Abstract
Ring D-modified gibberellin (GA) A5 and A20 derivatives are structurally similar to GA20 and GA9 (the precursors to growth-active GA1 and GA4) and, when applied to higher plants, especially grasses, can reduce shoot growth with concomitant reductions in levels of growth-active GAs and increases in levels of their immediate 3-deoxy precursors. The recombinant Arabidopsis GA 3β-hydroxylase (AtGA3ox1) protein was used in vitro to test a number of ring D-modified GA structures as possible inhibitors of AtGA3ox1. This fusion protein was able to 3β-hydroxylate the 3-deoxy GAs, GA9 and GA20, to GA4 and GA1, respectively, and convert the 2,3-didehydro GA, GA5, to its 2,3-epoxide, GA6. Michaelis-Menten constant (Km) values of 1.25 and 10 μm, respectively, were obtained for the GA9 and GA20 conversions. We utilized the enzyme's ability to convert GA20 to GA1 in order to test the efficacy of GA5, 16,17-dihydro GA5 (dihydro GA5), and a number of other ring D-modified GAs as inhibitors of AtGA3ox activity. For the exo-isomer of dihydro GA5, inhibition increased with the dose of dihydro GA5, with Lineweaver-Burk plots showing that dihydro GA5 changed only the Km of the enzyme reaction, not the Vmax, giving a dissociation constant of the enzyme-inhibitor complex (Ki) of 70 μm. Other ring D-modified GA derivatives showed similar inhibitory effects on GA1 production, with 16,17-dihydro GA20-13-acetate being the most effective inhibitor. This behavior is consistent with dihydro GA5, at least, functioning as a competitive substrate inhibitor of AtGA3ox1. Finally, the recombinant AtGA3ox1 fusion protein may be a useful screening tool for other effective 3β-hydroxylase inhibitors, including naturally occurring ones.
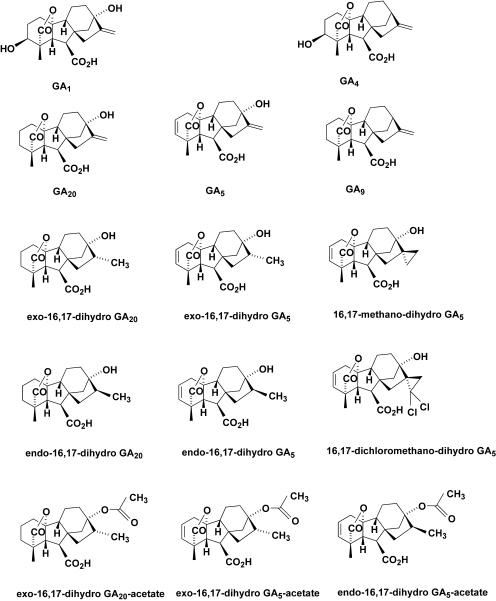
We now know, through the use of gibberellin (GA) biosynthesis mutants (for review, see Reid and Howell, 1995; Hedden and Proebsting, 1999), that GA1, GA4, and other 3β-hydroxylated GAs are active per se in stem elongation, while their immediate 3-deoxy precursors, i.e. GA20 and GA9, are not. The biosynthetic conversions of GA20 and GA9 to GA1 and GA4, respectively, are catalyzed by GA 3β-hydroxylases (Lester et al., 1997; Martin et al., 1997; Williams et al., 1998; Itoh et al., 2001). 16,17-Dihydro GA5 (Fig. 1) and other ring D-modified GA5 derivatives (Mander et al., 1995, 1998a, 1998b) are structurally very similar to GA20.When these ring D-modified GAs are applied to many higher plant species, and especially to grasses, they can effectively inhibit shoot growth (Evans et al., 1994a, 1994b; Takagi et al., 1994; Foster et al., 1997; King et al., 1997, 2004). Associated with the reduction in shoot elongation is a concomitant reduction in levels of endogenous 3β-hydroxylated GAs and an increase in one or more of the 3-deoxy precursors (Foster et al., 1997; Junttila et al., 1997; Zhou, 2000). For example, application of exo-16,17-dihydro GA5-13-acetate to wild oat reduces levels of endogenous GA1, GA3, and GA4 while elevating levels of their immediate 3-deoxy precursors, GA9 and GA20 (Zhou, 2000). It thus appears likely that 16,17-dihydro GA5 and allied ring D-modified GA derivatives gain their efficacy as growth-retarding compounds in grasses (Evans et al., 1994a; Mander et al., 1995; Foster et al., 1997; Zhou, 2000) by inhibiting per se the action of the GA 3β-hydroxylase(s) which catalyze(s) the conversion of GA20 to GA1 and GA9 to GA4, respectively.
Figure 1.
Structures of GA1, GA4, GA9, GA20, GA5, and several ring D-modified GA derivatives.
To examine this possibility, the Arabidopsis GA 3β-hydroxylase (AtGA3ox1) encoded by the GA4 gene was expressed in Escherichia coli. We then used this recombinant protein for a series of in vitro tests, both as the crude lysate (Zhou et al., 1998) and as a purified fusion protein (Zhou, 2000), to examine the ability of a range of concentrations of GA5, the endo- and exo-isomers of 16,17-dihydro GA5 and 16,17-dihydro GA20, three 13-O-acetyl derivatives of these two dihydro GAs, 16,17-methano-dihydro GA5 and 16,17-dichloromethano-dihydro GA5, to inhibit GA 3β-hydroxylase activity (i.e. inhibit conversion of GA20 to GA1 by AtGA3ox1). A parallel approach has also been taken by King et al. (2004) using cell lysates containing recombinant 3β- and 2β-hydroxylases from Pisum sativum, where their range of ring D-modified GA5 derivatives included the 17-ethyl, n-propyl, and n-butyl derivatives of dihydro GA5.
RESULTS
Williams et al. (1998) showed that AtGA3ox1, which is encoded by the GA4 gene, was a GA 3β-hydroxylase. To examine the inhibitory effects of ring D-modified GA derivatives, we also expressed this enzyme as a fusion protein in E. coli. Both the crude E. coli lysate and purified fusion protein, with the addition of appropriate cofactors, effectively 3β-hydroxylated 3-deoxy GAs (e.g. GA20 or GA9). However, in this paper we present only results obtained with the purified fusion protein.
Characterization of Bacterially Expressed AtGA3ox1
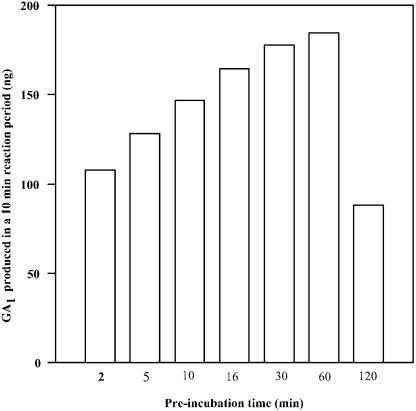
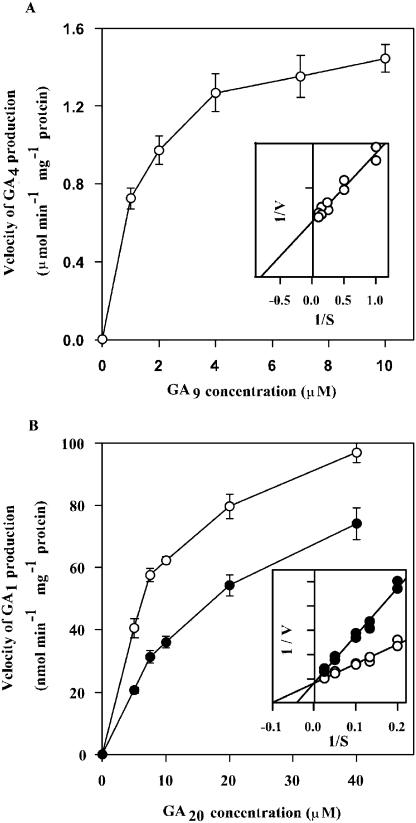
For longer term reactions, the fusion protein's ability to 3β-hydroxylate GA20 to GA1 and GA5 to GA6 was quite sensitive to pH, with enzyme activity being reduced appreciably below pH 6 or above pH 9. The optimal pH, then, for AtGA3ox1 activity, was determined to be 7.5, a finding similar to that obtained by Williams et al. (1998). When the AtGA3ox1 fusion protein was diluted in assay buffer containing essential cofactors, its activity at room temperature increased gradually up to hour 1 (Fig. 2). However, by hour 2, enzyme activity had dropped by nearly 50%. All subsequent assays were thus run with enzyme that had been preincubated at room temperature for 30 to 40 min. The identity and amounts of reaction products, GA4 and GA1, were determined by gas chromatography-mass spectrometry-selected ion monitoring (GC-MS-SIM) as the methyl ester trimethylsilyl ether (MeTMSi) derivatives, with quantification being accomplished through stable isotope dilution after addition of known amounts of [2H2]GA4 and [2H2]GA1 (Fujioka et al., 1988). Plots of the reciprocal of reaction rate against the reciprocal of substrate concentration were produced by linear regression analysis (Fig. 3, A and B). These yielded Km values of 1.25 μm (Vmax 800 nmol min−1 mg−1 protein) for GA9 and 10 μm (Vmax 62 nmol min−1 mg−1 protein) for GA20, respectively, values similar to those reported by Williams et al. (1998).
Figure 2.
Stability of recombinant AtGA3ox1 at room temperature. Enzyme (0.02 μg protein) was diluted in 80 μL Tris-HCl buffer, pH 7.5 containing 0.1 mm Fe2+, 5 mm ascorbate, 5 mm 2-oxoglutarate, 2 mm NADPH, and 0.2 mg BSA. This mixture was incubated at room temperature for varying periods before being mixed with 20 μL of a 50 μm solution of GA20 to form a reaction mixture. Quantification of GA1 was accomplished with the isotope dilution method using GC-MS-SIM (a known amount of [2H2]GA1 was added just prior to extracting the reaction mixture with EtOAc).
Figure 3.
Michaelis-Menten and Lineweaver-Burk (inset) plots for AtGA3ox1's conversion of GA9 to GA4 (A) and GA20 to GA1 (B). Enzyme (0.01 μg for A and 0.1 μg for B) was diluted in 90 μL Tris-HCl buffer, pH 7.5 containing 0.1 mm Fe2+, 5 mm ascorbate, 5 mm 2-oxoglutarate, 2 mm NADPH, and 0.2 mg BSA. This mixture was incubated at room temperature for 30 min before adding GA9 or GA20, with (black circles) or without (white circles) the addition of exo-16,17-dihydro GA5 at a final concentration of 100 μm to form a reaction mixture. The reaction period was 5 min. Quantification of GA4 and GA1 was accomplished by the isotope dilution method using GC-MS-SIM (a known amount of [2H2]GA4 or [2H2]GA1 was added to the reaction mixture just before extraction with EtOAc).
Mode of Action of 16,17-Dihydro GA5 in Inhibiting AtGA3ox1 Activity
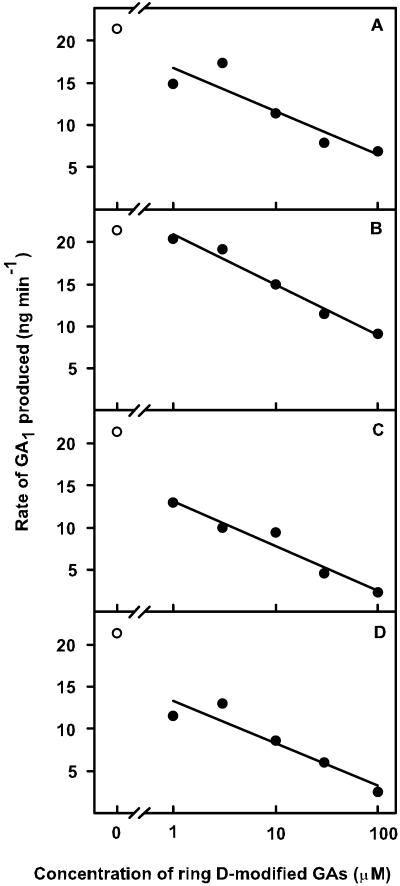
We initially tested the dose effects of four ring D-modified GA derivatives on the ability of AtGA3ox1 to produce GA1 from GA20 (Fig. 4). Enzyme activity decreased in a linear fashion as the log dose of exo-16,17-dihydro GA5 increased (Fig. 4B). A similar response occurred for endo-16,17-dihydro GA5, endo-16,17-dihydro GA20, and exo-16,17-dihydro GA20 (Fig. 4, A, C, and D, respectively). Then we utilized Michaelis-Menten plots to examine the effect of a 100 μm dose of the exo-isomer of dihydro GA5 on AtGA3ox1 activity (reaction velocity) across a range of GA20 concentrations (Fig. 3B, black circles). The Vmax in the presence of the dihydro GA5 remained the same as for GA20 alone, even though the slope increased (Fig. 3B). Hence, only the Km of the reaction was changed, not the Vmax. The dihydro GA5 molecule is thus functioning as a competitive substrate inhibitor in the AtGA3ox1 enzyme's conversion of GA20 to GA1. The dissociation constant of the enzyme-inhibitor complex (Ki) for exo-16,17-dihydro GA5 was calculated to be 70 μm (Fig. 3B).
Figure 4.
Effects of varying the dose (1, 3.3, 10, 33, and 100 μm) of ring D-modified GA derivatives on the production of GA1 from substrate GA20 in the presence of AtGA3ox1. The same amount of enzyme was added to each reaction mixture. The initial GA20 concentration was 10 μm. Cofactors added included 0.1 mm Fe2+, 5 mm ascorbate, 5 mm 2-oxoglutarate, 2 mm NADPH, and 2 mg BSA mL−1. The control is 10 μm GA20 alone, plus cofactors (white circle, upper left). A, endo-dihydro GA5 (r2 = 0.98); B, exo-dihydro GA5 (r2 = 0.82); C, endo-dihydro GA20 (r2 = 0.95); D, exo-dihydro GA20 (r2 = 0.89). The reaction period was 10 min. Quantification of GA1 was accomplished by GC-MS-SIM using a known amount of [2H2]GA1 which was added after stopping the reaction mixture, just before extraction with EtOAc.
We then tested the inhibitory effects of 16,17-dihydro GA5, 16,17-dihydro GA20, and a number of other ring D-modified GA5 derivatives, as well as GA5, all at 100 μm, on 3β-hydroxylation of 10 μm of GA20 by the fusion protein (Table I). Thus, when added to GA20, the aliquots of GA5, exo-16,17-dihydro GA5 and endo-16,17-dihydro GA5, all reduced GA1 production by nearly 50%. 16,17-Methano-dihydro GA5 and 16,17-dichloromethano-dihydro GA5, when added at 10-fold the concentration of GA20, reduced GA1 production by 70% (Table I). The most effective of the dihydro GAs, however, was dihydro GA20, where both the endo- and exo-isomers (Fig. 4, C and D), when added at 10-fold the GA20 concentration, reduced GA1 production by 79% (Table I).
Table I.
Inhibitory effects of GA5 and a range of ring D-modified GA5 and GA20 derivatives on 3β-hydroxylation of GA20 by recombinant AtGA3ox1
| GA1 Produced | |
|---|---|
| % of control | |
| GA20 alone | 100a |
| GA20 + GA5 | 52.4 ± 1.2 |
| GA20 + exo-16,17-dihydro GA5 | 51.4 ± 5.7 |
| GA20 + endo-16,17-dihydro GA5 | 51.2 ± 0.9 |
| GA20 + 16,17-dichloromethano-dihydro GA5 | 27.5 ±1.9 |
| GA20 + 16,17-methano-dihydro GA5 | 30.3 ± 6.0 |
| GA20 + exo-16,17-dihydro GA20 | 21.1 ± 2.6 |
| GA20 + endo-16,17-dihydro GA20 | 21.9 ± 0.4 |
| GA20 + exo-16,17-dihydro GA5-acetate | 3.6 ± 0.1 |
| GA20 + endo-16,17-dihydro GA5 acetate | 6.0 ± 0.3 |
| GA20 + exo-16,17-dihydro GA20-acetate | 0.26 ± 0.01 |
Initial GA20 concentration was 10 μm. Gibberellin A5 and the various GA derivatives were added at a concentration of 100 μm. Values are the mean of two replicate experiments that tested all compounds on the same day.
90 ng of GA1 was produced in 5 min.
Interestingly, addition of an acetate group at C-13 increased inhibition efficacy by approximately 10-fold, with the exo-isomers (most active) of each of dihydro GA5-Ac and dihydro GA20-Ac reducing GA1 formation by 96.4% and 99.7%, respectively (Table I).
Thus, GA5 and all of the ring D-modified GA5 or GA20 derivatives inhibited the conversion of GA20 to GA1 by AtGA3ox1, with exo-16,17-dihydro GA20-13-O-acetate being the most effective (Table I). However, only the exo-isomer of 16,17-dihydro GA5 has actually been shown to function as a competitive substrate inhibitor (Fig. 3).
Also of interest with regard to use of the 13-acetate derivatives of dihydro GA5 and dihydro GA20 is the fact that there was no production of dihydro GA5 or dihydro GA20 during the reaction. This was ascertained by GC-MS-SIM analysis of the reaction mixture. Thus, the exo-isomers of each of 16,17-dihydro GA5-13-O-acetate and 16,17-dihydro GA20-13-O-acetate, the most potent of the ring D-modified GAs, are per se active as inhibitors of the 3β-hydroxylase without conversion to their 13-hydroxyl forms. We also checked the reaction mixtures by GC-MS full scan and SIM for products of 16,17-dihydro GA5, i.e. dihydro GA6 or dihydro GA3, and found no evidence of its metabolism. However, when we checked the reaction mixture containing 16,17-dihydro GA20, putative 16,17-dihydro GA1 (tetrahydro GA3) was identified by GC-MS full scan based on its fragmentation pattern and retention time (Rt) relative to the Rt of [2H2]GA1 (which was present as an internal standard). Thus, the M+ (508) and characteristic m/z fragmentation ions 465, 450, and 209 for 16,17-dihydro GA1 were present at relative intensities shown by authentic 16,17-dihydro GA1. Additionally, the capillary GC Rt of authentic dihydro GA1, relative to the Rt of [2H2]GA1, was consistent with the Rt we found for the putative dihydro GA1. Based on use of the deuterated GA1 internal standard, it was apparent that the amount of putative dihydro GA1 formed increased with the reaction time, at least in the first 10 min.
DISCUSSION
The Km values of AtGA3ox1 fusion protein (in a pGEX-2T vector) for the conversions of GA9 to GA4 and GA20 to GA1 were approximately the same as those obtained by Williams et al. (1998) with lysate preparations and also roughly comparable to those seen for other GA3ox1 enzymes expressed in different vectors (Martin et al., 1997; King et al., 2004). However, purified AtGA3ox1 fusion protein was much more active than the earlier lysate preparations, with a Vmax of 800 and 62 nmol min−1 mg−1 protein for GA9 and GA20 at 20°C (Fig. 3). For example, at optimal pH, a 1-μg aliquot of enzyme, incubated for 1 min at room temperature with a 4 μm concentration of GA9 or 2 μm GA20 substrate in a final volume of 100 μL, produced 300 pmol of GA4 and 22 pmol of GA1, respectively (data not shown). This contrasts with only 5.5 and 1 pmol of GA4 and GA1, respectively, for 1 mg of protein incubated at 30°C for 1 min using 4 μm of GA9 and GA20 as substrate (Williams et al. 1998).
Our lysate preparations appeared to contain AtGA3ox1 inhibitors. That is, when we mixed 3 μL of AtGA3ox1 lysate with 87 μL of control lysate (prepared from bacteria transformed with the empty vector only) in a 100-μL reaction volume, the GA 3β-hydroxylase activity was reduced at least 15-fold (data not shown). Although this type of diminished activity seems most likely to be due to the presence of inhibitors of the hydroxylation reaction, one cannot rule out the possibility that bacterial proteases (which could digest AtGA3ox1) also occur in the lysate preparations. We also found that detergent can reduce the activity of the AtGA3ox1 fusion protein, e.g. Triton X-100 reduced enzyme activity by 75% (data not shown).
The AtGA3ox1 fusion protein is relatively unstable, with activity increasing initially with incubation time up to 1 h, but diminishing after that (Fig. 2). We cannot explain the initial gradual increase of enzyme activity. The AtGA3ox1 fusion protein prefers GA9 as a substrate, followed by GA20 and, finally, GA5. When similar amounts of GA9, GA20, and GA5 were pooled and then incubated with the enzyme, it produced predominantly GA4, only a small amount of GA1, and virtually no GA6 (data not shown). This trend in efficacy can also be seen from a comparison of the Km or Ki values, i.e. GA9, 1.25 μm; GA20, 10 μm; and GA5, 55 μm (the Km's of GA9 and GA20 were calculated from Fig. 3; the Ki of GA5 was estimated from Table I using 100 μm of GA5 incubated with 10 μm of GA20). This finding is in general agreement with earlier reports (Martin et al., 1997; Williams et al., 1998), although Kwak et al. (1988) found that a partially purified GA 3β-hydroxylase from immature seeds of Phaseolus vulgaris had a slightly higher affinity for GA20 than GA9. Thus, enzymes isolated from different plant species or even different tissues of the same plant may or may not show differences in substrate preference.
It is common that compounds with similar structures act as substrate competitors in biosynthetic steps. For the GA 3β-hydroxylase, we would expect that a range of GA structures might compete for the active site(s) with the preferred substrates, GA9 or GA20. Based on evidence in hand for rice (Takagi et al., 1994), sorghum (Foster et al., 1997), Lolium (Junttila et al., 1997), wild oat (Zhou, 2000), or a wild-type pea genotype (King et al., 2004), this type of competition is strongly implied for those ring D-modified GAs that can effectively inhibit shoot growth. Accompanying the inhibition of stem and leaf elongation that was obtained by exogenous application of 16,17-dihydro GA5 and/or its derivatives to various grass species were lowered levels of GA1 and/or GA4 and very elevated levels of GA20 and GA9 (King et al., 2004).
We thus tested the hypothesis that the ring D-modified GAs are acting as competitive substrate inhibitors by using the purified AtGA3ox1 fusion protein in an in vitro system that could rapidly convert GA20 to GA1 (see “Results”). Using the exo-isomer of 16,17-dihydro GA5 (Fig. 1) and expressing the results in a Lineweaver-Burk plot of enzyme activity, it was apparent that dihydro GA5 changed only the Km of the enzyme, with the Vmax remaining the same (Fig. 3B). Thus, dihydro GA5 is indeed a competitive substrate inhibitor of AtGA3ox1. In a parallel study with recombinant pea Le 3β-hydroxylase, where tritium release from [3H]GA20 was utilized as a measure of 3β-hydroxylation, King et al. (2004) provided evidence that implies a similar mechanism (competitive inhibition) for certain, but not all, of the ring D-modified GAs that they tested. However, their data did not allow them to determine whether or not the inhibition was truly competitive for these compounds. For the pea GA3ox, while GA5 was a fair inhibitor (it reduced 3β-hydroxylation to 40% of control at 100 μm), dihydro GA5 inexplicably showed very poor activity, although dihydro GA5-13-Ac was a good inhibitor, i.e. it reduced GA1 production to 30% of control at 10 μm (King et al., 2004). In contrast, for the Arabidopsis AtGA3ox1, GA5 and dihydro GA5 were equally effective, dihydro GA20 was very effective, and the 13-acetate derivatives of these two dihydro GAs were exceptionally effective enzyme activity inhibitors (Table I). Hence, ignoring purification differences and other variables, there may well be inherent differences between the Arabidopsis and pea 3β-hydroxylase enzymes in their ability to recognize ring D-modified GAs as competitive substrate inhibitors. The inhibition of GA 3β-hydroxylation by certain GAs (Kwak et al., 1988) and C/D-ring-rearranged GA isomers (Saito et al., 1992), using a partially purified bean GA 3β-hydroxylase, has also been described. It is thus possible that a wide range of GA5 or GA20 derivatives (Table I) will function as competitive substrate inhibitors not only of AtGA3ox1, but also of 3β-hydroxylases from other plant species.
Among the 16,17-dihydro GAs that we tested, exo-16,17-dihydro GA20-13-O-acetate was the most effective inhibitor of AtGA3ox1 (Table I). The exo-isomer of 16,17-dihydro GA5-O-13-acetate was also quite effective, with 1.7-fold greater inhibitory activity than the comparable endo-isomer (Table I). However, using the simpler structures (e.g. dihydro GA5 and dihydro GA20 which have a C-13 hydroxyl), there were essentially no differences in efficacy between the two exocyclic isomers (Table I).
The Ki value obtained for exo-dihydro GA5 in its inhibition of the GA20 to GA1 conversion is about 70 μm. In contrast, the Km values for GA9 and GA20 in their conversions to GA4 and GA1, respectively, are 1.25 and 10 μm (as calculated from Fig. 3). Thus, the inhibitory effect of dihydro GA5 on the in vitro activity of AtGA3ox1 is really quite limited. This can also be seen from results shown in Table I, where inhibitory activity of dihydro GA5 is compared with a range of ring D-modified GAs, including the highly effective 13-O-acetate- and 16,17-dichloromethano derivatives of dihydro GA5 and dihydro GA20. Even so, dihydro GA5 is a very effective inhibitor in situ of both growth and GA20 3β-hydroxylation in rice (Takagi et al., 1994), sorghum (Foster et al., 1997), Lolium (Junttila et al., 1997), and wild oat (Zhou, 2000).
Although GA5 and dihydro GA5 give similar inhibitory effects (Table I), GA5 stimulates stem elongation while dihydro GA5 usually inhibits it, especially in grasses (Evans et al., 1994b). The growth-promotive activity of GA5 can be explained in a number of ways. For example, GA5 may be per se active (as postulated by Spray et al. [1996]), or else growth-active metabolites of GA5 (e.g. GA3, GA6) are produced (Fujioka et al., 1990). We think that the latter hypothesis is more likely. This conclusion is based on in situ experiments that showed production of growth-active metabolites (see above), together with in vitro enzyme assays using AtGA3ox1 (GA5 converted to GA6; Williams et al., 1998, and herein) and OsGA3ox1 (GA5 converted to GA3; Itoh et al., 2001). That said, another explanation for the activity of GA5 is the possibility that GA5 may block GA20 2β-hydroxylation (which yields GA29, an inactive catabolite), an effect that would place appreciably increased amounts of GA20 into the biosynthetic pathway where it could be converted to growth-active GA1. Indeed, such an effect was seen for GA5 when it was used in vitro to inhibit the action of recombinant pea GA2ox1 enzyme (which catalyzes the GA20 to GA29 step; King et al., 2004).
Different dihydro GA5 derivatives have very different inhibitory effects on AtGA3ox1 activity, thus implicating structural differences between the derivatives at C-3, C-13, and C-16,17 as the causal factor(s). Relative to GA20, the C-2,3 double bond of GA5 reduces, by fivefold, the affinity of AtGA3ox1 for the GA molecule (Table I). In contrast, relative to GA5, the loss of the double bond at C-16,17, i.e. dihydro GA5, did not increase inhibition of AtGA3ox1's activity (Table I). However, it was somewhat surprising to note that for pea GA3ox1, dihydro GA5 was very much less effective as an inhibitor than GA5 (King et al., 2004). When one makes further modifications at C-16,17, a very enhanced inhibitory effect on activity of AtGA3ox1 can be obtained. For example, relative to 16,17-dihydro GA5, both 16,17-methano-dihydro GA5 and 16,17-dichloromethano-dihydro GA5 have much greater inhibitory effects on AtGA3ox1 activity (Table I). In a somewhat similar fashion, modifying the exocyclic 17-methyl (dihydro GA5) to exocyclic ethyl, n-propyl and n-butyl structures also gave enhanced inhibition of 3β-hydroxylation (relative to dihydro GA5) for the recombinant pea GA 3β-hydroxylase (King et al., 2004). Finally, replacing the 13-hydroxyl of GA5 with a 13-O-acetate substantially increased inhibitory activity against the pea GA 3β-hydroxylase (King et al., 2004). Also, replacing the 13-hydroxyl with a hydrogen, i.e. GA9, gives about a sevenfold higher affinity (relative to GA20) for AtGA3ox1 (Fig. 3; Williams et al., 1998) and a similar relationship occurs even with the pea 3β-hydroxylase (Martin et al., 1997).
Dihydro GA5-13-O-acetate and especially dihydro GA20-13-O-acetate gave exceptionally enhanced (10- to 100-fold increases) inhibition of AtGA3ox1 activity, relative to the dihydro GA analogs with a free hydroxyl at C-13 (Table I). However, we did not know whether the 13-acetates of 16,17-dihydro GA20 and dihydro GA5 function per se in the living plant, or first undergo hydrolysis of the 13-acetate group. A preliminary experiment with [3H]GA1-13-acetate in rice (D.W. Pearce, R.P. Pharis, and L.N. Mander, unpublished data) did show a rapid conversion of the 13-acetate to [3H]GA1. However, our in vitro work with AtGA3ox1 demonstrates that the 13-acetate forms of dihydro GA5 and dihydro GA20 exert their inhibitory effects directly (e.g. GC-MS-SIM of the reaction mixture showed no presence of a 13-hydroxylated dihydro GA [i.e. no dihydro GA5 or dihydro GA20] after the reaction was finished).
We also found that purified AtGA3ox1 did not produce detectable levels (based on GC-MS full-scan analysis of the reaction mixtures) of any metabolites of dihydro GA5. We had expected that there might be production of dihydro GA3 or dihydro GA6 based on earlier work with a cell-free system from immature bean seeds by Saito (1990) using C/D-rearranged GA5. King et al. (2004) also reported a finding similar to ours (no metabolism of 16,17-dihydro GA5) for the pea Le GA3ox. Hence, there may be inherent differences between species, or else the crude, cell-free system used by Saito et al. (1992) may have contained essential cofactors that were missing in our reaction mixture or in the reaction mixture of King et al. (2004). We did, however, find that 16,17-dihydro GA20 was metabolized in our reaction mixture to putative 16,17-dihydro GA1 by AtGA3ox1 and that the production of putative dihydro GA1 increased with time. Of interest here is the likelihood that production of dihydro GA1 would likely have resulted in a somewhat reduced level of enzyme-inhibitory activity by the dihydro GA20 in the reaction mixture (Table I), since dihydro GA1 has been shown to be growth promotive on stem elongation in Lolium (Evans et al., 1994b). The recombinant purified AtGA3ox1 system is a very useful tool in better understanding the interaction between substrate structure and enzyme function for the 3β-hydroxylation step in GA biosynthesis. Additionally, this system may be useful for screening GA 3β-hydroxylase inhibitors, including naturally produced inhibitors. Finally, the AtGA3ox1 fusion protein can also be used to synthesize stable isotope- or radioisotope-labeled GAs. Such a use of recombinant AtGA3ox1 has recently been described by Tudzynski et al. (2003).
MATERIALS AND METHODS
Expression of Arabidopsis AtGA3ox1 Protein in Escherichia coli
A sense primer (5′ to 3′, GGGGATCCATGCCTGCTATGTTAACAG) and an antisense primer (5′ to 3′, GGGGATCCTTCTTCTCTGTGATTTCTAA), with a BamHI restriction site for each primer, were designed based on the Arabidopsis GA4 cDNA sequence (Chiang et al., 1995). When pGEX-2T vector was used, the designed sense primer allowed the GST reading frame to read through the GA4 insert. PCR was then performed using the Arabidopsis GA4 cDNA clone pCD7 (a gift from Dr. H.M. Goodman, Harvard Medical School, Boston, MA) as the template. The reaction mixture (100 μL) contained 250 μm dNTPs, 2 mm MgCl2, 40 pmol of each primer, and 20 units of cloned Pfu DNA polymerase (Stratagene, La Jolla, CA). The reaction was heated to 94°C for 2 min, then subjected to 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min. The amplified PCR product was digested with BamHI and ligated into the dephosphorylated BamHI site of the pGEX-2T vector before E. coli DH5α cells were transformed. Isolated plasmids were examined with a PCR reaction using the sense and 3′ pGEX sequencing primers to see if they contained the correct insert oriented in the correct direction. The clone with the correct orientation was then sequenced. Transformed E. coli were inoculated into 5 mL of an LA broth (1% [w/v] NaCl; 1% [w/v] tryptone; and 0.5% [w/v] yeast extract containing 100 μg amphicillin mL−1;) and initially incubated at 37°C overnight with shaking. Then, after the addition of isopropyl-1-thio-α-d-galactopyranoside (IPTG) to a final concentration of 0.2, 0.4, 0.6, and 0.8 mm, the transformed E. coli cells were incubated at 28°C for 3 h. The bacteria were then spun down and to each of the pellets was added 1 mL of phosphate-buffered saline (PBS), containing 140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 1.8 mm KH2PO4, pH 7.3 with 50 μL 0.2 m phenylmethylsulfonyl fluoride The pellets were then sonicated to lyse the bacteria. About 20 μg of lysate protein from each pellet was analyzed on an SDS gel and we found that use of 0.4 mm IPTG induced the highest titer of fusion protein. The crude lysate containing the fusion protein was then tested to see if it could convert [2,3-3H]GA9 and [17,17-2H]GA9 to labeled-GA4. We used the bacterial lysate transformed with pGEX-2T vector alone as a control.
To make a large amount of recombinant AtGA3ox1 fusion protein, the transformed bacteria were inoculated into 100 mL of enriched medium (2% [w/v] tryptone; 1% [w/v] yeast extract; 50 mm KPi; 0.2% [w/v] glycerol; 0.5% [w/v] NaCl; pH 7.5) and incubated overnight at 37°C with shaking. Fresh medium (400 mL) was then used to dilute the bacteria and IPTG was added to a final concentration of 0.4 mm. After shaking at 28°C for 5 to 6 h, the bacteria were spun down at 4°C in 250-mL bottles at 8,000 rpm (approximately 5,000g) for 10 min. The pellet was suspended in 80 mL of PBS with 0.5 mm phenylmethylsulfonyl fluoride on ice. A French press was then used to lyse the bacteria in order to release the fusion protein and the lysate was centrifuged at 10,000 rpm (approximately 6,000g) for 10 min at 4°C to remove cell debris. The supernatant was passed through a preconditioned glutathione Sepharose 4B (2 mL) affinity column (Pharmacia, Piscataway, NJ). After washing the column with 20 mL of PBS, the flow was stopped and 10 mL of a freshly made reduced glutathione elution buffer (10 mm glutathione in 50 mm Tris, pH 7.5) was added. The affinity-column eluate was collected in 1-mL fractions and a Bio-Rad (Hercules, CA) protein assay was used to estimate protein titer for each fraction. The fusion protein eluted mainly in fractions 2 and 3, which were combined. Aliquots containing 100 μg of protein were taken and frozen in liquid N2 for storage at −60°C.
Enzyme Assays
When the crude lysate was used, 90 μL were mixed with 5 μL of the 3-deoxy GA substrates and another 5 μL of the necessary cofactors (10 mm Fe2+, 80 mm 2-oxoglutarate, 80 mm ascorbate, 2 mg bovine serum albumin [BSA] mL−1, and 1 mg catalase mL−1). When purified fusion protein was used, a thawed aliquot of the purified recombinant AtGA3ox1 fusion protein was added to 2 mL of Tris-HCl buffer (pH 7.5) containing 2 mg BSA mL−1, 2 mm dithiothreitol, 2 mm nicotinamide adenine dinucleotide phosphate, reduced (NADPH), 5 mm 2-oxoglutarate, 5 mm of ascorbic acid, and 0.1 mm Fe2+. The 3-deoxy GA substrates were first dissolved in methanol, then diluted to 10% methanol (v/v) with 50 mm Tris-HCl buffer (pH 7.5). Unless specified otherwise, all tests, including enzyme stability tests, were carried out at room temperature (20°C). The stability of the fusion protein was tested over 2, 5, 10, 16, 30, 60, and 120 min by taking an 80-μL aliquot of diluted protein from each incubation time and adding it to the 20 μL of 200 μm GA20 solution, followed by a 10-min reaction period. For kinetic studies, diluted protein was incubated at room temperature for 30 min before adding the 3-deoxy GA substrates to initiate the assay reaction. This was accomplished with and without the various ring D-modified GA5 derivatives and ring D-modified GA20 derivatives, and with GA5 (as putative competitive inhibitors of 3β-hydroxylation).
The assay reaction was stopped by adding 1 mL of cold 5% HOAc and mixing, yielding a pH of about 3. In order to measure the reaction product and/or precursor remaining, a known amount of internal standards was added and the mixture then partitioned 3× against 1 volume of H2O-saturated EtOAc. An aliquot of the combined EtOAc fractions was dried under N2, methylated with ethereal diazomethane, and trimethylsilyated by N,O,-bis(trimethylsilyl)trifluoroacetimide (BSTFA) with 1% trimethylchorosilane (TMCS) prior to analysis by GC-MS-SIM (Sheng et al., 1992). The GA substrate or product was then quantified by isotope dilution of the [2H2]GA (Fujioka et al., 1988). Identification was made by full-scan GC-MS.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. H.M. Goodman (Harvard Medical School and Department of Molecular Biology, Massachusetts General Hospital, Boston, MA) for GA4 clone and Prof. L.N. Mander (Research School of Chemistry, Australian National University, Canberra, A.C.T., Australia) for both the deuterated GAs and ring D-modified GAs.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041509.
References
- Chiang H-H, Hwang I, Goodman HM (1995) Isolation of the Arabidopsis GA4 locus. Plant Cell 7: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT, King RW, Mander LN, Pharis RP (1994. a) The relative significance for stem elongation and flowering in Lolium temulentum of 3β-hydroxylation of gibberellins. Planta 192: 130–136 [Google Scholar]
- Evans LT, King RW, Mander LN, Pharis RP, Duncan KA (1994. b) The differential effects of C-16,17-dihydro gibberellins and related compounds on stem elongation and flowering in Lolium temulentum. Planta 193: 107–114 [Google Scholar]
- Foster KR, Lee I-J, Pharis RP, Morgan PW (1997) Effects of ring D-modified gibberellins on gibberellin levels and development in selected Sorghum bicolor maturity genotypes. J Plant Growth Regul 16: 79–87 [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N (1988) Qualitative and quantitative analysis of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol 88: 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Phinney BO, Gaskin P, MacMillan J, Takahashi N (1990) Gibberellin A3 is biosynthesised from gibberellin A20 via gibberellin A5 in shoots of Zea mays L. Plant Physiol 94: 127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Proebsting WM (1999) Genetic analysis of gibberellin biosynthesis. Plant Physiol 119: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M (2001) Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc Natl Acad Sci USA 98: 8909–8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila O, King RW, Poole A, Kretschmer G, Pharis RP, Evans LT (1997) Regulation in Lolium temulentum of the metabolism of gibberellin A20 and gibberellin A1 by 16,17-dihydro-GA5 and by the growth retardant, LAB 198 999. Aust J Plant Physiol 24: 359–369 [Google Scholar]
- King RW, Blundell C, Evans LT, Mander LN, Wood JT (1997) Modified gibberellins retard growth of cool-season turfgrasses. Crop Sci 37: 1878–1883 [Google Scholar]
- King RW, Junttila O, Mander LN (2004) Gibberellin structure and function: biological activity and competitive inhibition of 3β-hydroxylase and 2β-oxidase enzymes. Physiol Plant 120: 287–297 [DOI] [PubMed] [Google Scholar]
- Kwak S-S, Kamiya Y, Sakurai A, Takahashi N, Graebe JE (1988) Partial purification and characterization of gibberellin 3ß-hydroxylase from immature seeds of Phaseolus vulgaris L. Plant Cell Physiol 29: 935–943 [Google Scholar]
- Lester DR, Ross JJ, Davies PJ, Reid JB (1997) Mendel's stem length gene (Le) encodes a gibberellin 3β-hydroxylase. Plant Cell 9: 1435–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander LN, Camp D, Evans LT, King RW, Pharis RP, Sherburn M, Twitchin B (1995) Designer gibberellins: The quest for specific activity. Acta Hortic 394: 45–55 [Google Scholar]
- Mander LN, Adamson G, Bhaskar VK, Twitchin B, Camp D, King RW, Evans LTE (1998. a) Effects of 17-alkyl-16,17-dihydrogibberellin A5 derivatives on growth and flowering in Lolium temulentum. Phytochemistry 49: 1509–1515 [DOI] [PubMed] [Google Scholar]
- Mander LN, Sherburn M, Camp D, King RW, Evans LT, Pharis RP (1998. b) Effects of D-ring modified gibberellins on flowering and growth in Lolium temulentum. Phytochemistry 49: 2195–2206 [Google Scholar]
- Martin DN, Proebsting WM, Hedden P (1997) Mendel's dwarfing gene: cDNAs from the Le alleles and function of expressed proteins. Proc Natl Acad Sci USA 94: 8907–8911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB, Howell SH (1995) Hormone mutants and plant development. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2, Kluwer Academic Publishers, The Netherlands, pp 448–485
- Saito T (1990) Purification of gibberellin biosynthetic enzymes and investigation of their inhibitors. PhD thesis. University of Tokyo, Tokyo, Japan
- Saito T, Kamiya Y, Yamane H, Sakurai A, Murofushi N, Takahashi N (1992) Effects of 3-methylgibberellin analogs on gibberellin 3β-hydroxylases and plant growth. Biosci Biotechnol Biochem 56: 1046–1052 [DOI] [PubMed] [Google Scholar]
- Sheng C, Bhaskar KV, Chu W-LA, Mander LN, Pearce DW, Pharis RP, Young S (1992) Identification of a novel gibberellin (GA85) in very young seedlings of Brassica campestris cv. Tobin. Biosci Biotechnol Biochem 56: 564–566 [DOI] [PubMed] [Google Scholar]
- Spray CR, Kobayashi M, Suzuki Y, Phinney BO, Gaskin P, MacMillan J (1996) The dwarf-1 (d1) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc Natl Acad Sci USA 93: 10515–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Pearce DW, Janzen LM, Pharis RP (1994) Effect of exo-16,17-dihydro-gibberellin A5 on gibberellin A20 metabolism in seedlings of dwarf rice (Oryza sativa L. cv. Tan-ginbozu). Plant Growth Regul 15: 207–213 [Google Scholar]
- Tudzynski B, Mihlan M, Rojas MC, Linnemannstones P, Gaskin P, Hedden P (2003) Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi. J Biol Chem 31: 28635–28643 [DOI] [PubMed] [Google Scholar]
- Williams J, Phillips AL, Gaskin P, Hedden P (1998) Function and substrate specificity of the gibberellin 3β-hydroxylase encoded by the Arabidopsis GA4 gene. Plant Physiol 117: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R (2000) The mode of action of ring D-modified gibberellin A5 and its derivatives in wild oat seedlings. PhD thesis. University of Calgary, Calgary, Alberta, Canada
- Zhou R, Yu M, Pharis RP (1998) Dihydro-GA5 and its derivatives competitively inhibit recombinant Arabidopsis GA 3β-hydroxylase. Intl Plant Growth Substances Assoc 16th Intl Conference, Tokyo, p 157 (Abstr No. 292)