Phytohormones are responsible for integrating many aspects of plant growth and development. They modulate how fast and in what direction an organ grows. And in many cases, they determine the point at which it will die. Hormones are the signals that integrate internal developmental and external environmental inputs and translate them into appropriate responses. Plants have many ways in which to modulate hormonal responses. Regulation can occur at the level of synthesis, transport, uptake, and turnover of the hormone. Regulation can also occur at the level of perception or signal transduction. Hormone sensitivity can, in turn, be regulated both spatially and temporally. For example, during organ abscission adjacent cells respond differentially to hormonal signals. In contrast, fruit ripening involves changes in sensitivity of the organ over time. Here, the term sensitivity refers to the response of a tissue or organ to a hormone. A change in sensitivity indicates that the concentration of hormone necessary to initiate a response is altered. For reasons described here, tomato (Lycopersicon esculentum) is an ideal system to study developmentally controlled changes in hormone responsiveness.
Ethylene is a small, readily diffusible hormone that has an important role integrating developmental events with external stimuli. It is a critical component of such diverse developmental processes as seed germination, fruit ripening, abscission, and senescence (Abeles et al., 1992). It is also an important stress hormone. Adverse biotic or abiotic stimuli usually lead to ethylene synthesis. This ethylene, in turn, slows down plant growth until the stress is removed. At the level of gene expression, ethylene induces transcription of many genes in response to a multitude of environmental and developmental stimuli.
Why do we study a signal transduction pathway in any organism but Arabidopsis? Let me count the ways. Perhaps the most obvious reason to study tomato, as opposed to Arabidopsis, is to exploit the anatomical differences. The role of ethylene in promoting ripening in fleshy fruits is well established. But other processes are ideally suited to tomato as well. For example, the anatomically distinct pedicel abscission zone is exquisitely sensitive to ethylene. Also, flower and, to a lesser extent, leaf senescence as well as adventitious root formation are all dependent upon ethylene signaling. But at a more fundamental level, we must assess the robustness of models developed in plants such as Arabidopsis. Indeed, with the critical role of ethylene in mediating environmental responses, we would not expect that an annual rosette plant would behave like a perennial vine. In the end, it is only by elaborating the differences between species that we come to understand the unifying principles.
In this article the focus is on ethylene signal transduction in tomato. Although multiple components of the pathway have been identified, we know the most about the receptors. Thus, we emphasize the earliest step in ethylene signal transduction. There appear to be significant differences in the way ethylene signaling is regulated in Arabidopsis and tomato. But the building blocks of ethylene signal transduction are very similar between the species. Thus, we exploit Arabidopsis genetics to identify the genes and then address the way they fit together in tomato.
A critical breakthrough in understanding ethylene signal transduction was the recognition that germination of seeds in the presence of ethylene could be used in large-scale screens for insensitive mutants (Bleecker et al., 1988). Seedlings of many dicotyledonous species germinated in the dark grow tall and spindly. In the presence of ethylene they undergo a triple response; relative to air-grown controls, hypocotyls and roots are shortened and thickened and the apical tip exhibits an exaggerated hook. Ethylene insensitive mutants are significantly taller than wild type and their elongation is directly proportional to the loss of ethylene response. Using this assay, several groups have extensively screened for mutants that define many of the elements comprising the ethylene signaling pathway (Bleecker et al., 1988; Guzman and Ecker, 1990).
Epistatic analysis has permitted researchers to place the Arabidopsis genetic elements in an order that provides a framework for experimentation (for review, see Wang et al., 2002). Briefly, the signaling pathway begins with a family of five receptors. Downstream from the receptors is the Raf-like protein kinase, CTR1. Genetic and biochemical evidence indicates that CTR1 interacts with ETR1 and is a negative regulator, as loss-of-function mutants exhibit constitutive activation of ethylene signaling. Since CTR1 is homologous to an MAPKKK, there may be roles in ethylene signaling for additional proteins homologous to MAPKK and MAP kinases. There is some evidence supporting roles for MAP kinases in ethylene signaling (Ouaked et al., 2003). Following this MAP kinase cascade is EIN2. It has homology to Nramp metal transporters. Loss-of-function mutants are completely ethylene insensitive. Its mode of action in ethylene signaling has yet to be determined. However, there is no doubt that its presence is absolute for ethylene signaling. At the end of the signaling pathway are the transcription factors EIN3 and ERF1. EIN3 binds to the promoter of ERF1. Loss-of-function mutations in EIN3 are partially ethylene insensitive, very likely because the gene is part of a family of at least three members. Conversely, EIN3 overproduction leads to constitutive ethylene responses. ERF1 is a member of a large family of transcription factors. Overexpression of ERF1 in transgenic plants recapitulates a partial constitutive ethylene response, indicating that ERF1 is a positive transcription factor, but there must be one or more additional transcription factors involved in the overall ethylene response (Berrocal-Lobo et al., 2002).
ARABIDOPSIS ETHYLENE RECEPTORS
Hormone signaling must necessarily start with the receptor and intuitively, it is logical that a receptor would be a key point of regulation. The ethylene receptor ETR1 was the first protein to be unambiguously identified as a phytohormone receptor. It was also the first protein with homology to His kinases to be identified in a higher eukaryote (Chang et al., 1993). ETR1 is homologous to the prokaryotic family of signal transducers known as two-component regulators. Mutant alleles of ETR1 confer dominant ethylene insensitivity. It functions as a dimer and exhibits copper-mediated high affinity ethylene binding (for review, see Bleecker and Kende, 2000). ETR1 is a member of a family of five proteins (ETR1, ETR2, EIN4, ERS1, and ERS2). These receptors can be structurally separated into three domains briefly summarized as follows:
The sensor domain contains three hydrophobic, putative transmembrane stretches. Ethylene binding occurs within this amino terminal hydrophobic region and all of the known ETR1 mutations are located within this portion of the protein. Three of the receptors, EIN4, ETR2 and ERS2 are predicted to have a fourth membrane-spanning domain. The amino terminal domain mediates dimerization and copper binding. The GAF domain, which is conserved among a range of diverse proteins, lies immediately C-terminal to the ethylene binding domain. Its function in ethylene signaling is unknown.
The kinase domain has extensive sequence homology to His kinases (HK). There are five subdomains that define the catalytic core of His kinases. While ETR1 and ERS1 contain all of these subdomains, the other three receptors lack one or more of them. Notably, ETR2 and ERS2 lack the His that is autophosphorylated. This His is not essential for the dominant ethylene insensitivity conferred by etr1-1 (Gamble et al., 2002). ETR1 has in vitro HK activity (Gamble et al., 1998) but is the only ethylene receptor with demonstrated HK activity. An ETR1 that lacks HK activity can complement ethylene hypersensitive loss-of-function mutants (Wang et al., 2003). We have shown that the other four Arabidopsis receptors have Ser/Thr kinase (STK) activity (P. Moussatche and H. Klee, unpublished data). This phosphorylation is consistent with a report of STK activity in a tobacco ethylene receptor (Xie et al., 2003) and the phosphorylation activity of phytochrome, a protein with homology to the ETR1 kinase domain (Yeh and Lagarias, 1998). STK activity explains the lack of conservation of the His kinase catalytic domains. The ethylene receptor family thus presents a snapshot into an active process of protein evolution. Linkage of any protein kinase activity to ethylene signal transduction has yet to be demonstrated.
The receiver domain has sequence identity to the output portion of bacterial two-component systems and contains an Asp that is active in phosphorelay in bacterial proteins. As in bacteria, some members of the plant ethylene receptor family are missing the receiver domain; ERS1 and ERS2 lack it while the other three contain it. That some of these proteins maintain the receiver domain with a high degree of conservation while others completely lack it suggests an important but undetermined function for this domain.
Based on structural and DNA sequence comparisons, the receptors have been classified as Subfamily I or Subfamily II. The Subfamily I receptors, ETR1 and ERS1, have the highest conservation of the His kinase elements. Overall sequence comparisons, including intron positioning, support this classification. Despite these structural and functional differences, most of the genetic evidence is consistent with redundant receptor function.
HOW DO THE RECEPTORS WORK?
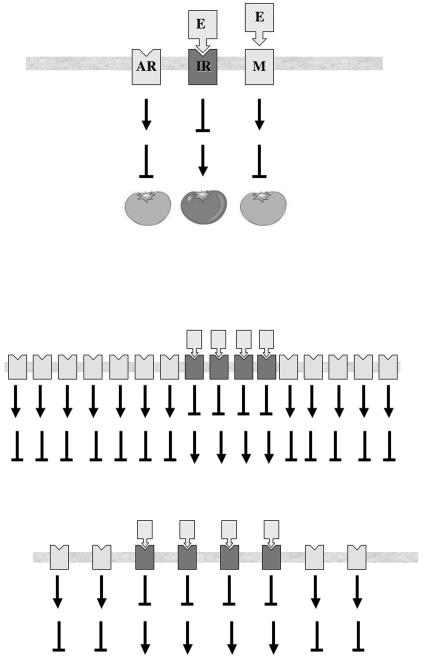
All of the receptor mutants display semidominant ethylene-insensitive phenotypes. Single gene knockouts, in contrast, have no obvious phenotype. Experiments using combinations of receptor knockouts indicate that they act as negative regulators of ethylene responses (Hua and Meyerowitz, 1998). Single and double loss-of-function mutants do not show obvious ethylene-related phenotypes; the only exception is an etr1/ers1 double mutant that reduces levels of the two Subfamily I receptors (Wang et al., 2003). This mutant and the triple mutants exhibit constitutive ethylene hypersensitivity. A quadruple mutant is more severe yet and does not reach maturity. It should be noted, however, that a careful analysis of an etr1 loss-of-function mutant indicated enhanced sensitivity to ethylene (Cancel and Larsen, 2002). The model, illustrated in Figure 1A, predicts a default state in which the receptor is actively suppressing expression of ethylene-inducible genes. Ethylene binding removes that suppression and ethylene responses can proceed. The most logical explanation is that kinase activity acts to suppress ethylene responses and a receptor incapable of binding ethylene cannot be inactivated. Based on this model, less receptor increases sensitivity to ethylene while more receptor would be predicted to reduce sensitivity. The triple and quadruple mutants respond to basal levels of ethylene constitutively made by plants because it takes less ethylene to inactivate the remaining receptors (Fig. 1B). This suggests that plants could modulate ethylene responses by altering the expression of receptor genes. There is, however, evidence that this basic model is not the entire story. In Arabidopsis, depletion of both Subfamily I receptors results in plants with severe ethylene hypersensitivity. This deficiency can be complemented by add-back of a transgene expressing a Subfamily I but not a Subfamily II receptor (Wang et al., 2003). This result suggests that there may be different roles for the two classes of receptor in Arabidopsis and that they may not have entirely redundant functions in vivo. As described below, our results are more consistent with redundant functions in tomato.
Figure 1.
Model for ethylene receptor action. A, In the absence of ethylene, receptors (AR) are actively suppressing ethylene responses such as fruit ripening. Upon ethylene binding, receptors become inactive (IR) and ethylene responses can proceed. Mutant receptors (M) cannot bind ethylene and continue to actively suppress downstream ethylene responses. B, Under normal circumstances, basal ethylene synthesis inactivates a small percentage of receptors (top). In loss-of-function mutants (bottom), the same amount of ethylene inactivates a much larger percentage of receptors, effectively lowering the threshold for initiating ethylene responses.
THE TOMATO SYSTEM
Genes encoding the two committed steps to ethylene synthesis, ACC synthase (ACS) and ACC oxidase, are under tight transcriptional control, being induced by multiple developmental and stress cues (Wang et al., 2002). Particularly relevant to discussion of developmental control of ripening is the concept of System 1 and System 2 ethylene synthesis. This terminology refers to the pattern of ethylene synthesis in immature versus mature fruits. Immature fruits produce low levels of ethylene and exogenous ethylene treatment does not stimulate further synthesis (System 1). In contrast, the ethylene produced by ripening fruits is autocatalytic, stimulating its own synthesis (System 2) (Yang, 1987). The difference between the two systems can be explained at a molecular level by the lack of induction of ACS transcription, which is rate-limiting, during System 1 versus induction during System 2. There are several interesting aspects of this phenomenon related to ethylene perception. First, immature fruits do recognize and respond to ethylene, as indicated by increased expression of certain ethylene-inducible genes. However, ethylene does not initiate ripening in immature fruits. Only mature fruits respond to ethylene by induction of a larger set of ripening-associated genes, indicating a developmental component to ethylene regulation. This suggests that combinations of transcription factors, both developmental and ethylene-regulated, control expression of some genes.
In addition to temporal control of fruit ethylene responses, there is spatial control. Fruits do not ripen uniformly. Ripening begins in internal tissue. It then proceeds toward external tissue progressing from the blossom end toward the calyx. Ethylene is a readily diffusible gas within the confines of a fruit. In fact, the skin of a tomato fruit is relatively impermeable to ethylene diffusion and the gas builds up to high internal levels throughout the fruit. Thus, differential spatial ripening within the fruit can only be explained by differential signal transduction. A further level of complexity in the developmental regulation of ethylene responses relates to the memory of immature fruits with respect to ethylene exposure. Although an immature fruit will not initiate ripening upon exposure to exogenous ethylene, that exposure will hasten the onset of ripening (Yang, 1987). Conversely, inhibition of System 1 ethylene synthesized by immature fruits delays the onset of ripening. Thus, tomato fruits possess a capacity to measure cumulative ethylene through development. When they reach a certain cumulative exposure to ethylene, ripening is initiated. The mechanisms mediating this developmental switch are unknown. However, many of the properties of ethylene receptors make them good candidates as developmental clocks.
While much is known about regulation of ethylene synthesis in tomato, less is known about its perception and signal transduction. We identified a tomato mutant that is altered in its ability to perceive ethylene. Never ripe (Nr) is a semidominant ethylene receptor mutant (Wilkinson et al., 1995). Analysis of Nr indicated a number of pleiotropic effects indicative of ethylene insensitivity throughout the plant (Lanahan et al., 1994). The mutant is greatly impaired in floral abscission and fruit ripening and exhibits significant delays in leaf and flower petal senescence. Identifying the molecular basis for the Nr mutant is a great example of translational biology. We knew the map position of Nr and could have initiated a time-consuming chromosome walk to identify the gene. But knowing that Nr is a semidominant ethylene insensitive mutant, we gambled that there would be an altered ethylene receptor. Several ETR1 homologs were cloned and mapped. One cosegregated with the Nr mutation. Sequencing the wild-type and mutant alleles led to identification of the mutation. Nr has subsequently proven to be a useful tool to assess the role of ethylene in a range of developmental (Clark et al., 1999; Hansen and Grossmann, 2000; Llop-Tous et al., 2000), gene expression (Nakatsuka et al., 1998), and stress (Ciardi et al., 2000; O'Donnell et al., 2001; Diaz et al., 2002) processes.
TOMATO ETHYLENE RECEPTORS
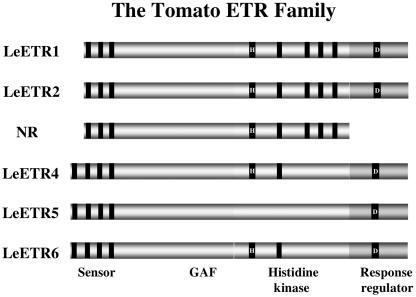
In tomato, there are six ethylene receptors (LeETR1–6; for review, see Klee and Tieman, 2002). The predicted structures of the tomato receptor family are very similar to those in Arabidopsis (Fig. 2). The tomato receptors are quite divergent, exhibiting less than 50% identity in primary sequence at the extremes. Three receptors have a potential extra amino terminal membrane-spanning domain. Only one, NR, lacks the receiver domain. Three (LeETR4–6) are missing one or more conserved HK domains, thus resembling the Subfamily II Arabidopsis receptors. Despite the extensive structural differences between them, all are receptors, as defined by their ability to bind ethylene (F. Rodriguez, A. Bleecker, and H. Klee, unpublished data).
Figure 2.
Schematic representation of the tomato ethylene receptor family. Black bars within the kinase domain indicate the presence of conserved His kinase elements. The His corresponding to the one phosphorylated in Arabidopsis ETR1 is indicated (H), where present.
Each tomato receptor gene has a distinct pattern of expression throughout development and in response to external stimuli (Klee, 2002). LeETR1 and LeETR2 are constitutively expressed in all tissues throughout development with LeETR1 expressed at about 5-fold higher level than LeETR2. In contrast, expression patterns of the other four genes are highly regulated. For example, during fruit development, ovaries express high levels of NR mRNA at anthesis. The level then drops approximately 10-fold until the onset of ripening whereupon it rises approximately 20-fold. The ripening-associated rise is an example of developmentally dependent ethylene inducibility, i.e. the gene is ethylene inducible during ripening but not in immature fruit (Wilkinson et al., 1995). As fruits approach maturity, expression of several receptors increases. NR and LeETR4 are also induced by pathogen infection. The pathogen inducibility of LeETR4 is associated with increased ethylene synthesis following infection (Ciardi et al., 2001).
IS RECEPTOR GENE EXPRESSION RELATED TO ETHYLENE SIGNALING?
Although there is evidence that expression of the Arabidopsis receptor genes is regulated by ethylene (Hua et al., 1998) and pathogen infection (O'Donnell et al., 2003), very little has been done to put patterns of expression in a biological context. With its multiple developmentally controlled ethylene responses, tomato is an excellent model in which to address such questions.
All of the genetic evidence supports a model in which ethylene receptors act as negative regulators. In this context, we consider the importance of receptor expression for regulating overall ethylene response. The model predicts an inverse correlation between receptor levels and ethylene sensitivity of a tissue. This model assumes that there is a threshold effect of ethylene. Once a certain percentage of receptors become inactivated by binding hormone, ethylene effects are manifested. More ethylene is required to inactivate high receptor levels than to inactivate low receptor levels. This is why receptor knockouts exhibit constitutive ethylene responsiveness despite unaltered levels of the hormone; basal levels of ethylene synthesis are sufficient to inactivate the full complement of receptors (Fig. 1B). A further factor to consider is that receptors apparently have a very long half-life for ethylene dissociation. The measured KD for yeast-expressed ETR1 was approximately 12 h (Schaller and Bleecker, 1995). This KD may be an underestimate since it does not factor in protein turnover. Association could be substantially longer than 12 h and may even be irreversible. Once a receptor has bound ethylene, it cannot repress ethylene-inducible genes for a long time, if ever. Therefore, synthesis of new receptor is an obvious way to turn off the response after it has been initiated. For terminal, irreversible responses such as abscission, flower senescence, or fruit ripening, there is no need to reverse the process. But ethylene also mediates environmental responses, many of which are transitory and must be terminated. Rapid return to normal growth likely requires new receptor synthesis. This model does not preclude specific functions for individual receptors. There could still be specific protein to protein interactions mediated by individual receptors. There may also be different efficiencies of signal transmission and/or differential turnover of individual receptors.
The available tomato data support the model. While most of the data are limited to RNA accumulation, we have measured the levels of NR protein with antibodies in both over- and underexpressing lines and there is a good correlation between RNA and protein levels for this receptor. Plants constitutively overexpressing the wild-type NR cDNA accumulate more protein and are less sensitive to ethylene, as measured by triple response and pathogen assays (Ciardi et al., 2000). Thus, more receptor decreases ethylene sensitivity. Conversely, gene-specific antisense reductions in LeETR1, LeETR2, or NR do not affect ethylene sensitivity. This lack of phenotype suggests a degree of redundancy built into the system, as in Arabidopsis. However, plants with reduced expression of the Subfamily 2 LeETR4 exhibit constitutive ethylene responses. The effects, including epinasty, flower abscission and premature fruit ripening, occur without any increase in ethylene synthesis (Tieman et al., 2000). Plants are more sensitive to ethylene. With respect to fruit ripening, loss of LeETR4 expression has profound effects. Lines with reduced LeETR4 expression initiate ripening earlier, ripen more quickly and synthesize more lycopene (Tieman et al., 2000). Some of the lines also synthesize significantly more ethylene than do wild-type fruits. Thus, alteration in LeETR4 expression is modifying the developmental control of fruit ripening.
Why do the single gene LeETR4 loss-of-function plants differ from those that are reduced in several of the other receptors? The explanation lies at least in part upon a response termed functional compensation (Tieman et al., 2000). In lines where NR expression has been reduced by 90%, LeETR4 expression increases to compensate for the NR reduction. In antisense LeETR4 lines, no such compensation occurs and overall receptor content is significantly reduced. Transcription of LeETR4 appears to be critically important. LeETR4 expression is regulated by either NR levels or overall ethylene sensitivity. There is clearly some mechanism for monitoring receptor levels or ethylene sensitivity. The observed functional compensation may be indicative of a fundamental difference between Arabidopsis and tomato. The data strongly suggest that Subfamily I and Subfamily II receptors are not functionally equivalent in Arabidopsis (Wang et al., 2003). So far in tomato, there does seem to be functional redundancy. NR is not essential for fruit ripening (Hackett et al., 2000) and loss of the Subfamily I NR can be compensated by increased expression of the Subfamily II LeETR4 (Tieman et al., 2000). Further, the severe ethylene hypersensitivity manifested in the LeETR4 loss-of-function lines can be complemented by add-back of a 35S-NR transgene. Clearly, the question of functional redundancy needs to be addressed in more detail.
Increased expression of LeETR4 in response to pathogen infection is an important aspect of disease response. This gene is induced during the hypersensitive response triggered by infection with Xanthomonas campestris pv. vesicatoria (Ciardi et al., 2000). In antisense lines with greatly reduced LeETR4 expression, an accelerated hypersensitive response occurs upon infection with an incompatible pathogen. Increased ethylene synthesis and pathogenesis-related gene expression are greater and more rapid in the infected antisense line, indicating a hastened defense response. However, this response has negative consequences since damage to the plant is not restricted to the immediate infection site. If the LeETR4 level does not increase in response to infection, the tissue is overly sensitive to ethylene and overshoots the appropriate level of defense response. Thus, the increase in receptor moderates the subsequent ethylene response to limit overall damage to the plant.
There are significant alterations in expression of multiple receptors in tomato, both during development and in response to external stimuli. In every known ethylene response, expression of one or more receptors increases. Even though reduced expression increases ethylene sensitivity, there are no examples where this response has been reported to occur. Rather, tomato initiates an ethylene response through the finely tuned system of ethylene synthesis. Once initiated, increased ethylene synthesis is followed by increased receptor synthesis. While it seems counterproductive to reduce hormone sensitivity following synthesis, this is a typical phytohormones response. A rapid increase in a hormone induces mechanisms to inactivate the response. Thus, it is normal for a plant to act to reduce a hormone response shortly after it is initiated. Increased ethylene receptor synthesis would be an effective means to achieve this outcome.
The patterns of receptor gene expression do not make sense in the context of fruit ripening. Receptor levels are generally high in ovaries at anthesis and decline until the onset of ripening, when there is a large increase that coincides with ripening-associated ethylene. Thus, at the time when ethylene exerts its greatest effect on fruit development, receptor gene expression is at its highest level. This higher rate of receptor synthesis must reduce ethylene responsiveness of the tissue. In the context of receptors as negative regulators of ethylene responses, this ripening-associated increase is paradoxical. However, during fruit ripening ethylene is synthesized far in excess of what is needed to drive the process forward. Any reduction in ethylene sensitivity caused by higher receptor gene expression would be more than offset by climacteric ethylene synthesis. This general pattern of receptor accumulation has been observed in multiple climacteric fruits (Sato-Nara et al., 1999; El-Sharkawy et al., 2003).
The constant low expression of receptor genes during the immature phase of fruit development could provide a mechanism for the fruits to monitor cumulative exposure to ethylene. If ethylene binding inactivates receptors for an extended period, eventually cumulative ethylene exposure would deplete the tissue of functional receptors. If ethylene synthesis exceeds receptor synthesis on a molar basis, the fruit will become progressively more sensitive to ethylene as they mature. When a fruit reaches a critical threshold of ethylene sensitivity, the ripening program would be triggered. Although speculative, the model is testable and consistent with what is known about gene regulation.
DOWNSTREAM SIGNALING ELEMENTS AND ETHYLENE SENSITIVITY
There is not a lot of information available on the downstream signaling elements involved in ethylene signaling. The available information argues against ripening control at the level of transcription of the downstream components. There are at least three genes encoding proteins with significant homology to CTR1 in tomato. One of these, LeCTR1, has been shown to functionally complement the Arabidopsis ctr1 mutation (Leclercq et al., 2002). Since CTR1 is a negative regulator of ethylene responses, its expression would decrease during ripening if it was a key regulatory element in ethylene signaling. In fact, the opposite is true; LeCTR1 is more highly expressed in ripening fruits than in unripe fruits. Thus, LeCTR1 behaves much like the ethylene receptors in that its expression goes up in response to ethylene. Transcriptional regulation of this gene cannot explain the observed differential ethylene responsiveness of fruits.
Genes encoding other downstream components of ethylene signaling that have been examined do not show any degree of transcriptional regulation. LeEIN2 is encoded by a single gene, as it is in Arabidopsis. Gene expression is unaltered during fruit development and is not ethylene inducible. Antisense reduction of expression delays ripening, as would be predicted upon loss of function (D. Tieman, J. Ciardi, and H. Klee, unpublished data). In the case of EIN3, there is a family of three tomato genes (Tieman et al., 2001). Each of these genes was shown to functionally complement the Arabidopsis ein3 mutation. The tomato genes appear to be functionally redundant and expression of all three must be reduced before ethylene signaling is measurably affected. There is little alteration in gene expression throughout growth and development and none of the genes is ethylene inducible. Thus, the evidence does not support any transcriptional regulation of CTR1, EIN2, or EIN3. However, it must be noted that Arabidopsis EIN3 is also not regulated by ethylene but the EIN3 protein clearly is regulated at the level of accumulation (Guo and Ecker, 2003; Potuschak et al., 2003). EIN3, and presumably the EIL proteins, is rapidly degraded by the ubiquitin/proteasome pathway in the absence of ethylene but accumulates to much higher levels in ethylene-treated plants. The F-box proteins that mediate this degradation are themselves positively transcriptionally regulated by ethylene. This mechanism of regulation appears critical to overall ethylene responses in Arabidopsis and there is certainly a need to test the model in other species.
CONCLUSIONS
The work that has been done over the last decade to define the ethylene signaling pathway in Arabidopsis has been nothing short of spectacular. It would have been impossible to assemble such a comprehensive understanding of the individual components in any other species. But once a framework has been established, it is essential that it be tested outside of a model organism. Ethylene plays such a critical role in integrating both developmental and environmental cues into overall growth that it would be naïve to assume any model developed for one organism would be universal. Indeed, tomato has evolved to use ethylene to control processes that do not even exist in Arabidopsis. In this regard, it is remarkable how much conservation of function does exist between the species. A snapshot taken today indicates that there are levels of control over ethylene signaling in tomato that have not been observed in Arabidopsis. Whether this is because the two species have evolved in different ways or because we simply haven't looked at Arabidopsis closely enough remains to be determined. In the end, critical comparisons of multiple species are likely to change our understanding of them all.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 [DOI] [PubMed] [Google Scholar]
- Bleecker A, Estelle M, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bleecker A, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Cancel J, Larsen P (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of products to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Jones JB, Klee HJ (2001) Reduced expression of the tomato ethylene receptor gene Leetr4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact 14: 487–495 [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123: 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol 121: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, ten Have A, van Kan J (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea. Plant Physiol 129: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Jones B, Li Z, Lelievre J, Pech JC, Latche A (2003) Isolation and characterization of four ethylene perception elements and their expression during ripening in pears with/without cold requirement. J Exp Bot 54: 1615–1625 [DOI] [PubMed] [Google Scholar]
- Gamble R, Coonfield M, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble R, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker J (2003) Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker J (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R, Ho C-H, Lin Z, Foote H, Fray R, Grierson D (2000) Antisense inhibition of the Nr gene restores normal ripening to the tomato Never-ripe mutant, consistent with the ethylene receptor-inhibition model. Plant Physiol 124: 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen Q, Bleecker A, Ecker J, Meyerowitz E (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H (2002) Control of ethylene-mediated processes in tomato at the level of receptors. J Exp Bot 53: 2057–2063 [DOI] [PubMed] [Google Scholar]
- Klee H, Tieman D (2002) The tomato ethylene receptor gene family: form and function. Physiol Plant 115: 336–341 [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ (1994) The Never ripe mutation blocks ethylene perception in tomato. Plant Cell 6: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq J, Adams-Philips L, Zegzouti H, Jones B, Latche A, Giovannoni J, Pech JC, Bouzayen M (2002) LeCTR1, a tomato CTR1-like gene, demonstrates ethylene signaling ability in Arabidopsis and novel expression patterns in tomato. Plant Physiol 130: 1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop-Tous I, Barry CS, Grierson D (2000) Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol 123: 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118: 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ (2001) Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J 25: 315–323 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Schmelz E, Moussatche P, Lund S, Jones JB, Klee HJ (2003) Susceptible to intolerance: a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J 33: 245–257 [DOI] [PubMed] [Google Scholar]
- Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22: 1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Sato-Nara K, Yuhashi K, Higashi K, Hosoya K, Kubota M, Ezura H (1999) Stage- and tissue-specific expression of ethylene receptor homologue genes during fruit development in muskmelon. Plant Physiol 119: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Tieman D, Ciardi J, Taylor M, Klee H (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout development. Plant J 26: 47–58 [DOI] [PubMed] [Google Scholar]
- Tieman DV, Taylor MG, Ciardi JA, Klee HJ (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97: 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li H, Ecker J (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hall A, O'Malley R, Bleecker A (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not require for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Xie C, Zhang JS, Zhou HL, Li J, Zhang Z, Wang D, Chen S (2003) Serine/threonine kinase activity in the putative histidine kinase-like ethylene receptor NTHK1 from tobacco. Plant J 33: 385–393 [DOI] [PubMed] [Google Scholar]
- Yang SF (1987) The role of ethylene and ethylene synthesis in fruit ripening. In W Thompson, E Nothnagel, R Huffaker, eds. Plant Senescence: Its Biochemistry and Physiology. The American Society of Plant Physiologists, Rockville, MD, pp 156–165
- Yeh K, Lagarias JC (1998) Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA 95: 13976–13981 [DOI] [PMC free article] [PubMed] [Google Scholar]