Abstract
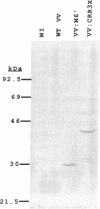
Previous studies have shown that when inoculated intranasally into mice, vaccinia virus (VV) recombinants expressing the carboxyl half of the Streptococcus pyogenes M protein [which contains the C-repeat region (CRR)] could elicit a protective immune response against subsequent challenge by both homologous and heterologous serotypes of pathogenic group A streptococci. In the present study, an insertion plasmid was constructed that contained three tandem in-frame repeats of a 310-base-pair DNA sequence encoding the CRR from streptococcal M6 protein under control of a constitutive viral promoter. The plasmid was used to introduce the bacterial sequences into the VV genome by homologous recombination. Surprisingly, the recombinant VV:CRR3X virus that was isolated appeared to represent not an individual recombinant virus but a complex mixture of variants that contained from 1 to greater than 20 tandem copies of the CRR region at the insertion site. This genomic complexity was mirrored at the transcriptional level in that a nested set of coterminal transcripts was detected in VV:CRR3X-infected cells, which increased in size from 1400 to 6600 bases by increments of approximately 300 bases. All transcripts containing two or more CRR inserts appeared functional, as Western (immuno) blot analyses of VV:CRR3X-infected cell extracts revealed a family of CRR-related proteins with apparent molecular masses that increased from 30 kDa upward in increments of 10 kDa. All data are consistent with the hypothesis that variation in the VV:CRR3X recombinants is from random crossover events that occur within the CRR region during viral DNA replication. These results suggest that the genomic diversity generated by the "recombinogenic" properties of vaccinia recombinants containing tandem foreign inserts could be used to facilitate induction of a broadly protective immune response against antigenically diverse pathogenic agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Moss B. Sequence homologies of diverse length tandem repetitions near ends of vaccinia virus genome suggest unequal crossing over. Nucleic Acids Res. 1982 Sep 25;10(18):5673–5679. doi: 10.1093/nar/10.18.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990 Aug 15;145(4):1251–1256. [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Fathi Z., Sridhar P., Pacha R. F., Condit R. C. Efficient targeted insertion of an unselected marker into the vaccinia virus genome. Virology. 1986 Nov;155(1):97–105. doi: 10.1016/0042-6822(86)90171-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Hodges W. M., Hruby D. E. Protection against streptococcal pharyngeal colonization with a vaccinia: M protein recombinant. Science. 1989 Jun 23;244(4911):1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. Identification of an immunodominant region. J Immunol. 1988 Nov 15;141(10):3592–3599. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Control of expression of the vaccinia virus thymidine kinase gene. J Virol. 1981 Nov;40(2):456–464. doi: 10.1128/jvi.40.2.456-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Hodges W. M., Wilson E. M., Franke C. A., Fischetti V. A. Expression of streptococcal M protein in mammalian cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5714–5717. doi: 10.1073/pnas.85.15.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Maki R. A., Miller D. B., Ball L. A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Manjula B. N., Johnston K. H., Hollingshead S. K., Scott J. R., Fischetti V. A. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. doi: 10.1084/jem.161.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford C. J., Edwards S. J., Smith G. L., Mitchell G. F., Moss B., Kemp D. J., Anders R. F. Anchoring a secreted plasmodium antigen on the surface of recombinant vaccinia virus-infected cells increases its immunogenicity. Mol Cell Biol. 1986 Sep;6(9):3191–3199. doi: 10.1128/mcb.6.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. N., Hruby D. E. Rifampicin prevents virosome localization of L65, an essential vaccinia virus polypeptide. Virology. 1989 May;170(1):227–237. doi: 10.1016/0042-6822(89)90370-x. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Franke C. A., Strauss J. H., Hruby D. E. Expression of Sindbis virus structural proteins via recombinant vaccinia virus: synthesis, processing, and incorporation into mature Sindbis virions. J Virol. 1985 Oct;56(1):227–239. doi: 10.1128/jvi.56.1.227-239.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. doi: 10.1126/science.191907. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W. The chain that links the heart to the throat. Circulation. 1973 Jul;48(1):9–18. doi: 10.1161/01.cir.48.1.9. [DOI] [PubMed] [Google Scholar]