Abstract
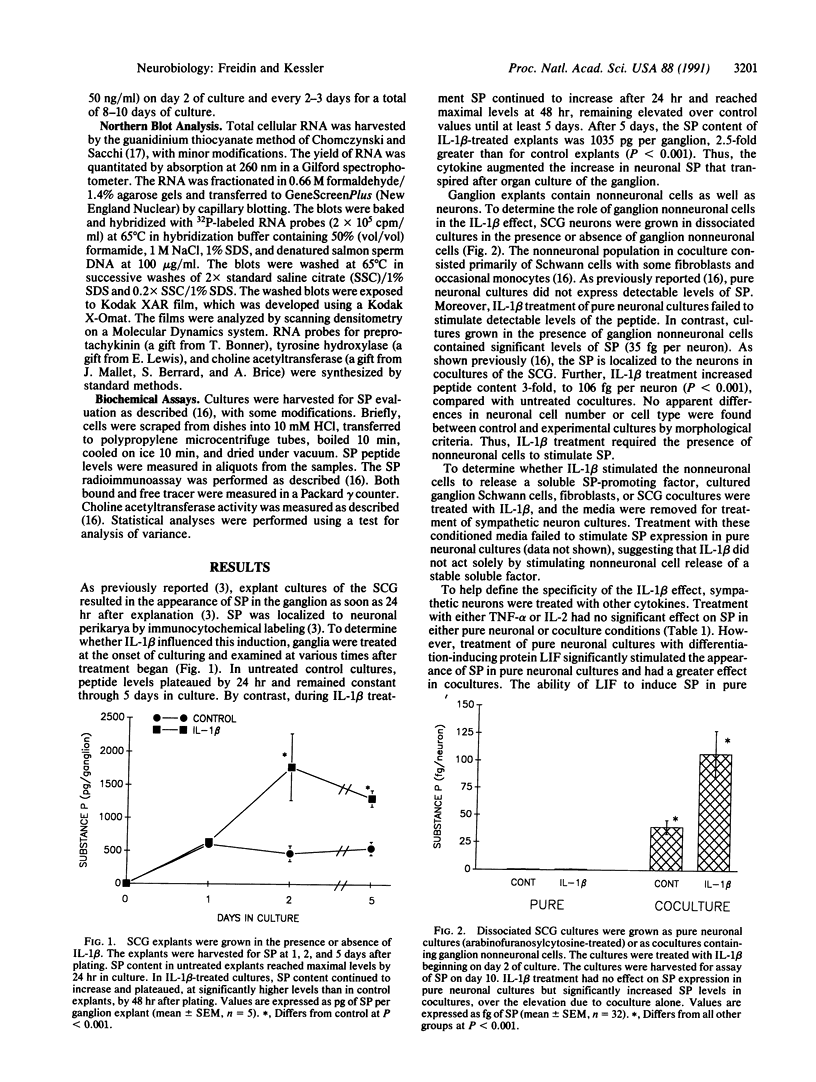
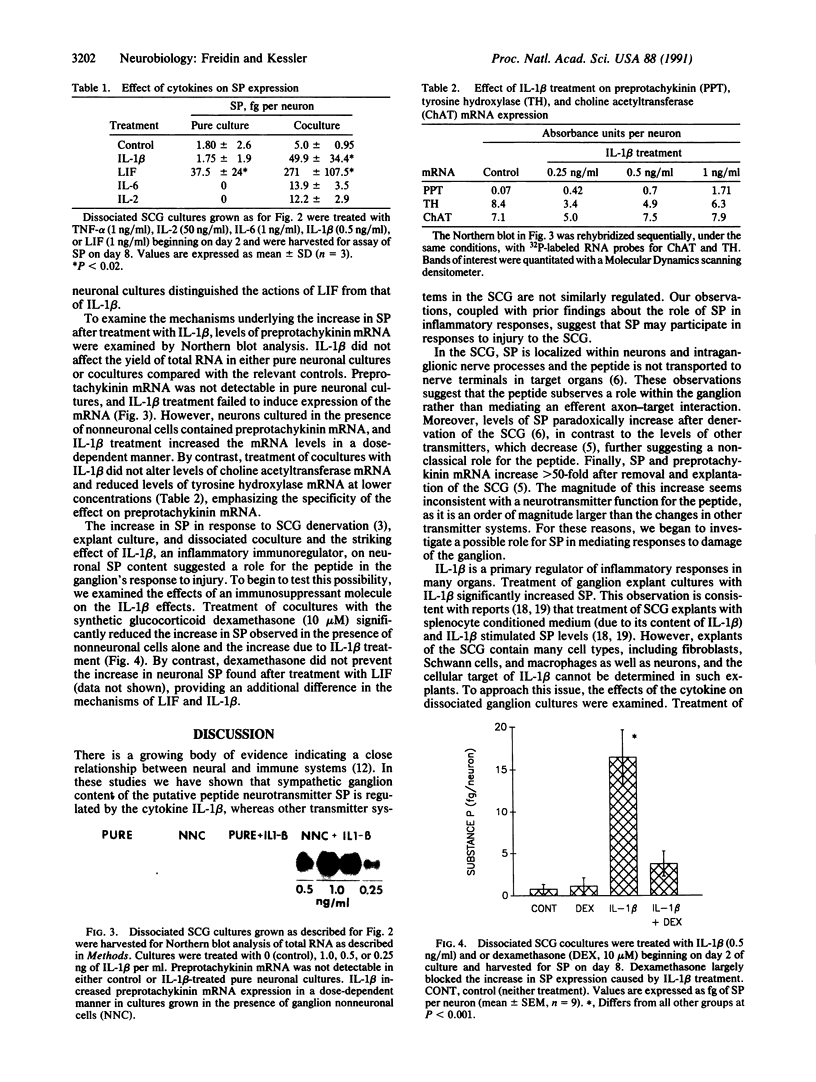
The nervous and immune systems interact in a bidirectional fashion. For example, the neuropeptide substance P (SP) has been implicated in a variety of immune responses. Conversely, cytokines, a class of immunoregulatory glycoproteins, affect the synthesis of neurotransmitters and neurotrophic factors. This paper examines the role of cytokines in regulating neuropeptide expression in sympathetic neurons. Exposure of cultured explants of the rat superior cervical ganglion to the cytokine interleukin 1 beta (IL-1 beta) increased levels of SP. IL-1 beta increased neuronal SP expression in dissociated cultures of ganglion neuronal and nonneuronal cells but had no effect on peptide content in pure neuronal cultures. By contrast, treatment with a differentiation-promoting protein, leukemia inhibitory factor, increased SP in both pure neuronal and mixed cultures, indicating a different mechanism of action for the two molecules. The specificity of the IL-1 beta effect was further demonstrated by the lack of response to treatment with other cytokines, including interleukin 2, interleukin 6, and tumor necrosis factor alpha. The cell type necessary for the IL-1 beta activity is probably the ganglion Schwann cell. Treatment with a synthetic immunosuppressant glucocorticoid, dexamethasone, blocked the increase in SP after treatment with IL-1 beta. These observations support the hypothesis that neuropeptide expression is regulated, in part, by interactions with specific immunoregulators. In addition, the data suggest a role for SP in mediating the response of the superior cervical ganglion to injury of the ganglion itself or to the fibers innervating it.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. E., Black I. B. Sympathetic neuron density differentially regulates transmitter phenotypic expression in culture. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4296–4300. doi: 10.1073/pnas.82.12.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Savage N. Interleukin-1 and its receptor. Crit Rev Immunol. 1989;9(1):1–20. [PubMed] [Google Scholar]
- Fukada K. Hormonal control of neurotransmitter choice in sympathetic neurone cultures. Nature. 1980 Oct 9;287(5782):553–555. doi: 10.1038/287553a0. [DOI] [PubMed] [Google Scholar]
- Jonakait G. M., Schotland S. Conditioned medium from activated splenocytes increases substance P in sympathetic ganglia. J Neurosci Res. 1990 May;26(1):24–30. doi: 10.1002/jnr.490260104. [DOI] [PubMed] [Google Scholar]
- Jonakait G. M., Schotland S., Hart R. P. Interleukin-1 specifically increases substance P in injured sympathetic ganglia. Ann N Y Acad Sci. 1990;594:222–230. doi: 10.1111/j.1749-6632.1990.tb40482.x. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Black I. B. Substance P and somatostatin regulate sympathetic noradrenergic function. Science. 1983 Sep 9;221(4615):1059–1061. doi: 10.1126/science.6192502. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Conn G., Hatcher V. B. Isolated plasma membranes regulate neurotransmitter expression and facilitate effects of a soluble brain cholinergic factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3528–3532. doi: 10.1073/pnas.83.10.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A. Differential regulation of peptide and catecholamine characters in cultured sympathetic neurons. Neuroscience. 1985 Jul;15(3):827–839. doi: 10.1016/0306-4522(85)90081-8. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Fisher M. C. Potentiation of IL-1-induced BALB/3T3 fibroblast proliferation by neuropeptides. J Immunol. 1988 Dec 15;141(12):4203–4208. [PubMed] [Google Scholar]
- Kimball E. S. Substance P, cytokines, and arthritis. Ann N Y Acad Sci. 1990;594:293–308. doi: 10.1111/j.1749-6632.1990.tb40489.x. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Hengerer B., Thoenen H. Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem. 1988 Nov 5;263(31):16348–16351. [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- McDonald J. K. NPY and related substances. Crit Rev Neurobiol. 1988;4(1):97–135. [PubMed] [Google Scholar]
- Nawa H., Patterson P. H. Separation and partial characterization of neuropeptide-inducing factors in heart cell conditioned medium. Neuron. 1990 Feb;4(2):269–277. doi: 10.1016/0896-6273(90)90101-k. [DOI] [PubMed] [Google Scholar]
- O'Byrne E. M., Blancuzzi V., Wilson D. E., Wong M., Jeng A. Y. Elevated substance P and accelerated cartilage degradation in rabbit knees injected with interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1990 Jul;33(7):1023–1028. doi: 10.1002/art.1780330715. [DOI] [PubMed] [Google Scholar]
- Pascual D. W., Blalock J. E., Bost K. L. Antipeptide antibodies that recognize a lymphocyte substance P receptor. J Immunol. 1989 Dec 1;143(11):3697–3702. [PubMed] [Google Scholar]
- Reusch M. K., Karasek M. A., Nickoloff B. J. Effect of neuropeptides present in skin on the proliferation of human peripheral blood mononuclear cells and T cells. Arch Dermatol Res. 1988;280(5):279–281. doi: 10.1007/BF00440600. [DOI] [PubMed] [Google Scholar]
- Saadat S., Sendtner M., Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J Cell Biol. 1989 May;108(5):1807–1816. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S. K., Cierpial M. A., Kilts C., Ritchie J. C., Weiss J. M. Brain IL-1-induced immunosuppression occurs through activation of both pituitary-adrenal axis and sympathetic nervous system by corticotropin-releasing factor. J Neurosci. 1990 Nov;10(11):3701–3706. doi: 10.1523/JNEUROSCI.10-11-03701.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall T. C., Martin J., Chen X. L., Ciarleglio A., Carpentier S., Henderson K., Knuepfer M., Beinfeld M., Naes L. Cardiovascular effects and modulation of noradrenergic neurotransmission following central and peripheral administration of neuropeptide Y. Synapse. 1988;2(3):299–307. doi: 10.1002/syn.890020320. [DOI] [PubMed] [Google Scholar]
- Wong V., Kessler J. A. Solubilization of a membrane factor that stimulates levels of substance P and choline acetyltransferase in sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8726–8729. doi: 10.1073/pnas.84.23.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Fukada K., Aebersold R., Korsching S., Fann M. J., Patterson P. H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]



