Abstract
[Purpose] Some patients with respiratory disease exhibit asymmetrical movement of the thorax. The purpose of this study was to investigate the relationship of thoracic configuration with changes in thoracic volume in 13 sedentary healthy men. [Subjects and Methods] In upright sitting, 84 reflective markers were placed on the anterior and posterior aspects of the trunk to record thoracic volume during quiet and volitional deep breathing. Using a three-dimensional motion analyzer, the difference in volume within the upper and lower hemithoraces was measured. For calculation of the thoracic volume six imaginary hexahedra were visualized for the upper and lower thorax using four reflective markers for each on the anterior and posterior aspects of the thorax. Each hexahedron was then divided into three imaginary triangular pyramids to calculate positional vectors. Finally, the volume for both the hexahedra and triangular pyramids was calculated. Four thoracic volumes were obtained. [Results] The findings showed that the left upper and right lower hemithorax yielded significantly larger thoracic volumes. [Conclusion] In conclusion the left upper and right lower hemithoraces were found to expand more than their corresponding sides. Understanding the characteristics of thoracic excursion during quiet and volitional deep breathing could be of value in assessment and instruction of breathing techniques to patients.
Keywords: Hemithorax, Thoracic volume, Three-dimensional motion analysis
INTRODUCTION
Patients often have a number of respiratory symptoms derived from a combination of pathophysiologic changes in the respiratory organs and possible changes in the mechanics of thoracic excursion that is often attributed to poor posture. Improvement in patient’s posture has often been found to alleviate some of the symptoms for patients with respiratory conditions1). A great number of the ventilatory muscles are also involved in postural control, especially those of the neck and trunk. Therefore, the position of the body may consequently affect respiratory function due to the effect of gravity. This change in posture may affect thoracic excursion and, subsequently, respiratory function, especially vital capacity and inspiratory reserve volume, which reflects on inspiratory capacity2). In fact, one such study verified that certain changes in the thoracic alignment produced a decrease in respiratory function and exercise tolerance3, 4).
The thoracic cage principally consists of the thoracic vertebrae, ribs and sternum5). If an incongruence, albeit a small one, occurs in this structure, its mechanics will be affected, resulting in a significant impact on the body. Therefore, it is clinically significant to be able to clarify the relationship between changes in thoracic configuration and respiratory function. Specifically, this may lead to an intervention for improvement in the patient’s posture.
We have previously reported on the effects of changes in chest volumes at specific locales in the thorax in the positions of upright sitting and stoop sitting6, 7). However, during assessment of patients with fatigue and dyspnea left-to-right asymmetry of respiratory muscle activity has also been frequently observed. This asymmetrical movement includes abnormal alignment of the thorax on the sagittal plane, changes in thoracic configuration in relation to the pelvis on the frontal plane and left-to-right discrepancy in chest expansion. There has been, to date, no study carried out on thoracic configuration in relation to the position of the pelvis on the frontal plane and left-to-right discrepancy in chest expansion. Therefore, the specific purpose of this study was to investigate the relationship between tidal volume and changes in volume of the upper and lower hemithoraces during two breathing patterns of quiet and volitional deep breathing.
SUBJECTS AND METHODS
The participants were 13 sedentary men with no history of smoking, respiratory or spinal condition. Sedentary men are defined as those who had partaken only in routine living activities. They had a mean age of 21.6 years (range=20 to 26; SD=1.5), mean body mass of 63.6 kg (range=48 to 76; SD=10.5), mean height of 170.0 cm (range=162 to 178; SD=0.1) and a body mass index of 21.9 kg/m2 (range=17.30 to 27.04; SD=3.0). As ethical considerations, the investigators explained to the participants the purpose and procedures of the study and obtained their written consent for their participation.
The participants were tested in an upright sitting position with a pelvic tilt of zero degree, feet flat on the floor and 90° flexion of the hip and knee joints. The pelvic angle was defined as an angle created by an intersecting imaginary line formed by infrared reflective markers attached to the skin over the anterior and posterior superior iliac spines together with an imaginary spatial horizontal line, which was determined by using a spirit level. A total of 84 infrared reflective markers each with a diameter of 9.5 mm were placed at specific points on the skin over the anterior and posterior aspects of the trunk. Their movement was used to record changes in thoracic volume. The precise positioning for both the anterior and posterior markers was determined from six midline markers placed vertically in line on six levels, these being the sternal notch, 3rd rib, xiphoid process, 8th rib, 10th rib and umbilicum, all of which are commonly used as a guide for palpation of chest movement. In relation to the midline markers of the trunk 78 markers, in order of their proximity to them, were placed medially, centrally and laterally on the anterior and posterior aspects of the trunk. Specifically, three markers were placed on either side of a midline marker, totaling seven markers in all. This procedure was repeated on the posterior aspect of the trunk at the corresponding six levels. The distance between each marker was based on the participant’s physique and set at 15% of the distance between the left and right acromion processes.
Using a three-dimensional motion analyzer Vicon MX (Vicon, Inc.), the difference in volume within the upper and lower hemithoraces was measured in the aforementioned testing position during quiet breathing and volitional deep breathing, each with five or more consecutive breaths. The oral instructions by the investigators were “Just relax and breathe as normal” for quiet breathing, and “Take a natural deep breath” for volitional deep breathing. An average value of three consistent breaths was taken for the calculation. In accordance with previous studies8,9,10,11,12,13,14,15), the changes in the thoracic volume were calculated from the amount of change in the movement of the infrared reflective markers attached to the body surface. Seven infrared cameras were used, and the data was stored on a personal computer with a sampling frequency of 100 Hz.
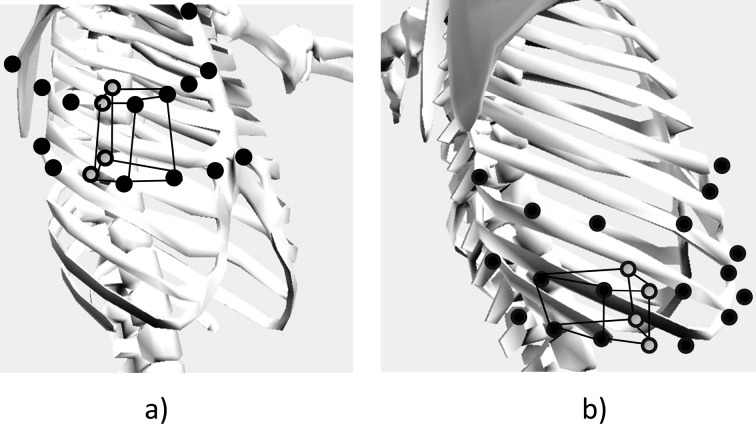
The thoracic volume was calculated in accordance with the method by Ferrigna et al. and Nakabo et al.10), Specifically, six imaginary hexahedra were visualized for the upper thorax with three for the right and three for the left using four markers for each on the anterior and posterior aspects at the levels of the sternal notch and third rib (Fig. 1a). Similarly, this was repeated for the lower thorax at the levels of the xiphoid process and tenth rib (Fig. 1b). Further, each of these imaginary hexahedra was, then, divided into three imaginary triangular pyramids to calculate positional vectors using the data obtained from the position of each marker in accordance with the Ferrigno et al.’s method8). Finally, the volume for both the hexahedra and triangular pyramids was calculated, culminating in any change of thoracic volume on a whole.
Fig. 1.
Illustration of imaginary hexahedra. a) One of six hexahedra indicating upper thorax demonstrated within anterior markers and midpoints. b) One of six hexahedra indicating lower thorax demonstrated within posterior markers and midpoints. Dots indicate markers from which the hexahedra were constructed. Circles indicate imaginary midpoints.
Four thoracic volumes were measured: a) the upper right and left hemithoraces’ volumes emanating from a midpoint on an imaginary line connecting from the sternal notch to the third rib; and b) the lower right and left hemithoraces’ volumes emanating from a midpoint on an imaginary line connecting from the xiphoid process to the tenth rib. The Vicon Body Builder and MATLAB (MathWorks, Inc., Natick, USA) were used for the calculation of the thoracic volumes.
For statistical analysis, the Wilcoxon rank-sum test was employed for comparison of the changes in volume of the bilateral hemithoraces. The level of significance was set at p<0.05, and, for the data analysis, the Statistics Package for Social Sciences version 23.0 (SPSS Japan Inc., Tokyo, Japan) for Windows was employed.
RESULTS
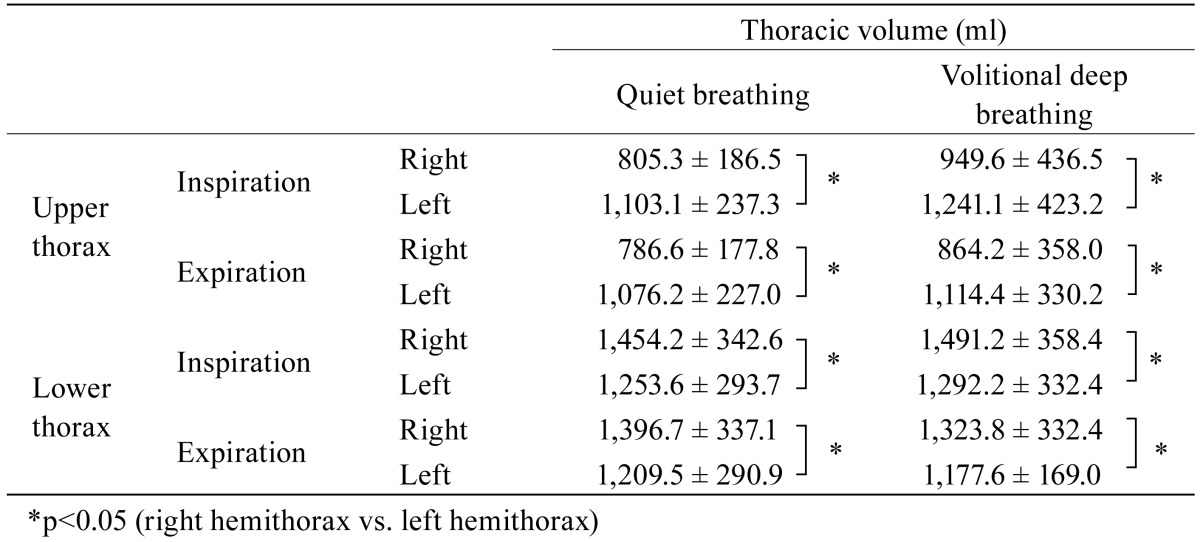
For quiet breathing the mean (± standard deviation) tidal volume was 632.1 (± 646.0) ml, and that for volitional deep breathing 1477.0 (± 706.0) ml. During quiet and volitional deep breathing the left upper hemithorax yielded a significantly larger change in thoracic volume compared to the right upper hemithorax (p=0.003 and p=0.002, respectively) (Table 1). For the lower thorax the right hemithorax yielded a significantly larger change in thoracic volume compared to the left hemithorax for both breathing patterns (p=0.002 and p=0.001, respectively) (Table 2 ). There was, however, no such correlation between the corresponding sides for the lower thoracic volume.
Table 1. Changes in the mean ± standard deviation volume of the right and left hemithoraces during quiet and volitional deep breathing (N=13).
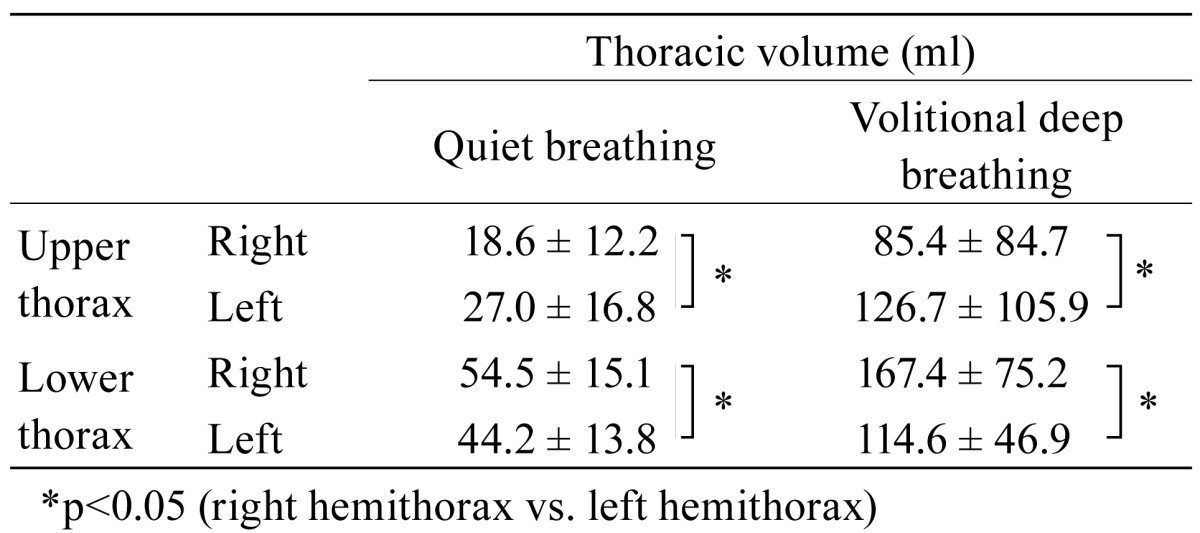
Table 2. Mean ± standard deviation thoracic volume during quiet and volitional deep breathing (N=13).
DISCUSSION
Changes in thoracic configuration were the same at maximum inspiratory and expiratory levels of quiet and volitional deep breathing (Table 2). The asymmetrical configuration could be a result of ventilatory dysfunction or spinal malalignment. However, ventilatory dysfunction and spinal malalignment were not the subject of this study. Therefore, future investigation and verification of these factors would be of interest to follow up.
For the thorax to exhibit such a configuration, the ribs of the left upper thorax may rotate posteriorly and those on the right anteriorly. Similarly, the ribs of the right lower thorax may rotate posteriorly and those of the left anteriorly. Furthermore, left-to-right differences in the lower hemithoraces’ volumes may exert a negative influence on diaphragmatic function, leading to reduced ventilation. Consequently, this may also lead to reduced thoracic mobility, affecting postural control with a shift in the center of gravity.
Thus, in clinical practice, attention should be paid to asymmetrical configuration of the thorax on a frontal plane when carrying out breathing and postural exercises, which may have a positive effect on thoracic mobility and ventilatory function.
This asymmetry in thoracic configuration can lead to incongruence within the costovertebral joints, resulting in a reduction of thoracic mobility. This may, in turn, increase breathing effort and respiratory rate, leading to dyspnea. Therefore, assessment of thoracic configuration can be a means of predicting whether the patient’s respiratory function would be compromised.
In conclusion, in upright sitting, the left upper hemithorax appears to expand more than the right, while the right lower hemithorax expands more than the left. It is, therefore, of importance to maintain mobility of the upper thorax so as to allow for a more efficient breathing pattern. The understanding of the characteristics of thoracic excursion during quiet and volitional deep breathing could be of value in assessment and instruction of breathing techniques to patients.
Limitations of this study were that our study population might have been too small, and the right-left difference in thoracic volume evidently did not have any effect on the inspiratory and expiratory air volumes.
Acknowledgments
Sincere thanks go to Dr. Shimpachiro Ogiwara, former Professor of Physical Therapy, University of Kanazawa, and Mrs. Sandra M. Ogiwara, CSP (UK), HT (UK), CPA (C) BScPT (C), for editorial assistance.
REFERENCES
- 1.Kakizaki F. (ed.): Methods for reconstruction of thoracic movement system. Tokyo: Miwa-Shoten, 2016, pp 202–217 (in Japanese). [Google Scholar]
- 2.Sasaki K, Kamiya A, Maruo T, et al. : The effect of reclining angles of wheelchair on spirometric indices and respiratory muscle activities. Rigakuryoho Kagaku, 2012, 27: 7–10(in Japanese). [Google Scholar]
- 3.Kusakari Y, Sasaki M: Influence of kyphotic posture on cardiopulmonary response and exercise tolerance. Rigakuryoho Kagaku, 2003, 18: 187–191(in Japanese). [Google Scholar]
- 4.Culham EG, Jimenez HA, King CE: Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine, 1994, 19: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 5.Schaffler A, Schmidt S: Biologie, Anatomie, Physiologie fur die pflegeberufe 2. (translated by Miki A and Inoue T). Tokyo: Nishimura, 1998, p 105. (in Japanese). [Google Scholar]
- 6.Shōbo A, Kakizaki F: Relationship between chest expansion and the change in chest volume. Rigakuryoho Kagaku, 2014, 29: 881–884(in Japanese). [Google Scholar]
- 7.Shobo A, Kakizaki F: Effects of two sitting positions on chest volume. Rigakuryoho Kagaku, 2015, 30: 499–502(in Japanese). [Google Scholar]
- 8.Ferrigno G, Carnevali P: Principal component analysis of chest wall movement in selected pathologies. Med Biol Eng Comput, 1998, 36: 445–451. [DOI] [PubMed] [Google Scholar]
- 9.Aliverti A, Ghidoli G, Dellacà RL, et al. : Chest wall kinematic determinants of diaphragm length by optoelectronic plethysmography and ultrasonography. J Appl Physiol 1985, 2003, 94: 621–630. [DOI] [PubMed] [Google Scholar]
- 10.Nakabo T, Yamamoto S: Influence of kyphosis on chest wall motion: comparison of slump sitting and straight sitting. Rigakuryoho Kagaku, 2009, 24: 697–701(in Japanese). [Google Scholar]
- 11.Wang HK, Lu TW, Liing RJ, et al. : Relationship between chest wall motion and diaphragmatic excursion in healthy adults in supine position. J Formos Med Assoc, 2009, 108: 577–586. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Takemasa S, Shinohara H: A study on the morphological changes with aging: discussion on the anthropometric measurements as indices for senility. Kobe Univ Repos, 1987, 3: 39–44(in Japanese). [Google Scholar]
- 13.Konno K, Mead J: Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol, 1967, 22: 407–422. [DOI] [PubMed] [Google Scholar]
- 14.Liu M: Kinematics of breathing. Sogo Rehabil, 1990, 18: 377–384. [Google Scholar]
- 15.Tabira K, Sekikawa N, Kozu R: Influence of chest expansion on pulmonary function and dyspnea in patients with chronic respiratory failure. Phys Ther Rev, 1998, 25: 376–380(in Japanese). [Google Scholar]