Abstract
A central problem in plant biology is how cell expansion is coordinated with wall synthesis. We have studied growth and wall deposition in epidermal cells of dark-grown Arabidopsis hypocotyls. Cells elongated in a biphasic pattern, slowly first and rapidly thereafter. The growth acceleration was initiated at the hypocotyl base and propagated acropetally. Using transmission and scanning electron microscopy, we analyzed walls in slowly and rapidly growing cells in 4-d-old dark-grown seedlings. We observed thick walls in slowly growing cells and thin walls in rapidly growing cells, which indicates that the rate of cell wall synthesis was not coupled to the cell elongation rate. The thick walls showed a polylamellated architecture, whereas polysaccharides in thin walls were axially oriented. Interestingly, innermost cellulose microfibrils were transversely oriented in both slowly and rapidly growing cells. This suggested that transversely deposited microfibrils reoriented in deeper layers of the expanding wall. No growth acceleration, only slow growth, was observed in the cellulose synthase mutant cesA6prc1-1 or in seedlings, which had been treated with the cellulose synthesis inhibitor isoxaben. In these seedlings, innermost microfibrils were transversely oriented and not randomized as has been reported for other cellulose-deficient mutants or following treatment with dichlorobenzonitrile. Interestingly, isoxaben treatment after the initiation of the growth acceleration in the hypocotyl did not affect subsequent cell elongation. Together, these results show that rapid cell elongation, which involves extensive remodeling of the cell wall polymer network, depends on normal cellulose deposition during the slow growth phase.
Plants have evolved a hydrostatic skeleton as an economic way to create the large surfaces needed for optimal capture of light; cells are filled with water and solutes reaching pressures of up to 10 bars. These high pressures are made possible by the presence of a tough extracellular matrix, the cell wall. How cells expand despite the presence of the cell wall is a central issue in plant biology. While driven by the osmotic pressure, cell expansion is thought to be controlled by changes in the extensibility of the wall (Taiz, 1984). The wall of growing cells behaves as a biphasic composite material, which in noncommelinoid cell walls consists of a load-bearing cellulose-xyloglucans network embedded in a pectin matrix. The latter controls elasticity, hydration, wall porosity, and presumably the mobility of wall-modifying enzymes, as well as the adhesion between cells (Willats et al., 2001; Chanliaud et al., 2002). Cell wall expansion is considered to be the result of a creep between cellulose microfibrils and xyloglucans (Pauly et al., 1999). Cell wall relaxing agents like expansins promote this creep and may therefore induce cell expansion in vivo (Cosgrove, 1999). The expression patterns and activities of these relaxing agents support the conclusions of early ultrastructural studies, referred to as the multinet growth hypothesis (Roelofsen, 1958; Green, 1960). This hypothesis arose from the observation that, in most elongating cells, polysaccharides are transversely oriented (with respect to the elongation axis) next to the plasma membrane and adopt a longitudinal orientation in deeper cell wall layers. The hypothesis proposes that the longitudinally oriented components initially had been transverse, indicating that remodeling (reorientation) is a key determinant of cell elongation. In this commonly accepted scheme, the synthesis of cell wall polysaccharides is thought to occur simultaneously with elongation, allowing its continuation, but is not believed to directly control cell elongation. Physiological observations also support this idea: e.g. in isolated pea epicotyl fragments, inhibition of cellulose synthesis by dichlorobenzonitrile (DCB) did not prevent the promotion of cell elongation by auxin over periods of several hours (Brummell and Hall, 1985).
In contrast to this passive role for cellulose synthesis in cell growth, the orientation of microfibrils plays a key role in the control of the growth direction. Indeed, the inhibition of cellulose deposition by either mutations or inhibitors dramatically influences organ growth (Fagard et al., 2000a). This effect was linked to the ability of the cellulose microfibrils to control the direction of cell expansion. Under normal conditions, transversely oriented microfibril arrays constrain growth in one direction (Takeda and Shiboaka, 1981; Verbelen and Stickens, 1995; Sugimoto et al., 2000), and upon inhibition of cellulose synthesis, randomization of the remaining microfibrils leads to isotropic cell expansion (Sugimoto et al., 2001). From these and other observations emerges the current view that cellulose deposition controls the direction but not the extent of cell expansion.
We previously described Arabidopsis mutants in the cellulose synthase isoform CESA6. Loss-of-function cesA6prc1 mutants show a reduced cellulose content and a growth defect in roots and dark-grown hypocotyls. One mutant allele, cesA6ixr2 (Desprez et al., 2002), does not show a growth phenotype but is resistant against the cellulose synthesis-inhibiting herbicide isoxaben. A second isoxaben-resistance locus IXR1 encodes cellulose synthase isoform CESA3 (Scheible et al., 2001). These and other observations suggest that both CESA6 and CESA3, presumably in a protein complex, constitute the target for isoxaben (Robert et al., 2004). In this study, we observed that, in contrast to cellulose-deficient mutants rsw1 (Sugimoto et al., 2001) and kob1 (Pagant et al., 2002), the cellulose synthesis defect in cesA6prc1 was not accompanied by a perturbation of the microfibril orientation. The dwarfism observed in these mutants was therefore not simply due to the fragilization of the cell wall and the ensuing loss of growth anisotropy, but may reflect a role for cellulose synthesis in the control of the extent of cell elongation. To investigate this possibility, we studied elongation kinetics and cell wall assembly in elongating cells of dark-grown Arabidopsis cv Columbia hypocotyls. We first showed that all hypocotyl cells elongated uniformly at a slow rate after germination and that at 48 h after seed imbibition, the growth of cells at the hypocotyl base accelerated. This acceleration propagated through the hypocotyl along an acropetal gradient. Our observations led to the following main conclusions: (1) cell wall synthesis was not coupled to the rate of cell elongation, (2) normal deposition of cellulose during the slow growth phase was a prerequisite for subsequent rapid growth, and (3) this rapid growth phase involved extensive remodeling of the polysaccharide network.
RESULTS
Cell Elongation Kinetics in Dark-Grown Hypocotyls
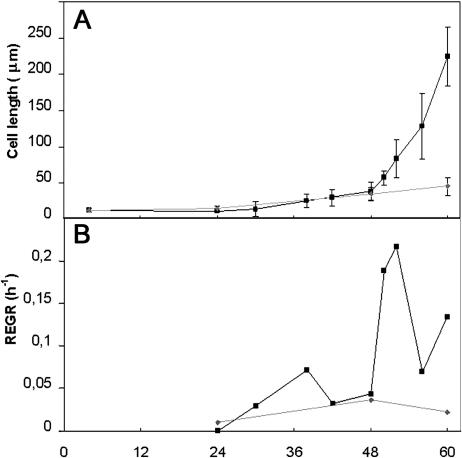
Cell elongation kinetics were investigated in dark-grown hypocotyls of wild-type Arabidopsis cv Columbia. Epidermal and cortical cells within this organ, except the cells that contribute to stomata formation, do not undergo cell divisions (Gendreau et al., 1997). In 3-d-old seedlings, each epidermal cell row of the hypocotyl contained 19.5 ± 0.8 cells. Epidermal cell length was measured in imbibed seeds (more precisely 4 h after imbibition) and at 24, 48, and 60 h after seed imbibition. We conducted cell measurements at two points along the length of the hypocotyl—at the 3rd and the 13th cells above the collet. In imbibed seeds, all epidermal cells were 11.7 ± 2 μm long. The increases in length of cells 3 and 13 over the first 60 h after imbibition are shown in Figure 1A.
Figure 1.
Kinetics of hypocotyl cell elongation of wild-type Arabidopsis cv Columbia seedlings grown in the dark. A, Cell length. B, REGR. Third epidermal cell (▪) and 13th epidermal cell (♦) as counted from the collet were measured.
Cell elongation was initiated throughout the hypocotyl at 24 h after imbibition. Between 24 and 48 h, both cell 3 and cell 13 showed a comparable slow growth rate of 1.0 and 0.8 μm/h, respectively (Fig. 1A), and both cells reached the same length at 48 h (38.4 ± 7.6 μm and 34.0 ± 9.6, respectively). Between 48 and 50 h, the growth of cell 3 accelerated, and at 60 h it had reached a length of 224.6 ± 51.5 μm. This corresponded to a higher relative elemental growth rate (REGR; see “Materials and Methods” for calculation) as shown in Figure 1B. By contrast, cell 13 maintained the same slow elongation rate, reaching a length of only 44.6 ± 12.8 μm at 60 h. The growth acceleration propagated following an acropetal gradient, and by 60 h the lower two-thirds of the hypocotyl had entered the rapid elongation phase (data not shown). The growth of cell 13 only underwent a growth acceleration at 80 h after it had reached a length of 82 ± 9 μm (data not shown).
Cell Wall Synthesis Is Not Coupled to the Cell Elongation Rate
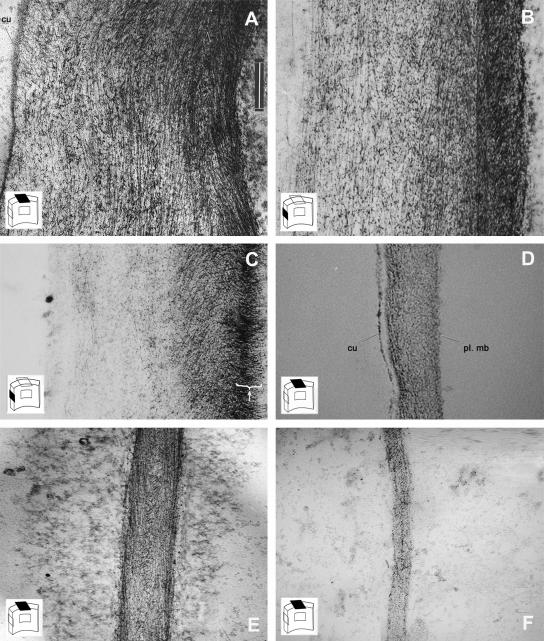
To study the structure of the wall at different cell elongation stages, we compared in 4-d-old seedlings slowly growing cells with a length of around 80 μm at the tip of the hypocotyl, with rapidly growing cells toward the base of the hypocotyl (approximately at the middle of the hypocotyl), which were around 400 μm long. All sections were first treated with methylamine, which extracts most of the pectins and hemicelluloses and induces only a very limited swelling of the cell walls (Reis et al., 1985). The remaining polysaccharides were stained with PATAg (periodic acid thiocarbohydrazide-silver proteinate; Roland et al., 1977, 1982). Transverse sections through the slowly growing cells revealed thick external epidermal walls (Fig. 2A; 1640 ± 361 nm) and 4-fold thinner radial or tangential cortical cell walls (Fig. 2E; 400 ± 80 nm).
Figure 2.
Wall architecture of wild-type hypocotyl cells at different stages of elongation in 4-d-old dark-grown seedlings. Ultrathin sections were extracted with methylamine and subsequently stained with PATAg to reveal nonextracted polysaccharides (mainly cellulose). The cartoon on the lower left of each photograph indicates the position of the section with respect to the cell wall orientation. A to D, External epidermal wall. E and F, Radial cortical walls. Transverse (A and E) and longitudinal (B) sections through slowly growing cells at the top of the hypocotyl. Longitudinal (C) and transverse (D and F) section through rapidly growing cells toward the hypocotyl base. Arrowhead in C shows the transverse orientation of the innermost polysaccharide layer in this section, which was slightly tangential to the plasma membrane. cu, cuticle; pl. mb, plasma membrane. Scale bar = 300 nm.
Polysaccharides in these walls showed a regular organization, appearing as dots or as fibers in homogeneous layers parallel to the plasma membrane. The fibers viewed on these sections, which presumably corresponded to cellulose microfibrils, were oriented transversely to the cell elongation axis. Interestingly, longitudinal sections of external epidermal walls of cells at the same growth stage also showed fibers parallel to the plasma membrane (Fig. 2B), which suggested that a subset of the cellulose microfibrils in these walls was oriented longitudinally to the cell elongation axis. We conclude that, in these slowly growing cells, the external epidermal cell walls consisted of successive polysaccharide layers with different orientations, a texture referred to as polylamellated. A comparable texture was also observed in the thinner cell walls of other tissues (cortex and endoderm; Fig. 2E).
Polylamellated architectures have been described for secondary cell walls, albeit with a more regular texture (Roland et al., 1987) and in primary cell walls in mung bean (Vigna radiata) hypocotyls (Satiat-Jeunemaitre, 1981; Roland et al., 1982). In rapidly growing cells, with a length of 400 μm or longer, both external epidermal cell walls (Fig. 2D) and cortical walls (Fig. 2F) were much thinner than corresponding walls in slowly growing cells.
Moreover, transverse sections of these walls showed little or no fibers, which corresponded to transverse cellulose microfibrils. Longitudinal sections (Fig. 2C; epidermal cell of 283 μm) confirmed the longitudinal orientation of the bulk of cell wall polysaccharides. Interestingly, some sections were slightly tangential to the cell surface and clearly showed that the innermost polysaccharide layers invariably had a transverse orientation (similar observations on other tangential sections through cortical cells, n = 7; epidermal cells, n = 5).
Together our observations showed that the slowly growing cells at the hypocotyl tip had accumulated a thick polylamellated wall, whereas the more elongated cells toward the base had a much thinner wall with axially oriented polysaccharides. Substantial cell wall synthesis therefore had taken place in slowly growing cells. The thinner wall in rapidly growing cells may hence reflect a higher polymer remodeling activity compared with slowly growing cells without a corresponding increase in cell wall synthesis.
Microfibrils Are Deposited in Transverse Arrays Both in Slowly and Rapidly Growing Cells, whereas Deeper Layers Exhibit Polylamellated or Longitudinal Texture
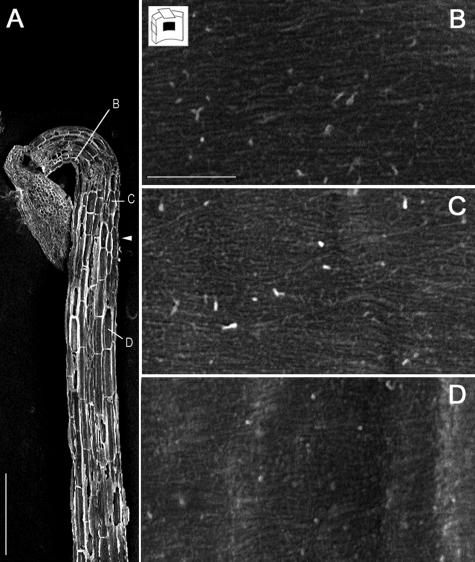
The presence of polysaccharide layers with different orientations in the walls of slowly growing cells implies either that successive cellulose microfibril layers are deposited with changing angles or that their orientation changes in deeper cell wall layers. To distinguish between these possibilities, we used field emission scanning electron microscopy (FESEM) to study in slightly younger wild-type seedlings (80 h old) the orientation of most recently deposited microfibrils in hypocotyl cells before and after growth acceleration. This method allowed the visualization of the tangential and radial walls of cortical cells and the radial walls of epidermal cells. All walls studied, in both cortical and epidermal cells (Fig. 3, B and C) and in both slowly and rapidly growing cells (Fig. 3D), displayed innermost microfibrils with a transverse orientation, which confirms the results obtained by transmission electron microscopy. Although the possibility that microfibrils had been deposited longitudinally at earlier cell growth stages, which are not represented in 80-h-old hypocotyls, cannot be ruled entirely, the most likely explanation is that the polylamellated architecture in slowly growing cells resulted from the reorientation of polysaccharides after their deposition in transverse arrays. Also, for the thinner walls in rapidly growing cells, it seems likely that transversely deposited microfibrils had reoriented toward a longitudinal orientation in deeper wall layers.
Figure 3.
Transversely oriented innermost cell wall layers in wild-type hypocotyl cells at different stages of elongation. Field emission scanning electron microscopy of 80-h-old dark-grown hypocotyls. A, Low magnification of the cryosectioned sample. The positions of higher magnifications presented in B, C, and D are shown. The arrowhead marks the approximate location of the growth acceleration zone. Scale bar in A = 300 μm. B and C, Slowly growing cells at the top of the hypocotyl. D, Rapidly growing cell further toward the hypocotyl base. B, Epidermal cell. C and D, Cortical cells. Scale bars in B, C, and D = 500 nm.
Normal Cellulose Deposition during the Slow Growth Phase Is Essential for Subsequent Growth Acceleration
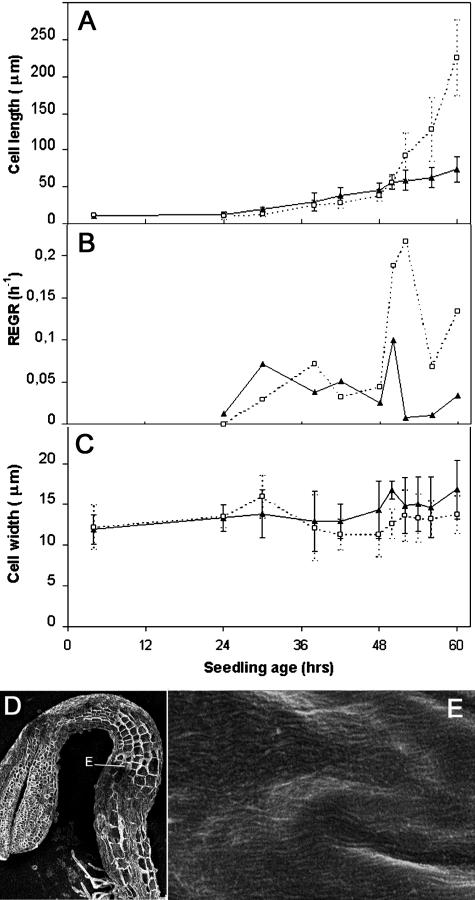
To study the role of cellulose synthesis in cell elongation, we first recorded cell elongation in dark-grown hypocotyls of the cesA6prc1-1 mutant. This mutant carries a premature stop codon in CESA6, one of the catalytic subunits of cellulose synthase, and shows reduced cellulose synthesis (Fagard et al., 2000b). Like in the wild type, cell elongation was initiated throughout the hypocotyl at 24 h after imbibition (Fig. 4A). During the slow growth phase (between 24 and 48 h), the elongation rate was indistinguishable from that of the wild type in cell 3 and cell 13. However, unlike the wild type, no acceleration occurred in cell 3 (or in any other hypocotyl cell; data not shown), which maintained an average slow elongation rate of 1.6 μm/h (Fig. 4A). cesA6prc1-1 cells did not show an increased radial expansion at this stage (Fig. 4C). Poor elongation in the cesA6prc1-1 mutant was therefore not due to a loss in cell growth anisotropy but to the inability to undergo a growth acceleration. Intriguingly, increased radial expansion was observed at later stages in mutant cortical cells (Fagard et al., 2000b).
Figure 4.
Kinetics of hypocotyl cell elongation (as compared with wild type) and orientation of innermost cellulose microfibrils in cellulose-deficient prc1-1 seedlings grown in the dark. Cell length (A), REGR (B), and cell width (C). D and E, FESEM of 80 h dark-grown prc1-1 cryosectioned hypocotyl. D, Low magnification (scale bar = 300 μm); the position of the higher magnification presented in E is shown. E, High magnification (same magnification as Fig. 3A). Third epidermal cells as counted from the collet were measured in prc1-1 (black triangle) and compared with wild type (white square; same magnification as Fig. 3, B–D).
The inhibition of cellulose synthesis in cellulose-deficient mutants such as cesA1rsw1-1 and kob1 (Pagant et al., 2002) or by the inhibitor DCB (Sugimoto et al., 2001) causes randomization of the remaining cellulose microfibrils in root cells. This aberrant cellulose deposition pattern was thought to cause reduced cell elongation and increased radial expansion. We therefore used FESEM to study microfibrils in cells at the top and at the base of 80-h-old cesA6prc1-1 hypocotyls. In contrast to other cellulose-deficient mutants or wild-type plants treated with DCB, the innermost microfibrils in this mutant were indistinguishable from those of the wild type (Fig. 4E) at all growth stages. The growth defect in cesA6prc1-1 therefore could not be explained simply by the disorganization of the microfibrils and the loss of growth anisotropy. The results rather suggest that ability of the cell to accelerate its growth is conditioned by the rate and/or extent of cellulose deposition in the wall. In this view, the simplest explanation for the absence of a growth acceleration in dark-grown hypocotyl cells of cesA6prc1-1 would be that in the wild type, CESA6 is expressed only from 48 h on and that CESA6-dependent cellulose synthesis is essential for the growth acceleration. This is unlikely the case since CESA6 mRNA levels appear and peak immediately after imbibition and no increase in mRNA levels is observed at 48 h (Fagard et al., 2000b). Nevertheless, posttranscriptional regulation of CESA6 cannot be ruled out. Ideally, the use of a conditional allele of CESA6 could indicate at what stage its activity is required for the growth acceleration. In the absence of such an allele, we took advantage of isoxaben, a cellulose synthesis inhibitor that targets both CESA3 and CESA6 and that phenocopies cesA6prc1-1 in the dark at a concentration of 4 nm (Desprez et al., 2002). Dark-grown seedlings were transferred at different ages to a medium with 4 nm isoxaben under a green safe light and allowed to develop for a further 2 d in the dark (Fig. 5B), after which hypocotyl length was measured (Fig. 5A).
Figure 5.
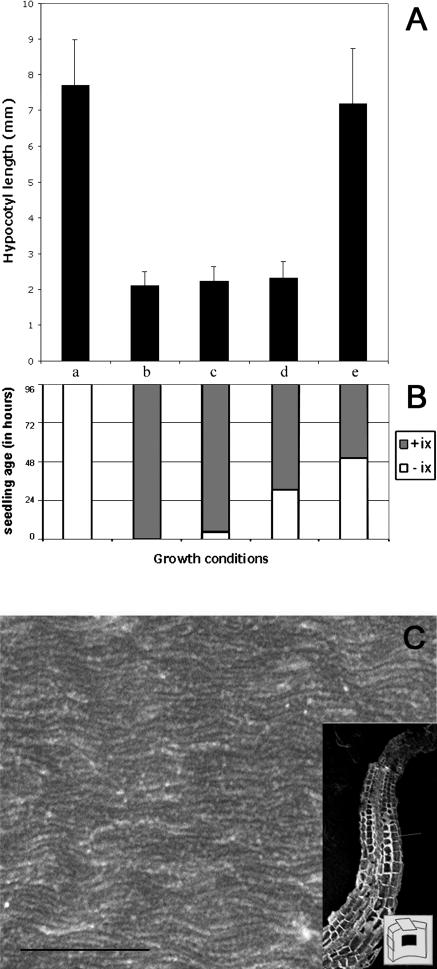
Effects of cellulose synthesis inhibition by isoxaben on hypocotyl elongation. A, Hypocotyl length of 4-d-old dark-grown seedlings. B, Diagram showing experimental growth conditions for each sample. Seedlings were grown for 4 d continuously in the absence (a) or presence (b) of isoxaben (ix); 4 h without, followed by 92 h with isoxaben (c); 30 h without, followed by 66 h with isoxaben (d); 50 h without, followed by 46 h with isoxaben (e). C, FESEM of innermost wall layer of an epidermal cell of a seedling grown in the presence of isoxaben. Scale bar of the high magnification = 500 nm. Inset shows a low-magnification image of the cryosectioned hypocotyl and the position of the cell in which the presented image was taken. The cartoon in the inset shows the location of the image with respect to the cell wall orientation. Isoxaben concentration in all experiments was 4 nm.
As described earlier, hypocotyl elongation was dramatically inhibited when seedlings were allowed to germinate on a medium containing isoxaben (Heim et al., 1990; Desprez et al., 2002). As in cesA6prc1-1, FESEM showed that the innermost microfibril orientation remained unaltered in isoxaben-treated seedlings (Fig. 5C). The hypocotyls of seedlings transferred to isoxaben at 4 h or 30 h were as short and radially expanded as those of seedlings that had germinated on isoxaben (Fig. 5A). Intriguingly, transfer to isoxaben at 50 h did not lead to an observable growth inhibition or to an increased radial expansion (data not shown). The absence of growth inhibition by isoxaben was not due to the loss of the sensitivity of cellulose synthesis to the inhibitor after 50 h. Indeed, in 4-d-old seedlings, cellulose synthesis, as measured by the incorporation of 14C-Glc into the acetic::nitric acid resistant fraction, was inhibited in less than 1 h after addition of isoxaben (G. Mouille, unpublished data).
Also, Fourier transform infrared microspectroscopy showed that hypocotyls of 4-d-old seedlings that had been transferred after 50 h to isoxaben contained less cellulose than those of untreated controls with the same length (data not shown). These results indicate first that perturbation of cellulose synthesis during the slow growth phase (between 24 and 48 h) by isoxaben or in the cesA6prc1-1 mutant abolished the capacity of the cell to accelerate its growth. The absence of a correlation between the hypocotyl length and the duration of the isoxaben treatment during the slow growth phase suggests that the effect of isoxaben on subsequent growth was qualitative and did not simply reflect the amount of cellulose that was available for wall expansion. Second, once the growth acceleration gradient was initiated, normal cellulose synthesis under the control of CESA6 and CESA3 was not necessary anymore for normal cell elongation to occur.
DISCUSSION
Two Cell Elongation Phases in Dark-Grown Hypocotyl Cells
A major finding of this work is that cells in dark-grown hypocotyls elongated in two distinct phases. The transition between these two phases was marked by a growth acceleration, which in our growth conditions occurred at 48 h at the hypocotyl base and propagated gradually toward the tip of the hypocotyl. Interestingly, two cell elongation phases also have been described in roots (Ishikawa and Evans, 1995; Sugimoto et al., 2001). Ishikawa and Evans distinguished a slow growth zone behind the meristem. In Arabidopsis, the REGR in this zone was approximately 0.06 h−1 (Sugimoto et al., 2001), which is similar to the rate observed in hypocotyls. Upon acceleration, which took place approximately 400 μm away from the tip, the REGR reached 0.6 h−1, which is 3-fold that of the hypocotyl measured in our system. Elongation rate differences may be due to differences in growth conditions (Granier et al., 2002) or may reflect intrinsic differences between the two organs. In roots, this slow elongation zone is implicated in modulating the growth responses to changing environments including gravitropism, thigmomorphogenesis, and various hormone responses (Ishikawa and Evans, 1995). Numerous signaling pathways converge to fine tune the processes taking place during this slow growth phase and that are critical for appropriate subsequent rapid growth. It will be interesting to see whether also in hypocotyls, hormones and light exert their effects during the slow growth phase. It is not clear whether a similar phase transition takes place in developing leaves (Granier et al., 2002).
Cell Wall Synthesis Is Not Coupled to Cell Growth
A second major conclusion of this work is that cellulose deposition is not correlated with the rate of cell elongation. In 4-d-old dark-grown hypocotyls, we observed a thick multilayered cell wall in cells prior to the growth acceleration and a much thinner wall in which polymers had adopted primarily an axial orientation in more elongated cells. At least in the cells of the upper part of the hypocotyl, the deposition of the thick cell wall therefore occurred prior to the growth acceleration, which is either during late embryogenesis or after seed imbibition. The expression pattern of CESA6, which is essential for the synthesis of cellulose prior to the growth acceleration, suggests that most of the cellulose synthesis occurred after embryogenesis. Indeed, CESA6 mRNA was not detected in developing embryos (Beeckman et al., 2002), and its level peaked at 24 h after seed imbibition (Fagard et al., 2000b). This was confirmed using promoter-β-glucuronidase fusions and in situ hybridization (G. Refrégier, unpublished data). The same peak in mRNA levels was observed for CESA1 (Fagard et al., 2000b).
This, together with the thinner epidermal cell wall observed in embryos compared with hypocotyl cells in the slow growth phase (Beeckman et al., 2002), strongly suggests that the deposition of the thick wall had taken place during the 24-h lag after seed imbibition and/or the slow growth phase prior to the growth acceleration. The fact that the walls of cells at different stages of growth did not have the same thickness, demonstrates that cell wall synthesis and cell elongation are independent processes. No feedback mechanism seems to exist that would link cell wall synthesis to the rate of cell elongation.
Absence of a Growth Acceleration in cesA6prc1-1 Mutants Is the Result of the Inhibition of Cellulose Synthesis during the Slow Growth Phase
The loss-of-function mutant prc1-1 for the cellulose synthase isoform CESA6 initiated growth at the same time and showed the same slow elongation rate as the wild type during the 48 h after seed imbibition. However, no subsequent growth acceleration was observed in the mutant. Paradoxically, this late growth phenotype appears to be the result of reduced cellulose synthesis during the initial slow growth phase. Indeed, isoxaben, which targets CESA6, presumably in a complex with CESA3, inhibits cellulose synthesis but only inhibited growth acceleration when applied during the slow growth phase. No effect on growth was observed when the inhibitor was applied once growth had accelerated in basal cells (Fig. 5). This indicates that, at this stage, cell elongation occurred independently from CESA6-mediated cellulose synthesis. The following picture emerges from these results: cell wall synthesis during the slow growth phase is essential for subsequent growth acceleration, and this acceleration involves the remodeling of previously deposited wall polymers. The absence of a correlation between hypocotyl length and the duration of the exposure to isoxaben during the slow growth phase indicates that the effect of isoxaben is qualitative, and not only due to the reduced amount of wall polysaccharides available for subsequent expansion. A simple interpretation of the effect of isoxaben is that it changes the ratio between cellulose and matrix polysaccharides of the layers that are deposited during the treatment. This perturbation would afterward either lead to a rigid layer blocking expansion even if deeper polymer layers still have the ability to be remodeled, or create a physical barrier, reducing the mobility of wall loosening agents that normally would migrate into the wall from the cytoplasmic side. Alternatively, a feedback mechanism may constitute a checkpoint, which senses the state of the newly deposited cell wall before triggering the wall loosening events that lead to the growth acceleration. Experiments are ongoing to distinguish between these possibilities.
Normal Orientation of Innermost Cellulose Microfibrils in cesA6prc1-1
A long-standing question is what controls growth anisotropy in plant cells. The innermost microfibrils, which are oriented transversely to the elongation axis, are thought to create an exoskeleton, which channels the turgor pressure of the cell in one direction. Recent studies indeed showed that innermost microfibrils were randomized in roots of cellulose-deficient mutants cesA1rsw1-1 (Sugimoto et al., 2001) and kob1-1 (Pagant et al., 2002), as well as in DCB-treated plants (Sugimoto et al., 2001), which occurred concomitantly with the loss of growth anisotropy. The orientation of the innermost microfibrils, however, is not the only factor that limits radial expansion. Indeed, mutants rsw4 and rsw7 show increased radial expansion with normally oriented microfibrils and microtubules (Wiedemeier et al., 2002). In addition, mor1 mutants, in which microtubules are depolymerized, show increased radial expansion without a change in microfibril orientation (Sugimoto et al., 2003). These observations indicate that other factors influence the extent of radial expansion. In this study we showed that the orientation of the microfibrils in cesA6prc1-1 also remained transverse in contrast to other cellulose-deficient mutants. Nevertheless, at later growth stages, cortical and endodermal hypocotyl cells showed a massive radial expansion (Fagard et al., 2000b). One possibility is that the increased matrix polysaccharide to cellulose ratio in the mutant favors slippage between microfibrils in the radial direction.
Another question is why microfibrils were randomized in cesA1rsw1-1, kob1, and DCB-treated roots but not in cesA6prc1-1 and isoxaben-treated cells. Most likely this is a quantitative rather than a qualitative effect since higher concentrations of isoxaben also led to the randomization of microfibrils (data not shown). Why exactly a reduced cellulose content leads to a randomization of the microfibrils is not known. An imbalance between forces that promote reorientation in growing cells and those promoting deposition of parallel arrays may lead to a premature randomization of the microfibrils.
Polylamellation in Primary Cell Walls Arises through an Unknown Mechanism
The thick external epidermal cell walls deposited prior to the growth acceleration observed in 4-d-old dark-grown hypocotyls consisted of consecutive layers of polysaccharides with different orientations. Cortical cell walls at this stage also showed a similar structure but with fewer polysaccharide layers. Interestingly, our results showed that, in both slowly and rapidly growing cells in 80-h-old hypocotyls, the microfibrils were deposited in arrays with a transverse orientation with respect to the elongation axis. Although we have not analyzed hypocotyl cells at earlier growth stages, so longitudinal and oblique deposition of microfibrils at those stages cannot be excluded, similar studies in roots also showed transversely oriented innermost microfibrils in cells at all growth stages (Sugimoto et al., 2001). The polylamellated architecture therefore may not be the result of changes in the direction of the movement of cellulose synthesizing complexes in the plasma membrane, but instead could arise as a result of the reorientation of cellulose microfibrils after their deposition in slowly growing cells. Remodeling agents such as expansins and/or xyloglucan transglycosylases hydrolysases (Cosgrove, 2000; Rose et al., 2002) may play a role in this process. Interestingly, recent work in petunia (Petunia hybrida) shows that the inhibition of an expansin isoform can lead to the inhibition of cellulose deposition, suggesting that this isoform plays a critical role in cell wall construction (Zenoni et al., 2004). In this scenario, it remains to be determined, however, how some polysaccharide layers can maintain a transverse orientation while others reorient to obtain the polylamellated architecture.
Two Possible Scenarios for Cell Wall Deposition and Remodeling during Cell Elongation
To explain our results, two scenarios can be proposed. The first scenario reconciles two distinct viewpoints on the synthesis and the dynamics of cell wall polymers in elongating cells. On one hand, there is the multinet growth hypothesis (Roelofsen, 1958), which draws the attention to microfibrils that reorient from transverse to axial in elongating cells but do not address the timing of the cell wall deposition. On the other hand, there is the work of Roland et al. (1982). Based on studies on mung bean hypocotyls, they described thick polylamellated, helicoidal walls in small cells toward the top of hypocotyls and much thinner walls in more elongated cells further toward their base. Their interpretation of these findings was that the highly structured helicoidal walls, which were deposited during early growth stages underwent extensive remodeling, leading to the dissipation of this structure during subsequent cell elongation. This viewpoint implicitly stated that cell wall synthesis occurred primarily before cell elongation. In our first scenario, substantial cell wall deposition occurs in all hypocotyl cells before and/or during a slow elongation phase (Fig. 6) leading to a thick polylamellated wall. Growth acceleration is caused by an increase in wall relaxation activity without a corresponding increase in cell wall synthesis. As a result, the polylamellated structure evolves into a simpler structure with mainly longitudinally oriented microfibrils.
Figure 6.
Two scenarios for cell wall synthesis and remodeling in elongating dark-grown hypocotyl cells. These scenarios were based on the following observations: embryonic cells are small with thin walls and they elongate slowly upon germination. Slowly growing cells at the top of 4-d-old hypocotyls have a thick polylamellated cell wall, especially the external epidermal walls. Rapidly growing cells around the middle of those hypocotyls have thin walls with primarily axially oriented polysaccharides. Inhibition of CESA6-dependent cellulose synthesis during the slow growth phase in the mutant or through administering isoxaben prevents cells from accelerating their growth. Isoxaben treatment after the initiation of the growth acceleration does not prevent further growth. In the first scenario, both cells at the top and the bottom of the hypocotyls follow the same growth steps: they accumulate a thick polylamellated wall during the slow growth phase (1a). During the rapid growth phase (1b), this wall is extensively remodeled through the action of wall relaxing agents. In the second scenario, cell wall thickness reflects the balance between wall synthesis and wall relaxation. Cell wall synthesis occurs continuously in all cells, an increase in the rate of wall relaxation and hence cell elongation is initiated at the hypocotyls basis at 48 h, whereas cells toward the top accelerate their growth later. As a result, smaller cells accumulate a thicker wall before their growth acceleration (1a), whereas cells toward the base maintain a thin wall before and after their growth acceleration (2). It is still conceivable in this scenario that the thick walls in smaller cells at the hypocotyl top undergo thinning after growth acceleration (1b). In this second scenario, the differential effect of isoxaben on cell elongation before and after growth acceleration is more difficult to explain. Question mark refers to a growth stage that has not been observed directly. The thicker side of the small cells represents the outer epidermal wall.
In the second scenario, wall synthesis occurs at a steady state in all hypocotyl cells and the thickness of the cell wall reflects the balance between cell wall synthesis and wall relaxation. As a result, slowly growing cells at the top accumulate thicker walls, whereas rapidly growing cells toward the hypocotyl base have thinner walls. Such thinner walls are therefore not necessarily the result of the thinning of thicker walls. In the latter scenario the differential effect on cell elongation of isoxaben administered before and after the initiation of the growth acceleration is more difficult to explain. We are currently carrying out kinetic experiments to distinguish between the two scenarios.
No matter which scenario is true, the observations described in this report provide a novel framework for further studies of the processes that coordinate wall synthesis and the elongation of plant cells.
MATERIALS AND METHODS
Growth Conditions
Arabidopsis seeds cv Columbia and of the mutant prc1-1 (Desnos et al., 1996), which had been backcrossed four times (Fagard et al., 2000b), were grown on nutrient agar-solidified medium without Suc according to Estelle and Somerville (Estelle and Somerville, 1987): 5 mm KNO3; 2.5 mm KH2PO4; 2 mm MgSO4(7H2O); 2 mm Ca(NO3)2; 70 μm H3BO3; 14 μm MnCl2; 0.5 μm CuSO4(5H2O); 0.2 mm Na2MoO4(2H2O); 10 μm NaCl; 1 μm ZnSO4(7H2O); 0.01 μm CoCl2(6H2O); 100 μg mL−1 myoinositol; 1 μg mL−1 calcium panthotenate; 1 μg mL−1 niacine; 1 μg mL−1 pyridoxine; 1 μg mL−1 thiamine HCl; 0.01 μg mL−1 biotine; 5 μg mL−1 ferric citrate (added after autoclave); 0.07% MES, pH 6; 8 μg mL−1 bromocresol purple; and 0.7% agar. For the experiment in which seedlings of different ages were transferred on isoxaben, plants were sown on a nylon mesh (NITEX 03-31/24, I OO4 M; Sefar, Thal, Switzerland) to prevent seedling damage during the transfer.
Germination was induced after 2 d of cold treatment (4°C) with fluorescent white light (150 μmol m−2 s−1 True Light; Philips, Eindhoven, The Netherlands) for 2 h. Darkness was obtained by wrapping the petri dishes in three layers of aluminum foil. Seedlings were placed under constant conditions (20°C, 70% humidity) in a growth cabinet (MLR 350H; Sanyo, Tokyo).
Measurements of Cell Length
Seedlings were fixed with a solution of ethanol:acetic acid (3:1) during 2 h with mild shaking, rinsed twice with water, cleared with 8 n NaOH for 2 h, and rinsed three times with water before mounting in 130% (w/v) chloral hydrate in 66% glycerol or in 0.05% (w/v) Calcofluor (fluorescent brightener, Sigma, St. Louis) in aqueous solution to highlight cell walls. For growth kinetics of hypocotyl cells, length of a minimum of 6 cells and of 40 cells for critical ages (beginning of rapid elongation) from different seedlings was measured by using a microscope and image analysis software Optimas (version 5.2; IMASYS, Surennes, France) as described previously (Gendreau et al., 1997). Values are means of one experiment. Each experiment was repeated at least once, which gave comparable results. The REGR was calculated with the formula: at tn, (ln Ln − ln Ln−1)/T, in which Ln is the average length of cells at tn, Ln−1 the average length of cells at tn−1, and T the time between tn and tn−1.
The length indicated in the text of cells of 80-h-old seedlings (cells from at least five different seedlings) was measured manually on low magnifications obtained by scanning electron microscopy.
Cell Wall Preparation for Scanning Electron Microscopy
Eighty-hour-old seedlings were prepared according to Sugimoto (Sugimoto et al., 2000). Briefly, seedlings were fixed for several hours in 4% formaldehyde PME (50 mm PIPES; 1 mm MgSO4; 5 mm EGTA, pH 7.2) buffer. Cryoprotection was achieved through 30 min incubation in 0.25% dimethyl sulfoxide and 0.5× PME. Whole hypocotyls of dark-grown prc1-1, or the upper half of the hypocotyl of dark-grown wild-type seedlings, were mounted in water for liquid nitrogen freezing. They were cryosectioned longitudinally at −120°C using an Ultracut E ultramicrotome with FC4 cryomicrotomy attachment (Leica Microsystems, Nussloch, Germany).
Sectioned seedlings were thawed in 50% dimethyl sulfoxide and 0.5× PME buffer and rinsed in PME buffer alone. Remaining cytoplasm and membranes of the cut cells were removed through 10 min incubation in 5% sodium hypochlorite. Specimens were rinsed in distilled water and postfixed in 0.5% OsO4 for 15 min. After rinsing in water, they were gradually dehydrated in ethanol and critical-point dried with CO2.
High Resolution Scanning Electron Microscopy
Dried specimens were fixed to stubs, using double-sided scarified sticky tape, and covered with approximately 1.5 to 2 nm platinum at an angle of 75° with 1.65 kV at 63 A during 30 s, under a vacuum of 10−5 Pa with an MED 010 (Balzers Union, Boiziau–France, Châtillon-sur-Cher, France), associated with an EVM 030 evaporation control unit (Balzers Union) and QSG 301 Quartz crystal thickness monitor (Balzers Union). Samples were observed at a distance of 7 mm with a Hitachi S4500 FESEM microscope (Hitachi, Elexience SA, Verrières-le-Buisson, France) at a power of 5 kV.
Transmission Electron Microscopy
Seedlings were grown for 4 d in the dark as described. They were prepared as described by Roland et al. (Reis, 1981). Upper- and second-third of wild-type hypocotyls were cut and marked at their lower side with ruthenium red powder before 2 h fixation in 3% glutaraldehyde 0.1 m cacodylate buffer, pH 7.4, followed by three washes with cacodylate buffer. Seedlings were postfixed with 1% OsO4 in 0.1 m cacodylate buffer and rinsed three times. To reveal cell wall structure, seedlings were then partially and aspecifically extracted for 10 h in 40% methylamine (Acros Organics, Noisy-le-Grand, France), under continuous mild stirring, and finally rinsed in water. They were dehydrated in graded ethanol series and embedded in Spurr resin, following manufacturer instructions (TAAB, Aldermaston, UK).
Ultrathin transverse sections (90–100 nm) were done at the level of the apical hook, which corresponds to cells still undergoing slow elongation (Gendreau et al., 1997), and at the first-third of the hypocotyl, which corresponds to cells undergoing rapid elongation (Gendreau et al., 1997).
Longitudinal sections of approximately 1 mm long were obtained from the upper part of the hypocotyl comprising the apical hook. The length of the observed cells was directly measured on low magnifications, which allowed determining the stage of elongation of these cells.
Sections were treated with PATAg, which specifically stains polysaccharides (Roland et al., 1982). Contrast was reinforced through uranyl acetate and lead citrate staining.
Observations were made with microscope EM 208 (Philips, Limeil-Brevannes, France) under a power of 100 kV.
Acknowledgments
We thank Béatrice Satiat-Jeunemaître for insightful advice and technical help, and Keiko Sugimoto for the teaching of the FESEM technique. We also thank the scanning electron microscope facilities of Institut National de la Recherche Agronomique in Jouy-en-Josas, France. Taka Hayashi is thanked for fruitful discussions. Thierry Genoud and Emma Pilling are thanked for critical reading of the manuscript.
This work was supported by the Ministère de la Recherche et Technologie (Ph.D. fellowship to G.R.), the Action Concertée Incitative, Développement et Physiologie intégrative (grant no. 47 to H.H.), and the European Economic Commission Framework 5 Program (GEMINI to H.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.038711.
References
- Beeckman T, Przemeck GK, Stamatiou G, Lau R, Terryn N, De Rycke R, Inze D, Berleth T (2002) Genetic complexity of cellulose synthase A gene function in Arabidopsis embryogenesis. Plant Physiol 130: 1883–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Hall JL (1985) The role of cell wall synthesis in sustained auxin-induced growth. Physiol Plant 63: 406–412 [Google Scholar]
- Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta 215: 989–996 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50: 391–417 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 [DOI] [PubMed] [Google Scholar]
- Desnos T, Orbovic V, Bellini C, Kronenberger J, Caboche M, Traas J, Höfte H (1996) Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in dark- and light-grown Arabidopsis seedlings. Development 122: 683–693 [DOI] [PubMed] [Google Scholar]
- Desprez T, Vernhettes V, Fagard S, Refrégier G, Desnos T, Aletti E, Py N, Pelletier S, Höfte H (2002) Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol 128: 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle MA, Somerville CR (1987) Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet 206: 200–206 [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refrégier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H (2000. a) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Höfte H, Vernhettes S (2000. b) Cell wall mutants. Plant Physiol Biochem 38: 1–11 [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Massonnet C, Turc O, Muller B, Chenu K, Tardieu F (2002) Individual leaf development in Arabidopsis thaliana: a stable thermal-time-based programme. Ann Bot (Lond) 89: 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PB (1960) Multinet growth in the cell wall of Nitella. J Biophys Biochem Cytol 7: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Skomp JR, Tschabold EE, Larrinua I (1990) Isoxaben Inhibits the Synthesis of Acid Insoluble Cell Wall Materials In Arabidopsis thaliana. Plant Physiol 93: 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1995) Specialized zones of development in roots. Plant Physiol 109: 725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Höfte H (2002) KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell 14: 2001–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M, Albersheim P, Darvill A, York WS (1999) Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J 20: 629–639 [DOI] [PubMed] [Google Scholar]
- Reis D (1981) Cytochimie ultrastructurale des parois en croissance par extractions ménagées. Effets comparés du méthylsulfoxyde et de la méthylamine sur le démasquage de la texture. Ann Sci Nat Bot Biol 13: 121–136 [Google Scholar]
- Reis D, Roland JC, Vian B (1985) Morphogenesis of twisted cell wall: chronology following an osmotic shock. Protoplasma 126: 36–46 [Google Scholar]
- Robert S, Mouille G, Höfte H (2004) Cellulose synthesis in primary walls: lessons from Arabidopsis mutants. Cellulose (in press)
- Roelofsen PA (1958) Cell-wall structure as related to surface growth. Acta Bot Neerl 7: 77–89 [Google Scholar]
- Roland JC, Reis D, Mosiniak M, Vian B (1982) Cell wall texture along the growth gradient of the mung bean hypocotyl: ordered assembly and dissipative processes. J Cell Sci 56: 303–318 [Google Scholar]
- Roland JC, Reis D, Vian B, Satiat-Jeunemaitre B, Mosiniak M (1987) Morphogenesis of plant cell walls at the supramolecular level: internal geometry and versatility of helicoidal expression. Protoplasma 140: 75–91 [Google Scholar]
- Roland JC, Vian B, Reis D (1977) Further observations on cell wall morphogenesis and polysaccharide arrangement during plant growth. Protoplasma 91: 125–141 [Google Scholar]
- Rose JK, Braam J, Fry SC, Nishitani K (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43: 1421–1435 [DOI] [PubMed] [Google Scholar]
- Satiat-Jeunemaitre B (1981) Texture et croissance des parois des deux épidermes du coléoptile de maïs. Ann Sci Nat Bot Biol 13: 163–176 [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville CR (2001) Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis ixr1 mutants. Proc Natl Acad Sci USA 98: 10079–10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO (2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15: 1414–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO (2000) New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol 124: 1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO (2001) Wall architecture in the cellulose-deficient rsw1 mutant of Arabidopsis thaliana: microfibrils but not microtubules lose their transverse alignment before microfibrils become unrecognizable in the mitotic and elongation zones of roots. Protoplasma 215: 172–183 [DOI] [PubMed] [Google Scholar]
- Taiz L (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol 35: 585–657 [Google Scholar]
- Takeda K, Shiboaka H (1981) Effects of gibberellin and colchicine on microfibril arrangement in epidermal cell walls of Vigna angularis Ohwi et Osashi epicotyls. Planta 151: 393–398 [DOI] [PubMed] [Google Scholar]
- Verbelen JP, Stickens D (1995) In vivo determination of fibril orientation in plant cell walls with polarization CSLM. J Microsc 177: 1–6 [Google Scholar]
- Wiedemeier AM, Judy-March JE, Hocart CH, Wasteneys GO, Williamson RE, Baskin TI (2002) Mutant alleles of Arabidopsis RADIALLY SWOLLEN 4 and 7 reduce growth anisotropy without altering the transverse orientation of cortical microtubules or cellulose microfibrils. Development 129: 4821–4830 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Zenoni S, Reale L, Tornielli GB, Lanfaloni L, Porceddu A, Ferrarini A, Moretti C, Zamboni A, Speghini A, Ferranti F, Pezzoti M (2004) Downregulation of the petunia hybrida α-expansin gene PhEXP1 reduces the amount of crystalline cellulose in cell walls and leads to phenotypic changes in petal limbs. Plant Cell 16: 295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]