Abstract
Background. The inconsistent finding was between hepatitis B virus (HBV) infections and cholangiocarcinoma (CCA). This meta-analysis is to explore this relationship in Asia. Methods. A literature search was performed using PubMed, Web of Science, and Cochrane Library to October 30, 2015. Pooled incidence rate and OR with 95% CI were calculated using STATA 11.0. Results. Thirty-nine studies were included. The pooled incidence rate of CCA patients with HBV infection was 31% (95% CI 22%–39%). The pooled OR showed increased risk of CCA incidence with HBV infection (OR = 2.72, 95% CI 1.90–3.88), especially in ICC (OR = 3.184, 95% CI 2.356–4.302), while it showed no risk in ECC (OR = 1.407, 95% CI 0.925–2.141). Also, the pooled OR showed increased risk of ICC and ECC incidence (OR = 6.857, 95% CI 4.421–10.633 and OR = 1.740, 95% CI 1.260–2.404) in patients with HBsAg+/HBcAb+. The pooled OR showed increased risk of ICC incidence (OR = 1.410, 95% CI 1.095–1.816) in patients with HBsAg−/HBcAb+. Conclusion. It is suggested that HBV infection is associated with an increased risk of CCA in Asia. Two HBV infection models (HBsAg+/HBcAb+ and HBsAg−/HBcAb+) increase the risk of CCA, and patients with HBsAg−/HBcAb+ also had a risk of ICC. This trial is registered with PROSPERO CRD42015029264.
1. Introduction
Cholangiocarcinoma (CCA) is the second commonest primary liver tumor worldwide after hepatocellular carcinoma (HCC) [1]. In the World Health Organization (WHO), CCA is classified as intrahepatic cholangiocarcinoma (ICC) or extrahepatic cholangiocarcinoma (ECC). It accounts for 3% of all gastrointestinal tumors [2]. During the past 3 decades, the overall incidence rate of CCA has appeared to have significantly increased [1]. Hepatobiliary malignancies account for 13% of cancer-related deaths and 10%–20% of these are attributable to CCA worldwide [3]. Currently, CCA has not been extensively investigated, possibly as a result of its presumptive rarity and worrisome prognosis at the time of diagnosis. It has been shown that CCA is characterized by a low survival rate with a median survival of less than 24 months after diagnosis [4].
Defining the risk factors for CCA benefits the search for better ways to prevent the occurrence of this disease. To date, it has been shown that viral infection (mainly hepatitis B and hepatitis C infection), gallstone formation, choledochoenteric anastomosis, oil sands carcinogenicity, chemicals and radiations exposure, biliary system development abnormalities, and so on are the major risk factors for CCA [5]. However, data from low hepatitis B (HBV) epidemic area and HBV infection is not a risk factor for CCA [6]. Thus, we conducted this meta-analysis for evaluating the association between HBV infection and CCA (including ICC and ECC) in Asian countries which are high HBV epidemic areas.
Also, two main treatment options in hepatitis B are available: interferon and nucleos(t)ide analogue (NUCs). There are five NUCs currently approved for HBV treatment: lamivudine (LAM), adefovir (ADV), telbivudine (LdT), entecavir (ETV), and tenofovir disoproxil fumarate (TDF). Treatment with TDF or ETV is more effective due to a high antiviral potency and has a high barrier to resistance reducing the risk of drug resistance and treatment failure [7]. So, HBV DNA could be inhibited during treatment. Even, some patients could achieve serological conversion of HBsAg (HBsAg−/HBcAb+) whereas, for patients with curative resection of HBV-related HCC, positive HBcAb is associated with higher risk of early recurrence and poorer survival [8] and hepatitis B reactivation in HBsAg−/HBcAb+ patients receiving rituximab for lymphoma [9]. According to those, the risk of cancer was still existing in patients with HBsAg−/HBcAb+. Thus, this meta-analysis was also conducted for the association between HBV infection model and CCA risk.
2. Methods
2.1. Search Strategy
We have registered this trial with PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) (trial number: CRD42015029264). Our study was performed according to the recommendations of the Moose [10] (see Supplementary Table 1 of the Supplementary Material available online at http://dx.doi.org/10.1155/2016/3417976). PubMed was searched combining the terms of (“hepatitis B” OR HBV OR CHB) AND (“bile duct neoplasms” OR cholangiocarcinoma).
2.2. Inclusion and Exclusion Criteria
For estimating the incidence rate of CCA, ICC, or ECC, the studies were included if they meet the criteria as follows: (i) studies only containing the cancer group; (ii) exposure to HBV infection; (iii) providing enough information for calculating the incidence rate; and (iv) the number of cases being not less than 50.
For estimating the OR of CCA, ICC, or ECC, the studies were included if they meet the criteria as follows: (i) exposure to HBV infection; (ii) the outcome being CCA, ICC, or ECC incidence; (iii) providing risk estimates with 95% confidence interval (CI) or available information to calculate them; and (iv) published full-text report in English language.
Abstracts and reviews, letters, case reports, and studies that did not provide sufficient data to calculate the risk estimates were excluded. Two investigators (Hao Zhang and Biqing Zhu) independently selected studies, and any discrepancies were resolved by the third investigator (Wenting Zeng).
2.3. Quality Assessment
The quality of the included studies was assessed independently by two authors (Hao Zhang and Jianxin Liang) using the Newcastle-Ottawa Scale (NOS) [46]. The NOS is for observed studies and consists of 3 parameters of quality: selection, comparability, and exposure/outcome assessment. The NOS assigns a maximum of 4 points for selection, 2 for comparability, and 3 for exposure or outcome. We assigned NOS scores of 1–3, 4–6, and 7–9 for low-, intermediate-, and high-quality studies. Discrepancies were settled by consensus after joint reevaluation of the original studies with the third author (Jianxin Liang).
2.4. Data Extraction
The following information was extracted from each study: first author, publication time, the sample size, country, number of exposures in cases and controls; risk estimate; and 95% CI. The data were collected independently by two investigators (Hao Zhang and Biqing Zhu). When the literature citations were controversial, these investigators discussed them and reached a consensus on inclusion or exclusion.
2.5. Data Analysis
The overincidence rate of CCA, ICC, and ECC with HBV infection was calculated by effect size (ES) and the corresponding 95% confidence interval (95% CI). The risk of HBV infection outcomes was estimated by odds ratio (OR) with the corresponding 95% CI. It was considered statistically significant when P < 0.05. In the forest plots, OR > 1 represented a risk effect and OR < 1 represented a protective effect. Statistical heterogeneity of results was appraised using a Chi-Square based Q test and I 2 statistic. Only when the analysis fullfilled both P > 0.10 and I 2 < 50%, the heterogeneity was considered not significant. The fixed-effects model was used when literature heterogeneity did not exist; otherwise, the random-effects model was employed. Sensitivity analysis was conducted by modification of the inclusion criteria of this meta-analysis. The pooled proportion of vertical transmission of toxoplasmosis was calculated by STATA 11.0 software (Stata Corporation, College Station, TX, USA) and the publication bias was considered significant when P value was less than 0.05 in either Begg's test or Egger's test.
3. Results
3.1. Selection of Studies
A flow diagram of the selection of the studies is shown in Figure 1. A total of 39 studies with CCA matched the inclusion criteria in this meta-analysis, including 21 studies [11–31] for estimating the incidence rates of CCA, 14 studies [32–45] for unadjusted OR, and 7 studies [32, 33, 36, 39, 41, 42, 44] for adjusted OR analysis (Tables 1 and 2). Among the 39 studies, 26 studies were for ICC, including 13 studies [11–14, 16, 17, 19, 22, 23, 25, 26, 29, 31] for estimating the incidence rates of ICC, 11 studies [32, 34–36, 39–42, 44, 45, 47] for unadjusted OR analysis, and 7 studies [32, 35, 36, 39, 41, 42, 44] for adjusted OR analysis (Tables 3 and 4). Nine studies were for ECC, including 3 studies [11, 15, 20] for estimating the incidence rates of ECC, 5 studies [32, 35, 40, 43, 47] for unadjusted OR analysis, and 1 study [35] for adjusted OR analysis (Tables 4 and 5). The main features of included studies were shown in Tables 1 –5. Among the included studies, 4 studies are from Korea [24, 29, 32, 43], 4 from Japan [11, 28, 30, 45], 3 from Thailand [18, 30, 38], and 24 from China [12–17, 19–23, 25–27, 33–37, 39–43]. For analyzing the association between HBV infection and CCA risk, twelve studies (75%) were of high quality and the other four (25%) were acceptable based on the NOS scores.
Figure 1.
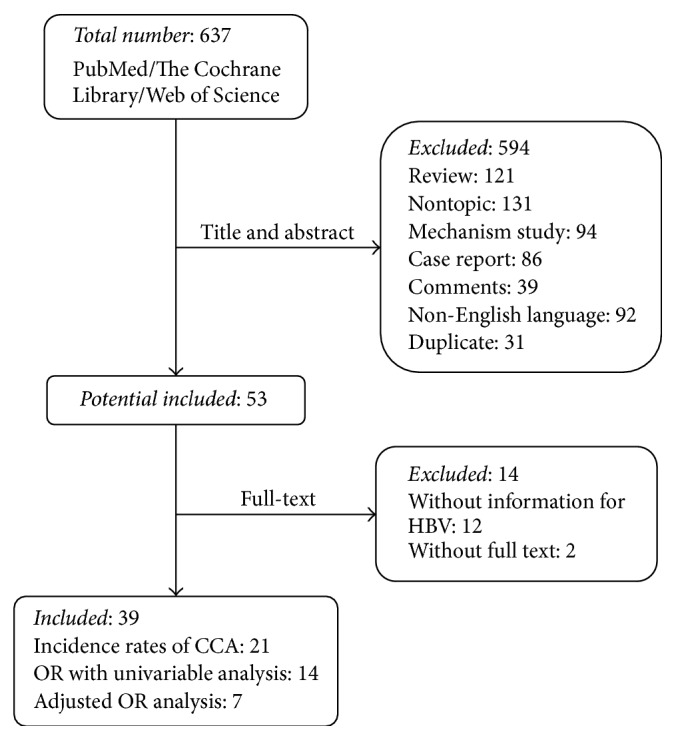
Flow diagram of selection and disposition of studies.
Table 1.
Incidence rate of studies of HBV infection and CCA.
| Authors | Year | Country | HBV infection cases (n) | CCA cases (n) | Reference |
|---|---|---|---|---|---|
| Matsumoto et al. | 2014 | Japan | 29 | 145 | [11] |
| Luo et al. | 2014 | China | 608 | 1233 | [12] |
| Liu et al. | 2013 | China | 37 | 81 | [13] |
| Yin et al. | 2013 | China | 139 | 411 | [14] |
| Zhou et al. | 2013 | China | 88 | 239 | [15] |
| Wu et al. | 2013 | China | 97 | 138 | [16] |
| Lee et al. | 2013 | China (Taiwan) | 29 | 127 | [17] |
| Barusrux et al. | 2012 | Thailand | 24 | 295 | [18] |
| Yin et al. | 2012 | China | 131 | 386 | [19] |
| Qu et al. | 2012 | China | 19 | 305 | [20] |
| Wu et al. | 2012 | China | 18 | 66 | [21] |
| Jiang et al. | 2011 | China | 44 | 102 | [22] |
| Zhou et al. | 2011 | China | 87 | 155 | [23] |
| Park et al. | 2011 | Korea | 32 | 160 | [24] |
| Zhou et al. | 2010 | China | 154 | 317 | [25] |
| Zhou et al. | 2008 | China | 64 | 131 | [26] |
| Zuo et al. | 2007 | China | 20 | 59 | [27] |
| Fukuhara et al. | 2001 | Japan | 4 | 53 | [28] |
| Lee et al. | 2006 | Korea | 11 | 79 | [29] |
| Pinyosophon and Wiwanitkit | 2002 | Thailand | 5 | 55 | [30] |
| Asayama et al. | 2002 | Japan | 8 | 68 | [31] |
Table 2.
Characteristics of studies of HBV infection and CCA risk.
| Authors | Year | Country | Cases (n) | Controls (n) | Control description | Adjusted OR (95% CI) | Study quality | Reference |
|---|---|---|---|---|---|---|---|---|
| Lee et al. | 2015 | Korea | 276 | 552 | Hospital-based control | 4.12 (2.01–8.44) |
7 | [32] |
| Zhou et al. | 2014 | China | 126 | 504 | Hospital-based control | 19.245 (13.260–27.931) |
7 | [33] |
| Li et al. | 2014 | China | 183 | 549 | Hospital-based control | NR | 8 | [34] |
| Chang et al. | 2013 | China (Taiwan) | 5157 | 8716 | Population-based control | NR | 9 | [35] |
| Peng et al. | 2011 | China | 98 | 196 | Hospital-based control | 2.75 (1.27–5.95) |
8 | [36] |
| Cai et al. | 2011 | China | 313 | 608 | Hospital-based control | NR | 7 | [37] |
| Srivatanakul et al. | 2010 | Thailand | 106 | 106 | Population-based control | NR | 6 | [38] |
| Zhou et al. | 2010 | China | 317 | 634 | Hospital-based control | 9.669 (6.329–14.77) |
8 | [39] |
| Tao et al. | 2010 | China | 190 | 380 | Hospital-based control | NR | 8 | [40] |
| Lee et al. | 2009 | China (Taiwan) | 160 | 160 | Hospital-based control | 4.985 (2.775–8.945) |
6 | [41] |
| Zhou et al. | 2008 | China | 312 | 438 | Hospital-based control | 8.876 (5.973–13.192) |
6 | [42] |
| Hsing et al. | 2008 | China | 134 | 762 | Population-based control | NR | 8 | [43] |
| Lee et al. | 2008 | Korea | 622 | 2488 | Hospital-based control | 2.3 (1.6–3.3) |
7 | [44] |
| Yamamoto et al. | 2004 | Japan | 50 | 200 | Hospital-based control | NR | 6 | [45] |
NR: not reported.
Table 3.
Incidence rate of studies of HBV infection and ICC/ECC.
| Authors | Year | Country | Cancer group | HBV infection cases (n) | CCA cases (n) | Reference |
|---|---|---|---|---|---|---|
| Matsumoto et al. | 2014 | Japan | ICC | 16 | 50 | [11] |
| Luo et al | 2014 | China | ICC | 608 | 1233 | [12] |
| Liu et al. | 2013 | China | ICC | 37 | 81 | [13] |
| Yin et al. | 2013 | China | ICC | 139 | 411 | [14] |
| Zhou et al. | 2013 | China | ECC | 88 | 239 | [15] |
| Wu et al. | 2013 | China | ICC | 97 | 138 | [16] |
| Lee et al. | 2013 | China (Taiwan) | ICC | 29 | 127 | [17] |
| Yin et al. | 2012 | China | ICC | 131 | 386 | [19] |
| Qu et al. | 2012 | China | ECC | 19 | 305 | [20] |
| Jiang et al. | 2011 | China | ICC | 44 | 102 | [22] |
| Zhou et al. | 2011 | China | ICC | 87 | 155 | [23] |
| Zhou et al. | 2010 | China | ICC | 154 | 317 | [25] |
| Zhou et al. | 2008 | China | ICC | 64 | 131 | [26] |
| Lee et al. | 2006 | Korea | ICC | 11 | 79 | [29] |
| Asayama et al. | 2002 | Japan | ICC | 8 | 68 | [31] |
Table 4.
Characteristics of studies of HBV infection and ICC risk.
| Authors | Year | Country | Cases (n) | Controls (n) | Control description | Adjusted OR (95% CI) | Study quality | Reference |
|---|---|---|---|---|---|---|---|---|
| Lee et al. | 2015 | Korea | 83 | 166 | Hospital-based control | 5.27 (1.93–14.38) |
7 | [32] |
| Li et al. | 2014 | China | 183 | 549 | Hospital-based control | NR | 8 | [34] |
| Chang et al. | 2013 | China (Taiwan) | 2978 | 11912 | Population-based control | 3.5 (2.9–4.3) |
9 | [35] |
| Wu et al. | 2012 | China | 102 | 835 | Hospital-based control | NR | 7 | [47] |
| Peng et al. | 2011 | China | 98 | 196 | Hospital-based control | 2.75 (1.27–5.95) |
8 | [36] |
| Zhou et al. | 2010 | China | 317 | 634 | Hospital-based control | 9.669 (6.329–14.77) |
8 | [39] |
| Tao et al. | 2010 | China | 61 | 380 | Hospital-based control | NR | 8 | [40] |
| Lee et al. | 2009 | China (Taiwan) | 160 | 160 | Hospital-based control | 4.985 (2.775–8.945) |
6 | [41] |
| Zhou et al. | 2008 | China | 312 | 438 | Hospital-based control | 8.876 (5.973–13.192) |
6 | [42] |
| Lee et al. | 2008 | Korea | 622 | 2488 | Hospital-based control | 2.3 (1.6–3.3) |
7 | [44] |
| Yamamoto et al. | 2004 | Japan | 50 | 200 | Hospital-based control | NR | 6 | [45] |
NR: not reported.
Table 5.
Characteristics of studies of HBV infection and ECC risk.
| Authors | Year | Country | Cases (n) | Controls (n) | Control description | Adjusted OR (95% CI) | Study quality | Reference |
|---|---|---|---|---|---|---|---|---|
| Lee et al. | 2015 | Korea | 193 | 386 | Hospital-based control | NR | 7 | [32] |
| Chang et al. | 2013 | China (Taiwan) | 2179 | 8716 | Population-based control | 2.6 (2.0–3.4) | 9 | [35] |
| Zhou et al. | 2013 | China | 239 | 478 | Hospital-based control | NR | 7 | [15] |
| Wu et al. | 2012 | China | 86 | 835 | Hospital-based control | NR | 7 | [47] |
| Tao et al. | 2010 | China | 129 | 380 | Hospital-based control | NR | 8 | [40] |
| Hsing et al. | 2008 | China | 134 | 762 | Population-based control | NR | 8 | [43] |
NR: not reported.
3.2. HBV Infection and the Risk of CCA
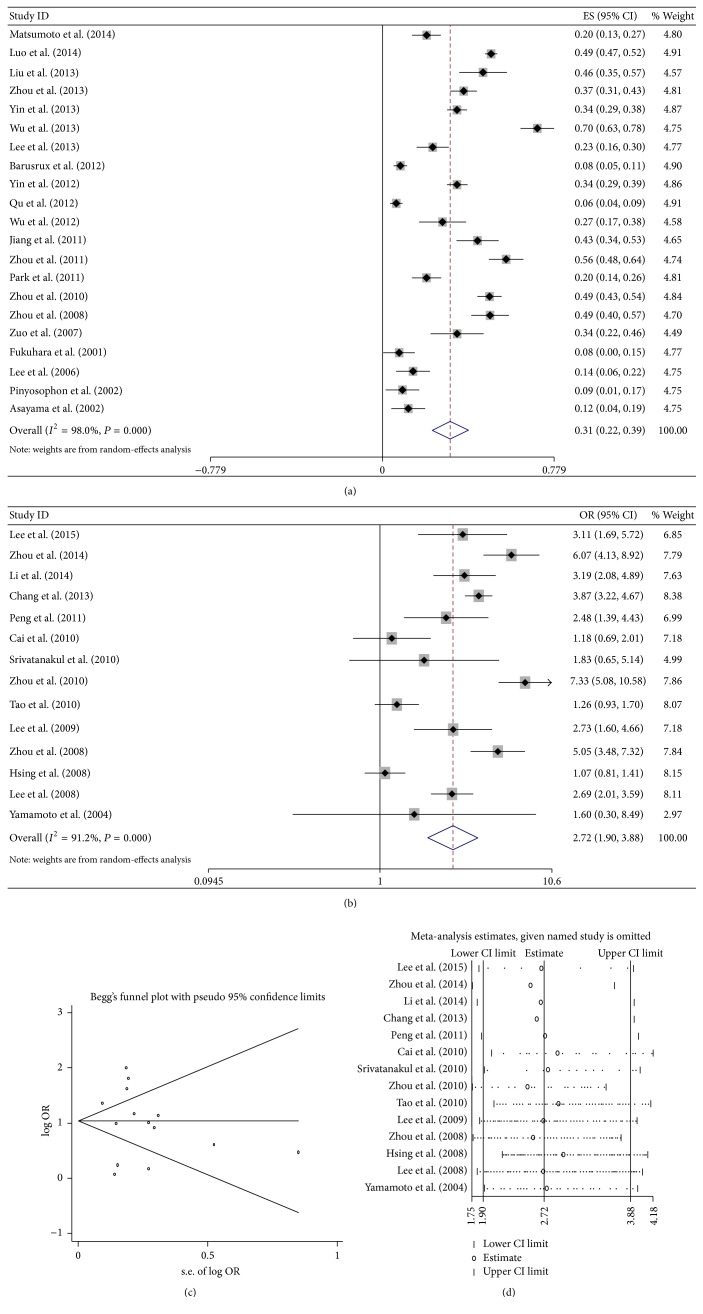
For pooled analysis of incidence rates of CCA with HBV infection, 21 studies with 6 253 participants were included [11–31]. All these studies were from Asia, including 3 from Japan, 2 from Thailand, one from Korea, and 15 from China. The overall pooled incidence rates were 31% (95% CI 22%–39%), calculated with the random-effects model (P < 0.001, I 2 = 98.0%, Figure 2(a)). In China, the pooled incidence rates were 37.6% (95% CI 26.8%–48.3%, P < 0.001), calculated with the random-effects model (P < 0.001, I 2 = 98.5%).
Figure 2.
Meta-analysis of the association between HBV infection and CCA. (a) Pooled incidence of CCA in Asia; (b) forest plots of the association between HBV infection and CCA with unadjusted OR; (c) sensitivity analysis of the association between HBV infection and CCA; and (d) forest plots of the association between HBV infection and CCA with adjusted OR.
For pooled analysis of unadjusted OR of CCA with HBV infection, 14 studies with 24 337 participants including controls were analyzed [32–45]. The meta-analysis in a random-effects model (P < 0.001, I 2 = 91.2%) found statistically significant increased risk of CCA incidence with HBV infection (OR = 2.72, 95% CI 1.90–3.88, P < 0.001, Figure 2(b)). No publication bias was observed by Begg's test (P = 0.443) and Egger's test (P = 0.774). The sensitivity analysis showed that the result that HBV infection was a high risk for patients progressing into CCA was not changed (Figure 2(c)).
For pooled analysis of adjusted OR of CCA with HBV infection, 7 studies with 6,883 participants including controls were analyzed [32, 33, 36, 39, 41, 42, 44]. The meta-analysis in a random-effects model (P < 0.001, I 2 = 92.2%) also found statistically significant increased risk of CCA incidence with HBV infection (OR = 5.903, 95% CI 3.110–11.207, P < 0.001, Figure 2(d)).
3.3. HBV Infection and the Risk of ICC
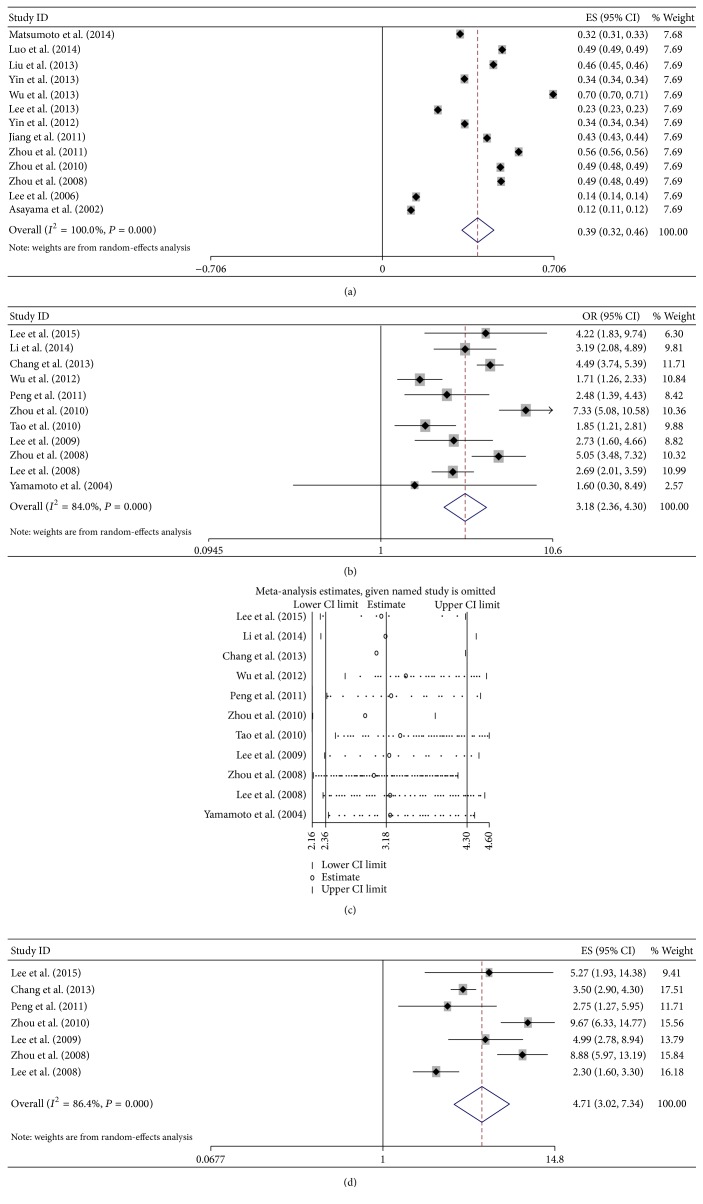
For pooled analysis of incidence rates of ICC with HBV infection, 13 studies with 4,871 participants were included [11–14, 16, 17, 19, 22, 23, 25, 26, 29, 31]. All these studies were from Asia, including 2 were from Japan, 1 from Korea, and 10 from China. The overall pooled incidence rates were 39% (95% CI 32%–46%, P < 0.001), calculated with the random-effects model (P < 0.001, I 2 = 100%, Figure 3(a)).
Figure 3.
Meta-analysis of the association between HBV infection and ICC. (a) Pooled incidence of ICC in Asia; (b) forest plots of the association between HBV infection and ICC with unadjusted OR; (c) sensitivity analysis of the association between HBV infection and ICC; and (d) forest plots of the association between HBV infection and ICC with adjusted OR.
For pooled analysis of unadjusted OR of ICC with HBV infection, 11 studies with 22 924 participants including controls were analyzed [32, 34–36, 39–42, 44, 45, 47]. The meta-analysis in a random-effects model (P < 0.001, I 2 = 84.0%) found statistically significant increased risk of ICC incidence with HBV infection (OR = 3.184, 95% CI 2.356–4.302, P < 0.001, Figure 3(b)). No publication bias was observed by Begg's test (P = 0.755) and Egger's test (P = 0.428). The sensitivity analysis showed that the result that HBV infection was a high risk for patients progressing into ICC was not changed (Figure 3(c)).
For pooled analysis of adjusted OR of ICC with HBV infection, 7 studies with 20,564 participants including controls were analyzed [32, 35, 36, 39, 41, 42, 44]. The meta-analysis in a random-effects model (P < 0.001, I 2 = 86.4%) also found statistically significant increased risk of ICC incidence with HBV infection (OR = 4.709, 95% CI 3.020–7.341, P < 0.001, Figure 3(d)).
3.4. HBV Infection and the Risk of ECC
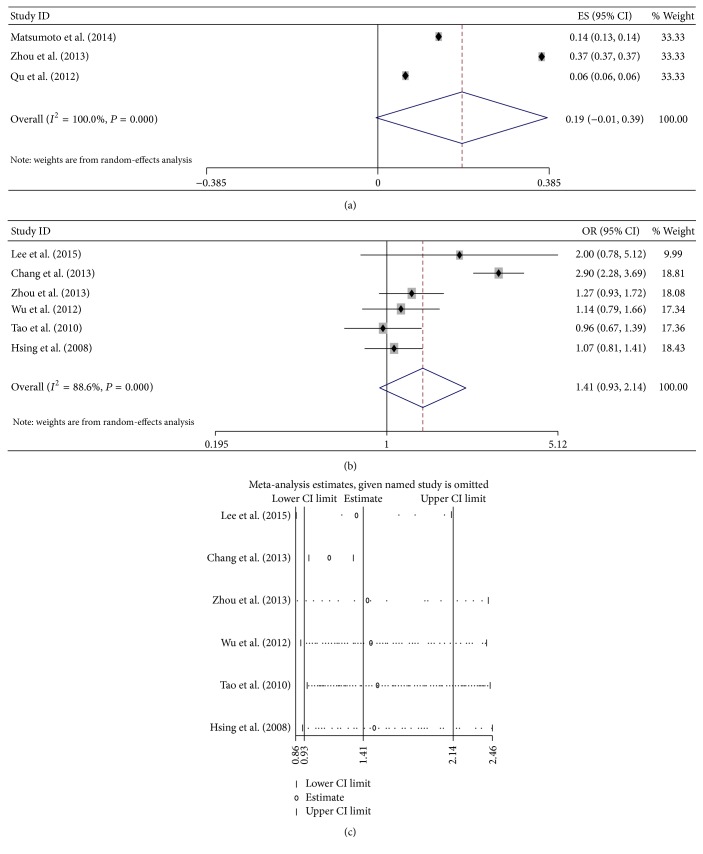
For pooled analysis of incidence rates of ECC with HBV infection, 2 studies with 483 participants were included [11, 20]. These studies were from Japan and China. The overall pooled incidence rates were 18.9% (95% CI −0.7%–38.5%, P = 0.059), calculated with the random-effects model (P < 0.001, I 2 = 100%, Figure 4(a)).
Figure 4.
Meta-analysis of the association between HBV infection and ECC. (a) Pooled incidence of ECC in Asia; (b) forest plots of the association between HBV infection and ECC with unadjusted OR; and (c) sensitivity analysis of the association between HBV infection and ECC.
For pooled analysis of unadjusted OR of ECC with HBV infection, 6 studies with 14 517 participants including controls were analyzed [15, 32, 35, 40, 43, 47]. The meta-analysis in a random-effects model (P < 0.001, I 2 = 88.6%) found no statistically significant increased risk of ECC incidence with HBV infection (OR = 1.407, 95% CI 0.925–2.141, P = 0.110, Figure 4(b)). No publication bias was observed by Begg's test (P = 1.0) and Egger's test (P = 0.579). The sensitivity analysis showed that the result that HBV infection was not a high risk for patients progressing into ECC was not changed (Figure 4(c)).
However, the adjusted OR of ECC with HBV infection found statistically significant increased risk of ECC incidence with HBV infection (OR = 2.6, 95% CI 2.0–3.4), which was only in one study [35].
3.5. HBV Infection Status and the Risk of Cancer
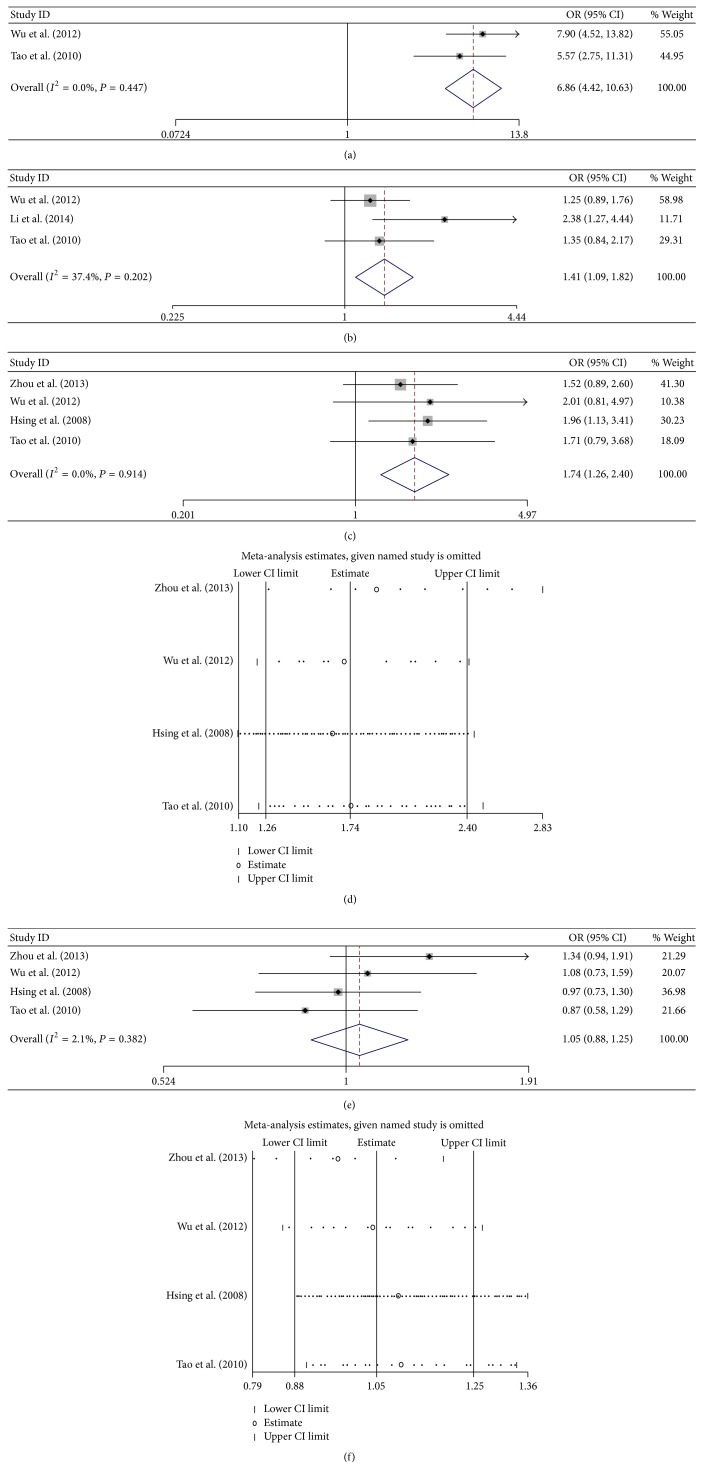
Two HBV infection models, which were HBsAg+/HBcAb+ and HBsAg−/HBcAb+, were included in this meta-analysis. Patients with HBsAg+/HBcAb+ showed a high risk of ICC (OR = 6.857, 95% CI 4.421–10.633) with calculating in a fix-effects model (P = 0.447, I 2 = 0%, Figure 5(a)). As only two studies were analyzed, the publication bias and sensitivity analysis cannot be performed. The risk of ICC was increased in patients with HBsAg−/HBcAb+ (OR = 1.410, 95% CI 1.095–1.816) with calculating in a fix-effects model (P = 0.202, I 2 = 37.4%, Figure 5(b)). No publication bias was observed by Begg's test (P = 0.96) and Egger's test (P = 0.270). As only three studies were analyzed, the sensitivity analysis cannot be performed.
Figure 5.
Meta-analysis of the association between HBV infection models and cancer. (a) Forest plots of the association between HBsAg+/HBcAb+ and ICC; (b) forest plots of the association between HBsAg−/HBcAb+ and ICC; (c) forest plots of the association between HBsAg+/HBcAb+ and ECC; (d) sensitivity analysis of the association between HBsAg+/HBcAb+ and ECC; (e) forest plots of the association between HBsAg−/HBcAb+ and ICC; and (f) sensitivity analysis of the association between HBsAg−/HBcAb+ and ECC.
Patients with HBsAg+/HBcAb+ showed a high risk of ECC (OR = 1.740, 95% CI 1.260–2.404) with calculating in a fix-effects model (P = 0.914, I 2 = 0%, Figure 5(c)). No publication bias was observed by Begg's test (P = 0.734) and Egger's test (P = 0.627). The sensitivity analysis showed that the result that HBV infection was a high risk for patients progressing into ECC was not changed (Figure 5(d)). The risk of ECC was not increased of patients with HBsAg−/HBcAb+ (OR = 1.049, 95% CI 0.881–1.249) with calculating in a fix-effects model (P = 0.382, I 2 = 2.1%, Figure 5(e)). No publication bias was observed by Begg's test (P = 0.734) and Egger's test (P = 0.993). As only three studies were analyzed, the sensitivity analysis cannot be performed. The sensitivity analysis showed that the result that HBV infection was not a high risk for patients progressing into ECC was not changed (Figure 5(f)).
4. Discussion
HBV is widely epidemic in Asian countries [48]. Previous study has concluded that HBV infection is associated with an increased risk of ICC [49]. This meta-analysis was employed to estimate the CCA incidence rate in HBV infection patients and the CCA risk of HBV infection models in Asia, including ICC and ECC.
In this study, HBV infection leading to CCA was about 31%, and this rate (39%) was also high in China. HBV infection, where pooled OR is 2.72 (95% CI 1.90–3.88) and adjusted OR is 5.903 (95% CI 3.110–11.207), is a risk factor of CCA. This phenomenon also occurred in the patients with ICC. As all we know, HBV is a strong risk factor for HCC. And hepatocytes and cholangiocytes have the same progenitor cell; therefore, it can be postulated that HBV can induce carcinogenesis in both hepatocytes and cholangiocytes by the same mechanism [50]. Furthermore, studies show that HBV has been suggested to be involved in the pathogenesis of ICC through the inflammatory process [51, 52], which further supports the potential role of HBV infection in the pathogenesis of cholangiocarcinoma. Also, recent clinical surveys in China have detected HBV DNA in the tissue species from bile duct cancer.
Interestingly, the risk of ICC was also excluded in patients with the model of HBsAg−/HBcAb+ that termed non-B non-C. The main mechanism may be that these patients were occult HBV infection. It is reported that even a history of HBV infection (positive HBcAb+ with HBsAg−) can be a risk factor for HCC [53]. HBcAb positive status may reflect occult HBV infection and could be associated with an increase in the risk of carcinogenesis [54]. Also, a recent study reported that positive HBcAb is associated with a higher risk of early intrahepatic recurrence and poorer relapse-free survival of HBV-related HCC patients after curative resection [8], which means that HBcAb may play a role in the HCC patients.
Although the incidence rate of ECC was not significantly different in HBV infection patients and HBV infection was not a risk of patients progressing into ECC, the HBV infection status was much different. Patients with HBsAg+/HBcAb+ showed a high risk of ECC, while patients with HBsAg−/HBcAb+ showed no risk of ECC. The mechanism may be associated with HBsAg status. Though few studies have explained the link between HBsAg and ECC, a recent report showed that HBsAg could stimulate proliferation and functional modification of hepatocytes via LEF-1 through the Wnt pathway at the premalignant stage of HCC [55]. Also, high HBsAg levels were associated with late recurrence (after 2 years) after curative resection in HBV-related HCC [56]. So, HBsAg might use a similar action to affect ECC.
In general, our meta-analysis results were similar to those recently published by others about this subject [57]. However, this meta-analysis has several weaknesses that should be considered. First, the included studies were all observational studies that might introduce selection and recall biases. Second, lots of results were based on the random-effect model analysis, which might not reduce the strength of evidence. Third, a small number of studies were used to analyze the HBV infection status and the risk of cancer. Finally, the included studies were mostly from China. Thus, in the future, more studies from other counties need to be performed to explain the link between HBV infection and risk of CCA.
5. Conclusion
This study suggests that HBV infection is associated with an increased risk of CCA in Asia. The two HBV infection models (HBsAg+/HBcAb+ and HBsAg−/HBcAb+) increase the risk of CCA, while the model of HBsAg−/HBcAb+ increases the risk of ICC. More studies, especially from low epidemic areas, were needed to explore the connection between HBV infection model and CCA risk.
Supplementary Material
Table 1: A Proposed Reporting Checklist for Authors, Editors, and Reviewers of Meta-analyses
Acknowledgments
This study was supported by Science and Technology Planning Project of Guangdong Province to Wenting Zeng (2013B022000101).
Competing Interests
On behalf of all authors, the corresponding author states that there is no conflict of interests.
Authors' Contributions
Hao Zhang and Biqing Zhu collected the data. Biqing Zhu did the statistical analysis. Hao Zhang wrote the article. He Zhang and Jianxin Liang contributed to conception and design of the study and revised the article. Wenting Zeng is guarantor of the article. All authors approved the final version of the manuscript. Hao Zhang and Biqing Zhu contributed equally to this work.
References
- 1.Khan S. A., Davidson B. R., Goldin R. D., et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 2.Khan S. A., Thomas H. C., Davidson B. R., Taylor-Robinson S. D. Cholangiocarcinoma. The Lancet. 2005;366(9493):1303–1314. doi: 10.1016/s0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Rizvi S., Gores G. J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2, article 10 doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Bahrani R., Abuetabh Y., Zeitouni N., Sergi C. Cholangiocarcinoma: risk factors, environmental influences and oncogenesis. Annals of Clinical and Laboratory Science. 2013;43(2):195–210. [PubMed] [Google Scholar]
- 6.Shaib Y. H., El-Serag H. B., Davila J. A., Morgan R., McGlynn K. A. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 7.EASL Clinical Practice Guidelines: management of chronic hepatitis B virus infection. Journal of Hepatology. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Li T., Wang S.-K., Zhou J., et al. Positive HBcAb is associated with higher risk of early recurrence and poorer survival after curative resection of HBV-related HCC. Liver International. 2016;36(2):284–292. doi: 10.1111/liv.12898. [DOI] [PubMed] [Google Scholar]
- 9.Mozessohn L., Chan K. K. W., Feld J. J., Hicks L. K. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. Journal of Viral Hepatitis. 2015;22(10):842–849. doi: 10.1111/jvh.12402. [DOI] [PubMed] [Google Scholar]
- 10.Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Journal of the American Medical Association. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K., Onoyama T., Kawata S., et al. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Internal Medicine. 2014;53(7):651–654. doi: 10.2169/internalmedicine.53.1410. [DOI] [PubMed] [Google Scholar]
- 12.Luo X., Yuan L., Wang Y., Ge R., Sun Y., Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: A Large Single-Center Cohort Study. Journal of Gastrointestinal Surgery. 2014;18(3):562–572. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu R.-Q., Shen S.-J., Hu X.-F., Liu J., Chen L.-J., Li X.-Y. Prognosis of the intrahepatic cholangiocarcinoma after resection: hepatitis B virus infection and adjuvant chemotherapy are favorable prognosis factors. Cancer Cell International. 2013;13(1, article 99) doi: 10.1186/1475-2867-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X., Zheng S.-S., Zhang B.-H., et al. Elevation of serum γ-glutamyltransferase as a predictor of aggressive tumor behaviors and unfavorable prognosis in patients with intrahepatic cholangiocarcinoma: analysis of a large monocenter study. European Journal of Gastroenterology and Hepatology. 2013;25(12):1408–1414. doi: 10.1097/meg.0b013e328364130f. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Zhou Q., Lin Q., et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. International Journal of Cancer. 2013;133(8):1867–1875. doi: 10.1002/ijc.28196. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z.-F., Yang N., Li D.-Y., Zhang H.-B., Yang G.-S. Characteristics of intrahepatic cholangiocarcinoma in patients with hepatitis B virus infection: clinicopathologic study of resected tumours. Journal of Viral Hepatitis. 2013;20(5):306–310. doi: 10.1111/jvh.12005. [DOI] [PubMed] [Google Scholar]
- 17.Lee C.-H., Hsieh S.-Y., Chang C.-J., Lin Y.-J. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. Journal of Gastroenterology and Hepatology. 2013;28(1):122–127. doi: 10.1111/j.1440-1746.2012.07289.x. [DOI] [PubMed] [Google Scholar]
- 18.Barusrux S., Nanok C., Puthisawas W., Pairojkul C., Poovorawan Y. Viral hepatitis B, C infection and genotype distribution among cholangiocarcinoma patients in northeast Thailand. Asian Pacific Journal of Cancer Prevention. 2012;13(1):83–87. doi: 10.7314/apjcp.2012.13.kksuppl.83. [DOI] [PubMed] [Google Scholar]
- 19.Yin X., Zhang B.-H., Qiu S.-J., et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Annals of Surgical Oncology. 2012;19(9):2869–2876. doi: 10.1245/s10434-012-2328-0. [DOI] [PubMed] [Google Scholar]
- 20.Qu Z., Cui N., Qin M., Wu X. Epidemiological survey of biomarkers of hepatitis virus in patients with extrahepatic cholangiocarcinomas. Asia-Pacific Journal of Clinical Oncology. 2012;8(1):83–87. doi: 10.1111/j.1743-7563.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y., Wang T., Ye S., et al. Detection of hepatitis B virus DNA in paraffin-embedded intrahepatic and extrahepatic cholangiocarcinoma tissue in the northern Chinese population. Human Pathology. 2012;43(1):56–61. doi: 10.1016/j.humpath.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B.-G., Ge R.-L., Sun L.-L., Zong M., Wei G.-T., Zhang Y.-J. Clinical parameters predicting survival duration after hepatectomy for intrahepatic cholangiocarcinoma. Canadian Journal of Gastroenterology. 2011;25(11):603–608. doi: 10.1155/2011/917097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H.-B., Wang H., Li Y.-Q., et al. Hepatitis B virus infection: a favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World Journal of Gastroenterology. 2011;17(10):1292–1303. doi: 10.3748/wjg.v17.i10.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H., Choi K. H., Choi S.-B., et al. Clinicopathological characteristics in combined hepatocellular-cholangiocarcinoma: a single center study in Korea. Yonsei Medical Journal. 2011;52(5):753–760. doi: 10.3349/ymj.2011.52.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H.-B., Wang H., Zhou D.-X., et al. Etiological and clinicopathologic characteristics of intrahepatic cholangiocarcinoma in young patients. World Journal of Gastroenterology. 2010;16(7):881–885. doi: 10.3748/wjg.v16.i7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y.-M., Yang J.-M., Li B., et al. Clinicopathologic characteristics of intrahepatic cholangiocarcinoma in patients with positive serum a-fetoprotein. World Journal of Gastroenterology. 2008;14(14):2251–2254. doi: 10.3748/wjg.14.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo H.-Q., Yan L.-N., Zeng Y., et al. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary and Pancreatic Diseases International. 2007;6(2):161–165. [PubMed] [Google Scholar]
- 28.Fukuhara T., Sharp G. B., Mizuno T., et al. Liver cancer in atomic-bomb survivors: histological characteristics and relationships to radiation and hepatitis B and C viruses. Journal of Radiation Research. 2001;42(2):117–130. doi: 10.1269/jrr.42.117. [DOI] [PubMed] [Google Scholar]
- 29.Lee W.-S., Lee K.-W., Heo J.-S., et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surgery Today. 2006;36(10):892–897. doi: 10.1007/s00595-006-3276-8. [DOI] [PubMed] [Google Scholar]
- 30.Pinyosophon A., Wiwanitkit V. The prevalence of hepatitis B seropositivity among patients with cholangiocarcinoma. Viral Immunology. 2002;15(4):655–657. doi: 10.1089/088282402320914593. [DOI] [PubMed] [Google Scholar]
- 31.Asayama Y., Aishima S.-I., Taguchi K.-I., et al. Coexpression of neural cell adhesion molecules and bcl-2 in intrahepatic cholangiocarcinoma originated from viral hepatitis: relationship to atypical reactive bile ductule. Pathology International. 2002;52(4):300–306. doi: 10.1046/j.1440-1827.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee B. S., Park E.-C., Park S. W., Nam C. M., Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a casecontrol study in Korea. World Journal of Gastroenterology. 2015;21(2):502–510. doi: 10.3748/wjg.v21.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y.-M., Zhang X.-F., Wu L.-P., Sui C.-J., Yang J.-M. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World Journal of Gastroenterology. 2014;20(35):12615–12620. doi: 10.3748/wjg.v20.i35.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y. Q., Wang H., Li D. P., et al. Occult hepatitis B virus infection in Chinese cryptogenic intrahepatic cholangiocarcinoma patient population. Journal of Clinical Gastroenterology. 2014;48(10):878–882. doi: 10.1097/MCG.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 35.Chang J. S., Tsai C.-R., Chen L.-T. Medical risk factors associated with cholangiocarcinoma in Taiwan: A Population-Based Case-Control Study. PLoS ONE. 2013;8(7, article e69981) doi: 10.1371/journal.pone.0069981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng N.-F., Li L.-Q., Qin X., et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of Hepatitis B virus. Annals of Surgical Oncology. 2011;18(5):1258–1266. doi: 10.1245/s10434-010-1458-5. [DOI] [PubMed] [Google Scholar]
- 37.Cai W.-K., Sima H., Chen B.-D., Yang G.-S. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World Journal of Gastroenterology. 2011;17(2):249–253. doi: 10.3748/wjg.v17.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivatanakul P., Honjo S., Kittiwatanachot P., Jedpiyawongse A., Khuhaprema T., Miwa M. Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pacific Journal of Cancer Prevention. 2010;11(4):985–988. [PubMed] [Google Scholar]
- 39.Zhou H., Wang H., Zhou D., et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. European Journal of Cancer. 2010;46(6):1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Tao L.-Y., He X.-D., Qu Q., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: case-control study in China. Liver International. 2010;30(2):215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee C. H., Chang C. J., Lin Y. J., Yeh C. N., Chen M. F., Hsieh S. Y. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. British Journal of Cancer. 2009;100(11):1765–1770. doi: 10.1038/sj.bjc.6605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y.-M., Yin Z.-F., Yang J.-M., et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World Journal of Gastroenterology. 2008;14(4):632–635. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsing A. W., Zhang M., Rashid A., et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. International Journal of Cancer. 2008;122(8):1849–1853. doi: 10.1002/ijc.23251. [DOI] [PubMed] [Google Scholar]
- 44.Lee T. Y., Lee S. S., Jung S. W., et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: A Case-Control Study. American Journal of Gastroenterology. 2008;103(7):1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto S., Kubo S., Hai S., et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Science. 2004;95(7):592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells G. A., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2012. [Google Scholar]
- 47.Wu Q., He X.-D., Yu L., Liu W., Tao L.-Y. The metabolic syndrome and risk factors for biliary tract cancer: a case-control study in China. Asian Pacific Journal of Cancer Prevention. 2012;13(5):1963–1969. doi: 10.7314/apjcp.2012.13.5.1963. [DOI] [PubMed] [Google Scholar]
- 48.Sankaranarayanan R., Ramadas K., Qiao Y.-L. Managing the changing burden of cancer in Asia. BMC Medicine. 2014;12(1, article 3) doi: 10.1186/1741-7015-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y., Zhao Y., Li B., et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12, article 289 doi: 10.1186/1471-2407-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alison M. R. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Reviews. 2005;1(3):253–260. doi: 10.1385/scr:1:3:253. [DOI] [PubMed] [Google Scholar]
- 51.Liu X.-F., Zou S.-Q., Qiu F.-Z. Pathogenesis of cholangiocarcinoma in the porta hepatis and infection of hepatitis virus. Hepatobiliary and Pancreatic Diseases International. 2003;2(2):285–289. [PubMed] [Google Scholar]
- 52.Tomimatsu M., Ishiguro N., Taniai M., et al. Hepatitis C virus antibody in patients with primary liver cancer (hepatocellular carcinoma, cholangiocarcinoma, and combined hepatocellular- cholangiocarcinoma) in Japan. Cancer. 1993;72(3):683–688. doi: 10.1002/1097-0142(19930801)72:3<683::aid-cncr2820720310>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H., Iwasaki Y., Nouso K., et al. Possible contribution of prior hepatitis B virus infection to the development of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2005;20(6):850–856. doi: 10.1111/j.1400-1746.2005.03823.x. [DOI] [PubMed] [Google Scholar]
- 54.Reddy A., May E., Ehrinpreis M., Mutchnick M. Latent hepatitis B is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C. World Journal of Gastroenterology. 2013;19(48):9328–9333. doi: 10.3748/wjg.v19.i48.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian X., Li J., Ma Z.-M., Zhao C., Wan D.-F., Wen Y.-M. Role of hepatitis B surface antigen in the development of hepatocellular carcinoma: regulation of lymphoid enhancer-binding factor 1. Journal of Experimental and Clinical Cancer Research. 2009;28(1, article 58) doi: 10.1186/1756-9966-28-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohn W., Paik Y.-H., Kim J. M., et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Annals of Surgical Oncology. 2014;21(7):2429–2435. doi: 10.1245/s10434-014-3621-x. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Li J., Li P., et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. Journal of Gastroenterology and Hepatology. 2012;27(10):1561–1568. doi: 10.1111/j.1440-1746.2012.07207.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: A Proposed Reporting Checklist for Authors, Editors, and Reviewers of Meta-analyses