Abstract
Background and Aim
Tumour testing of colorectal cancers (CRC) for mismatch repair (MMR) deficiency is an effective approach to identify carriers of germline MMR gene mutation (Lynch syndrome). The aim of this study was to identify MMR gene mutation carriers in two cohorts of population-based CRC utilising a combination of tumour and germline testing approaches.
Methods
CRCs from 813 patients diagnosed with CRC <60 years of age from the Australasian Colorectal Cancer Family Registry (ACCFR) and from 826 patients from the Melbourne Collaborative Cohort Study (MCCS) were tested for MMR protein expression using immunohistochemistry (IHC), microsatellite instability (MSI), BRAFV600E somatic mutation and for MLH1 methylation. MMR gene mutation testing (Sanger sequencing and MLPA) was performed on germline DNA of patients with MMR-deficient tumours and a subset of MMR-proficient CRCs.
Results
Of the 813 ACCFR probands, 90 probands demonstrated tumour MMR-deficiency (11.1%) and 42 had a MMR gene germline mutation (5.2%). For the MCCS, MMR-deficiency was identified in the tumours of 103 probands (12.5%) and 7 had a germline mutation (0.8%). All the mutation carriers were diagnosed prior to 70 years of age. Probands with a MMR-deficient CRC without MLH1 methylation and a gene mutation were considered Lynch-like and comprised 41.1% and 22.3% of the MMR-deficient CRCs for the ACCFR and MCCS, respectively.
Conclusions
Identification of MMR gene mutation carriers in Australian CRC-affected patients is optimised by IHC screening of CRC diagnosed before 70 years. A significant proportion of MMR-deficient CRCs will have unknown aetiology (Lynch-like) proving problematic for clinical management.
Keywords: Colorectal cancer, Lynch syndrome, Mismatch repair protein expression, immunohistochemistry, microsatellite instability, MLH1, MSH2, MSH6, PMS2, MLH1 methylation, BRAFV600E
Introduction
Worldwide, colorectal cancer (CRC) is a leading cause of cancer related deaths1. Australia and New Zealand have the highest incidence of CRC with age-standardized rates of 44.8 and 32.2 per 100,000 in men and women, respectively1. The lifetime risk of CRC to age 75 years in Australia is 1 in 18 (5.5%) for men and 1 in 26 (3.9%) for women, and is the second most common cause of cancer-related death after lung cancer2.
One way to reduce the incidence of CRC is to identify high risk individuals in the population and target them for screening and increased surveillance. Lynch syndrome is an autosomal dominant cancer predisposition disorder, defined by the identification of a pathogenic germline mutation in one of the DNA MMR genes MLH1, MSH2, MSH6, or PMS2 or in EPCAM, a gene upstream of MSH2. MMR gene mutation carriers have a high risk of developing cancers within the large intestine and the endometrium, and also from the urinary tract, pancreas, hepatobiliary tract, stomach, small intestine and ovaries3. Up to 6% of all CRCs can be attributed to Lynch syndrome making it the most common hereditary CRC condition4. The identification of MMR gene mutation carriers enables appropriate risk management strategies that can improve patient outcomes.
Tumours arising in individuals with a MMR gene mutation demonstrate high levels of microsatellite instability (MSI) secondary to altered DNA MMR mechanisms. Testing tumours for evidence of this MMR deficiency by immunohistochemistry (IHC) is widely used by pathologists to screen for Lynch syndrome5. Two population-based studies have previously reported the identification of Lynch syndrome in Australia, one from Victoria in CRC patients diagnosed before 45 years6 and one from Western Australia in CRC patients diagnosed before 60 years7. In this study, we describe the identification of Lynch syndrome in one early-onset CRC cohort and one later onset CRC cohort both derived from Victoria, Australia.
Methods
Study Participants
Participants were identified from two different Australian studies: the Australasian Colorectal Cancer Family Registry (ACCFR) and the Melbourne Collaborative Cohort Study (MCCS). In the ACCFR, population-based incident CRC cases were recruited, independent of family history of cancer, in Victoria between 1997 and 20078. Of these, we identified 959 probands with a primary adenocarcinoma of the colon or rectum during two recruitment periods. Phase I recruitment (1997 to 2001) included all patients with a CRC diagnosed between 18 and 44 years of age and 50% of patients with CRC diagnosed between the ages of 45-59 years. Phase II recruitment (2001 to 2006) included all patients with a CRC diagnosed between 18 and 49 years of age. A proportion of these cases presented in this study (71 individuals from phase II recruitment) were reported in a previous study6.
The MCCS is a prospective cohort study of 41,514 people (17,045 males and 24,469 females) recruited between 1990 and 19949. Participants diagnosed with an incident CRC were aged 41 to 86 years. By 31 December 2009, 1046 participants had a first histopathological diagnosis of invasive adenocarcinoma of the colon or rectum in Victoria following the baseline study visit (a total of 1101 CRCs). A further 25 subjects had a clinical diagnosis only and were not considered as cases. Insufficient sample remained for 50 (5%) tumours, and the sample could not be obtained or was not sent for testing for 181 (17%) of the eligible tumours. In total, data were available for 851 of the eligible tumours from 826 of the MCCS participants.
Written informed consent was obtained from all participants to collect a blood sample and tumour pathology materials. The study protocols were approved by Human Research Ethics Committees at the University of Melbourne (ACCFR) and the Cancer Council Victoria (MCCS).
Family History of CRC and Extra-Colonic Cancers
For the ACCFR, information on personal and family history of CRC and other cancers in first- and/or second-degree relatives was obtained from completion of baseline and follow-up questionnaires completed at recruitment and 5 yearly thereafter, respectively. Cancers were verified, where possible using pathology reports, medical records, cancer registry reports, and/or death certificates8. Information on cancer family history was used to determine if the proband’s family history met Amsterdam I or II criteria or the revised Bethesda guidelines10, 11. Information on family history of CRC was not available for the MCCS probands.
Pathology Review
Primary CRC tissue was available for 813 of the probands from the ACCFR Jeremy Jass Memorial Tissue Bank and 851 from the MCCS for standardised pathological review and molecular characterisation12. Tumours from the ileo-caecal junction through the caecum, ascending colon, hepatic flexure, and transverse colon were grouped as right-sided (proximal). Tumours from the splenic flexure, descending, sigmoid colon and recto-sigmoid junction were classified as left-sided (distal); tumours from the rectum were considered as a third distinct group. Tumour TNM stage was derived from the pathological stage of the tumour and of the lymph node status, and the clinical metastatic stage.
DNA Mismatch Repair Deficiency and Molecular Testing
MMR-deficiency was determined primarily by IHC staining for the four MMR proteins as previously described13, 14. Microsatellite instability (MSI) was determined from a ten-marker panel in 794 tumours collected from the MCCS and for 555 tumours collected from the ACCFR (predominantly Phase I recruited participants and for CRCs showing loss of MMR protein expression), as previously described5, 13. Tumours were categorised as: 1) MMR-deficient if they were MSI-H and/or showed loss of expression of one or more of the MMR proteins by IHC; or 2) MMR-proficient if tumours were MSS (microsatellite stable) or MSI-L (low-level MSI) and/or showed normal retained expression of all four MMR proteins by IHC. CRCs for which both MSI and MMR IHC testing were completed (ACCFR 555/823, 67.4%; MCCS 794/851, 93.3%) demonstrated 95.7% and 98.9% concordance for MMR-deficiency, respectively. For the discordant cases, CRCs were categorised as MMR-deficient if one of the tests was positive (MSI-H or abnormal IHC). In addition, tumours demonstrating loss of the MLH1 and PMS2 by IHC were characterised for methylation of the MLH1 promoter region using the MethyLight assay as previously described15, 16. All CRCs were tested for the BRAFV600E somatic mutation using a fluorescent allele-specific PCR assay as previously described17.
Germline Mutation Testing
Germline MMR gene mutation testing was performed using Sanger sequencing and Multiplex Ligation Dependent Probe Amplification (MLPA), including testing for 3′ deletions in EPCAM as previously described8, 14, 15, 18. For the ACCFR, the following probands were tested: 1) MMR-deficient CRCs (n=90), 2) a subset of MSI-L CRCs (n=40), and 3) a subset of probands with MMR-proficient CRC who met Revised Bethesda guidelines, Amsterdam I or II criteria or who had a family history of CRC (n=35). For MCCS, only probands with MMR-deficiency and negative for MLH1 promoter methylation were screened for germline mutations (n=32). A subset of probands with MLH1 methylation positive CRCs from both studies were tested for germline MMR gene mutations (n=19). Germline variants were classified for pathogenicity based on the InSiGHT database classifications (http://insight-group.org/variants/classifications/) where classes 4 and 5 were considered pathogenic19. For variants not yet classified by InSiGHT, we considered a variant as pathogenic if it resulted in a stop codon, frameshift, large deletion, or if it removed a canonical splice site. Individuals with a MMR-deficient CRC, negative for the BRAFV600E mutation and MLH1 promoter methylation, but without an identified MMR gene germline mutation were considered as Lynch-like syndrome cases20.
Statistical Analysis
Statistical analyses were conducted using SPSS statistics software version 19.0 (SPSS Inc., Chicago, IL). Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using non-parametric Wilcoxon-Mann-Whitney rank-sum test. All p-values were two-tailed and p-values less than 0.05 were considered to be statistically significant to test null hypothesis.
Results
Primary CRC tissue (n=823) was available for molecular characterisation from 813 ACCFR probands where MMR-deficiency was identified in 94 tumours from 90 probands (11.1%). For the MCCS CRC-affected probands (n=1046), molecular pathology results were obtained for 851 CRCs from 826 probands (79%). MMR-deficiency was identified in the tumours of 103 participants (12.5%). The characteristics of the ACCFR and MCCS study participants overall and by their tumour MMR status are shown in Tables 1 and 2, respectively.
Table 1.
Proband characteristics and tumour histological features from the Australian Colon Cancer Family Registry (ACCFR)
| Number (%) | |||
|---|---|---|---|
|
All
(823 CRC, 813 probands) |
MMR-Proficient
(729 CRC, 723 probands) |
MMR-Deficient
(94 CRC, 90 probands) |
|
| Gender | |||
| Female | 395 (49) | 352 (49) | 43 (48) |
| Male | 418 (51) | 371 (51) | 47 (52) |
| Age at diagnosis† (years) | |||
| Median (range) | 47 (18-59) | 47 (18-59) | 43 (18-59) |
| Mean ± Standard deviation | 46.3 ± 8.0 | 46.7 ± 7.7 | 43.1 ± 9.1 |
| 18 – 30 | 25 (3) | 18 (2.5) | 7 (7.4) |
| 31 – 40 | 153 (18.6) | 129 (17.7) | 24 (25.5) |
| 41 – 50 | 374 (45.4) | 332 (45.5) | 42 (44.7) |
| 51- 60 | 268 (32.6) | 249 (34.2) | 19 (20.2) |
| 61- 70 | 3 (0.4) | 1 (0.1) a | 2 (2.1) b |
| 71-80 | 0 | 0 | 0 |
| >80 | 0 | 0 | 0 |
| Family History | |||
| Amsterdam I | 38 (5) | 26 (4) | 12 (13) |
| Amsterdam II | 11 (1) | 5 (1) | 6 (7) |
| Amsterdam I or II | 49 (6) | 31 (4) | 18 (20) |
| Revised Bethesda | 602 (74) | 514 (71) | 90 (100) |
| Tumour location | |||
| Proximal colon | 214 (28) | 155 (23) | 59 (66) |
| Distal colon | 231 (30) | 216 (32) | 15 (17) |
| Rectum | 317 (42) | 302 (45) | 15 (17) |
| Missing | 61 | 56 | 5 |
| Histological type | |||
| Adenocarcinoma | 724 (91) | 658 (93) | 66 (74) |
| Mucinous carcinoma | 70 (9) | 47 (7) | 23 (26) |
| Signet ring cell carcinoma | 4 | 4 | 0 |
| Missing | 25 | 20 | 5 |
| Histological grade (adenocarcinoma) | |||
| Low grade | 591 (82) | 543 (83) | 48 (73) |
| High grade | 126 (18) | 108 (17) | 18 (27) |
| Missing | 7 | 7 | 0 |
| Stage (TNM) | |||
| Stage I | 134 (18) | 118 (17) | 16 (19) |
| Stage II | 259 (34) | 208 (31) | 51 (61) |
| Stage III | 323 (42) | 307 (45) | 16 (19) |
| Stage IV | 48 (6) | 47 (7) | 1 (1) |
| Missing | 59 | 49 | 10 |
| Tumour margin | |||
| Expanding | 447 (63) | 376 (60) | 71 (89) |
| Infiltrating | 261 (37) | 252 (40) | 9 (11) |
| Missing | 115 | 101 | 14 |
| Peritumoural lymphocytes | |||
| Present | 320 (43) | 266 (41) | 54 (67) |
| Absent | 412 (57) | 385 (59) | 27 (33) |
| Missing | 91 | 78 | 13 |
| Crohn's-like reaction | |||
| Present | 145 (20) | 101 (16) | 44 (55) |
| Absent | 578 (80) | 542 (84) | 36 (45) |
| Missing | 100 | 86 | 14 |
| Tumour-infiltrating lymphocytes | |||
| Present | 166 (22) | 100 (15) | 66 (76) |
| Absent | 605 (78) | 584 (85) | 21 (24) |
| Missing | 52 | 45 | 7 |
| BRAFV600E mutation | |||
| Positive | 57 (7) | 44 (6) | 13 (15) |
| Negative | 735 (93) | 659 (94) | 76 (85) |
| Missing | 31 | 26 | 5 |
Age at diagnosis of first CRC for metachronous CRC
proband had MMR-proficient CRC diagnosed at 53 years and metachronous MMR-proficient CRC diagnosed at 61 years.
proband had MMR-proficient CRC diagnosed at 58 years and two metachronous MMR-deficient CRCs diagnosed at 62 years.
Table 2.
Proband characteristics and tumour histological features from the Melbourne Collaborative Cohort Study (MCCS)
| Number (%) | |||
|---|---|---|---|
|
All
(851 CRC, 826 probands) |
MMR-Proficient
(740 CRC, 723 probands) |
MMR-Deficient
(111 CRC, 103 probands) |
|
| Gender | |||
| Female | 398 (48) | 334 (46) | 64 (62) |
| Male | 428 (52) | 389 (54) | 39 (38) |
| Age at diagnosis† (years) | |||
| Median (range) | 70 (41-86) | 69 (42-86) | 72 (41-83) |
| Mean ± Standard deviation | 68.5 ± 8.2 | 68.2 ± 8.2 | 70.3 ± 8.2 |
| 18 – 30 | 0 | 0 | 0 |
| 31 – 40 | 0 | 0 | 0 |
| 41 – 50 | 23 (2.7) | 17 (2.3) | 6 (5.4) |
| 51- 60 | 117 (13.8) | 111 (15) | 6 (5.4) |
| 61- 70 | 327 (38.4) | 291 (39.3) | 36 (32.4) |
| 71-80 | 347 (40.8) | 293 (39.6) | 54 (48.7) |
| >80 | 37 (4.3) | 28 (3.8) | 9 (8.1) |
| Tumour location | |||
| Proximal colon | 301 (37) | 212 (30) | 89 (84) |
| Distal colon | 215 (26) | 208 (29) | 7 (7) |
| Rectum | 299 (37) | 289 (41) | 10 (9) |
| Missing | 36 | 31 | 5 |
| Histological type | |||
| Adenocarcinoma | 749 (90) | 671 (93) | 78 (72) |
| Mucinous carcinoma | 66 (8) | 42 (6) | 24 (22) |
| Signet ring cell carcinoma | 10 (2) | 5 (1) | 5 (5) |
| Undifferentiated | 4 (0) | 3 (0) | 1 (1) |
| Missing | 22 | 19 | 3 |
| Histological grade (adenocarcinoma) | |||
| Low grade | 669 (81) | 605 (84) | 64 (60) |
| High grade | 155 (19) | 113 (16) | 42 (39) |
| Undifferentiated | 4 (0) | 3 (0) | 1 (1) |
| Missing | 23 | 19 | 4 |
| Tumour margin | |||
| Expanding | 522 (71) | 444 (69) | 78 (80) |
| Infiltrating | 214 (29) | 195 (31) | 19 (20) |
| Missing | 115 | 101 | 14 |
| Peritumoural lymphocytes | |||
| Present | 160 (22) | 114 (18) | 46 (46) |
| Absent | 583 (78) | 528 (82) | 55 (54) |
| Missing | 108 | 98 | 10 |
| Crohn's-like reaction | |||
| Present | 130 (18) | 83 (13) | 47 (48) |
| Absent | 593 (82) | 542 (87) | 51 (52) |
| Missing | 128 | 115 | 13 |
| Tumour-infiltrating lymphocytes | |||
| Present | 185 (23) | 113 (16) | 72 (69) |
| Absent | 608 (77) | 576 (84) | 32 (31) |
| Missing | 58 | 51 | 7 |
| BRAFV600E mutation | |||
| Positive | 134 (17) | 74 (11) | 60 (58) |
| Negative | 657 (83) | 613 (89) | 44 (42) |
| Missing | 60 | 53 | 7 |
Age at diagnosis of first CRC for metachronous CRC
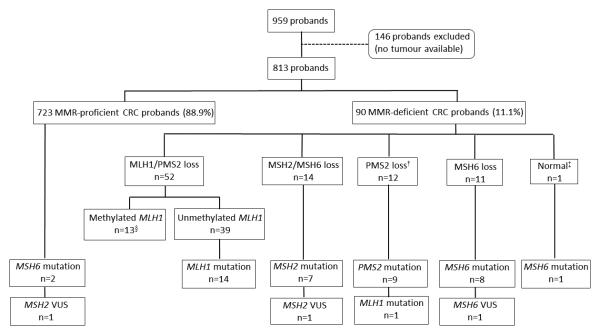
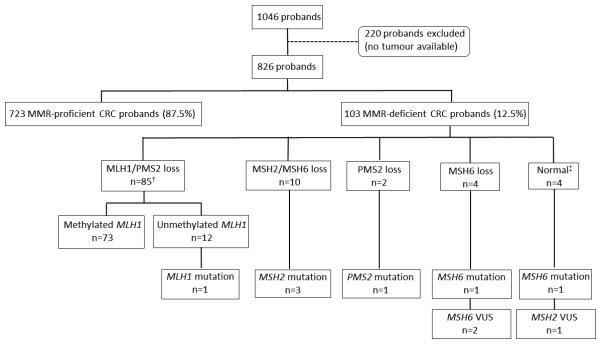
Detail for the patterns of abnormal MMR expression and germline mutations are given in Table 3, Supplementary Table 1 and in the flow diagrams (Figures 1 and 2). Methylation of the MLH1 gene promoter was observed in 25% and 85.7% of MLH1/PMS2-deficient CRCs from the ACCFR and MCCS, respectively. Of the MLH1/PMS2-deficient CRCs that were positive for MLH1 methylation, only 61.5% and 77.8% were also positive for the BRAFV600E somatic mutation (ACCFR and MCCS studies, respectively). No pathogenic MLH1 gene mutations were identified out of 10 (ACCFR) and 9 (MCCS) probands with MLH1 methylated CRCs that were tested. MLH1 methylation was not observed in 47 MMR-proficient CRCs tested from the ACCFR.
Table 3.
Results from MMR tumour and germline testing from both ACCFR and MCCS.
| ACCFR | % | MCCS | % | |
|---|---|---|---|---|
| Probands recruited | 959 | 1046 | ||
| Probands with CRC tissue for MMR IHC | 813 | 100 | 826 | 100 |
| Probands with MSI tested CRC | 551 | 67.8 | 794 | 96.1 |
| Probands with MMR-proficient CRC | 723 | 88.9 | 723 | 87.5 |
| Probands with MMR-deficient CRC (All MMR- deficient CRCs i.e. synchronous/metachronous) |
90 (94) | 11.1 | 103 (111) | 12.5 |
| Germline MMR gene mutation testing results | ||||
| MLH1/PMS2 loss† | 52 (53) | 57.8 | 84 (90) | 81.6 |
| MLH1 mutation-neg, MLH1 methylated (BRAF V600E mutation positive)‡ |
13 (8) | 25 | 72 (56) | 85.7 |
| MLH1 mutation-pos, MLH1 unmethylated | 14 | 26.9 | 1 | 1.2 |
| MLH1 VUS-pos, MLH1 unmethylated | 0 | 0 | 0 | 0 |
| MLH1 mutation-neg, MLH1 unmethylated | 24 | 46.2 | 11 | 13.1 |
| MLH1 mutation-unknown, MLH1
unmethylated§ |
1 | 1.9 | 0 | 0 |
| MSH2/MSH6 loss | 14 | 15.6 | 10 | 9.7 |
| MSH2 mutation-positive | 7 | 50 | 3 | 30 |
| MSH2 VUS-positive | 1 | 7.1 | 0 | 0 |
| MSH2 mutation-negative | 5 | 35.8 | 7 | 70 |
| MSH2 mutation-unknown§ | 1 | 7.1 | 0 | 0 |
| MSH6 only loss | 11 | 12.2 | 4 | 3.9 |
| MSH6 mutation-positive | 8 | 72.7 | 1 | 25 |
| MSH6 VUS-positive¶ | 1 | 9.1 | 2 | 50 |
| MSH6 mutation-negative | 2 | 18.2 | 1 | 25 |
| MSH6 mutation-unknown§ | 0 | 0 | 0 | 0 |
| PMS2 only loss | 11 | 12.2 | 2 | 1.9 |
| PMS2 mutation-positiveb | 8 | 72.7 | 1 | 50 |
| MLH1 mutation-positive | 1 | 9.1 | 0 | 0 |
| PMS2 mutation-negative | 1 | 9.1 | 0 | 0 |
| PMS2 mutation-unknown§ | 1 | 9.1 | 1 | 50 |
| PMS2 and MSH2/MSH6 loss | 1 | 1.1 | 0 | 0 |
| PMS2 mutation-positive, MLH1
unmethylated, MLH1, MSH2, MSH6 mutation-negative |
1 | 100 | 0 | 0 |
| MLH1/PMS2 and MSH2/MSH6 loss | 0 | 0 | 1 | 0.9 |
| MLH1, PMS2, MSH2, MSH6 mutation- negative, MLH1 methylated |
0 | 0 | 1 | 100 |
| MSI-H and normal MMR protein expression | 1 | 1.1 | 4 | 3.9 |
| MSH6 mutation-positive, MLH1, MSH2, and PMS2 mutation-negativea |
1 | 100 | 1 | 25 |
| MSH2 VUS-positive, MLH1 and MSH6
mutation negative |
0 | 0 | 1 | 25 |
| MLH1, MSH2 and MSH6 mutation-negative | 0 | 0 | 2 | 50 |
| MSI-L and normal MMR protein expression | 53 | NT | ||
| MLH1 and MSH2 mutation-negative | 25 | |||
| MLH1, MSH2 and MSH6 mutation-negative | 15 | |||
| MMR mutation testing not performed | 13 | |||
| MSS and/or normal MMR protein expression | 670 | NT | ||
| MSH6 mutation-positive | 2 | |||
| MSH2 VUS-positive | 1 | |||
| MLH1, MSH2 and MSH6 mutation-negative | 32 | |||
| MMR mutation testing not performed | 635 | |||
1x tumour showed loss of MLH1/PMS2 and MSH6 protein expression in ACCFR and 3x tumours showed loss of MLH1/PMS2 and MSH6 expression in the MCCS.
10/13 individuals were tested for MLH1 mutations and 3/13 had no blood-derived DNA available for MLH1 mutation testing for the ACCFR. For the MCCS, 9 out of 72 CRCs demonstrating MLH1 methylation were tested for MLH1 germline mutations. Neither ACCFR nor MCCS probands with MLH1 methylated CRCs were found to carry a germline MLH1 mutation in those who were screened.
no blood-derived DNA available for germline mutation testing and therefore mutation carrier status is unknown
MSH6 c.2341C=T p.Pro781Ser is classified as a class 3 variant with insufficient evidence for pathogenicity. CRC demonstrated solitary loss of MSH6 expression and MSI-H.
MSH6 c.3107_3108delTG p.(Phe1037Leufs*2) identified in proband from ACCFR and from MCCS that were subsequently identified as the same individual with MSI-H CRC.
PMS2 c.802dup p.(Tyr268Leufs*31) identified in proband from ACCFR and from MCCS that were subsequently identified as the same individual with CRC showing solitary loss of PMS2 expression.
NT= none tested
Figure 1.
Flow diagram of the study with results of germline mismatch repair gene mutations by pattern of abnormal immunohistochemical expression in the ACCFR.
† One tumour showed loss of PMS2 and MSH2/MSH6 expression.
‡ One tumour showed normal expression of MMR proteins but an MSI-H phenotype.
§ One tumour showed loss of MLH1/PMS2 and MSH6 expression. From the 13 tumours with methylation of MLH1, 8 were also positive for the BRAFV600E somatic mutation.
Figure 2.
Flow diagram of the study with results of germline mismatch repair gene mutations by pattern of abnormal immunohistochemical expression in the MCCS.
† One tumour loss of MLH1/PMS2 and MSH2/MSH6 expression and was MLH1 methylated.
‡ These tumours showed high levels of MSI (MSI-H) but showed normal expression of all four MMR proteins
A MMR gene mutation was identified in 42 ACCFR probands (5.2%), representing 46.5% of the MMR-deficient CRCs. Two of the mutation carriers were identified among individuals with MMR-proficient tumours, both carried MSH6 mutations. None of the 40 ACCFR probands with an MSI-L CRC were found to have a germline MMR gene mutation. Comparing these results to the MCCS findings, only 7 probands had a pathogenic germline mutation (0.8%), representing 5.8% of the MMR-deficient CRCs. Of the 47 non-overlapping MMR gene mutation carriers identified in this study, 13 (27.7%) met Amsterdam I criteria, 4 (8.5%) met Amsterdam II criteria, 26 (55.3%) met Revised Bethesda guidelines and 1 (2.1%) had no clinical family history.
A number of atypical patterns of MMR-deficiency were observed across both studies namely, the loss of expression of 3 or more MMR proteins within a single CRC (Table 3). Four CRCs (all females, diagnosis ages were 59, 73, 75, and 77 years) demonstrated loss of MLH1 and PMS2 as well as loss of MSH6. All four tumours were right-sided, demonstrated MLH1 promoter methylation, the BRAFV600E somatic mutation and the absence of MLH1 or MSH6 germline mutations. A single CRC demonstrated loss of MSH2 and MSH6 as well as loss of PMS2 expression while retaining MLH1 expression. A germline mutation in PMS2 was identified while no MSH2 or MSH6 germline mutation was identified to account for the loss of MSH2/MSH6 expression. A female MCCS proband developed synchronous ascending colon tumours at 77 years of age where one CRC demonstrated loss of expression of all 4 MMR proteins while the other showed loss of MLH1/PMS2 expression only. Both of these CRCs demonstrated MLH1 promoter hypermethylation and the BRAFV600E somatic mutation however no MSH2 or MSH6 germline mutation was identified. One additional MCCS proband developed a MLH1/PMS2 deficient CRC in the caecum at 72 years of age resulting from MLH1 promoter methylation and was diagnosed 6 months later with a rectal adenocarcinoma that demonstrated loss of MSH2 and MSH6 protein expression however no MSH2 or MSH6 germline mutation was identified.
Probands with MMR-deficient CRCs that underwent germline mutation testing but had no pathogenic mutation identified, or had a VUS identified or did not show evidence of MLH1 methylation were, therefore, considered suspected Lynch or Lynch-like cases. For the ACCFR 37/90 (41.1%) MMR-deficient CRCs were considered Lynch-like while for the MCCS 26/103 (25.2%) of the MMR-deficient CRCs were considered Lynch-like. The combined Lynch-like syndrome probands from the ACCFR and MCCS had a median age of diagnosis of 50 years which was older than the median age of MMR gene mutation carriers (p=0.002) and younger than the median age of MMR-proficient cases (p=0.02) and MLH1 methylation cases (p<0.001) (Table 4). There was no evidence for a difference in the tumour characteristics of MMR gene mutation carriers and Lynch-like syndrome probands (Table 4).
Table 4.
Comparison of proband and tumour features between cases with a MMR-proficient CRC, a MMR-deficient CRC with MLH1 methylation, MMR gene mutation carrier (Lynch syndrome) and Lynch-like syndrome from the ACCFR and MCCS probands combined.
| Number (%) | ||||
|---|---|---|---|---|
|
MMR- Proficient 1469 CRC, 1446 probands) |
MLH1
methylation (91 CRC, 85 probands) |
MMR-gene
mutation (49 CRC, 45 probands) |
Lynch-like
syndrome (63 CRC, 63 probands) |
|
| Gender | ||||
| Female | 686 (47.4) | 61 (71.8) | 15 (33.3) | 31 (49.2) |
| Male | 760 (52.6) | 24 (28.2) | 30 (66.7) | 32 (50.8) |
| Age at diagnosis (years) | ||||
| Mean ± Standard deviation | 57.5 ± 13.4 | 69.6 ± 8.8 | 43.1 ± 8.8 | 52.9 ± 16.3 |
| Mean (range) | 57 (18-85) | 72 (46-83) | 43 (22-69) | 50 (18-81) |
| 18-30 | 18 (1.2) | 0 | 3 (6.1) | 4 (6.3) |
| 31-40 | 129 (8.8) | 0 | 11 (22.4) | 13 (20.6) |
| 41-50 | 349 (23.8) | 4 (4.4) | 28 (57.2) | 15 (23.8) |
| 51-60 | 360 (24.5) | 10 (10.9) | 5 (10.2) | 9 (14.3) |
| 61-70 | 292 (19.9) | 26 (28.6) | 2 (4.1) | 10 (15.9) |
| 71-80 | 293 (19.9) | 43 (47.3) | 0 | 11 (17.5) |
| >80 | 28 (1.9) | 8 (8.8) | 0 | 1 (1.6) |
| Family History † | ||||
| Amsterdam I | 24 (3) | 0 (0) | 13 (31) | 1 (3) |
| Amsterdam II | 5 (1) | 1 (8) | 4 (10) | 1 (3) |
| Amsterdam I or II | 29 (4) | 1 (8) | 17 (40) | 2 (5) |
| Revised Bethesda | 512 (71) | 13 (100) | 42 (100) | 37 (100) |
| Tumour location | ||||
| Proximal colon | 367 (26.5) | 76 (90.5) | 32 (69.6) | 39 (61.9) |
| Distal colon | 424 (30.7) | 5 (5.9) | 8 (17.4) | 9 (14.3) |
| Rectum | 591 (42.8) | 3 (3.6) | 6 (13) | 15 (23.8) |
| Missing | 87 | 7 | 3 | 0 |
| Histological type | ||||
| Adenocarcinoma | 1329 (93) | 58 (67.4) | 32 (69.6) | 52 (82.5) |
| Mucinous carcinoma | 89 (6.2) | 22 (25.6) | 14 (30.4) | 10 (15.9) |
| Signet ring cell carcinoma | 9 (0.6) | 5 (5.8) | 0 | 0 |
| Undifferentiated | 3 (0.2) | 1 (1.2) | 0 | 1 (1.6) |
| Missing | 39 | 5 | 3 | 0 |
| Histological grade (adenocarcinoma) | ||||
| Low grade | 1148 (83.7) | 45 (52.9) | 32 (69.6) | 46 (73) |
| High grade | 221 (16.1) | 39 (45.9) | 14 (30.4) | 17 (27) |
| Undifferentiated | 3 (0.2) | 1 (1.2) | 0 | 0 |
| Missing | 26 | 6 | 3 | 0 |
| Stage (TNM) † | ||||
| Stage I | 117 (17) | 2 (17) | 8 (21) | 7 (20) |
| Stage II | 207 (31) | 8 (66) | 22 (56) | 22 (63) |
| Stage III | 307 (45) | 2 (17) | 9 (23) | 5 (14) |
| Stage IV | 47 (7) | 0 | 0 | 1 (3) |
| Missing | 49 | 1 | 6 | 0 |
| Tumour margin | ||||
| Expanding | 820 (64.7) | 65 (81.3) | 36 (90) | 46 (83.6) |
| Infiltrating | 447 (35.3) | 15 (18.7) | 4 (10) | 9 (16.4) |
| Missing | 202 | 11 | 9 | 8 |
|
Peritumoral
lymphocytes |
||||
| Present | 380 (29.4) | 37 (44.6) | 24 (60) | 37 (64.9) |
| Absent | 913 (70.6) | 46 (55.4) | 16 (40) | 20 (35.1) |
| Missing | 176 | 8 | 9 | 6 |
| Crohn's-like reaction | ||||
| Present | 184 (14.5) | 40 (48.2) | 17 (43.6) | 34 (61.8) |
| Absent | 1084 (85.5) | 43 (51.8) | 22 (56.4) | 21 (38.2) |
| Missing | 201 | 8 | 10 | 8 |
|
Tumour-infiltrating
lymphocytes |
||||
| Present | 213 (15.5) | 60 (70.6) | 30 (68.2) | 47 (78.3) |
| Absent | 1160 (84.5) | 25 (29.4) | 14 (31.8) | 13 (21.7) |
| Missing | 96 | 6 | 5 | 3 |
Results include only ACCFR probands as no data was available for the MCCS probands
The combined data from the two cohorts demonstrated that all the mutation carriers identified in this study were diagnosed prior to 70 years of age and that 95.7% of carriers were identified prior to a diagnosis age of 60 years (Table 5). When considering the group of individuals with MMR-deficient CRCs who were positive for MLH1 methylation and, therefore, would not have germline mutation testing in the clinical setting, 56.5% and 83.5% of all the MLH1 methylated MMR-deficient CRCs were identified in the age at CRC diagnosis groups of >70 years and >60 years, respectively. The yield of MMR mutation carriers relative to the number of MMR IHC tests that were performed demonstrated that testing: 1) all CRCs, 2) only those diagnosed <70 years or 3) those diagnosed <60 years resulted in yields of 2.9%, 3.7% and 4.7%, respectively, for a total of 1639, 1267 and 951 MMR IHC tests performed for those same three scenarios. The sensitivity, specificity, positive and negative predictive values and positive likelihood ratio for MMR IHC testing overall and by differing age at CRC diagnosis thresholds are shown in Supplementary Table 2.
Table 5.
The number of MMR gene mutation carriers identified within groups defined by age at first CRC diagnosis relative to the number of MLH1 methylated CRCs and the number of MMR-deficient and MMR-proficient CRCs identified from both ACCFR and MCCS studies combined. The proportion of carriers identified by the total number of MMR IHC tumour tests for each age group are presented.
|
Age at first
CRC diagnosis (years) |
MMR
mutation carriers† |
MLH1
methylated CRCs |
Lynch-like |
MMR- deficient CRCs |
MMR- proficient CRCs |
Total |
Proportion of
carriers from total tested |
|---|---|---|---|---|---|---|---|
| Mean ± Standard deviation |
43.1 ± 8.8 | 69.6 ± 8.8 | 52.9 ± 16.3 | 57.7 ± 16 | 57.5 ± 13.4 | ||
| 18-30 | 3 (6.7%) | 0 | 4 (6.3%) | 7 (3.6%) | 18 (1.2%) | 25 | 12% |
| 31-40 | 11 (24.4%) | 0 | 13 (20.6%) | 24 (12.4%) | 127 (8.8%) | 151 | 7.2% |
| 41-50 | 25 (55.6%) | 4 (4.7%) | 15 (23.8%) | 44 (22.8%) | 347 (24.0%) | 391 | 6.4% |
| 51-60 | 4 (8.9%) | 10 (11.8%) | 9 (14.3%) | 23 (11.9%) | 359 (24.8%) | 382 | 1.0% |
| 61-70 | 2 (4.4%) | 23 (27.1%) | 10 (15.9%) | 35 (18.1%) | 281 (19.4%) | 316 | 0.6% |
| 71-80 | 0 | 40 (47.1%) | 11 (17.5%) | 51 (26.4%) | 286 (19.8%) | 337 | 0 |
| 81-86 | 0 | 8 (9.4%) | 1 (1.6%) | 9 (4.7%) | 28 (1.9%) | 37 | 0 |
| Total Probands | 45 | 85 | 63 | 193 | 1446 | 1639 |
VUS were excluded
Discussion
In this study, we report the results of tumour and germline mutation testing of CRC-affected individuals from two Australian cohorts to identify Lynch syndrome. From the ACCFR, where probands were diagnosed with CRC before age 60, we identified MMR deficiency in 11.1% of cases of which 14.4% were secondary to MLH1 methylation. A MMR gene mutation was identified in 40 probands with a MMR-deficient CRC and in 2 probands with a MMR-proficient CRC for a total prevalence of 5.2%. From the MCCS, where all incident CRCs were recruited from probands aged 41 to 86, the proportion of MMR deficiency was 12.5% of which 85.2% were caused by MLH1 methylation; Lynch syndrome was diagnosed in 7 probands (0.8%). Previous studies looking at Lynch syndrome in incident CRC probands from Australian cohorts described 17% (18/105) of CRC-affected individuals diagnosed under the age of 45 years6 and 2.7% (36/1344) of CRCs diagnosed before 60 years of age7. Compared to these studies, our study included individuals older than 60 years at CRC diagnosis to address the potential benefit of universal CRC screening by MMR IHC. The results for the detection of Lynch syndrome in the ACCFR were twice as high compared with the Western Australian cohort (5.2% versus 2.6%) where both studies were restricted to investigating CRCs diagnosed before 60 years of age, however, the recruitment of only 50% of patients with CRC diagnosed between the ages of 45-59 years in Phase I of the ACCFR study may have led to some bias in the number of mutation carriers identified.
Interestingly, 3 ACCFR cases with normal MMR IHC expression were MSH6 mutation carriers, although 2/3 did demonstrate high levels of MSI. This is in agreement with previous studies which reported that a substantial proportion of tumours in MSH6 mutation carriers showed low level or absence of MSI and retained IHC expression of MSH6, in particular for missense mutations21, 22. These observations and our own findings showing that patients with MSH6 mutations maybe missed by IHC and MSI testing suggests the prevalence of Lynch syndrome caused by MSH6 mutations is probably underestimated in the population.
Most MLH1-deficient CRCs are sporadic, caused by somatic MLH1 promoter methylation. Two molecular genetic tests are currently used to identify these cases: MLH1 promoter methylation and BRAFV600E mutation testing. In our study, BRAFV600E was observed in 61.5% and 77.8% of CRCs positive for MLH1 methylation from the ACCFR and MCCS studies, respectively, suggesting that using BRAFV600E instead of MLH1 methylation as a negative MMR mutation predictor would result in ~20-40% of CRCs with MLH1 and PMS2 loss of protein expression being incorrectly referred for germline mutation testing. However, in most pathology departments, BRAFV600E testing is the most accessible and therefore preferred strategy showing less variability in methodologies with non-quantitative results. Recently, IHC with a specific antibody for the BRAF V600E protein has become available to be used in as a promising surrogate marker for sequencing. However, despite some initial encouraging results,23 BRAF V600E IHC should be used with caution due to the variable reliability for determining the BRAFV600E status in CRC with sensitivity and specificity as low as 59% and 51%, respectively (reviewed in 24), and the increasing recognition of other BRAF mutations (non V600E) in up to 23% of CRC that would not be identified with this mutation-specific antibody25-27.
Universal testing, or reflex testing of all newly diagnosed CRC tumours for MMR protein status, has been recommended or endorsed by several organisations as the preferred approach to identify Lynch syndrome28-31_ENREF_37. Universal testing has been shown to reduce morbidity and mortality from Lynch syndrome in relatives28. Compared with selective strategies, universal testing is more sensitive to identify Lynch syndrome patients and is cost-effective32-34. In this study, when the results from both cohorts were combined, the number of MMR gene mutation carriers identified peaked in the 41-50 years age group. Based on our data, adopting a universal tumour testing approach compared with a strategy that tested only CRCs diagnosed ≤60 years would result in 688 additional MMR IHC tests and 65 additional MLH1 methylation tests for the identification of two mutation carriers. An upper age threshold of 70 years would mean 372 fewer MMR IHC tests and 57 fewer MLH1 methylation tests for no gains in identified mutation carriers. The use of upper age cutoffs to limit the testing of older CRC patients who are less likely to have Lynch syndrome has received support35. Although the study by Moreira and colleagues36 found that universal testing was the most sensitive, a model where all CRC diagnosed <70 years and only those CRCs diagnosed >70 years that met Bethesda guidelines were tested resulted in a sensitivity of 95.1% (versus 100% sensitivity for universal MMR IHC) with ~35% fewer MMR IHC tests being performed.
In the latest 2012 edition of the Cancer Protocol for CRC, recommendation from the Royal College of Pathologists of Australasia (RCPA) is to perform IHC for MMR protein expression in all CRC cases diagnosed in patients less than 50 years of age and in patients meeting the revised Bethesda criteria37. Despite low numbers of identified Lynch syndrome in individuals older than 50 years, our findings suggest that the RCPA could increase their age limit recommendations to test all CRCs diagnosed <70 years of age, and be performed in conjunction with MLH1 promoter methylation or BRAFV600E mutation testing for MLH1-deficient CRCs, as the optimal strategy to identify MMR gene mutation carriers. A cost-effectiveness study in Australia would be useful to support this suggestion. If laboratory resources are limited, MMR IHC tumour testing of all CRC cases diagnosed ≤60 years of age in conjunction with MLH1 promoter methylation testing would also be an efficient strategy, although less sensitive and has been recommended for Lynch syndrome screening in endometrial cancer16.
Supplementary Material
Acknowledgements
The authors thank all study participants of the Australasian Colorectal Cancer Family Registry Cohort and the Melbourne Collaborative Cohort and staff for their contributions to this project.
This work was supported by grant UM1 CA167551 from the National Cancer Institute and through cooperative agreements with Australasian Colorectal Cancer Family Registry (U01 CA074778 and U01/U24 CA097735) and was conducted under Colon-CFR approval C-AU-0312-01. The Melbourne Collaborative Cohort Study for colorectal cancer was funded by NHMRC project grant 509348 (PI-Dallas English) “Risk Factors for Molecular Subtypes of Colorectal Cancer”. Aung Ko Win is an Australian National Health and Medical Council (NHMRC) Early Career Fellow. Melissa C. Southey is a NHMRC Senior Research Fellow. Mark A. Jenkins is a NHMRC Senior Research Fellow. John L. Hopper is a NHMRC Senior Principal Research Fellow and Distinguished Visiting Professor at Seoul National University, Korea. Christophe Rosty is the Jass Pathology Fellow. Daniel D. Buchanan is a University of Melbourne Research at Melbourne Accelerator Program (R@MAP) Senior Research Fellow.
Footnotes
Disclosure Statement:
The authors declare they hold no conflict of interest with respect to this work.
Disclaimer: The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Cancer Family Registries, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the Cancer Family Registry. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- [2].AIHW . Cancer in Australia: an overview, 2007. AIHW (Australian Institute of Health and Welfare) & AACR (Australasian Association of Cancer Registries); Canberra: 2010. [Google Scholar]
- [3].Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–64. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- [6].Southey MC, Jenkins MA, Mead L, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–32. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- [7].Schofield L, Watson N, Grieu F, et al. Population-based detection of Lynch syndrome in young colorectal cancer patients using microsatellite instability as the initial test. Int J Cancer. 2009;124:1097–102. doi: 10.1002/ijc.23863. [DOI] [PubMed] [Google Scholar]
- [8].Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- [9].Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- [10].Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- [11].Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rosty C, Young JP, Walsh MD, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26:825–34. doi: 10.1038/modpathol.2012.240. [DOI] [PubMed] [Google Scholar]
- [13].Cicek MS, Lindor NM, Gallinger S, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn. 2011;13:271–81. doi: 10.1016/j.jmoldx.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Walsh MD, Buchanan DD, Cummings MC, et al. Lynch syndrome-associated breast cancers: clinicopathologic characteristics of a case series from the colon cancer family registry. Clin Cancer Res. 2010;16:2214–24. doi: 10.1158/1078-0432.CCR-09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buchanan DD, Sweet K, Drini M, et al. Risk factors for colorectal cancer in patients with multiple serrated polyps: a cross-sectional case series from genetics clinics. PLoS ONE. 2010;5:e11636. doi: 10.1371/journal.pone.0011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clendenning M, Walsh MD, Gelpi JB, et al. Detection of large scale 3' deletions in the PMS2 gene amongst Colon-CFR participants: have we been missing anything? Fam Cancer. 2013;12:563–6. doi: 10.1007/s10689-012-9597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thompson BA, Spurdle AB, Plazzer JP, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2013 doi: 10.1038/ng.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Buchanan DD, Rosty C, Clendenning M, Spurdle AB, Win AK. Clinical problems of colorectal cancer and endometrial cancer cases with unknown cause of tumor mismatch repair deficiency (suspected Lynch syndrome) Appl Clin Genet. 2014;7:183–93. doi: 10.2147/TACG.S48625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berends MJ, Wu Y, Sijmons RH, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet. 2002;70:26–37. doi: 10.1086/337944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Berends MJ, Mensink RG, et al. Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet. 1999;65:1291–8. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toon CW, Walsh MD, Chou A, et al. BRAFV600E Immunohistochemistry Facilitates Universal Screening of Colorectal Cancers for Lynch Syndrome. Am J Surg Pathol. 2013;37:1592–602. doi: 10.1097/PAS.0b013e31828f233d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Estrella JS, Tetzlaff MT, Bassett RL, Jr., et al. Assessment of BRAF V600E Status in Colorectal Carcinoma: Tissue-Specific Discordances between Immunohistochemistry and Sequencing. Mol Cancer Ther. 2015;14:2887–95. doi: 10.1158/1535-7163.MCT-15-0615. [DOI] [PubMed] [Google Scholar]
- [25].Loes IM, Immervoll H, Angelsen JH, et al. Performance comparison of three BRAF V600E detection methods in malignant melanoma and colorectal cancer specimens. Tumour Biol. 2015;36:1003–13. doi: 10.1007/s13277-014-2711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lasota J, Kowalik A, Wasag B, et al. Detection of the BRAF V600E mutation in colon carcinoma: critical evaluation of the imunohistochemical approach. Am J Surg Pathol. 2014;38:1235–41. doi: 10.1097/PAS.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carter J, Tseng LH, Zheng G, et al. Non-p.V600E BRAF Mutations Are Common Using a More Sensitive and Broad Detection Tool. Am J Clin Pathol. 2015;144:620–8. doi: 10.1309/AJCP85ATMJOZOUDJ. [DOI] [PubMed] [Google Scholar]
- [28].Evaluation of Genomic Applications in P, Prevention Working G Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109:1159–79. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- [30].Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209–17. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62. doi: 10.1038/ajg.2014.435. quiz 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- [33].Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Genetic/Familial high-risk assessment: colorectal, Version2. 2014 http://wwwnccnorg/professionals/physician_gls/f_guidelinesasp#genetics_colon.
- [36].Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–65. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Australasia TRCoPo Colorectal Cancer Structured Reporting Protocol. 2012 Available from: https://wwwrcpaeduau/Library/Practising-Pathology/Structured-Pathology-Reporting-of-Cancer/Cancer-Protocols. [cited Oct 2015]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.