Abstract
Objective We have recently discovered new gastric lesions with black spots. There have been no reports about black spots and their clinicopathological features. We therefore report the clinicopathological features of black spots and assess their causes and mechanisms.
Methods Sixty-four patients with black spots among 26,620 Japanese patients that underwent endoscopy between May 2012 and October 2014 were enrolled. Endoscopic findings of black spots were defined as black pigmentations in the gastric mucosa by conventional endoscopy. We investigated the clinicopathological characteristics, including gender, age, underlying diseases and medications, endoscopic and pathologic findings of patients with black spots.
Results The prevalence of patients with black spots was 0.24%. Of sixty-four cases, 44 (68.8%) were taking proton pump inhibitors (PPIs). Eight (12.5%) were taking corticosteroids. There were 10 cases (15.6%) with decreased renal function. All black spots were identified only in the fundic gland region. Forty-one (64.1%) patients had multiple (more than ten) black spots. There were two different types: black spots on the flat mucosa and black spots on fundic gland polyps. Pathologically, parietal cell protrusions, fundic gland cysts and brownish pigmentation in fundic gland cysts were seen in 26 (76.5%), 23 (67.6%) and 6 (17.6%) patients, respectively.
Conclusion We herein describe gastric black spots as a new gastric mucosal finding that arises only in the fundic gland region. The black spots are pathologically brownish pigmentations in fundic gland cysts. Adverse events of PPIs and parietal cell protrusion caused by PPI use are strongly considered to be one of the etiologies of black spots.
Keywords: black spot, drug-related adverse reaction, fundic gland polyposis, parietal cell protrusion, proton pump inhibitor
Introduction
Proton pump inhibitor (PPI) use has been suggested to cause various endoscopic gastric mucosal changes, such as fundic gland polyps (FGPs) (1-5). Histologically, PPI causes parietal cell protrusion (PCP) and hypertrophy (6-9). PCP is recognized as prominent intraluminal protrusions of the parietal cell cytoplasma resulting in a serrated glandular lumen (6-10). As systemic diseases causing gastric mucosal changes, liver cirrhosis and renal failure lead to portal hypertensive gastropathy, gastric antral vascular ectasia and gastric erosions (11-18).
We recently discovered new lesions that we call black spots (BSs). This new gastric mucosal finding is distinct from previously identified black lesions such as melanoma and pseudomelanosis (19-23). To the best of our knowledge, BSs have not been reported to date.
We therefore reported the clinicopathological features of BS in 64 cases and assessed its causes and mechanisms.
Materials and Methods
Selection of cases and ethical concerns
We examined 26,620 Japanese patients that underwent endoscopy for abdominal symptoms or as a part of gastric cancer surveillance at three hospitals between May 1, 2012 and October 31, 2014. Two of these hospitals were university hospitals in central cities and the other was in a rural area. We searched for cases with gastric BSs during this period. Sixty-four patients were endoscopically diagnosed with BSs and were included in this study. BSs were defined as black pigmentations in the gastric mucosa by conventional endoscopy (Fig. 1-3). The study was approved by the medical ethical committees of the patient-including hospitals on October 31, 2014.
Figure 1.
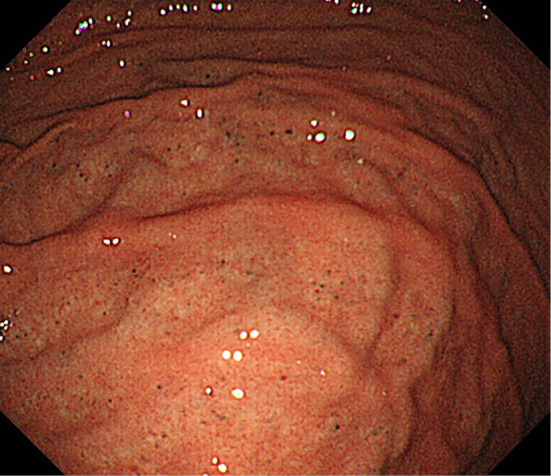
(Case 1: Proton pump inhibitor and calcium channel blocker). Endoscopic features of black spots (BSs). BSs are seen on the flat mucosa.
Figure 2.
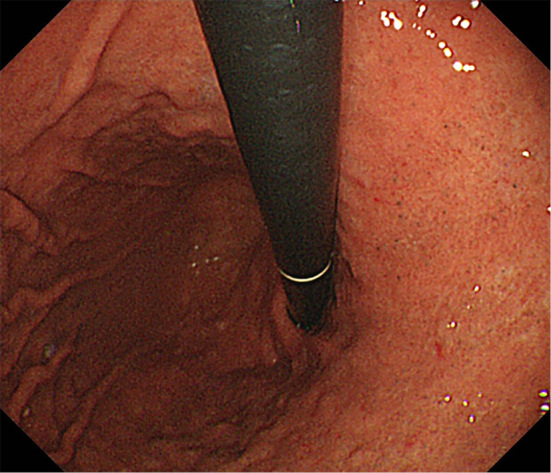
(Case 2: Proton pump inhibitor). Endoscopic features of black spots (BSs). BSs are seen on the flat mucosa.
Figure 3.
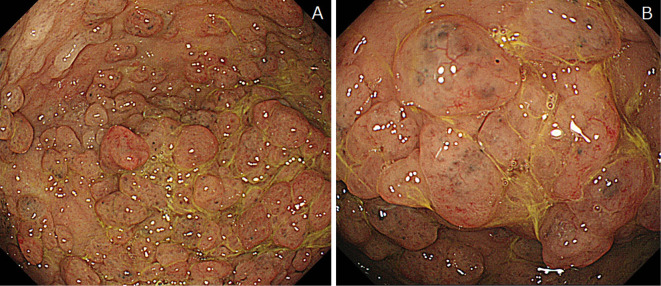
(Case 3: Familial adenomatous polyposis and proton pump inhibitor). Endoscopic features of black spots (BSs). A: BSs are seen on fundic gland polyps. B: The same lesion seen at higher magnification.
Evaluation of clinical and endoscopic findings
The following clinical characteristics were collected from the medical records: gender, age, underlying diseases, medicines and endoscopic findings. In this study, decreased renal function was defined as an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2 or nephrotic syndrome (24). Regarding endoscopic findings, the following were investigated: the number of BSs, the location of BSs, the presence of atrophy and multiple white and flat lesions (MWFLs), and whether BSs were present on the flat mucosa or FGPs. Gastric mucosal atrophy was evaluated according to the endoscopic atrophy border scale described by Kimura et al. (25). “Multiple” BSs were defined as more than ten BSs. MWFL has been recognized to be induced by PPI usage in the gastric fundus and foveolar epithelial hyperplasia (26).
Evaluation of histologic findings
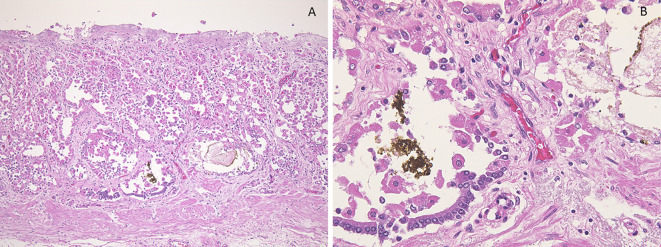
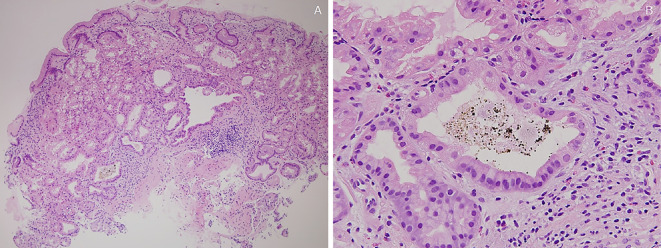
One autopsy sample was obtained from gastric BS lesions and 33 patients underwent biopsies from gastric BS lesions. Each specimen was embedded in paraffin wax and cut into 4 μm thick sections. The sections were stained with Hematoxylin and Eosin (H&E) staining. The presence or absence of PCPs (defined as prominent intraluminal protrusions of the parietal cell cytoplasm resulting in a serrated glandular lumen) (Fig. 4A, 5A and 6A), fundic gland cysts (FGCs) (defined as gland dilation and cystic changes with tinning of the aligning parietal and chief cells) (Fig. 4, 5A and B, 6) and brownish pigmentation in FGC (Fig. 4, 5A and B, 6), were determined in H&E-stained sections (10). The sections were stained with Prussian blue, copper, and Fontana-Masson's stains and immunohistochemically stained for MUC1, MUC2, MUC5AC and MUC6. In addition, the sections were observed using the polarizing observation method.
Figure 4.
(Case 4). Histologic findings of black spot (BS) (pathological anatomy). A: Parietal cell protrusions, fundic gland cysts (FGCs) and brownish pigmentation in the FGC [Hematoxylin and Eosin (H&E) staining, 40×]. B: Brownish pigmentation in the FGC (H&E staining, 400×).
Figure 5.
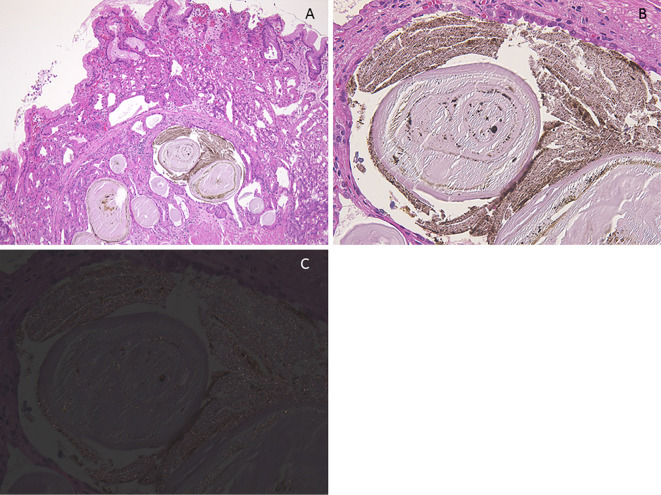
(Case 5). Histologic findings of black spot (BS). A: Parietal cell protrusions, fundic gland cysts (FGCs) and brownish pigmentation in the FGCs [Hematoxylin and Eosin (H&E) staining, 40×]. B: Amorphous eosinophilic contents and brownish pigmentation in the FGCs (H&E staining, 400×). C: Brownish pigmentation includes tiny birefringent crystals (polarizing observation method, 400×).
Figure 6.
(Case 6). Histologic findings of black spot (BS). A: Parietal cell protrusions, fundic gland cysts (FGCs) and brownish pigmentation in the FGC [Hematoxylin and Eosin (H&E) staining, 40×]. B: Brownish pigmentation in the FGC (H&E staining, 400×).
Results
Clinical presentation
Sixty-four patients were included in this study. The prevalence of patients with BSs was 0.24%. Demographic details and the data of underlying diseases are summarized in Table 1. There were 10 patients (15.6%) with decreased renal function.
Table 1.
Demographic Details and the Data of Underlying Diseases in 64 Patients with Black Spots.
| Age, y | |
| Mean | 71.8 |
| Minimum | 38 |
| Maximum | 92 |
| Sex, no. (%) | |
| Male | 39 (60.9%) |
| Female | 25 (39.1%) |
| Comorbidities, no. (%) | |
| Hypertension | 24 (37.5%) |
| Hyperlipidemia | 12 (18.8%) |
| Diabetic mellitus | 12 (18.8%) |
| Decreased renal function | 10 (15.6%) |
| Familial adenomatous polyposis | 2 (3.1%) |
Table 2 shows the data of medications. Forty-four patients (68.8%) were taking PPI. Furthermore, the number of patients that were taking calcium channel blockers and corticosteroids were 26 (40.6%) and 8 (12.5%) patients, respectively.
Table 2.
Data of Medicines in 64 Patients with Black Spots.
| Medicine, no. (%) | |
| Proton pump inhibitor | 44 (68.8%) |
| Histamine H2-receptor antagonists | 2 (3.1%) |
| Calcium channel blocker | 26 (40.6%) |
| ARB/ACEI | 18 (28.1%) |
| Loop diuretic | 9 (14.1%) |
| Statin | 13 (20.3%) |
| Aspirin | 9 (14.1%) |
| Corticosteroid | 8 (12.5%) |
| Magnesium oxide | 11 (17.2%) |
| Sennoside | 5 (7.8%) |
| Calcium polystyrene sulfonate | 2 (3.1%) |
ARB: angiotensin II receptor blocker, ACEI: angiotensin converting enzyme inhibitor, Loop diuretic: sodium potassium chloride symporter inhibitor
Endoscopic features
The endoscopic lesion characteristics are summarized in Table 3. All BSs were identified only in the fundic gland region. There were no cases with BSs in the pyloric gland region. Forty-one (64.1%) cases had multiple BSs. We observed two different types: BS on the flat mucosa (Fig. 1 and 2) and BS on FGPs (Fig. 3). BSs on FGPs were identified in 11 patients (17.2%). MWFLs accompanied BSs in 31 patients (48%).
Table 3.
Endoscopic Features in 64 Patients with Black Spots.
| Location of BSs, no. (%) | |
| Antrum | 0 (0%) |
| Corpus | 63 (98.4%) |
| Fundus | 42 (65.6%) |
| Background mucosa of BSs, no. (%) | |
| Flat | 54 (84.3%) |
| Fundic gland polyp | 11 (17.2%) |
| Multiple, no. (%) | 41 (64.1%) |
| Atrophy, no. (%) | 45 (70.3%) |
| Multiple white flat lesion accompanied by BSs, no. (%) | 31 (48.4%) |
BS: black spot
Pathologic features
PCPs, FGCs and eosinophilic materials and brownish pigmentation in FGCs are shown in Fig. 4 (an autopsy case) 5 and 6 (biopsy cases). Brownish pigmentation included tiny birefringent crystals in the polarizing observation method (Fig. 5C). Brownish pigmentations and eosinophilic materials were negative for Prussian blue, copper, and Fontana-Masson's stains. Eosinophilic materials were slightly positive for MUC5AC and MUC6, but negative for MUC1 and MUC2. On the other hand, brownish pigmentation was negative for all MUC stains.
Histopathology was analyzed in 34 of 64 patients. Table 4 reveals that the number of patients with PCPs, FGCs and brownish pigmentation in FGCs were 26 (76.5%), 23 (67.6%) and 6 (17.6%) patients, respectively.
Table 4.
Pathologic Features of Black Spots (n=34).
| Parietal cell protrusions, no. (%) | 26 (76.5%) |
| Fundic gland cysts, no. (%) | 23 (67.6%) |
| Brownish pigmentation in fundic gland cyst, no. (%) | 6 (17.6%) |
Discussion
In this study, we reported four important clinicopathologic observations. First, we discovered gastric BSs, which are new gastric mucosal lesions. Second, BSs arose only in the fundic gland region. Third, BSs were observed as pathologically brownish pigmentation in FGCs. Lastly, the effect of PPIs and PCPs was thought to be one of the mechanisms.
To the best of our knowledge, this is the first report describing gastric BS as a new gastric mucosal lesion. BSs were located only in the fundic gland region, where there are many parietal cells. There were no cases with BSs in the pyloric gland region. As other clinical features, some BSs were observed on FGPs in 11 (17.2%) cases. In addition, the ratio of the cases with a decreased renal function or the use of corticosteroids or calcium channel blockers tended to be high.
Pathologically, BSs were composed of a brownish substance and eosinophilic materials in FGCs, which were thought to result from the accumulation of secretion from lining cells of FGCs. Moreover, PCPs and FGCs were seen in many cases. Pseudomelanosis, which appears similar to BS when observed through endoscopy, is a potential differential diagnosis, however, it histologically presents as a dark brown substance inside the macrophage lysosomes in the lamina propria (22,23). Therefore, the pathological findings of BSs differed from that of pseudomelanosis.
The effect of PPIs and PCPs was thought to be one of the mechanisms. The ratio of PPI usage was 68.8% in this study, which tended to be much higher than the general ratio, 30.8% in Japan (27). Of the 17 cases whose PPI treatment length were known to us, 15 (88.2%) had treatment for over 1 year and long-term PPI therapy tended to cause BSs. We could not find any difference in the number or location of BSs according to the type of PPI. Active secretion of hydrochloric acid from the secretory canaliculi is inhibited by PPI usage and the acid accumulates in parietal cells, which results in PCPs (6). PCPs in the shallow mucosa could cause blockage of the isthmus, increasing resistance to outflow from the gland, which leads to FGCs formation in the deeper zone of the mucosa (28). Then, brownish substances are thought to be stored in FGCs, which can be seen as BSs endoscopically. Moreover, PCP development has been known to be related to serum gastrin elevation (6). Renal failure and the exogenous administration of corticosteroids have also been shown to increase serum gastrin (29,30). Therefore, renal failure and corticosteroid administration can also be considered to be the causes of PCPs and BSs, which is in agreement with our clinical results. On the other hand, almost 20% of BSs were not linked to parietal cell protrusions. The following reason is plausible: the formation of FGCs, which can store pigmentation, could lead to BSs without the involvement of PCPs. FGPs and Helicobacter pylori eradication are possible causes of the formation of FGCs without PCPs (6).
However, there are several limitations associated with this study. We could not conduct a comparative study between cases with BSs and control subjects. On the one hand, we actively searched and recorded medicines taken by those with BSs. On the other hand, we did not actively record medicines taken by patients without BSs, which is a bias. Therefore, we cannot conclude the exact relationship between BSs and PPI usage. In addition, we did not investigate the relevance of H. pylori infection or eradication on BSs formation in all cases. H. pylori eradication especially is known to enhance the development of FGCs; therefore, it can be one of the reasons that BSs and FGCs were observed in cases without PPI usage (6). The content of BSs has not been clarified. It is necessary to analyze the content itself via mass spectrometry or electron microscopy.
In conclusion, this is the first report describing gastric BS, a new gastric lesion. BSs arose only in the fundic gland region. BSs were observed as pathologically brownish pigmentation in FGCs. The effect of PPIs and PCPs were thought to be one of the mechanisms. Therefore, these new lesions, BSs, might also be one of the adverse events induced by long-term PPI use.
The authors state that they have no Conflict of Interest (COI).
References
- 1. el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol 92: 1858-1860, 1997. [PubMed] [Google Scholar]
- 2. Jalving M, Koornstra JJ, Wesseling J, Boezen HM, DE Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther 24: 1341-1348, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Hongo M, Fujimoto K. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol 45: 618-624, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Graham JR. Gastric polyposis: onset during long-term therapy with omeprazole. Med J Aust 157: 287-288, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Choudhry U, Boyce HW Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol 110: 615-621, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Cats A, Schenk BE, Bloemena E, et al. . Parietal cell protrusions and fundic gland cysts during omeprazole maintenance treatment. Hum Pathol 31: 684-690, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Stolte M, Bethke B, Rühl G, Ritter M. Omeprazole-induced pseudohypertrophy of gastric parietal cells. Z Gastroenterol 30: 134-138, 1992. [PubMed] [Google Scholar]
- 8. Driman DK, Wright C, Tougas G, Riddell RH. Omeprazole produces parietal cell hypertrophy and hyperplasia in humans. Dig Dis Sci 41: 2039-2047, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol 38: 527-536, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Krishnamurthy S, Dayal Y. Parietal cell protrusions in gastric ulcer disease. Hum Pathol 28: 1126-1130, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Benzo J, Matos M, Milanes C, Arminio A, Stempel CA. Endoscopic findings in the upper digestive tract in patients with terminal chronic kidney failure. G E N 48: 34-38, 1994. (in Spanish, Abstract in English). [PubMed] [Google Scholar]
- 12. Sotoudehmanesh R, Ali Asgari A, Ansari R, Nouraie M. Endoscopic findings in end-stage renal disease. Endoscopy 35: 502-505, 2003. [DOI] [PubMed] [Google Scholar]
- 13. McCormack TT, Sims J, Eyre-Brook I, et al. . Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut 26: 1226-1232, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toyonaga A, Iwao T, Shimotsuura Y, Tanikawa K. Endoscopic, histologic and haemodynamic studies on portal hypertensive gastric mucosa. J Gastroenterol Hepatol 4 (Suppl 1): 132-135, 1989. [PubMed] [Google Scholar]
- 15. D'Amico G, Montalbano L, Traina M, et al. . Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology 99: 1558-1564, 1990. [DOI] [PubMed] [Google Scholar]
- 16. Toyonaga A, Iwao T. Portal-hypertensive gastropathy. J Gastroenterol Hepatol 13: 865-877, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology 87: 1165-1170, 1984. [PubMed] [Google Scholar]
- 18. Ward EM, Raimondo M, Rosser BG, Wallace MB, Dickson RD. Prevalence and natural history of gastric antral vascular ectasia in patients undergoing orthotopic liver transplantation. J Clin Gastroenterol 38: 898-900, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Lagoudianakis EE, Genetzakis M, Tsekouras DK, et al. . Primary gastric melanoma: a case report. World J Gastroenterol 12: 4425-4427, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamura K, Kondo K, Moritani S. Primary malignant melanoma of the stomach: report of a case. Surg Today 42: 195-199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jelincic Z, Jakic-Razumovic J, Petrovic I, Cavcic I, Unusic J, Trotic R. Primary malignant melanoma of the stomach. Tumori 91: 201-203, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Antaki F, Irwin BC, Levi E. Rare occurrence of gastric pseudomelanosis. Gastrointest Endosc 69: 599; Author reply 599-600, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Weinstock LB, Katzman D, Wang HL. Pseudomelanosis of stomach, duodenum, and jejunum. Gastrointest Endosc 58: 578, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Matsuo S, Imai E, Horio M, et al. . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1: 87-97, 1969. [Google Scholar]
- 26. Haruma K, Kato M, Inoue K, Murakami K, Kamada T In: Ien no Kyoto Bunrui. Nihon Medical Center, 2014: 91-93 (in Japanese). [Google Scholar]
- 27. Miyamoto M, Haruma K, Okamoto T, Higashi Y, Hidaka T, Manabe N. Continuous proton pump inhibitor treatment decreases upper gastrointestinal bleeding and related death in rural area in Japan. J Gastroenterol Hepatol 27: 372-377, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Synnerstad I, Holm L. Omeprazole induces high intraglandular pressure in the rat gastric mucosa. Gastroenterology 112: 1221-1230, 1997. [DOI] [PubMed] [Google Scholar]
- 29. Kes P. Serum gastrin concentration in chronic renal failure. Acta Med Croatica 46: 47-58, 1992. [PubMed] [Google Scholar]
- 30. Okahata H, Nishi Y, Muraki K, et al. . In vitro study of antral gastrin biosynthesis in response to weaning and corticosteron acetate in rats. Acta Endocrinol (Copenh) 111: 539-545, 1986. [DOI] [PubMed] [Google Scholar]