Abstract
Signal recognition by seven-transmembrane (7TM) cell-surface receptors is typically coupled by heterotrimeric G-proteins to downstream effectors in metazoan, fungal, and amoeboid cells. Some responses perceived by 7TM receptors in amoeboid cells and possibly in human cells can initiate downstream action independently of heterotrimeric G-proteins. Plants use heterotrimeric G-protein signaling in the regulation of growth and development, particularly in hormonal control of seed germination, but it is not yet clear which of these responses utilize a 7TM receptor. Arabidopsis GCR1 has a predicted 7TM-spanning domain and other features characteristic of 7TM receptors. Loss-of-function gcr1 mutants indicate that GCR1 plays a positive role in gibberellin- (GA) and brassinosteroid- (BR) regulated seed germination. The null mutants of GCR1 are less sensitive to GA and BR in seed germination. This phenotype is similar to that previously observed for transcript null mutants in the Gα-subunit, gpa1. However, the reduced sensitivities toward GA and BR in the single gcr1, gpa1, and agb1 (heterotrimeric G-protein β-subunit) mutants are additive or synergistic in the double and triple mutants. Thus, GCR1, unlike a typical 7TM receptor, apparently acts independently of the heterotrimeric G-protein in at least some aspects of seed germination, suggesting that this alternative mode of 7TM receptor action also functions in the plant kingdom.
Signaling through heterotrimeric G-proteins is highly conserved among divergent eukaryotes.G-proteins physically couple the recognition of many extracellular signals by cell-surface receptors to activation of enzyme activities in the cytoplasm. In the classical paradigm, ligand binding to its cognate GPCR activates receptor-mediated GDP/GTP exchange on the α-subunit (Gα), causing dissociation of Gα from the βγ dimer (Gβγ). Activated Gα-subunits, Gβγ, or both then bind to downstream target proteins, which results in the relevant cellular responses (Gilman, 1987). There are 23 Gα-, 6 Gβ-, and 12 Gγ-subunits in humans (Vanderbeld and Kelly, 2000). In contrast to humans, the Arabidopsis genome contains genes encoding only one prototypical G-protein α-subunit (GPA1), oneG-protein β-subunit (AGB1), and two G-proteinγ-subunits (AGG1 and AGG2), indicating that the repertoire of heterotrimeric G-protein complexes in plants is smaller (Assmann, 2002; Jones, 2002). Studies on the null alleles of GPA1 and AGB1 suggest that plants use heterotrimeric G-protein signaling in many growth and developmental processes (Ullah et al., 2001, 2002, 2003; Wang et al., 2001; Chen et al., 2003).
No classical GPCR has been definitively identified in plants. To date, the most promising candidate for a plant GPCR remains GCR1, independently cloned by two groups (Josefsson and Rask, 1997; Plakidou-Dymock et al., 1998). GCR1 encodes a protein with predicted seven membrane-spanning domains and has some sequence similarity to Dictyostelium cAMP receptors. GCR1 was originally proposed to be a receptor for cytokinins (Plakidou-Dymock et al., 1998), but this notion has not been supported (Humphrey and Botella, 2001; Kanyuka et al., 2001). Although a ligand for GCR1 has not been identified, there is biochemical evidence that GCR1 does physically interact with GPA1 (Pandey and Assmann, 2004).
Colucci et al. (2002) reported that one of the phenotypes caused by overexpression of GCR1 in Arabidopsis is loss of seed dormancy. Expression of a germination marker, a phosphatase PP2A subunit, correlated with GCR1 overexpression (Colucci et al., 2002). In a follow-up study using BY2 cells overexpressing GCR1, Apone et al. (2003) concluded that GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C.
Based on the observations that GCR1 is physically coupled to GPA1 (Pandey and Assmann, 2004) and that a heterotrimeric G-protein complex is involved in control of seed germination (Ullah et al., 2002; Iwasaki et al., 2003; Lapik and Kaufman, 2003) as well as indications from the GCR1 overexpression phenotypes, we addressed the role of GCR1 in seed germination. Assays with loss-of-function alleles of GCR1 were used to assess results based on ectopic expression of GCR1 and to gain insight into the possible physiological function of GCR1. The characterization here of gcr1 mutants clearly supports a role for GCR1 in seed germination. Epistasis analysis using double and triple mutants of gcr1 and G-protein α- and β-subunit genes extends this conclusion and indicates that some aspects of GCR1 function may act in parallel to the heterotrimeric G-protein complex.
RESULTS
GCR1 Encodes a Seven-Transmembrane Receptor Homolog
At the time GCR1 was cloned (Josefsson and Rask, 1997; Plakidou-Dymock et al., 1998), genome sequence information was limited and computational approaches for structure prediction were less sophisticated than at present. We revisited the topic of GCR1 homology to the GPCR model using current bioinformatic tools. Our analysis is based on the fact that the seven-transmembrane (7TM) domain is the most conserved feature for all GPCRs and that computational algorithms to detect predicted 7TM models have greatly improved. As predicted by a transmembrane domain hidden Markov model (Krogh et al., 2001), the probability of GCR1 containing a 7TM domain is near 1.0 (Supplemental Fig. 1A, which can be viewed at www.plantphysiol.org). The predicted overall topology of GCR1, as generated by the residue-based diagram editor Web server (Konvicka et al., 2000), is reminiscent of GPCRs that contain a 7TM domain with a preferred orientation of an extracellular N terminus and an intracellular C terminus (Supplemental Fig. 1B). The predicted topology of GCR1 places Cys residues (Cys-80 and Cys-151) near the entry to transmembrane domain-3 and the second extracellular loop, respectively, in similar positions to a common disulfide linkage found frequently in GPCRs of all subfamilies. National Center for Biotechnology Information standard protein-protein BLAST (blastp) analyses confirmed that GCR1 contains a domain that is conserved in pfam00002, 7tm_2, 7TM receptor, secretin family (data not shown). GCR1 also contains a 7TM domain that is conserved in pfam01534, frizzled, frizzled/smoothened family membrane region. Proteins related to Drosophila frizzled are receptors for the Wnt signaling molecules. The smoothened receptor mediates hedgehog signaling. GCR1 showed the highest overall similarity to cAMP receptors or cAMP receptor-like GPCR, CR1A (accession no. AAO62367) and CAR3 (accession no. P35352) in Dictyostelium discoideum, and TasA (accession no. BAA99285) in Polysphondylium pallidum (Supplemental Fig. 1C). GCR1 has 25% identity with CR1A within 247 amino acids, 24% with CAR3 within 260 amino acids, and 24% with TasA within 258 amino acids. GCR1 also showed similarity to the rhodopsin family of GPCRs (data not shown). A database search of Arabidopsis open reading frames using full-length GCR1 failed to yield homologs, indicating that GCR1 is a single gene in Arabidopsis. A GCR1 homolog is present in rice (Oryza sativa; Kato et al., 2003).
gcr1 Null Alleles in Arabidopsis
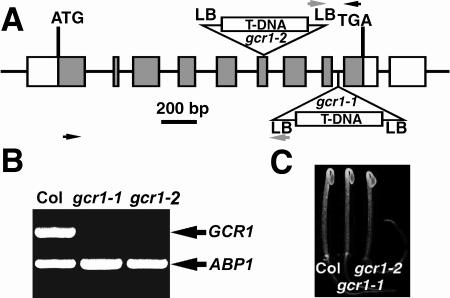
gcr1-1, which harbors a T-DNA insert in the eighth intron of GCR1 coding sequence, was obtained by screening deconvoluted pools of DNA from T-DNA transformed plants as described in “Materials and Methods.” A second allele (gcr1-2) was obtained from the Salk Institute sequence-indexed insertion mutant collection (http://signal.salk.edu/cgi-bin/tdnaexpress), and the T-DNA insertion was confirmed to be in the sixth exon of the GCR1 coding sequence (Fig. 1A). Reverse transcription (RT)-PCR of cDNA isolated from homozygous mutant plants failed to detect the full-length GCR1 transcripts, indicating that individuals homozygous at the mutant GCR1 locus are transcript null (Fig. 1B). Another independent allele, gcr1-3 in the Wassilewskija (WS) ecotype, is described elsewhere (Pandey and Assmann, 2004).
Figure 1.
T-DNA insertion mutant alleles of GCR1 in Arabidopsis. A, T-DNA insertion sites in GCR1. LB, T-DNA left border. Gray boxes represent exons. The T-DNA insert is not drawn to scale. The gray arrows at LB indicate the T-DNA left border primer, and the black arrows indicate the GCR1 specific primers used for mutant isolation. B, RT-PCR analysis for GCR1 transcript. Total RNA was isolated from 10-d-old, light-grown seedlings. The GCR1 transcript was present in total RNA from wild-type Arabidopsis but absent in the gcr1-1 and gcr1-2 mutants. As a control, Arabidopsis ABP1 primers that amplify a 554-bp product were added together with GCR1 primers in each PCR reaction. C, Two-day-old, dark-grown gcr1 mutant seedlings.
gcr1 gpa1 Mutants Have gpa1 Leaf Morphology and Plant Architecture
If GCR1 is coupled by a heterotrimeric G-protein complex, gcr1 mutants should share some or all phenotypes exhibited by loss-of-function G-protein subunit mutants. To address the genetic interaction between GCR1 and heterotrimeric G-protein subunits, we examined single, double, and triple gcr1, gpa1, and agb1 mutants in the Columbia (Col-0) ecotype background. When grown in darkness for 2 d, gpa1 and agb1 mutants have shorter hypocotyls and partially-opened hooks, as reported previously (Ullah et al., 2001, 2003; Jones et al., 2003), whereas gcr1 mutants have the wild-type (Col-0) traits (Figs. 1C and 2, A and B). The phenotypes of shorter hypocotyl and partially-opened hook were also observed in gcr1 gpa1 double, agb1 gcr1 double, and agb1 gcr1 gpa1 triple mutants (Fig. 2, A and B).
Figure 2.
gcr1 gpa1 double mutant resembles gpa1 single mutant in morphology. A, gcr1 gpa1 double mutant phenotypes of 2-d-old, dark-grown seedlings. B, Hypocotyl lengths of 2-d-old, dark-grown gcr1 gpa1 double mutant seedlings. Shown are the means ± se of 20 seedlings. C, gcr1 gpa1 double mutant phenotypes of 43-d-old, light-grown plant. Shown below are the 10th rosette leaves from wild-type (Col-0) and mutant plants.
When grown in light, gpa1 and agb1 mutants have leaves with rounded lamina, whereas gcr1 mutant leaf morphology is that of a wild type (Fig. 2C). This round leaf phenotype is also found in gcr1 gpa1, agb1 gcr1, and agb1 gcr1 gpa1 double or triple mutants (Fig. 2C). No additional vegetative phenotypes were observed in gcr1 gpa1, agb1 gcr1, and agb1 gcr1 gpa1 mutants.
Colucci et al. (2002) showed that plants ectopically expressing GCR1 flower early providing a clear prediction that plants lacking a functional GCR1 would flower late. However, gcr1 null mutants typically do not flower later than wild-type plants, and, under certain conditions, actually flower slightly, but statistically earlier, than wild-type plants (data not shown).
gcr1 Null Alleles Have Altered Sensitivities to GA and BR in Seed Germination
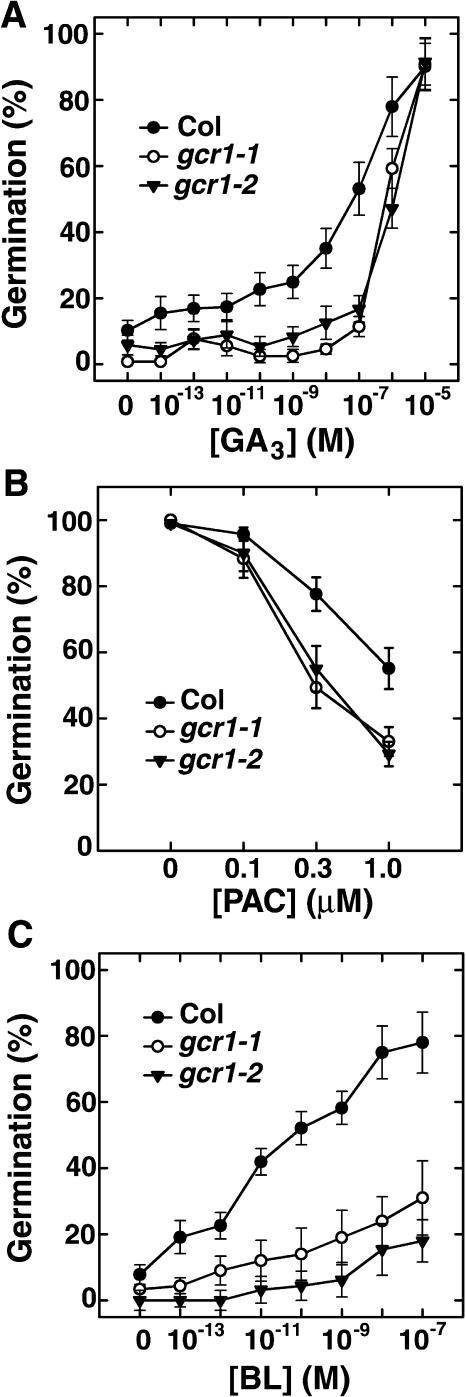
Seed germination is regulated by many signals in a G-protein-dependent manner. Previously, we found that gpa1 mutant seeds are less responsive to GA, and that seeds ectopically expressing GPA1 are at least one million-fold more responsive to GA yet still require GA for germination (Ullah et al., 2002). We hypothesized that the GPA1 heterotrimeric complex operates upon the GA pathway to control germination, and that this potentiation is directly mediated by BR (Ullah et al., 2002). A role for GCR1 in seed germination has also been proposed (Colucci et al., 2002). Therefore, to directly test if gcr1 seeds are altered in the GA response, seeds were pretreated with the GA biosynthesis inhibitor, paclobutrazol (PAC), to reduce the endogenous GA pool, then sown on plates supplemented with defined concentrations of GA or BR. gcr1 seeds are less responsive to exogenous GA and are hypersensitive to PAC (Fig. 3).
Figure 3.
Null alleles of GCR1 have altered sensitivity to GA3 and BL in seed germination. A, Sensitivity of gcr1 mutant seeds to GA3. B, Sensitivity of gcr1 mutant seeds to the GA biosynthesis inhibitor PAC. C, Sensitivity of gcr1 mutant seeds to BL. Wild-type (Col-0) and mutant seeds from matched seed lots in A and C were pretreated with 8 μm PAC. After 3 d at 23°C in darkness, germination was scored and expressed as a percent of total seeds. Wild-type and mutant seeds from matched lots in B were sterilized and sown on plates supplemented with 1% Glc and the indicated concentration of PAC. After 3 d at 23°C in dark, germination was scored and expressed as a percent of total seeds. Shown are means of three replicates ± se.
BR either potentiates GA signaling or blocks the abscisic acid (ABA) inhibition of germination (Leubner-Metzger, 2001; Steber and McCourt, 2001), and GPA1 appears to couple BR signaling in germination (Ullah et al., 2002). Therefore, the sensitivity of gcr1 mutant seeds to BR was also tested in seed germination. As shown in Figure 3C, gcr1 mutant seeds have reduced responsiveness to BR. The altered sensitivities to BR and GA of the Col-0 alleles of mutant gcr1 are recapitulated in a WS allele gcr1 mutant (gcr1-3; Supplemental Fig. 2, A and B).
gcr1 gpa1 Double Mutants Have Additive or Synergistic GA and BR Responses in Seed Germination
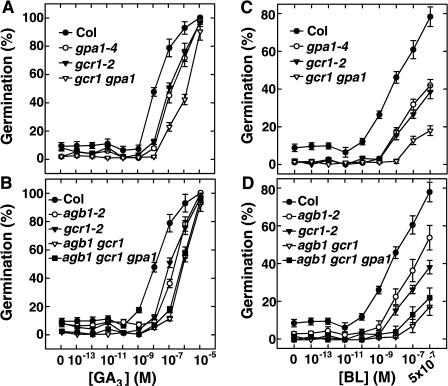
Because gpa1 and gcr1 mutants have reduced sensitivities to GA and BR in seed germination, we investigated whether GCR1 and GPA1 are genetically coupled in seed germination pathway(s) mediated by GA and BR. As shown in Figure 4, the sensitivities of gcr1 gpa1 double mutants to GA and BR in seed germination were additive or synergistic (Fig. 4, A and C). In the GA sensitivity assay, 50% germination occurred at approximately 10−7 m for both gpa1 and gcr1 single mutants, whereas gcr1 gpa1 double mutants required at least 10-fold more GA to reach 50% germination. In the BR sensitivity assay, both gpa1 and gcr1 single mutants showed reduced sensitivity to BR compared to wild type. While for wild-type seed 50% germination was obtained at 10−8 m brassinolide (BL), germination for the double mutants could not be reached even when BL was applied at concentrations as high as 5 × 10−7 m. Germination rescued by BL for the gpa1 and gcr1 single mutants was intermediate to wild type and gpa1 gcr1 double mutants (Fig. 4C). Similar additive or synergistic effects were also observed in agb1 gcr1 double mutants and agb1 gcr1 gpa1 triple mutants (Fig. 4, B and D). Not shown in Figure 4, for purpose of clarity, is that the sensitivities of the gpa1 agb1 double mutant to GA and BR are the same as that of the agb1 single mutant.
Figure 4.
gcr1 gpa1 and gcr1 agb1 double mutants have additive or synergistic reduced responses to GA3 and BL in seed germination. A, The sensitivity of gcr1 gpa1 double mutant to GA3. B, The sensitivities of the agb1 gcr1 double mutant and agb1 gcr1 gpa1 triple mutant to GA3. C, The sensitivity of gcr1 gpa1 double mutant to BL. D, The sensitivities of agb1 gcr1 double mutant and agb1 gcr1 gpa1 triple mutant to BL. Wild-type (Col-0) and mutant seeds from matched seed lots were pretreated with 8 μm PAC at 4°C for 2 d in the dark, washed, then sown on one-half-strength Murashige and Skoog (with Gamborg's vitamins) plates supplemented with the indicated concentration of GA3 or BL. After 3 d at 23°C in darkness, germination was scored and expressed as a percent of total seeds. All germination assays were repeated at least twice. Shown are means ± se.
GCR1 Subcellular and Tissue Localization
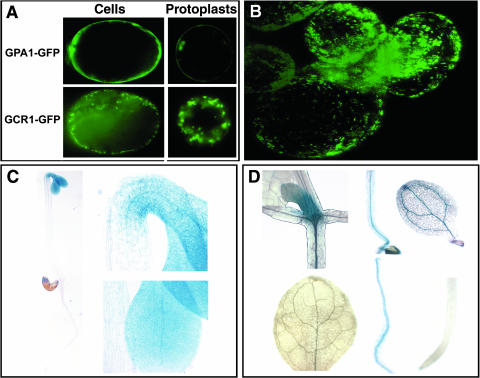
Humphrey and Botella (2001) previously showed that GCR1 is localized to the outer edge of the leaf epidermal cells of Arabidopsis plants. Here, we utilize three-dimensional imaging of Arabidopsis suspension cells expressing a GCR1-GFP fusion to increase spatial resolution of GCR1 subcellular localization. GCR1-GFP fluorescence could be detected in the region near the plasma membrane of the intact cell and protoplast, but not in the same pattern as that of GPA1-GFP fluorescence, which is distributed evenly across the plasma membrane (Fig. 5A). In contrast, GCR1-GFP fluorescence appeared in a punctuate pattern, implying an association with particular membrane structures or possible internalization (Fig. 5B), a pattern also found with some mammalian GPCR-GFP fusions (Chun et al., 1994; Tarasova et al., 1997; Kallal et al., 1998).
Figure 5.
GCR1 localization and expression in Arabidopsis. A, GCR1 subcellular localization. 35S::GCR1-GFP and 35S::GPA1-GFP binary constructs were transformed separately into Arabidopsis suspension cells. GFP was visualized by fluorescence microscopy both in intact cells and protoplasts. B, Z-stack image of GCR1-GFP in Arabidopsis suspension cells. C, GCR1::GUS expression in2-d-old, dark-grown Arabidopsis seedlings. The regions of hook and cotyledons are highlighted. D, GCR1::GUS expression in10-d-old, light-grown Arabidopsis seedlings. Top (left to right): young leaves, hypocotyl, and cotyledon; bottom (left to right): rosette leaf, root, and root tip.
To visualize tissue and organ distribution of GCR1 expression, a GCR1::GUS (β-glucuronidase) reporter comprised of genomic DNA 932 bp upstream of the GCR1 start codon fused with GUS was introduced into Col-0 plants. In dark-grown, 2-d-old seedlings, the GCR1::GUS transcriptional fusion transgene was expressed in the cotyledons and the hook (Fig. 5C). In light-grown, 10-d-old seedlings, GUS staining was detected in young leaves and vascular tissues of cotyledons, hypocotyl, and root (Fig. 5D), whereas no GUS staining was detected in the root tip, a predominant site of expression of GPA1 (Huang et al., 1994). We did not observe any GUS staining in the vegetative tissues or flowers in mature plant, confirming that GCR1 transcript expression is low (Plakidou-Dymock et al., 1998; Humphrey and Botella, 2001; Colucci et al., 2002).
DISCUSSION
We provide genetic evidence that GCR1 is an important component for multiple signaling pathways in seed germination. We found that loss-of-function of GCR1 confers altered sensitivities to GA and BR in germination response. Subcellular localization of GFP-tagged GCR1 shows that GCR1 appears in a punctuate pattern around the plasma membrane. This distribution pattern does not preclude the possibility that GCR1 functions as a receptor. From recent studies on metazoan GPCRs, it is now known that some GPCRs are not confined to the cell surface as earlier thought, but are located throughout the cell cortex (Daly and McGrath, 2003). The subcellular localization of the receptor molecules also depends on the cell type, environmental conditions, and concentration of the agonist. Some receptors cluster in the plasma membrane prior to agonist induced internalization (Drmota et al., 1998; McLean and Milligan, 2000). Because both GPA1-GFP and GCR1-GFP expressions were driven by 35S promoter, we do not rule out the possibility that the expression patterns we observed were due to ectopic expressions of the fusion proteins. Furthermore, because we have not yet shown that the GCR1-GFP fusion protein is able to rescue fully the gcr1 mutant phenotypes, we remain cautious in interpreting the internalization pattern. With this precautionary comment in mind, we note that this pattern is common to many well-studied GPCR in mammals (Tan et al., 2004).
We have previously shown that plants and seeds harboring null alleles of GPA1, the single gene encoding a canonical Gα in Arabidopsis, have altered sensitivities to a number of plant hormones (Ullah et al., 2001, 2002, 2003). Gα mutants are less sensitive to GA and BR in seed germination (Ashikari et al., 1999; Ueguchi-Tanaka et al., 2000; Ullah et al., 2002), and rice plants harboring the d1 mutation in the rice Gα gene, RGA1, are dwarf (Fujisawa et al., 1999). These data all point to a role of heterotrimeric G-protein in GA signaling (Iwasaki et al., 2003).
GA is a critical player in seed germination (Olszewski et al., 2002). We found that similar to gpa1, gcr1 mutants also have reduced sensitivity to GA in seed germination (Fig. 3). Our loss-of-function data are consistent with the gain-of-function data that overexpression of GCR1 abolishes seed dormancy and enhances the expression of germination-associated genes (Colucci et al., 2002). In view of these data and the observation that GCR1 physically interacts with GPA1 (Pandey and Assmann, 2004), we used genetic tools to address whether GCR1 is coupled to GPA1 in seed germination responses. The most obvious expectation based on the germination response to single gcr1 or gpa1 mutants would be that the gcr1 gpa1 double mutants would display an epistatic phenotype. However, gcr1 gpa1 double mutants as well as agb1 gcr1 double mutants and agb1 gcr1 gpa1 triple mutants have mostly additive or synergistic effects of reduced sensitivity to GA and BR in germination (Fig. 4). The simplest explanation of our results to explain the additive/synergistic phenotypes of double and triple mutants would be that GCR1 acts in a parallel pathway with G-proteins in seed germination (Fig. 6).
Figure 6.
Schemes of GCR1 modes of action. GCR1 positively regulates seed germination, by coupling or modulating BR potentiation of GA-stimulated germination, but acts in a pathway independent of GPA1 and AGB1. GPA1 and AGB1 also negatively regulate the antagonistic effect of ABA on GA-stimulated germination. Glc delays seed germination, but not necessarily via ABA.
Considering the importance of GA in controlling seed germination (Koornneef et al., 2002; Olszewski et al., 2002; Peng and Harberd 2002; Ogawa et al., 2003) and now the evidence that GCR1 is an element in the transduction of these signals, it was not surprising to find putative cis-elements controlling GCR1 expression that are found in GA-inducible genes (Table I). An analysis of the GCR1 promoter using the PLACE Signal Scan Search (Higo et al., 1999) also revealed cis-acting regulatory DNA elements associated with dehydration and ABA responsiveness.
Table I.
Analysis of cis-acting regulatory DNA elements in GCR1 promoter region
| Factor or Site Name | Signal Sequence | Repeat Time(s) | Function |
|---|---|---|---|
| TAAAGSTKST1 | TAAAG | 1 | Found in promoter of KST1 (encodes a K+ influx channel of guard cells); target site for trans-acting StDof1 protein controlling guard cell-specific gene expression |
| ABRELATERD1 | ACGTG | 1 | ABA response element-like sequence; required for etiolation-induced expression of erd1 (early responsive to dehydration) |
| MYCATERD1 | CATGTG | 1 | MYC recognition sequence; necessary for expression of erd1 in dehydrated Arabidopsis |
| ACGTATERD1 | ACGT | 6 | Required for etiolation-induced expression of erd1 |
| MYCCONSENSUSAT | CANNTG | 2 | MYC recognition site found in the promoters of the dehydration-responsive gene rd22 |
| MYCATRD22 | CACATG | 1 | Binding site for MYC in dehydration-responsive gene rd22 |
| LTRECOREATCOR15 | CCGAC | 3 | Core of low temperature responsive element (LTRE) of cor15a gene; ABA responsiveness |
| ACGTABOX | TACGTA | 2 | Responsible for sugar repression |
| AMYBOX1 | TAACARA | 2 | Amylase box; conserved sequence found in 5′-upstream region of α-amylase gene |
| CGACGOSAMY3 | CGACG | 3 | Found in the GC-rich regions of the rice Amy3D and Amy3E amylase genes; may function as a coupling element for the G box element |
| SP8BFIBSP8BIB | TACTATT | 3 | One of SPBF binding sites (SP8b); found in promoter of β-amylase |
| MYBGAHV | TAACAAA | 2 | Central element of GA response complex in high-pI α-amylase gene; partially involved in sugar repression |
| C8GCARGAT | CWWWWWWWWG | 6 | Binding site of plant MADS-domain protein AGL15 |
| WBOXATNPR1 | TTGAC | 3 | W-box found in the promoter of Arabidopsis NPR1 gene; recognized specifically by SA-induced WRKY DNA binding proteins |
In bold are the signal sequences related to dehydration response or amylase activity.
The possibility remains that GPA1 interacts indirectly with GCR1 in GA and BR regulation of seed germination since a network of other proteins that might interact with GPA1 or GCR1 are expected to be involved in control of germination in response to hormonal signals. For example, recently a GPA1 interacting protein, AtPirin1, was identified in a yeast two-hybrid screen (Lapik and Kaufman, 2003). AtPirin1 encodes a cupin-domain protein. Interestingly, seed germination was also delayed in Atpirin1 mutants. GPCRs in mammalian systems have also been shown to interact with other GPCRs or receptor-like kinases that also modulate their activities (Hall et al., 1999).
Because GCR1 and GPA1 physically interact (Pandey and Assmann, 2004), GCR1 presumably operates as a bona fide GPCR under some circumstances. However, the genetic evidence presented here indicates that GCR1 and GPA1 signals are not coupled in GA and BR-mediated seed germination responses. Recently, it has been shown that the slime mold CAR and human 5HT GPCRs also operate independently of their cognate G-protein in a subset of pathways (Heuss and Gerber, 2000; Brzostowski and Kimmel, 2001; Kimmel and Parent, 2003). However, due to the large number of the G-protein complexes in these animal systems (multiple α-subunits) the evidence is indirect. With single prototypical α- and β-subunits in Arabidopsis, the work presented here provides compelling evidence for GCR1 action independent of a conventional G-protein heterotrimer. Our work shows that what has been termed action at zero G by Alan Kimmel is more than an anomaly because the phenomenon, if not the mechanism as well, is apparently evolutionarily conserved from plants to metazoans.
MATERIALS AND METHODS
Isolation of gcr1 Mutants
The accession number for GCR1 is At1g48270. Deconvoluted pools of DNA from T-DNA transformed plants were originally used to screen for insertion in the Arabidopsis GCR1 gene. A total of 60,000 T-DNA insertion lines in Col-0 (Alonso et al., 2003) were screened by PCR using GCR1- andT-DNA-specific primers. A single putative insertion line was identified independently in two sets of screening by using primers specific for the GCR1 5′-UTR (untranslated region) or 3′-UTR together with a T-DNA left border-specific primer. PCR primers used were as follows: for GCR1 5′-UTR(5′-CGAACACGAACAGCGGAAATCGTCAATTC-3′); for 3′-UTR region (5′-CTAGAGGAAACTTACCAATCTCTCCATC-3′); and a T-DNA left border primer (5′-GGCAATCAGCTGTTGCCCGTCTCACTGGTG-3′). A single insertion in the eighth intron of GCR1 was isolated, and the insertion was confirmed by sequencing. This gcr1 mutant allele was designated as gcr1-1. The second gcr1 mutant allele (gcr1-2) was obtained from the Salk Institute sequence-indexed insertion mutant collection (http://signal.salk.edu/cgi-bin/tdnaexpress). The T-DNA insertion site in this allele was at the sixth exon of GCR1. Plants homozygous for gcr1 were isolated, and the insertion was confirmed by sequencing. Loss of detectable GCR1 transcripts in gcr1-1 and gcr1-2 mutants was verified by RT-PCR. Total RNA was isolated from seedlings grown in light for 10 d. GCR1 primers flanking the insertion site (5′-GTCGGCGGTTCTCACAGCCGGCGGAGGCT-3′ and 5′-GGTCCTCGGTCTTGAGTGATACCATTTCGC-3′) and Arabidopsis ABP1 primers (5′-TGATCGTACTTTCTGTTGGTTCC-3′ and 5′-CCAATAGTAAGGGAACTTCAGCC-3′) were added together in each PCR reaction. The gcr1-3 insertion mutant (WS background) is described elsewhere (Pandey and Assmann, 2004). It should be clarified that GCR1, which is located on chromosome 1, was originally incorrectly mapped to chromosome 5 (Plakidou-Dymock et al., 1998).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining permission will be the responsibility of the requestor.
GCR1 Constructs
The open reading frame of GCR1 was amplified by PCR from a cDNA library made from seedlings grown in light for 10 d and cloned into the pENTR/D-TOPO vector (Invitrogen, Carlsbad, CA), subcloned into Gateway plant transformation destination vector pGWB5 (Research Institute of Molecular Genetics, Matsue, Japan) by an LR recombination reaction, and transformed into Arabidopsis suspension cells by Agrobacterium-mediated transformation (Ferrando et al., 2000). In this vector, expression of GCR1-GFP was driven by the 35S promoter of the cauliflower mosaic virus.
To create the GCR1::GUS fusion construct, genomic DNA 932 bp upstream of the GCR1 start code was cloned into the pENTR/D-TOPO vector, subcloned into the Gateway plant transformation destination vector pBGWFS7 (Karimi et al., 2002), and transformed into Arabidopsis (Col-0) by Agrobacterium-mediated transformation (Bechtold and Pelletier, 1998).
Assays
Sterilized wild-type and mutant seeds from matched lots were pretreated with 8 μm PAC (Chem Service, West Chester, PA) in the dark at 4°C for 2 d, washed 6 times with deionized water, sown on plates containing one-half-strength Murashige and Skoog basal medium with Gamborg's vitamins (ICN Biomedicals, Aurora, OH), 1% Suc, 0.5% phytoagar (Research Products International, Mt. Prospect, IL), pretreated with 75 μmol m−2 s−1 light for 6 h, and treated with different concentrations of GA3 or BL. After 3 d in darkness at 23°C, the percentage of germination was scored. Germination is defined here as an obvious protrusion of the radicle through the seed coat. Each experiment was repeated at least twice. A minimum of 50 seeds was scored for each treatment of each genotype.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AAO62367, P35352, BAA99285, and At1g48270.
Supplementary Material
Acknowledgments
We thank Miin-Feng Wu and Jiansheng Liang (both University of North Carolina) for technical assistance and Tsuyoshi Nakagawa (Shimane University, Nishikawatsu, Japan) for making certain plant destination vectors freely available to us and the plant biology community.
This work was supported by the NIGMS (grant no. GM65989–01 to A.M.J.) and by the NSF (grant no. MCB–0209711 to A.M.J.; grant no. MCB–0209694 to S.M.A.; grant no. 0115103 to J.R.E.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.038992.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Apone F, Alyeshmerni N, Wiens K, Chalmers D, Chrispeels MJ, Colucci G (2003) The G-protein-coupled receptor GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C. Plant Physiol 133: 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Natl Acad Sci USA 96: 10284–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM (2002) Heterotrimeric and unconventional GTP binding proteins in plant signaling. Plant Cell 14: S355–S373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Brzostowski JA, Kimmel AR (2001) Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci 26: 291–297 [DOI] [PubMed] [Google Scholar]
- Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP (2003) A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 301: 1728–1731 [DOI] [PubMed] [Google Scholar]
- Chun K, Liyanage UK, Lisanti MP, Lodish HF (1994) Signal tranduction of a G-protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci USA 91: 11728–11732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci G, Apone F, Alyeshmerni N, Chalmers D, Chrispeels MJ (2002) GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering. Proc Natl Acad Sci USA 99: 4736–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC (2003) Fluorescent ligands, antibodies, and proteins for the study of receptors. Pharmacol Ther 100: 101–118 [DOI] [PubMed] [Google Scholar]
- Drmota T, Gould GW, Milligan G (1998) Real time visualization of agonist-mediated redistribution and internalization of a green fluorescent protein-tagged form of the thyrotropin-releasing hormone receptor. J Biol Chem 273: 24000–24008 [DOI] [PubMed] [Google Scholar]
- Ferrando A, Farras R, Jasik J, Schell J, Koncz C (2000) Intron-tagged epitope: a tool for facile detection and purification of proteins expressed in Agrobacterium-transformed plant cells. Plant J 22: 553–560 [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y (1999) Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci USA 96: 7575–7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649 [DOI] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Lefkowitz RJ (1999) Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol 145: 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuss C, Gerber U (2000) G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci 23: 469–475 [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weiss CA, Ma H (1994) Regulated expression of the Arabidopsis G protein α subunit gene GPA1. Int J Plant Sci 155: 3–14 [Google Scholar]
- Humphrey TV, Botella JR (2001) Re-evaluation of the cytokinin receptor role of the Arabidopsis gene GCR1. J Plant Physiol 158: 645–653 [Google Scholar]
- Iwasaki Y, Fujisawa Y, Kato H (2003) Function of heterotrimeric G protein in gibberellin signaling. J Plant Growth Regul 22: 126–133 [Google Scholar]
- Josefsson LG, Rask L (1997) Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur J Biochem 249: 415–420 [DOI] [PubMed] [Google Scholar]
- Jones AM (2002) G-protein-coupled signaling in Arabidopsis. Curr Opin Plant Biol 5: 402–407 [DOI] [PubMed] [Google Scholar]
- Jones AM, Ecker JR, Chen JG (2003) A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol 131: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallal L, Gagnon AW, Penn RB, Benovic JL (1998) Visualization of agonist-induced sequestration and down-regulation of a green fluorescent protein-tagged beta2-adrenergic receptor. J Biol Chem 273: 322–328 [DOI] [PubMed] [Google Scholar]
- Kanyuka K, Couch D, Hooley R (2001) A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Curr Biol 11: 535. [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kato H, Oki K, Yamamoto S, Fujisawa Y, Iwasaki Y (2003) Isolation and characterization of OsGCR1 encoding a putative G protein-coupled receptor in rice (abstract no. 885). In Plant Biology 2003, July 25–30, 2003, Honolulu, HI. American Society of Plant Biologists, Rockville, MD, p 185
- Kimmel AR, Parent CA (2003) The signal to move: D. discoideum go orienteering. Science 300: 1525–1527 [DOI] [PubMed] [Google Scholar]
- Konvicka K, Campagne F, Weinstein H (2000) Interactive construction of residue-based diagrams of proteins: the RbDe web service. Protein Eng 13: 395–396 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5: 33–36 [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Lapik YR, Kaufman LS (2003) The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 15: 1578–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G (2001) Brassinosteroids and gibberellins promote tobacco seed germination by distinct pathways. Planta 213: 758–763 [DOI] [PubMed] [Google Scholar]
- McLean AJ, Milligan G (2000) Ligand regulation of green fluorescent protein-tagged forms of the human β1-and β2-adrenoceptors; comparison with the unmodified receptors. Br J Pharmacol 130: 1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Assmann SM (2004) The Arabidopsis putative G-protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16: 1616–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP (2002) The role of GA-mediated signalling in the control of seed germination. Curr Opin Plant Biol 5: 376–381 [DOI] [PubMed] [Google Scholar]
- Plakidou-Dymock S, Dymock D, Hooley R (1998) A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Curr Biol 8: 315–324 [DOI] [PubMed] [Google Scholar]
- Steber CM, McCourt P (2001) A role for brassinosteroids in germination in Arabidopsis. Plant Physiol 125: 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE (2004) Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609 [DOI] [PubMed] [Google Scholar]
- Tarasova NI, Stauber RH, Choi JK, Hudson EA, Czerwinski G, Miller JL, Pavlakis GN, Michejda CJ, Wank SA (1997) Visualization of G-protein-coupled receptor trafficking with the aid of the green fluorescent protein: endocytosis and recycling of cholecystokinin receptor type A. J Biol Chem 272: 14817–14824 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M (2000) Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci USA 97: 11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- Vanderbeld B, Kelly GM (2000) New thoughts on the role of βγ subunit in G-protein signal transduction. Biochem Cell Biol 78: 537–550 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.