraser, as noted in the figure.
Figure 2.
Selected images of a bovine cortical bone sample acquired with 2D dual echo saturation recovery UTE with a TSR of 100 ms, TEs of 8 µs (A) and 2.5 ms (B), 2D dual echo UTE with a TR of 100 ms and TEs of 8 µs (C) and 2.5 ms (D), and 3D dual echo Cones with a TR of 20 ms and TEs of 8 µs (E) and 2.5 ms (F). Both 2D UTE and 3D Cones show similar bi-component T2* decay for cortical bone (bound water with a shorter T2* of 0.26/0.27 ms and pore water with a longer T2* of 2.40/3.11 ms) and single-component T2* decay for rubber. Single-component exponential recovery curve fitting shows a T1TW of 264±30 ms (J), a T1PW of 586±24 ms (K), and a T1rubber of 193±6 ms (L) with the SR-UTE approach, a T1TW of 207±6 ms (M), a T1PW of 548±8 ms (N), and a T1rubber of 185±4 ms (O) with the UTE-VTR approach, and a T1TW of 193±5 ms (P), a T1PW of 521±15 ms (Q), and a T1rubber of 166±3 ms (R).
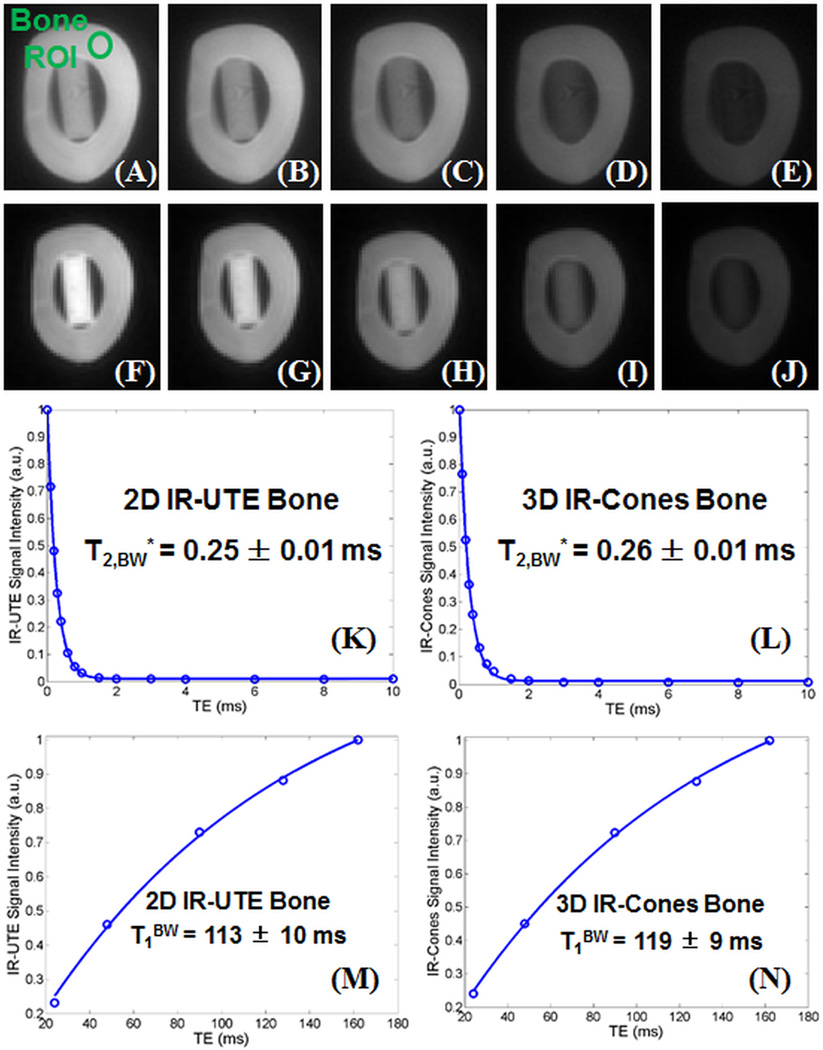
Figure 3 shows representative 2D IR-UTE and 3D IR-Cones images of the same bovine cortical bone sample, as well as bi-component fitting of T2* data and single-component fitting of T1 data from the same ROI above. Both bi-component fitting and single-component fitting of IR-UTE and 3D IR-Cones images show similar results with a single T2* of ~0.25 ms, which is similar to the short T2* value derived from bi-component fitting of 2D UTE and 3D Cones imaging. The same single component T2* decay behavior was observed with different TR and TI combinations in 2D IR-UTE and 3D IR-Cones imaging of all bone samples. These results demonstrate that pore water with longer T2*s was suppressed and bound water with much shorter T2* was selectively imaged. T1BW from the 3D IR-Cones approach (119±9 ms) is comparable to that of 113±10 ms from the 2D IR-UTE approach.
Figure 3.
2D IR-UTE imaging of a bovine cortical bone sample with a series of TR/TI combinations of 400/164 ms (A), 300/129 ms (B), 200/91 ms (C), 100/48 ms (D), 50/24 ms (E), and 3D IR-Cones imaging with the same TR/TI combinations of 400/164 ms (F), 300/129 ms (G), 200/91 ms (H), 100/48 ms (I) and 50/24 ms (J). Excellent single-component fitting of images acquired with different TEs was achieved for cortical bone with a T2*BW of 0.25±0.01 ms derived from 2D IR-UTE images (K) and a T2*BW of 0.26±0.01 ms derived from 3D IR-Cones images (L). Exponential fitting of images acquired with different TR/TI combinations was achieved for cortical bone with a T1BW of 113±10 ms derived from 2D IR-UTE images (M) and a T1BW of 119±9 ms derived from 3D IR-Cones images (N).
Table 1 shows the mean and standard deviation of T1TW, T1PW and T1BW. All the measurements were consistent between the different techniques. T1 values from the literature were also summarized. Our measurements were largely comparable with these from the literature, especially these from NMR spectroscopy studies [22, 25]. Those results also suggest that both T1PW and T1BW have strong field dependence.
Table 1.
Measurement of T1s of pore water (T1PW), bound water (T1BW) and total water (T1TW) in bovine cortical bone (n=8) using 2D non-selective saturation recovery UTE (SR-UTE), 2D UTE with variable TRs, 2D IR-UTE with variable TR and TI combinations, 3D UTE single-echo Cones with variable TRs, 3D dual echo Cones with variable TRs, and 3D IR-Cones with variable TR and TI combinations, respectively. T1TW, T1PW and T1BW values reported in the literature are also listed for comparison.
| Authors | Field Strength |
Sequences | Pore Water T1 (T1PW, ms) |
Bound Water T1 (T1BW, ms) |
Total Water T1 (T1TW, ms) |
|---|---|---|---|---|---|
| Chen et al. | 3 T | 2D dual-echo SR-UTE | 574 ± 36 | – | 256 ± 28 |
| Chen et al. | 3 T | 2D dual-echo UTE-VTR | 560 ± 32 | – | 214 ± 25 |
| Chen et al. | 3 T | 2D IR-UTE Variable TR/TI | – | 122 ± 9 | – |
| Chen et al. | 3 T | 3D dual-echo Cones-VTR | 545 ± 28 | - | 208 ± 22 |
| Chen et al. | 3 T | 3D IR-Cones Variable TR/TI | – | 131 ± 12 | – |
| Reichert et al.35 | 1.5 T | 2D SR-UTE | – | – | 140 – 260 |
| Techawiboonwong.18 | 3 T | 2D SR-UTE | – | – | 398 ± 7 |
| Han et al.36 | 3 T | 3D UTE Variable Flip Angle | – | – | ~120 |
| Han et al.37 | 3 T | 3D UTE Actual Flip Angle | – | – | ~210 |
| Cao et al.38 | 4.7 T | 3D UTE-VTR | – | – | ~3600 |
| Du et al.32 | 3 T | 2D SR-UTE | – | – | 223 ± 11 |
| Rad et al.19 | 3 T | 3D Hybrid UTE with Two TRs | – | – | 302 ± 45 |
| Horch et al.12 | 4.7 T | IR CPMG | ~1000 | ~350 | – |
| Horch et al.25 | 4.7 T | IR CPMG | 551 ± 120 | 357 ± 10 | – |
| Seifert et al.22 | 1.5 T | SR CPMG | 651 ± 273 | 82.6 ± 10.4 | – |
| Seifert et al.22 | 3 T | SR CPMG | 880 ± 281 | 145 ± 25 | – |
| Seifert et al.22 | 7 T | SR CPMG | 1790 ± 470 | 400 ± 68 | – |
| Seifert et al.22 | 9.4 T | SR CPMG | 1300 ± 370 | 358 ± 240 | – |
| Akbari et al.33 | 1.5 T | 3D GRE Variable TR | 111 – 243 | – | – |
| Chen et al.39 | 3 T | 2D SR-UTE & IR-UTE | 527 ± 28 | 116 ± 6 | 243 ± 37 |
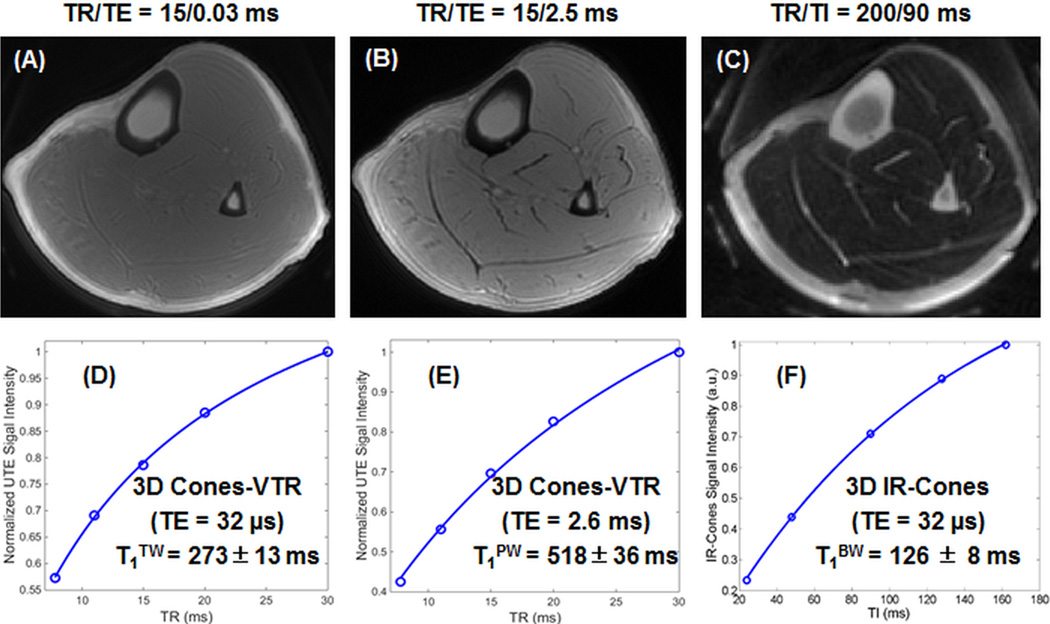
Figure 4 shows selected images of the tibial midshaft of a 33-year-old healthy volunteer using 3D dual echo Cones with variable TR and single-echo IR-Cones with variable TR/TI combinations. Cortical bone is barely visible with the 3D Cones sequence due to the high signal from surrounding long T2 muscle and marrow fat. The 3D IR-Cones sequence efficiently suppressed signals from the surrounding long T2 muscle and marrow fat, providing improved dynamic range for cortical bone with relatively high SNR of 22.1~72.8 (higher SNR for longer TR/TI). Fitting of the signal recovery curve shows a short T1TW of 273±13 ms for water (combined bound and pore water) in cortical bone with a TE of 32 µs, a T1PW of 518±36 ms for pore water in cortical bone with a TE of 2.5 ms. Fitting of the 3D IR-Cones images with different TR and TI combinations shows a T1BW of 126±8 ms for bound water in cortical bone. Fitting residues are typically less than 2% of the total signal, suggesting the effectiveness of the IR-Cones variable TR/TI approach in measuring T1BW. T1TW, T1PW and T1BW in the tibial midshafts of healthy volunteers are largely consistent with those obtained from bovine cortical bone samples.
Figure 4.
Representative 3D dual echo Cones-VTR imaging of the tibial midshaft of a 33 year old healthy volunteer with a TR of 15 ms, dual TEs of 32 µs (A) and 2.5 ms (B) in 2.5 min scan time, as well as 3D IR-Cones imaging with a TR of 200 ms and a TI of 90 ms in 2 min scan time (C). Single-component exponential recovery curve fitting of dual echo 3D Cones images with variable TRs shows a T1TW of 273±13 ms (D) and a T1PW of 518±36 ms (E), while fitting of IR-Cones images with variable TR/TI combinations shows a T1BW of 126±8 ms (F) for cortical bone.
Table 2 shows the mean and standard deviation of T1TW, T1PW and T1BW calculated from 3D dual-echo Cones acquisitions with variable TRs, as well as 3D single IR-Cones acquisitions with variable TR and TI combinations in healthy volunteers. On average, a mean T1TW of 246±32 ms, a mean T1pw of 524±46 ms and a mean T1BW of 134±11 ms were observed for the tibial midshafts of the eight healthy volunteers. These values were again largely consistent with the values obtained from bovine cortical bone samples as shown in Table 1.
Table 2.
Measurement of T1s of pore water (T1PW), bound water (T1BW) and total water (T1TW) in tibial midshaft of healthy volunteers (n=8) using 3D dual echo Cones with variable TRs, and 3D IR-Cones with variable TR and TI combinations, respectively.
| Sequences | Pore Water T1 (T1PW, ms) |
Bound Water T1 (T1BW, ms) |
Total Water T1 (T1TW, ms) |
|---|---|---|---|
| 3D dual-echo Cones Variable TR | 524 ± 46 | - | 246 ± 32 |
| 3D IR-Cones Variable TR/TI | - | 134 ± 11 | - |
Discussion
T1 relaxation, also known as spin-lattice relaxation, describes the recovery of longitudinal magnetization after the application of a radiofrequency pulse. The mechanisms of T1 relaxation in cortical bone are poorly understood and for reasons not currently known, most recent studies suggest that T1 of cortical bone (assuming a single T1 component) is much shorter than that of long T2 tissues, including muscle, liver, and grey and white matter [18–20, 33]. In five different healthy volunteers (mean age 29 years), we previously obtained a mean total water T1 measurement of 223±11 ms using a saturation recovery 2D-UTE technique, comparing closely to 246±32 ms obtained with variable TR 3D-Cones in this study [33]. Reichert et al used a saturation recovery technique and a 2D-UTE technique in vivo on a 1.5T system and found a range of 140–260 ms for T1 measurement of total water [37]. Rad et al. employed a hybrid 3D UTE imaging with two different TRs approach to map T1 and reported a total water T1 of 302±45 ms at 3T [19]. Using a saturation recovery technique and 3D-UTE imaging on a 3T scanner, Techawiboonwong et al found mean total water T1 of 398±7 ms in human tibial cortex specimens [18]. It is expected that the donor specimens used in their study (mean age at death of 67 years) would yield higher T1 values than in our volunteers since our volunteers were younger and presumably have lower cortical porosity than elderly specimens. More recently, Han et al investigated the temperature dependence of T1 in cortical bone at 3T using a varying flip angle approach (i.e., 8° and 44°) and found a linear relationship with T1 increased from ~120 ms at 25.1 °C to ~155 ms at 70.1 °C [38]. The same group also reported an actual flip angle imaging (AFI) UTE technique to improve T1 measurement for short T2 tissues, and reported a short T1 value of ~210 ms for cortical bone at 3T [39]. The rapid T1 relaxation of bone provides a unique opportunity since quantification can potentially be performed without significantly prolonging imaging protocol. However, there are some studies suggesting that bone has a long T1, instead of a short T1. For example, Cao et al. reported a very long T1 of 3.6 s for cortical bone at 4.7T [40]. This result is consistent with the long T1 values expected for solid-state materials. Clearly more research is needed to further validate T1 measurements of bone water using both high performance NMR spectrometers as well as clinical MR scanners.
A number of recent studies have demonstrated that the different water components in cortical bone have very distinct T2 and T2* relaxation times. Pore water has a long T2 (> 100 ms) but a short T2* (~a few milliseconds), while bound water has much reduced T2 and T2* (~a few hundred microseconds) [10–14,17]. The distinct T2* values suggest that the exchange rate between bound and pore water is relatively slow. As a result, one would also expect that bound and pore water should have distinct T1 values. However, limited studies have been reported on this topic. Only a few groups to date have investigated techniques to measure T1s of bound and pore water in cortical bone. Using inversion recovery Carr-Purcell-Meiboom-Gill (CPMG) sequences and fitting with a 2D T1–T2 spectrum, Horch et al found shorter T1 values of ~350 ms for bound water and ~1 s for pore water [12]. In another study by the same group, a mean T1BW of 357±10 ms and a mean T1PW of 551±120 ms were reported for human cortical bone samples at 4.7T [25]. Chen et al reported a mean T1BW of 116 ± 6 ms and a mean T1PW of 527±28 ms for bovine cortical bone samples at 3T [41]. Seifert et al reported a mean T1PW of 880 ± 281 ms and a mean T1BW of 145±25 ms at 3T [22]. More recently, Akbari et al proposed the use of a clinical gradient echo sequence with a relatively short echo time of ~1.29 ms and variable TRs (i.e., TR = 20 and 60 ms) to measure T1 of pore water. They reported a relatively short T1 of 111–243 ms for T1 of pore water at 1.5T [34]. T1BW value from our study is very close to that reported by Seifert et al. Meanwhile, the mean T1pw values of 880±281 ms at 3T and 1790±470 ms at 7T from the Seifert study are significantly higher than the mean T1PW values of 545±28 ms at 3T from our study, 551±120 ms at 4.7T from the Horch study, and 111–243 ms at 1.5T from the Akbari study. The variability may be due to multiple factors, including differences in field strengths (T1 is field strength dependent) and type of specimen (T1PW in human cortical bone with larger pores is expected to be longer than in bovine cortical bone with smaller pores). More work is needed to validate the different techniques to measure T1PW and T1BW.
In this study, we have demonstrated that the fast, volumetric 3D-Cones sequences provide additional opportunities for quantification since ultrashort echo times can now be employed in a time and SNR efficient manner. In bovine bone samples, the dual-echo variable TR 3D-Cones sequence produced similar results compared with the non-selective 2D UTE sequence using both dual-echo TSR and variable TR techniques for the quantification of total and bound water. For T1 measurements of bound water, the IR technique using the 3D-Cones sequence yielded a mean measurement of 144 ms compared with 116 ms obtained using the 2D-UTE sequence. This may be due to the differences in spatial resolution, echo times and sampling window. The 2D-UTE sequence with shorter echo time and sampling window is more efficient in capturing signal from a larger proportion of the rapidly decaying bound water component. Furthermore, the 2D UTE sequence is non-slice selective. B1 variation across the specimen thickness (~6 cm) will also affect T1 measurement.
This study has a number of limitations. First, a single component model was used for T1 calculation of total water, which is only an approximation. Multicomponent analysis would be helpful to elucidate the fractions and T1 values of the bound and free water pools [22]. However, the accuracy of multi-component fitting is dependent on the quality of data including SNR, number of sampling points (or echo times), and separation of relaxation times. The 3D-Cones sequence may be well suited for multi-component modeling and this deserves additional study. Second, errors in T1PW measurements would lead to imperfect nulling of pore water, resulting in long T2 signal contamination in IR-UTE imaging of bound water, and thus errors in T1BW estimation using the IR-Cones acquisitions with variable TR and TI combinations. Third, flip angle errors were not considered in the T1 quantification in this study. B1 mapping or actual flip angle imaging (AFI) techniques would likely improve the accuracy of T1TW, T1PW and T1BW measurements [39]. Fourth, mapping of bound and pore water concentrations were not performed in this study. With T2* and T1 values of both bound and pore water components known, accurate measurement of their absolute concentration can be easily achieved through comparison of bone signal with that of a reference phantom with known proton density. Validation studies as well as clinical applications of total, bound and pore water mapping will be performed in future studies.
Acknowledgments
The authors acknowledge grant support from GE Healthcare, NIH (1R01 AR062581 and 1R01 AR068987) and the VA Clinical Science R&D Service (5IK2CX000749), as well as the ‘The International Postdoctoral Exchange Fellowship Program (No. 20130021)’ from China.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 72013;29, 2000: highlights of the conference. South Med J. 2001;94:569–573. [PubMed] [Google Scholar]
- 2.Hahn M, Vogel M, Pompesius-Kempa M, Delling G. Trabecular bone pattern factor--a new parameter for simple quantification of bone microarchitecture. Bone. 1992;13:327–330. doi: 10.1016/8756-3282(92)90078-b. [DOI] [PubMed] [Google Scholar]
- 3.Bousson V, Peyrin F, Bergot C, Hausard M, Sautet A, Laredo JD. Cortical bone in the human femoral neck: three-dimensional appearance and porosity using synchrotron radiation. J Bone Miner Res. 2004;19:794–801. doi: 10.1359/JBMR.040124. [DOI] [PubMed] [Google Scholar]
- 4.Bousson V, Bergot C, Meunier A, Barbot F, Parlier-Cuau C, Laval-Jeantet AM, Laredo JD. CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity. Radiology. 2000;217:179–187. doi: 10.1148/radiology.217.1.r00se11179. [DOI] [PubMed] [Google Scholar]
- 5.Pistoia W, van Rietbergen B, Ruegsegger P. Mechanical consequences of different scenarios for simulated bone atrophy and recovery in the distal radius. Bone. 2003;33:937–945. doi: 10.1016/j.bone.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie RO, Buehler MJ, Hansma P. Plasticity and toughness in bone. Physics Today. 2009;62:41–47. [Google Scholar]
- 8.Cowin SC. Bone poroelasticity. Journal of Biomechanics. 1999;32:217–238. doi: 10.1016/s0021-9290(98)00161-4. [DOI] [PubMed] [Google Scholar]
- 9.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19:731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 10.Wang XD, Ni QW. Determination of cortical bone porosity and pore size distribution using a low field pulsed NMR approach. Journal of Orthopaedic Research. 2003;21:312–319. doi: 10.1016/S0736-0266(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 11.Nyman JS, Ni Q, Nicolella DP, Wang X. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008;42:193–199. doi: 10.1016/j.bone.2007.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horch RA, Nyman JS, Gochberg DF, Dortch RD, Does MD. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magn Reson Med. 2010;64:680–687. doi: 10.1002/mrm.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz E, Chung CB, Bae WC, Statum S, Znamirowski R, Bydder GM, Du J. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed. 2012;25:161–168. doi: 10.1002/nbm.1728. [DOI] [PubMed] [Google Scholar]
- 14.Biswas R, Bae W, Diaz E, Masuda K, Chung CB, Bydder GM, Du J. Ultrashort echo time (UTE) imaging with bi-component analysis: bound and free water evaluation of bovine cortical bone subject to sequential drying. Bone. 2012;50:749–755. doi: 10.1016/j.bone.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horch RA, Gochberg DF, Nyman JS, Does MD. Non-invasive predictors of human cortical bone mechanical properties: T(2)-discriminated H NMR compared with high resolution X-ray. PLoS One. 2011;6:e16359. doi: 10.1371/journal.pone.0016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae WC, Chen PC, Chung CB, Masuda K, D'Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27:848–857. doi: 10.1002/jbmr.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Hermida JC, Diaz E, Corbeil J, Znamirowski R, D’Lima DD, Bydder GM. Assessment of cortical bone with clinical and ultrashort echo time sequences. Magn Reson Med. 2013;70:697–704. doi: 10.1002/mrm.24497. [DOI] [PubMed] [Google Scholar]
- 18.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortial bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008;248:824–833. doi: 10.1148/radiol.2482071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rad HS, Lam SCB, Magland JF, Ong H, Li C, Song HK, Love J, Wehrli FW. Quantifying cortical bone water in vivo by three-dimensional ultra-short echo time MRI. NMR Biomed. 2011;24:855–864. doi: 10.1002/nbm.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Seifert AC, Rad HS, Bhagat Y, Rajapakse CS, Sun W, Benny Lam SC, Wehrli FW. Cortical Bone Water Concentration: Dependence of MR Imaging Measures on Age and Pore Volume Fraction. Radiology. 2014;272:796–806. doi: 10.1148/radiol.14132585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Diaz E, Carl M, Bae W, Chung C, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012;67:645–649. doi: 10.1002/mrm.23047. [DOI] [PubMed] [Google Scholar]
- 22.Seifert AC, Wehrli SL, Wehrli FW. Bi-component T2* analysis of bound and pore bone water fractions fails at high field strengths. NMR Biomed. 2015;28:861–872. doi: 10.1002/nbm.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric cortical bone porosity assessment with MR imaging: validation and clinical feasibility. Radiology. 2015 May 19;:141850. doi: 10.1148/radiol.15141850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Ma L, Chang E, Shao H, Chen J, Chung CB, Bydder GM, Du J. Effects of inversion time on inversion recovery prepared ultrashort echo time (IR-UTE) imaging of bound and pore water in cortical bone. NMR Biomed. 2015;28:70–78. doi: 10.1002/nbm.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horch R, Gochberg D, Nyman J, Does M. Clinically-compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn Reson Med. 2012;68:1774–1784. doi: 10.1002/mrm.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manhard MK, Horch RA, Gochberg DF, Nyman JS, Does MD. In vivo quantitative MR imaging of bound and pore water in cortical bone. Radiology. 2015;277:221–229. doi: 10.1148/radiol.2015140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manhard MK, Horch RA, Harkins KD, Gochberg DF, Nyman JS, Does MD. Validation of quantitative bound- and pore-water imaging in cortical bone. Magn Reson Med. 2014;71:2166–2171. doi: 10.1002/mrm.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55:575–582. doi: 10.1002/mrm.20796. [DOI] [PubMed] [Google Scholar]
- 29.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multi-spoke inversion recovery pulse sequence. Magn Reson Med. 2015 Aug 26; doi: 10.1002/mrm.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Bydder M, Takahashi AM, Chung CB. Two-dimensional ultrashort echo time imaging using a spiral trajectory. Magn Reson Imaging. 2008;26:304–312. doi: 10.1016/j.mri.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Tsai CM, Nishimura DG. Reduced aliasing artifacts using variable-density k-space sampling trajectories. Magn Reson Med. 2000;43:452–458. doi: 10.1002/(sici)1522-2594(200003)43:3<452::aid-mrm18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Wanspaura JP, Daniel BL, Pauly JM, Butts K. Temperature mapping of frozen tissue using eddy current compensated half excitation RF pulses. Magn Reson Med. 2001;46:985–992. doi: 10.1002/mrm.1285. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010;207:304–311. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Akbari A, Abbasi-Rad S, Rad HS. T1 correlates age: a short-TE MR relaxometry study in vivo on human cortical bone free water at 1.5T. Bone. 2015 doi: 10.1016/j.bone.2015.10.006. (in press) [DOI] [PubMed] [Google Scholar]
- 35.Du J, Sheth V, He Q, Carl M, Chen J, Corey-Bloom J, Bydder GM. Measurement of T1 of the ultrashort T2* components in white matter of the brain at 3T. PLOS One. 2014:e103296. doi: 10.1371/journal.pone.0103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larson PE, Conolly SM, Pauly JM, Nishimura DG. Using adiabatic inversion pulses for long-T2 suppression in ultrashort echo time (UTE) imaging. Magn Reson Med. 2007;58:952–961. doi: 10.1002/mrm.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichert ILH, Robson MD, Gatehouse PD, He T, Chappell KE, Holmes J, Girgis S, Bydder GM. Magnetic resonance imaging of cortical bone with ultrashort TE (UTE) pulse sequences. Magn Reson Imaging. 2005;23:611–618. doi: 10.1016/j.mri.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Han M, Scott S, Ozhinsky E, Salgaonkar V, Jones P, Larson P, Diederich C, Krug R, Rieke V. Assessing temperature dependence of T1 in cortical bone using ultrashort echo-time MRI. Journal of Therapeutic Ultrasound. 2015;3(Suppl 1):P5. doi: 10.1002/mrm.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han M, Larson P, Krug R, Rieke V. Proceedings of ISMRM 23rd Annual Meeting. Toronto, Canada: 2015. Jun, Actual flip angle imaging to improve T1 measurement for short T2 tissues; p. P501. [Google Scholar]
- 40.Cao H, Nazarian A, Ackerman JL, Snyder BD, Rosenberg AE, Nazarian RM, Hrovat MI, Dai G, Mintzopoulos D, Wu Y. Quantitative (31)P NMR spectroscopy and (1)H MRI measurements of bone mineral and matrix density differentiate metabolic bone diseases in rat models. Bone. 2010;46:1582–1590. doi: 10.1016/j.bone.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Grogan SP, Shao H, D'Lima D, Bydder GM, Wu Z, Du J. Evaluation of bound and pore water in cortical bone using ultrashort-TE MRI. NMR Biomed. 2015;28:1754–1762. doi: 10.1002/nbm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]