Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive method to modulate cortical excitability. This technique is a promising emerging tool to treat several neuropathologies, including addiction. We have previously shown in mice that repeated tDCS normalizes pathological behaviors associated with chronic nicotine exposure. Here, we evaluated, in adult female mice, the impact of tDCS on cocaine-induced behavior and gene regulation in corticostriatal circuits implicated in psychostimulant addiction. Anodal tDCS was applied transcranially over the frontal cortex. Three weeks after repeated tDCS, we investigated the induction of a gene expression marker (Zif268) by cocaine (25 mg/kg) in 26 cortical and 23 striatal regions using in situ hybridization histochemistry. We also assessed place preference conditioning by cocaine (5, 10, and 25 mg/kg). tDCS pretreatment increased basal expression and attenuated cocaine (25 mg/kg)-induced expression of Zif268 in specific corticostriatal circuits. Cocaine-induced locomotor activation (25 mg/kg) and place preference conditioning (5 and 25 mg/kg) were also reduced. These results demonstrate that tDCS can attenuate molecular and behavioral responses to cocaine for several weeks. Together, our findings provide pre-clinical evidence that such electrical brain stimulation may be useful to modify the psychostimulant addiction risk.
Keywords: cocaine, conditioned place preference, corticostriatal circuits, gene expression, neuromodulation, tDCS
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive and painless neuromodulatory technique that uses weak constant electrical current to stimulate specific areas of the cerebral cortex. During the last decade, tDCS has emerged as a successful approach to alleviate symptoms of various psychiatric and neurological conditions, including depression (e.g. Berlim et al., 2013; Brunoni et al., 2012; Kuo et al., 2014), memory disorders (e.g. Bennabi et al., 2014) and addiction (Feil and Zangen, 2010). For example, studies found that repeated tDCS over the prefrontal cortex decreased craving for, and the consumption of, cigarettes (Boggio et al., 2009; Fecteau et al., 2014; Fregni et al., 2008) and alcohol (Boggio et al., 2008; Klauss et al., 2014) in chronic users. The mechanisms underlying these behavioral modifications are unknown. We recently developed an animal model for tDCS (Pedron et al., 2014) to investigate the neuronal processes affected by this technique. Consistent with the above clinical findings, our early work shows that repeated tDCS for five days in mice has antidepressant-like properties, improves working memory, and decreases nicotine-induced place preference conditioning, three weeks after tDCS (Pedron et al., 2014).
The potential effects of tDCS in cocaine addiction remain poorly explored (Conti and Nakamura-Palacios, 2014). However, reduced cocaine craving has been reported after another kind of non-invasive cortical stimulation, repeated transcranial magnetic stimulation (rTMS) (Camprodon et al., 2007; Politi et al., 2008), suggesting that modifying cortical activity may also alter psychostimulant-induced processes. The mechanisms underlying these effects of tDCS or rTMS, and whether or not other subcortical addiction-related brain structures are also impacted, remain unclear.
It has been shown that corticostriatal circuits play a critical role in several aspects of addiction, including abnormal reward processing, habit formation, and compulsive behavior (Berke and Hyman, 2000; Everitt and Robbins, 2005; Wise, 2009). A large literature implicates changes in gene regulation in specific corticostriatal circuits in addiction (Renthal and Nestler, 2008). Among the many genes affected by psychostimulants such as cocaine in the cortex and striatum is Zif268 (Steiner and Van Waes, 2013), which encodes a transcription factor (Knapska and Kaczmarek, 2004) that is critical for cocaine-induced behavioral changes (Lee et al., 2005; Theberge et al., 2010; Valjent et al., 2006)
In the present study, we determined, in mice, whether tDCS can modify cocaine-induced behavior and/or normal or cocaine-induced gene regulation in the cortex and striatum, using Zif268 as a gene regulation marker. Our mapping study also assessed the spread of such molecular changes across different corticostriatal circuits.
Materials and methods
Subjects
Swiss female mice (8 weeks at the beginning of tDCS; Janvier, France) were housed 8–10 per cage under standard laboratory conditions (12:12-hour light/dark cycle; lights on at 7:00 am) with food and water available ad libitum. Prior to the surgery, mice were allowed 1 week of acclimation, during which they were repeatedly handled. In experiment 1 (locomotor activity, gene expression), animals were divided into eight experimental groups: Sham-Veh (Vehicle) (N=8), tDCS-Veh (N=6), Sham-5 (Cocaine 5 mg/kg, i.p.) (N=9), tDCS-5 (N=10), Sham-10 (Cocaine 10 mg/kg) (N=9), tDCS-10 (N=10), Sham-25 (Cocaine 25 mg/kg) (N=8), and tDCS-25 (N=8). In experiment 2 (place preference conditioning by cocaine), animals were divided into eight groups: Sham-Veh (N=8), tDCS-Veh (N=10), Sham-5 (N=14), tDCS-5 (N=12), Sham-10 (N=14), tDCS-10 (N=14), Sham-25 (N=13), and tDCS-25 (N=12). All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the University of Franche-Comté Animal Care and Use Committee (CEBEA-58).
Surgery
A tubular plastic jack (internal diameter: 2.1 mm) was surgically fixed onto the skull one week before the stimulation protocol began (Fig. 1A). Animals were anesthetized with ketamine hydrochloride/xylazine (80/12 mg/kg; i.p.) and were placed in a stereotaxic apparatus. The center of the plastic jack was positioned over the left frontal cortex 1 mm rostral and 1 mm left of bregma (Fig. 2) and fixed with a coating of glass ionomer cement (GC Fuji I, Leuven, Belgium) (Pedron et al., 2014). After surgery, all animals were allowed to recover for 1 week before undergoing tDCS. During this period and tDCS, mice were placed in individual cages.
Figure 1.
(A) Experimental design (experiments 1 and 2). (B) Total distance traveled (mean ± SEM, in cm) in a novel open field is shown for animals that were subjected to tDCS (twice daily, 5 days) or Sham stimulation and, 3 weeks after stimulation, received an injection of cocaine (Coc) (5, 10, or 25 mg/kg) or vehicle (Veh), followed by the 40 min behavioral test. (C) Time course of the distance traveled is given for each group. ** p<0.01 and *** p<0.001, versus respective control group (Sham-Veh or tDCS-Veh); &&& p<0.001, Sham-25 versus tDCS-25. N=6–10 per group.
Figure 2.
(A) Schematic illustration of the 26 cortical areas (Paxinos and Franklin, 2001) and the 23 striatal sectors (mostly defined by their predominant cortical inputs, Willuhn et al., 2003) used to measure Zif268 expression. Gene expression was assessed in coronal sections from four rostrocaudal levels: frontal, rostral, middle, and caudal (ranging from approximately +1.98 to −0.22 mm relative to bregma; Paxinos and Franklin, 2001). Horizontal black lines indicate the position of the anode. Cortical areas (from medial to lateral): IL, infralimbic; PrL, prelimbic; Cg, cingulate; M2, secondary motor; M1, primary motor; SS, somatosensory; I/LO, insular/lateral orbital; I, insular; Pir, piriform. Striatal sectors: m, medial; dm, dorsomedial; d, dorsal; dl, dorsolateral; dc, dorsal central; c, central; vc, ventral central; vl, ventrolateral; v, ventral. Nucleus accumbens: mC, medial core; lC, lateral core; mS, medial shell; vS, ventral shell; lS, lateral shell. (B) The center of the stimulation electrode (anode, filled circle) was positioned over the left frontal cortex 1 mm rostral and 1 mm left of bregma. The anode (diameter: 2.1 mm) had a contact area of 3.5 mm2, and the cathode (rubber-plate electrode, 9.5 cm2) was positioned onto the ventral thorax (not shown). A 2 × 20 minutes/day constant current of 0.2 mA, with a linear fade in/fade out of 10 sec, was applied transcranially using a direct current stimulator (DC-Stimulator Plus), on 5 consecutive days. The position of the assessed brain sections (frontal to caudal) relative to the stimulation electrode is also shown.
Stimulation protocol
The plastic jack was filled with saline (NaCl 0.9 %) to establish a contact area of 3.5 mm2 with the skull. The stimulation electrode (anode, diameter: 2.1 mm; DIXI Medical, Besançon, France) was then screwed into the jack. A larger conventional rubber-plate electrode (cathode, 9.5 cm2; Physiomed Elektromedizin AG, Schnaittach, Germany) served as the counterelectrode and was placed onto the ventral thorax (Pedron et al., 2014). On 5 consecutive days, an anodal constant current (0.2 mA; 2 × 20 minutes/day, 5 hours interstimulation interval) was applied transcranially over the frontal cortex, using a DC-Stimulator Plus (NeuroConn, Ilmenau, Germany) with a linear fade in/fade out (10 sec ramp). Animals were awake and restrained during tDCS to prevent possible interactions between tDCS effects and anesthetic drugs. The design of the custom-made restraining box is shown in Pedron et al., 2014. Control (Sham) animals were subjected to the same procedure (surgery, restraining box, electrode connected to the jack), except the current was not delivered.
Our protocol of stimulation was chosen based on earlier clinical studies (Boggio et al., 2009; Ferrucci et al., 2009; Rigonatti et al., 2008), as well as our previous work in mice (Pedron et al., 2014). We evaluated effects on behavior and gene expression three weeks after tDCS ended, since this time window showed robust effects of tDCS on behavior (Pedron et al., 2014).
Experiment 1: Locomotor activity and gene expression
Drug treatment and behavioral testing
Three weeks after the last tDCS session, animals received a single injection of either cocaine (5, 10, or 25 mg/kg in 0.02% ascorbic acid, i.p.,1 ml/kg, Sigma-Aldrich, France) or vehicle. Immediately after the injection, the animal was placed in a circular open-field (diameter 47 cm) for 40 minutes in low-light conditions (40 lux). Locomotor activity was analyzed using a video-tracking system (Ethovision, Noldus, France). The parameter assessed was the distance traveled in the open-field. The mice were then killed with CO2. The brain was rapidly removed, frozen in isopentane cooled on dry ice, and stored at −30°C until cryostat sectioning.
Tissue preparation and in situ hybridization histochemistry
Only the mice treated with cocaine 25 mg/kg or vehicle were used in the gene expression study. Coronal sections (12 μm) were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA), dried on a slide warmer and stored at −30°C. In preparation for the in situ hybridization histochemistry, the sections were fixed in 4% paraformaldehyde/0.9 % saline for 10 minutes at room temperature, incubated in a fresh solution of 0.25 % acetic anhydride in 0.1 M triethanolamine/0.9 % saline (pH 8.0) for 10 minutes, dehydrated, defatted for 2 × 5 minutes in chloroform, rehydrated and air-dried. The slides were then stored at −30°C until hybridization. An oligonucleotide probe (48-mer, Invitrogen, Rockville, MD, USA) was labeled with [α-33P]-dATP, as described earlier (Van Waes et al., 2014). The probe had the following sequence: Zif268 (Egr1), complementary to bases 352–399, GenBank accession number M18416. One hundred microliters of hybridization buffer containing labeled probe (~3 × 106 cpm) was added to each slide. The sections were coverslipped and incubated at 37°C overnight. After incubation, the slides were first rinsed in four washes of 1X saline citrate (150 mM sodium chloride, 15 mM sodium citrate), and then washed three times for 20 minutes each in 2X saline citrate/50 % formamide at 40°C, followed by two washes of 30 minutes each in 1X saline citrate at room temperature. After a brief water rinse, the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak, Rochester, NY, USA) for 6 days.
Analysis of autoradiograms
Gene expression in the cortex was measured in a total of 26 regions (infralimbic, prelimbic, cingulate, secondary motor, primary motor, somatosensory, lateral orbital, insular and piriform, based on (Paxinos and Franklin, 2001) in coronal sections from four rostrocaudal levels: frontal, approximately at +1.98 mm relative to bregma; rostral, +1.18 mm; middle, +0.74 mm; and caudal, −0.22 mm, Fig. 2A). Striatal gene expression was determined at the rostral, middle and caudal levels in a total of 23 sectors mostly defined by their predominant cortical inputs (Willuhn et al., 2003). Eighteen of these sectors represented the caudate-putamen (medial, dorsomedial, dorsal, dorsolateral, dorsal central, central, ventral central, ventrolateral, ventral) and five the nucleus accumbens (medial core, lateral core, medial shell, ventral shell, and lateral shell, Fig. 2A) (Van Waes et al., 2010).
Hybridization signals on film autoradiograms were measured by densitometry (ImageJ, Wayne Rasband, Bethesda, MD, USA). The films were captured using a light table (Northern Light, Imaging Research, St. Catharines, Ontario, Canada) and a Sony CCD camera (Imaging Research). The ‘mean density’ value of a region of interest was measured by placing a template over the captured image. Mean densities were corrected for background by subtracting mean density values measured over white matter (corpus callosum) of the same hemisphere. Values from corresponding regions in the two hemispheres were averaged when no significant differences in the patterns of Zif268 induction by tDCS or cocaine were detected between the left (stimulated) and right (contralateral) sides. The illustrations of film autoradiograms displayed in Fig. 3 are computer-generated images, and are contrast-enhanced. The maximal hybridization signal is black.
Figure 3.
Illustrations of film autoradiograms depict Zif268 expression in coronal sections from the middle striatum in Sham or tDCS mice treated with vehicle (Veh) or cocaine (25 mg/kg, i.p.). Horizontal black lines illustrate the position of the anode. The maximal hybridization signal is in black.
Experiment 2: Place preference conditioning by cocaine
Animals performed the conditioned place preference (CPP) test three weeks after tDCS, as previously described (Pedron et al., 2014). Three doses of cocaine were tested (5, 10, or 25 mg/kg in 0.02% ascorbic acid, i.p.). Two groups that received vehicle injections in both compartments were used as controls (Sham-Veh and tDCS-Veh). Briefly, the CPP apparatus consists of two main compartments linked by a corridor displaying each different features: visual (wall patterns) and tactile (floor texture). On day 1 (pre-conditioning, D1), mice were placed in the corridor and allowed free access to the compartments for 10 minutes. The time spent in each compartment was recorded using the Ethovision system. On days 2–4 (conditioning phase) mice received an injection of cocaine or vehicle (one of each per day, interval between the injections: 6 hours) and were immediately confined into one of the two conditioning compartments for 15 minutes (drug pairing occurred in the least preferred compartment). On day 5 (post-conditioning, D5), mice were again allowed free access to both compartments for 10 minutes, without drug treatment. The percentage of time spent in the drug-paired compartment was calculated for the pre-conditioning (D1) and the post-conditioning (D5) phases as follows: drug-paired compartment (sec) / (drug-paired compartment + vehicle-paired compartment (sec)) x 100. A significant increase in the percentage of time spent in the drug-paired compartment between the pre-conditioning session (D1) and the post-conditioning session (D5) indicates that the substance induces a place preference.
Statistical analysis
The results were expressed as mean ± standard error of the mean. Significance was set at p ≤ 0.05. For locomotor activity two-factor ANOVAs with factors drug (Veh, 5, 10, 25) and stimulation (Sham, tDCS) were performed. To compare Zif268 densities, we first used three-factor ANOVAs (one ANOVA per cortical area or striatal sector) with factors drug (Veh, 25), stimulation (Sham, tDCS) and side of the brain (right, left; within factor). Then, for the averaged values (right/left hemisphere), two-factor ANOVAs were performed with factors drug and stimulation. Finally, for the CPP experiment, we performed for each dose (0, 5, 10, and 25 mg/kg) two-factor ANOVAs with factors stimulation (Sham, tDCS) and time (pre-(D1), post-(D5) conditioning; within factor). Newman-Keuls post-hoc tests were used to describe differences between individual groups (Statistica, StatSoft, USA). For illustrations of topographies (maps, Fig. 4A and 5A), the increase in gene induction (versus respective control group) in a given region was expressed as the percentage of the maximal increase observed (% max). The regional distribution of Zif268 induction in the cortex and striatum was compared by Pearson’s correlations, for different experimental conditions.
Figure 4.
Topography of tDCS-induced Zif268 expression in the striatum (in vehicle-treated mice). (A) Maps depict the distribution of increases in Zif268 expression at the rostral, middle, and caudal levels of the striatum, 3 weeks after tDCS and following the behavioral test (ipsi- and contralateral values averaged). The values (difference tDCS Veh minus Sham-Veh) are expressed relative to the maximal increase observed (% of maximum). Sectors with significant differences (p<0.05) are shaded as indicated. Sectors without significant effect are in white. (B) Mean density values (mean ± SEM) for Zif268 expression in Sham (white) and tDCS (black) mice 3 weeks after tDCS and following the behavioral test are depicted for the 6 middle striatal sectors. # p<0.05, ## p<0.01, and ### p<0.001, versus Sham-Veh. N=6–8 per group.
Figure 5.
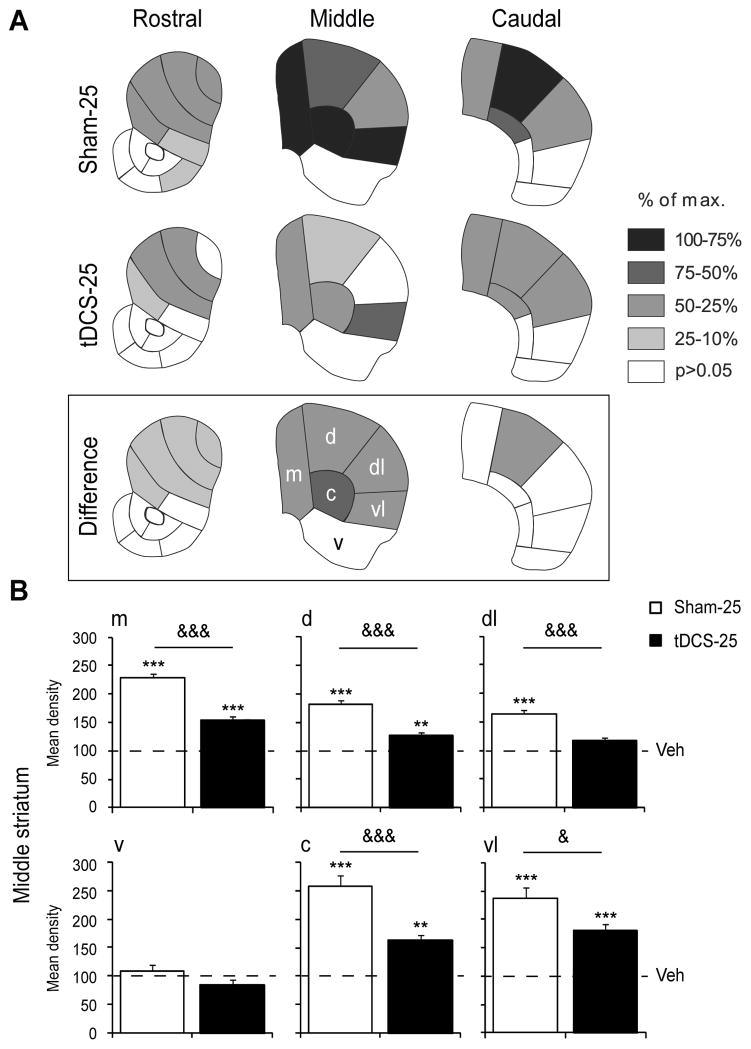
tDCS attenuates cocaine-induced Zif268 expression in specific areas of the striatum. (A) Maps depict the distribution of Zif268 expression induced by cocaine (25 mg/kg, i.p.; ipsi- and contralateral values averaged) at the rostral, middle, and caudal levels of the striatum, for cocaine-treated Sham (Sham-25) and tDCS (tDCS-25) mice. The data are expressed relative to the maximal increase observed (% of maximum). Sectors with significant differences versus respective Veh controls (i.e. Sham-Veh or tDCS-Veh) are shaded as indicated. Sectors without significant effects are in white. The “difference” (box) indicates significant differences in Zif268 induction between cocaine alone (Sham-25) and tDCS+cocaine (tDCS-25) groups. (B) Mean density values (mean ± SEM) (expressed as percentage of respective control groups, Veh=100%) for Zif268 expression are depicted for the 6 middle striatal sectors. ** p<0.01 and *** p<0.001, versus respective Veh control group; & p<0.05 and &&& p<0.001, Sham-25 versus tDCS-25. N=6–8 per group.
Results
Experiment 1: Locomotor activity and gene expression
tDCS attenuated cocaine-induced locomotor activity for the highest dose of cocaine
Cocaine increased locomotor activity in both Sham and tDCS mice (Drug effect: F(3,59)=80.97, p<0.001; Fig. 1B,C, Newman-Keuls post-hoc analyses ** p<0.01 and *** p<0.001 versus respective control group (Sham-Veh or tDCS-Veh)). tDCS had a differential effect on locomotor activity depending on the dose of cocaine tested (tDCS x drug interaction: F(3,59)=3.87, p<0.05). Newman-Keuls post-hoc analyses revealed that tDCS alone had no effect on locomotor activity (tDCS-Veh versus Sham-Veh, p=0.50). For the lower doses, tDCS had also no impact on cocaine-induced locomotor activity (tDCS versus Sham: 5 mg/kg, p=0.84; 10 mg/kg, p=0.93). However, tDCS reduced cocaine-induced locomotor activity for the highest cocaine dose tested (25 mg/kg, p<0.001).
No asymmetrical effects of tDCS or cocaine on Zif268 expression in the left versus right hemisphere
Although the stimulation electrode was positioned asymmetrically on the skull (1 mm left of bregma, Fig. 2B), there was no difference between the left (stimulated) and right (contralateral) hemisphere in the expression of Zif268, with or without cocaine (Fig. 3). That is, for each area of cortex and striatum, there was no significant interaction with the hemisphere (right or left). Values from corresponding regions in the two hemispheres were therefore averaged for the rest of the study.
tDCS increased basal Zif268 expression
Three weeks after tDCS, vehicle-treated animals subjected to tDCS displayed enhanced expression of Zif268 compared to sham controls in the striatum (Fig. 3 and 4, supplementary Table 2) and to a lesser degree in the cortex (supplementary Table 1). Among the cortical areas, a statistically significant increase was seen in the piriform cortex on rostral, middle and caudal levels (p<0.05) and in the cingulate cortex (frontal level, p<0.05; supplementary Table 1). A similar tendency was also present in the motor cortex (M1 and M2 on the middle level, p=0.06 and 0.07, respectively), in the infralimbic and prelimbic cortices on the frontal level (each p=0.07) and in the cingulate cortex on the rostral level (p=0.07; supplementary Table 1).
The striatum was more affected by tDCS than the cortex. In tDCS-treated animals, significantly increased Zif268 expression was observed on the levels situated under the electrode (i.e., in rostral and middle sections, Fig. 4A and B, supplementary Table 2), in dorsal sectors of the striatum (rostral: dorsolateral and dorsomedial; middle: medial, dorsal, dorsolateral, and central). Notably, tDCS had no significant effect in the nucleus accumbens (supplementary Table 2).
tDCS attenuated cocaine-induced Zif268 expression
Since significant differences were observed between Sham and tDCS groups in vehicle-treated animals (Fig. 4), the effects of cocaine were expressed relative to the values in the respective Veh control groups (the supplementary Tables 1 and 2 present the absolute values for comparison). In Sham animals, the single cocaine injection induced a minor but statistically significant increase in Zif268 mRNA expression in a few areas of the cortex (frontal: cingulate; middle: M1, M2; p<0.05, supplementary Table 1), with similar tendencies in other areas. In the striatum, cocaine produced a more robust augmentation in Zif268 expression on all three rostrocaudal levels (Fig. 3 and 5, supplementary Table 2). A significant increase in Zif268 mRNA levels was observed in 15 of the 23 sectors (Fig. 5A). Gene induction varied considerably between different striatal regions. The most robust increase was observed on the middle (Fig. 5B) and caudal levels, in striatal sectors that receive cingulate, motor and sensorimotor cortical inputs (i.e., middle: medial, dorsal, central, and lateral sectors; caudal: dorsal sector, Willuhn et al., 2003). In contrast, in accordance with previous findings in the rat (Unal et al., 2009), the nucleus accumbens displayed modest or no drug effects. Cocaine significantly increased Zif268 expression only in the lateral shell (p<0.05).
In the tDCS group, cocaine had no significant effect on Zif268 expression in the cortex. Thus, Zif268 induction in the cingulate and motor cortex was prevented in animals that received tDCS three weeks before the cocaine treatment (supplementary Table 1). Consistent with this result, in tDCS-treated animals (tDCS-25), cocaine-induced Zif268 expression in the striatum was markedly attenuated compared to the Sham control (Sham-25). This was reflected, for one, by a lower proportion of the 23 striatal sectors displaying significantly increased Zif268 expression in the tDCS group than in the Sham group (relative data, 11 sectors versus 15 sectors; Fig. 5A, tDCS-25). Direct statistical comparisons showed that Zif268 induction was significantly weaker in tDCS-25 animals in 10 striatal sectors (Fig. 5A, Difference and Fig. 5B). tDCS had no significant effect on cocaine-induced Zif268 expression in the nucleus accumbens (Fig. 5A, Difference).
Correlation analysis was used to compare tDCS- and cocaine-induced increases in Zif268 expression between striatal sectors and their respective cortical input regions (see Cotterly et al., 2007; Van Waes et al., 2010; Willuhn et al., 2003; Yano and Steiner, 2005 for more details). Our results for vehicle-treated mice show that, despite the modest effects in the cortex, tDCS-induced Zif268 expression in the 23 striatal sectors was positively correlated with that in their anatomically connected cortical areas (Veh animals, r=0.415, p<0.05). Similarly, in these striatal sectors, the magnitude of the reduction in cocaine-induced gene expression produced by tDCS was highly correlated with that in their connected cortical input regions (r=0.627, p<0.001). Therefore, tDCS produced coordinated molecular changes in cortical and striatal nodes of specific corticostriatal circuits.
Experiment 2: Place preference conditioning by cocaine
tDCS abolished cocaine-induced place preference conditioning for 5 and 25 mg/kg
Vehicle injections did not produce any place preference (ANOVA all effects: p>0.05, Fig. 6, Veh). In sham groups, cocaine induced a place preference with all doses tested (5 mg/kg: p<0.05, 10: p<0.001, 25: p<0,01), in accordance with previous studies in mice (e.g. Iniguez et al., 2015; Zhang et al., 2002). In contrast, animals subjected to repeated anodal tDCS failed to show cocaine-induced place preference for the doses of 5 (p=0.98) and 25 mg/kg (p=0.53). Only the 10 mg/kg dose produced statistically significant place preference conditioning (p<0.001, Fig. 6). For the dose of 25 mg/kg, the percentage of time spent in the drug-paired compartment on the post-conditioning day (D5) was significantly lower in tDCS than in sham mice (p<0.05).
Figure 6.
Conditioned place preference induced by cocaine (5, 10, or 25 mg/kg) or vehicle (Veh, control groups) 3 weeks after Sham (white) or tDCS (black) stimulation. A significant increase in the time spent in the drug-paired compartment between day 1 (D1, pre-conditioning session) and day 5 (D5, post-conditioning session) indicates that cocaine induced a place preference. For the Sham groups, cocaine induced a place preference with all doses tested. In contrast, in the tDCS groups, cocaine induced a place preference only with the 10 mg/kg dose. $ p<0.05, $$ p<0.01, and $$$ p<0.001, D1 versus D5; & p<0.05, Sham-25 D5 versus tDCS-25 D5. N=8–14 per group.
Discussion
Our findings show that tDCS produces long-lasting modifications in behavioral responses and gene regulation in corticostriatal circuits induced by cocaine. Thus, repeated anodal tDCS over the frontal cortex increased “basal” expression of the marker gene Zif268 and attenuated cocaine-induced gene regulation, locomotion and place preference conditioning, three weeks after tDCS pretreatment.
Cortical and subcortical effects of tDCS in vehicle controls
One aim of this study was to determine possible tDCS effects on normal gene regulation (i.e., in vehicle controls) and to map the distribution of such effects in the cortex and striatum. Our findings in vehicle-treated mice show that repeated tDCS produced increased expression of Zif268, mostly in the striatum, three weeks after tDCS pretreatment. Future studies will have to determine whether these increased Zif268 mRNA levels represented upregulated gene expression that endured for three weeks, or whether they reflected an altered responsiveness to experimental conditions such as handling or behavioral testing, or other neuronal changes (e.g., increased arousal; see Steiner and Van Waes, 2013), in the affected corticostriatal circuits. Regardless of the underlying cause, these findings demonstrate long-lasting effects of tDCS on gene regulation in these circuits.
Although tDCS preferentially impacted dorsal striatal regions under the stimulation electrode, these effects were not strictly related to the position of the electrode. For one, gene regulation changes in striatum (and cortex) were symmetrically distributed in the two hemispheres, despite the asymmetrical electrode placement (over left cortex). Moreover, in the cortex, the most robust increase in Zif268 expression was present in the piriform (olfactory) cortex, a ventral brain region. In the striatum, regions on the middle level were considerably more affected than those on the rostral level, despite the similar position of these levels relative to the electrode placement. These findings indicate that specific neuronal circuits, rather than just proximal cortical and striatal tissues, are modified by tDCS. This conclusion is supported by our correlation analysis that showed that changes in gene expression were correlated between cortical areas and their connectionally (functionally) related striatal target sectors (Veh animals, r=0.415, p<0.05) (Willuhn et al., 2003) and that these effects preferentially occurred in sensorimotor circuits (Steiner and Van Waes, 2013).
The finding that the asymmetrically positioned electrode produced bilaterally symmetrical gene regulation patterns (with and without cocaine) was somewhat unexpected. The contralateral cortical and striatal effects may have been mediated by the pronounced interhemispheric cortico-cortical and cortico-striatal projection systems. Alternatively, they may have resulted in part from bilateral current spread. tDCS is known to be less focal than rTMS (Miniussi et al., 2008; Nitsche et al., 2007), and the current may thus have spread over both hemispheres to some degree. However, the finding that distinct corticostriatal circuits were affected, without clear relationship to their distance from the electrode (see above), seems to argue against such a nonspecific effect as the sole factor. This is an important question that will have to be addressed in future studies.
Gene regulation effects of cocaine are attenuated by tDCS
The main goal of this study was to determine whether tDCS might modulate gene regulation by cocaine. Our results show diminished induction of Zif268 by cocaine in cortex and striatum when examined three weeks after the repeated tDCS pretreatment. Again the magnitude of this effect was correlated between cortical regions and their striatal targets (r=0.627, p<0.001), indicating that specific circuits were affected. The tDCS-mediated decrease in gene induction was maximal in (but not limited to) sensorimotor and associative corticostriatal circuits, which are known to be involved in habit formation and compulsive aspects of drug taking (Everitt and Robbins, 2005).
The basis for this altered gene regulation is presently unclear. Early studies in animals using direct current stimulation (current applied directly to the cortex) (Bindman et al., 1964; Purpura and McMurtry, 1965), as well as more recent data on tDCS in humans (Nitsche and Paulus, 2000), suggest that anodal stimulation increases neuronal excitability, which may increase transmitter release in the striatum. Repeated tDCS may thus induce synaptic plasticity (Stagg and Nitsche, 2011) that is usually associated with such changes in neuronal activity and their molecular sequelae, including altered gene regulation. Altered neuronal responsiveness in these corticostriatal circuits is consistent with the here observed reduced behavioral responses, which are modulated by neuronal activity in these circuits, including place preference conditioning (Ilango et al., 2014).
The acute induction of immediate-early genes such as Zif268 by psychostimulants serves as a marker that predicts long-term neuroadaptations after repeated drug exposure, as this acute response is correlated with various neuronal changes after repeated drug treatments (Steiner and Van Waes, 2013). However, Zif268 is also directly implicated in various plasticity processes, including several long-term neurobehavioral changes induced by psychostimulants. For example, this transcription factor likely mediates some drug-induced neuroplastic changes (Knapska and Kaczmarek, 2004). Indeed, previous work demonstrated that Zif268 is critical for place preference conditioning by cocaine (Valjent et al., 2006) and for reconsolidation of cocaine memories (Lee et al., 2005; Theberge et al., 2010). Zif268 also contributes to processes underlying cocaine-induced behavioral sensitization (Valjent et al., 2006).
Our present findings of an association between diminished Zif268 induction and attenuated locomotor activity and place preference conditioning by cocaine are consistent with these earlier findings. Whether directly affecting the underlying neuronal mechanisms or serving as a marker, the attenuated Zif268 response after repeated tDCS may indicate a “protective” effect of tDCS against drug-induced neuronal changes subsequent to tDCS treatment.
Behavioral effects of cocaine are attenuated by tDCS
The impact of tDCS on cocaine-induced behavioral effects was dependent on the dose of cocaine. For locomotor activity, the tDCS effect was selectively observed with the highest dose (25 mg/kg). It could be argued that an increase in focused stereotypies, which are associated with certain psychostimulants, might have contributed to the reduced locomotion in these animals. We did not measure stereotypies and, therefore, cannot ruled out (or confirm) a contribution of tDCS-induced stereotypies. It is noteworthy that a previous study in female (but not male) rats found comparable amounts of stereotypies for cocaine doses of 10, 20 and 40 mg/kg (Walker et al., 2001), while our effect was observed for the dose of 25 mg/kg only. Future studies will have to clarify how tDCS modulates cocaine-induced locomotor activity and/or stereotypies.
In the CPP paradigm, which aims to evaluate the motivational properties of cocaine, tDCS suppressed place preference conditioning for the lowest and highest dose of cocaine (5 and 25 mg/kg), but not 10 mg/kg. There is evidence that place preference conditioning by cocaine displays an inverted U-shaped dose-response function, such that very low or high doses of cocaine do not induce place preference (Hnasko et al., 2007). The optimal dose of cocaine for inducing place preference in mice seems to be situated between 7.5 and 15 mg/kg (Hnasko et al., 2007). Therefore, the present tDCS treatment seems to have attenuated place preference conditioning for suboptimal doses of cocaine (5 and 25 mg/kg), but was not sufficient to modify place preference conditioning induced by an optimal dose (10 mg/kg).
Regarding the underlying mechanisms, cocaine-induced behavior in the CPP paradigm is determined by opponent processes (“rewarding” versus “aversive” properties of the drug), which appear to have different neuronal substrates (Lammel et al., 2012). Thus, lower-dose conditioning (left limb of inverted U) is taken to reflect a rewarding effect of the drug, while upper-dose conditioning (right limb) is governed by increasing aversion. It is therefore tempting to speculate that tDCS may attenuate “reward” and/or increase “aversion” by (differentially) modifying their underlying neuronal systems. A potential beneficial effect of tDCS on addiction processes will have to be verified in other drug addiction-related paradigms such as the cocaine self-administration model.
Conclusions
Overall, our results indicate that repeated tDCS pretreatment produces long-lasting modifications in the molecular and behavioral sensitivity to cocaine, especially for a high dose (25 mg/kg). These findings suggest the intriguing possibility that tDCS pretreatment might attenuate the addiction liability of psychostimulants such as cocaine by attenuating the drugs’ molecular impact. This technique of neuromodulation, which is non-invasive, easy to use and affordable, might therefore be useful as an intervention to protect vulnerable individuals from getting addicted.
Supplementary Material
Acknowledgments
This work was supported in part by the University of Franche-Comté (VVW) and the city of Besançon (SP, VVW), and by the National Institutes of Health Grant DA011261 (HS). We would like to thank Marie-Ange Bolard for animal care, Romain Monier and Aurélie Salvadori for excellent technical assistance, and Dr Adriana Caballero for proofreading and editing the manuscript. The authors declare no biomedical financial interests or potential conflicts of interests.
Footnotes
Conflicts of interest: none
Authors contribution
VVW, HS, and EH were responsible for the study concept and design. SP, JB and PA contributed to the acquisition of animal data. SP, VVW and HS assisted with data analysis and interpretation of findings. SP, VVW and HS drafted the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y, Van Waes V. Transcranial direct current stimulation for memory enhancement: from clinical research to animal models. Front Syst Neurosci. 2014;8:159. doi: 10.3389/fnsys.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47:1–7. doi: 10.1016/j.jpsychires.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463:82–86. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Fregni F, Boggio PS, Priori A. Transcranial direct current stimulation for the treatment of major depressive disorder: a summary of preclinical, clinical and translational findings. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:9–16. doi: 10.1016/j.pnpbp.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM. Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul. 2014;7:130–132. doi: 10.1016/j.brs.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Cotterly L, Beverley JA, Yano M, Steiner H. Dysregulation of gene induction in corticostriatal circuits after repeated methylphenidate treatment in adolescent rats: differential effects on zif 268 and homer 1a. Eur J Neurosci. 2007;25:3617–3628. doi: 10.1111/j.1460-9568.2007.05570.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118:215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Riggs LM, Nieto SJ, Wright KN, Zamora NN, Cruz B, Zavala AR, Robison AJ, Mazei-Robison MS. Fluoxetine exposure during adolescence increases preference for cocaine in adulthood. Sci Rep. 2015;5:15009. doi: 10.1038/srep15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, de Almeida Correia Santos G, Fregni F, Nitsche MA, Miyuki Nakamura-Palacios E. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol. 2014;17:1793–1803. doi: 10.1017/S1461145714000984. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2014;85(Pt 3):948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, Oliveri M, Pascual-Leone A, Paulus W, Priori A, Walsh V. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1:326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinate. 2. Academic Press; 2001. [Google Scholar]
- Pedron S, Monnin J, Haffen E, Sechter D, Van Waes V. Repeated transcranial direct current stimulation prevents abnormal behaviors associated with abstinence from chronic nicotine consumption. Neuropsychopharmacology. 2014;39:981–988. doi: 10.1038/npp.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigonatti SP, Boggio PS, Myczkowski ML, Otta E, Fiquer JT, Ribeiro RB, Nitsche MA, Pascual-Leone A, Fregni F. Transcranial direct stimulation and fluoxetine for the treatment of depression. Eur Psychiatry. 2008;23:74–76. doi: 10.1016/j.eurpsy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Steiner H, Van Waes V. Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants. Prog Neurobiol. 2013;100:60–80. doi: 10.1016/j.pneurobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Beverley JA, Willuhn I, Steiner H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: effects on zif 268 and homer 1a. Eur J Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Marinelli M, Steiner H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur J Neurosci. 2010;32:435–447. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Vandrevala M, Beverley J, Steiner H. Selective serotonin re-uptake inhibitors potentiate gene blunting induced by repeated methylphenidate treatment: Zif268 versus Homer1a. Addict Biol. 2014;19:986–995. doi: 10.1111/adb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–662. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.