Abstract
Auxin affects many aspects of plant growth and development. We previously used chemical genetics to dissect auxin-signaling mechanisms and identified a small molecule, sirtinol, that constitutively activated auxin signaling (Y. Zhao et al. [2003], Science 301: 1107–1110). Here we describe the isolation, characterization, and cloning of an Arabidopsis mutant Atcand1-1 that emerged from a genetic screen for mutants insensitive to sirtinol. Loss-of-function mutants of AtCAND1 were resistant to sirtinol and auxin, but not to gibberellins or brassinolide. Atcand1 displayed developmental phenotypes similar to those of axr1, namely, short petioles, downwardly curling leaves, short inflorescence, and reduced fertility. AtCAND1 is homologous to human CAND1, a protein that is composed almost entirely of HEAT-repeat units and has been implicated in regulating the assembly and disassembly of the SCF protein degradation machinery. Taken together with previous biochemical studies, this work helps to elucidate the roles of AtCAND1 in protein degradation and auxin signaling.
Auxin is essential for plant growth and development, and it participates in processes ranging from embryogenesis and seedling growth up to flowering and senescence. There are two commonly known responses when plants are treated with auxin. The first is the rapid degradation of the transcription repressor AUX/IAA proteins by a ubiquitin-related pathway (Abel et al., 1994; Dharmasiri and Estelle, 2002). The second is the subsequent induction of a certain subset of genes, curiously including those that encode AUX/IAA proteins (Hagen and Guilfoyle, 1985; Theologis et al., 1985). A large part of the transcriptional response is believed to be mediated by the binding of auxin response factors (ARFs; Ulmasov et al., 1997; Guilfoyle et al., 1998) to auxin response elements (Ballas et al., 1993; Ulmasov et al., 1995) found upstream of auxin-inducible genes. In the basal state, AUX/IAA proteins sequester ARFs by heterodimerizing with them and hence prevent ARFs from homodimerizing and activating auxin-inducible genes (Ulmasov et al., 1999; Worley et al., 2000; Reed, 2001; Rogg and Bartel, 2001). Therefore, the model for auxin-mediated gene expression rests on the controlled degradation of the inhibitory AUX/IAA proteins to release the ARFs, enabling them to dimerize with each other and to activate transcription from auxin response elements.
Following a poorly defined but presumed modification in response to auxin, AUX/IAA proteins become substrates for the SCFTIR (Skp1p, Cdc53p/cullin, and F-box protein) protein degradation complex. TIR1 (an F-box protein) has been shown to have an auxin-dependent physical interaction with AUX/IAA proteins, through which they are recruited to the SCF complex where they are targeted for degradation (Gray et al., 2001). In Arabidopsis, mutations in any of the SCFTIR components, including ask1-1 (a Skp1-like protein; Gray et al., 1999), axr6 (cullin1; Hobbie et al., 2000; Hellmann et al., 2003), and tir1 (an F-box protein; Ruegger et al., 1998), all confer resistance to auxin.
In addition to the core components of the SCFTIR1 complex, genetic screens for auxin-resistant mutants have produced several other genes, such as axr1 (Lincoln et al., 1990; Leyser et al., 1993) and rce1 (Dharmasiri et al., 2003), that are known to participate in auxin-related protein degradation, but are not part of the SCF complex per se. AXR1 and RCE1 are involved in the conjugation of RUB1 (related to ubiquitin) to AXR6 (Gray et al., 2002). The precise biochemical significance of RUB1-cullin1 conjugation is not yet clear; however, in animal systems, this process, commonly known as neddylation, has been shown to promote SCF complex assembly and therefore lead to ubiquitin chain formation upon target proteins (Cope and Deshaies, 2003). The role of neddylation in auxin signaling is perhaps somewhat more complex: Mutations that disrupt the process in either direction, i.e. RUB1 conjugation or deconjugation from AXR6, all lead to auxin resistance (Leyser et al., 1993; Schwechheimer et al., 2001). The regulation of the cullin subunit of the SCF complex is further complicated by the fact that the HEAT-repeat protein CAND1 (Cullin-associated and neddylation dissociated) has been shown in human cells to preferentially sequester the unneddylated form of cullin1, thereby preventing it from binding to SKP1 and the F-box component SKP2 (Liu et al., 2002; Zheng et al., 2002; Oshikawa et al., 2003). Therefore, a better understanding of CAND1 and other regulatory mechanisms imposed on protein degradation may provide further insight into auxin signaling in higher plants.
Using chemical genetics (Blackwell and Zhao, 2003), we have previously identified a small molecule, sirtinol, that activates auxin-inducible genes, causes degradation of AUX/IAA proteins, and leads to auxin-related developmental phenotypes (Zhao et al., 2003). Here we describe the use of sirtinol to isolate, characterize, and clone Arabidopsis CAND1 (AtCAND1). Loss-of-function alleles of AtCAND1 conferred resistance to sirtinol and auxin, but not to GA3 or brassinolide. AtCAND1 encodes a protein composed almost entirely of HEAT-repeat units that is highly homologous to the human CAND1. Together with previous biochemical studies, this work helps to elaborate on roles of protein degradation in auxin signaling.
RESULTS
Isolation of New Sirtinol-Resistant Mutants
It is known from our previous work that all auxin-signaling mutants tested are resistant to sirtinol (Zhao et al., 2003), but those mutants did not emerge from our initial sirtinol-resistant mutant screen. Perhaps the sirtinol concentration (25 μm) used in the previous screen was too high. Therefore, we carried out a genetic screen for mutants resistant to sirtinol at lower concentration (20 μm). As expected, new alleles of the known auxin-resistant mutants (axr1, axr2, axr3, and axr6) emerged from the screen (data not shown). A new mutant (A1-1) was also identified, and linkage analysis placed its locus at the top of chromosome II, where there are no previously identified auxin-resistant genes.
When A1-1 (hereafter referred to as Atcand1-1) was backcrossed to either wild-type Columbia or Landsberg, the resulting F1 plants were all sensitive to sirtinol, indicating that Atcand1-1 was recessive. About 25% of the F2 population resulting from self-fertilization of F1 plants of the Atcand1-1 backcross were sirtinol resistant, suggesting that the observed phenotype arises from a mutation in a single gene.
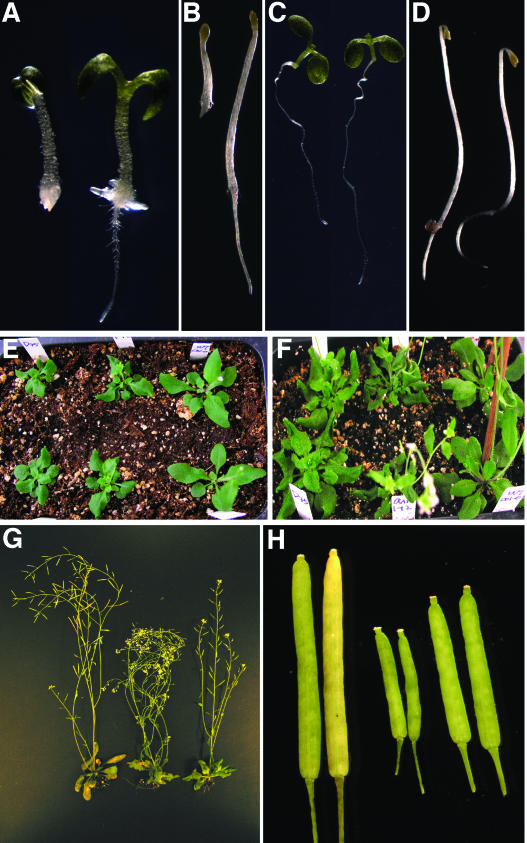
In the presence of sirtinol, light-grown Atcand1-1 displayed significant root elongation whereas the wild-type control lacked primary roots (Fig. 1A). In the dark, sirtinol had little effect on Atcand1-1 hypocotyl and primary root development, but suppressed apical hook formation. This is in contrast to the wild type for which both hypocotyl elongation and root elongation were suppressed by sirtinol (Fig. 1B). In the absence of sirtinol, both light-grown and dark-grown Atcand1-1 seedlings grew normally and there were no apparent differences between Atcand1-1 and the wild-type controls (Fig. 1, C and D). However, the young adult plants of Atcand1-1 displayed strong developmental phenotypes that closely resemble those observed in the well-characterized auxin-resistant mutant axr1 (Lincoln et al., 1990), namely, short petioles and irregular rosette leaves that have the tendency to curl downward (Fig. 1, E and F). Like axr1 mutants, Atcand1-1 had short inflorescences and reduced fertility relative to wild type, but, in contrast, it produced far more seeds than the severe axr1-12 allele. The siliques of Atcand1-1 were shorter than those of the wild type but longer than those of axr1-12, which may be related to its intermediate fertility (Fig. 1, G and H).
Figure 1.
Distinct phenotypes of a sirtinol-resistant mutant Atcand1-1. A, Atcand1-1 had an elongated primary root (right), whereas the wild-type control (left) had essentially no primary roots when grown on 10 μm sirtinol under white light for 5 d. B, Atcand1-1 was also resistant to sirtinol in the dark. Atcand1-1 grown on 5 μm sirtinol in total darkness for 3 d developed normal hypocotyls and roots (right). C, Atcand1-1 (right) and wild type (left) grown on 0.5× MS in light for 7 d. D, Atcand1-1 (right) and wild type grown on 0.5× MS for 3 d in the dark. E and F, Adult Atcand1-1 plants grown in a greenhouse. Left, Atcand1-1; middle, axr1-12; and right, wild type. G, Inflorescences and a mature plant of Atcand1-1. Left, wild type; middle, axr1-12; and right, Atcand1-1. H, Siliques of Atcand1-1. Left, wild type; middle, axr1-12, and right, Atcand1-1.
Atcand1-1 Is an Auxin-Resistant Mutant
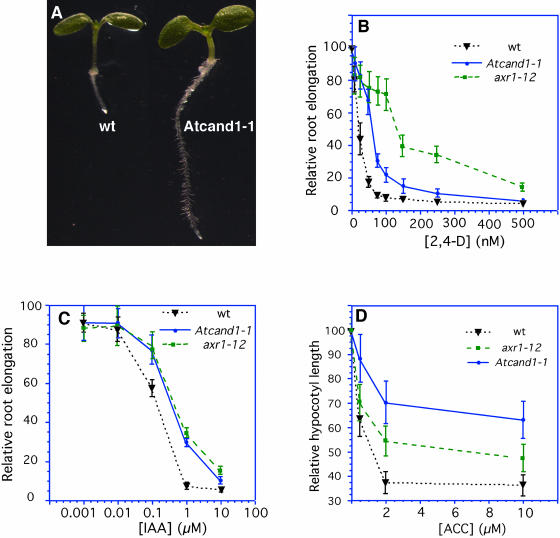
The main response of Arabidopsis seedlings to exogenous auxin is inhibition of primary root elongation. Unlike the wild-type controls, Atcand1-1 displayed elongated primary roots when grown on media containing 100 nm 2,4-dichlorophenoxyacetic acid (2,4-D) (Fig. 2A). Comparative root elongation assays were performed at various concentrations of 2,4-D and indole-3-acetic acid (IAA), and we found that Atcand1-1 is approximately three times less sensitive to both 2,4-D and IAA than the wild type (Fig. 2, B and C).
Figure 2.
Atcand1-1 is resistant to exogenous auxin in root elongation assays. A, Seedlings were germinated and grown on 100 nm 2,4-D under white light for 5 d. Atcand1-1 (right) displayed elongated primary roots. B, Effects of 2,4-D on root elongation. Both axr1-12 and Atcand1-1 displayed decreased sensitivities to exogenous auxin. C, Effects of IAA on root elongation. Note that the x axis is in log scale. D, Effects of ethylene biosynthetic precursor ACC on hypocotyl elongation in the dark.
Positional Cloning of Atcand1-1
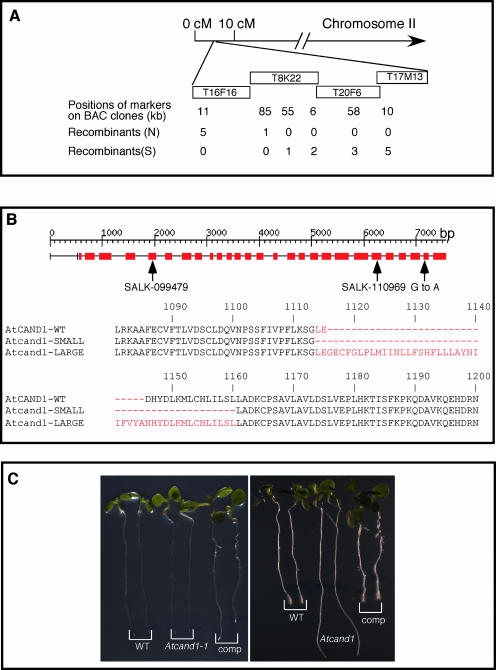
The Atcand1-1 mutant was mapped to a 30-kb interval between markers T8K22A and T8K22B at the top of chromosome II (Fig. 3A). DNA sequencing of the open reading frames in that interval identified a single G-to-A transversion in the gene At2g02560 (Fig. 3B). The mutation occurred at the splice junction of exon 26 and intron 25 (Fig. 3B) and led to aberrant mRNA processing. Two At2g02560 transcripts were found in the Atcand1-1 mutant, one smaller and one larger than the wild type (Fig. 3B). The former species was predominant (over 90% of the total At2g02560 mRNA) and led to a 17 amino acid-residue deletion near the C terminus. The latter species led to a 30 amino acid-residue insertion in the same region (Fig. 3B). Either or both species may be compromised in function relative to wild-type At2g02560.
Figure 3.
Cloning of Atcand1-1 mutant. A, Cloning of Atcand1-1 by map-based cloning. cM, Centimorgan; BAC, bacterial artificial chromosome. B, The nature and molecular consequences of Atcand1 mutations. The intron/exon diagram shown here was downloaded from TIGR database (www.tigr.org). The SALK numbers represent T-DNA insertion lines and the insertion locations were indicated. The G-to-A conversion occurred in Atcand1-1. C, Complementation of Atcand1-1 with a genomic fragment of At2g02560 plus its regulatory sequences. Left, Seedlings were just transferred to media containing 100 nm 2,4-D; right, plants shown at left grown on 2,4-D for 3 d.
A genomic fragment containing the entire coding region of At2g02560 plus an additional 2.5 kb of upstream sequence was able to restore auxin and sirtinol sensitivity to Atcand1-1 transgenic plants (Fig. 3C), providing strong evidence that the mutation in At2g02560 was responsible for the phenotypes observed.
At2g02560 is a single-copy gene in Arabidopsis and was annotated as TIP120A (TATA-box-binding-protein interacting protein) in the Arabidopsis genome database. Further sequence analysis indicated that At2g02560 is the Arabidopsis homolog of human CAND1 (GenBank accession no., NM_018448), hence the name Atcand1-1. The overall sequence identities and similarities between the amino acid sequences of At2g2560 and human CAND1 were 40% and 58%, respectively.
Human CAND1 contains approximately 25 HEAT-repeat units (for Huntingtin, Elongation factor 3, Protein phosphatase 2A, TOR1), a structural motif composed of two anti-parallel interacting helices (Liu et al., 2002; Zheng et al., 2002). A HEAT-repeat matrix often serves as flexible scaffolding on which other proteins can assemble. The fact that CAND1 interacts preferentially with unneddylated cullin in human cells (Liu et al., 2002; Zheng et al., 2002) suggests that AtCAND1 may use a similar mechanism to affect SCF complex assembly in plants, thus potentially regulating the degradation of AUX/IAA proteins.
AtCAND1 Is Ubiquitously Expressed
Total RNA prepared from Arabidopsis seedlings, roots, leaves, stems, flowers, and siliques was used to analyze tissue specificity of AtCAND1 expression. AtCAND1 was expressed in every tissue throughout the plant, consistent with its role in fundamental cellular processes (data not shown).
Identification of Additional Alleles of Atcand1
To identify additional alleles of Atcand1, we searched the SALK T-DNA database (http://signal.salk.edu/cgi-bin/tdnaexpress) for insertional mutants. Three T-DNA lines were identified and seeds for the lines were ordered from the Arabidopsis Stock Center. Upon genotyping these lines, we only identified T-DNA insertions in two lines, SALK-099479 and SALK-110969. According to the data base annotation, T-DNA line SALK-099479 contains an insertion in exon 5 and the T-DNA line SALK-110969 has an insertion in exon 22 (Fig. 3B); these lines were renamed Atcand1-2 and Atcand1-3, respectively. Both insertional alleles were resistant to sirtinol and auxin (Fig. 4, A and B). The adult plants of these alleles had phenotypes similar to those of Atcand1-1, namely, shorter petioles, downward curled leaves, and overall smaller stature (Fig. 4, C and D). In contrast to Atcand1-1, the phenotypes of the insertional alleles were more severe: the inflorescences were shorter and they were almost completely sterile.
Figure 4.
Analysis of T-DNA insertion alleles of Atcand1. A, Atcand1-2 (right) and wild type grown on 10 μm sirtinol for 6 d. B, Atcand1-2 (right) grown on 100 nm 2,4-D for 7 d. C, Adult plants of Atcand1 mutants. Left, Atcand1-1; middle, Atcand1-2; and right, wild type. Both Atcand1-2 and Atcand1-1 had short petiole and curly leaves, but Atcand1-2 had stronger phenotypes. D, Mature plants of Atcand1 mutants. Left, Atcand1-1; and right, Atcand1-2. Atcand1-2 was essentially sterile.
Responses of Atcand1-1 to Other Plant Hormones
Previous studies had shown that genes involved in regulating AXR6 modification and/or SCF complex assembly are often involved in multiple hormone and cellular signaling processes. For example, axr1, the first cloned auxin-resistant mutant, has been shown to be resistant to jasmonic acid (Tiryaki and Staswick, 2002). We examined Atcand1-1 in this light. Among all the hormones tested (jasmonic acid, ethylene, GAs, and brassinolide), we found that Atcand1-1 was slightly resistant to jasmonic acid (data not shown). In the presence of 1-aminocyclopropane-1-carboxyclic acid (ACC), an ethylene biosynthesis precursor, Atcand1-1 and axr1-12 displayed longer hypocotyls and roots (Fig. 2D). There was essentially no difference between Atcand1-1 and wild type in response to GA and brassinolide.
DISCUSSION
The Atcand1 mutants identified in this work demonstrate that AtCAND1 plays an important role in auxin signaling and plant development. Loss-of-function alleles of AtCAND1 were all resistant to exogenous auxin and had deformed leaves and reduced fertility. The resemblance of Atcand1 phenotypes to those of axr1 mutants suggests that both AXR1 and AtCAND1 participate in regulating a common process. AXR1 has homology to the ubiquitin-activating enzyme E1 and has been shown to promote neddylation of AXR6. Defects in AXR1 appear to reduce its capacity to neddylate AXR6 (Dharmasiri et al., 2003), which in turn leads to a smaller population of active SCF complexes and, therefore, less efficient AUX/IAA protein degradation.
CAND1 is believed to negatively regulate SCF complex assembly by preventing SKP1 and SKP2 (the F-box component) from associating with the cullin template (Liu et al., 2002; Zheng et al., 2002). Although appearing somewhat paradoxical, loss-of-function mutations in AXR1 (failure to activate cullin) and CAND1 (failure to sequester deactivated cullin) both lead to auxin resistance and similar developmental phenotypes. Moreover, transgenic Arabidopsis plants with reduced levels of CSN5, a gene involved in deneddylation (i.e. cullin deactivation), were also resistant to auxin and displayed other auxin-related developmental phenotypes (Schwechheimer et al., 2001). In this light, it has been proposed that the neddylation/deneddylation cycle, not simply neddylation in and of itself, is the important factor in ubiquitin chain formation and elongation (Schwechheimer et al., 2001; Gray et al., 2002).
Together with the previous findings that F-box proteins themselves are also short-lived (Zhou and Howley, 1998; Wirbelauer et al., 2000) and that the loss of CAND1 led to down-regulation of the F-box protein SKP2 and less degradation of the target protein p27 (Zheng et al., 2002), our finding that Atcand1 is auxin resistant suggests a mechanism whereby auxin responsiveness is regulated according to the timing and/or level of AXR6 neddylation. Assuming that the SCF complex is not only important for degrading target proteins, such as the AUX/IAAs, but that it also is involved in destabilizing the carrier F-box proteins as was the case in animal systems, if AXR6 were to remain neddylated, whether by mutations in itself or loss-of-function mutations in CSN5, one of the consequences would be the depletion of F-box proteins such as TIR1. Therefore, the capacity to bring AUX/IAA proteins to the SCF complex is decreased, which mimics the loss of function of TIR1 and translates to an auxin-resistant phenotype. It follows that, when AtCAND1 is mutated, F-box proteins could be depleted because AXR6 may no longer be sequestered in the deactivated, unneddylated form, giving rise to auxin resistance. In summary, it seems possible that, in axr1, the SCFTIR1 complex cannot be activated and, in csn5 and Atcand1, more of the SCFTIR1 complex remains neddylated, destabilizing the F-box proteins. In either scenario, an auxin-resistant phenotype is observed because of the failure to degrade AUX/IAA proteins.
MATERIALS AND METHODS
Mutagenesis and the Sirtinol-Resistant Mutant Screen
Ethylmethane sulfonate-mutagenized Arabidopsis Columbia M2 seeds were purchased from Lehle Seeds (Round Rock, Texas). The M2 seeds were germinated and grown on 0.5× Murashige and Skoog medium (MS) containing 20 μm sirtinol under white light (16-h-light/8-h-dark cycle) for 6 d. Seedlings with elongated roots or normal cotyledons and hypocotyls were selected as putative sirtinol-resistant mutants and directly transplanted to soil. Seeds from the putative mutants were retested on 20 μm sirtinol for sensitivity to sirtinol by measuring root elongation.
Initial Characterization of Sirtinol-Resistant Mutants
The identified sirtinol-resistant mutants were backcrossed to wild-type Columbia and Landsberg to segregate away from background mutations, to determine whether the mutants were recessive or dominant, and to generate F2 populations for identifying chromosome locations of the mutations. For each mutant, 48 sirtinol-resistant F2 plants from the F1 of a Landsberg erecta-cross were used to identify linkages to known markers. If a mutant was linked to a marker where a known auxin-resistant gene is located, that gene was sequenced in the mutant. If a mutation was found in the gene, the mutant was assigned as low priority. For example, we had many sirtinol-resistant mutants that were linked to the marker nga63 on chromosome I, and axr1 is located nearby. We have sequenced six mutants that were linked to nga63, and all had mutations in axr1. Only mutants that appear to be linked to loci different from previously identified genes were subject to further characterization.
Positional Cloning
Atcand1-1 was cloned using a map-based cloning strategy (Lukowitz et al., 2000). Single sequence length polymorphism and cleaved amplified polymorphic sequence markers were designed according to the polymorphisms between Columbia and Landsberg ecotypes provided by Monsanto (http://www.arabidopsis.org).
To confirm that the Atcand1-1 phenotypes resulted from the mutation in At2g02560, a 12.3-kb genomic fragment, including the entire coding region of At2g02560 and 2.5 kb upstream of the coding region, was cloned into the binary vector pPZP211. The resulting construct was introduced to Agrobacterium tumefaciens GV3101 and transformed to Atcand1-1 using the floral dipping method (Clough and Bent, 1998). Seeds from Atcand1-1 plants transformed with the complementation construct were sown on 0.5× MS containing 50 μg/mL kanamycin, stratified at 4°C for 2 d, and grown for about 1 week before sirtinol- or auxin-resistant tests were carried out. Kanamycin-resistant transgenic seedlings were transferred to 0.5× MS containing 100 nm 2,4-D to measure relative root elongation.
RNA Isolation and RT-PCR Analysis
Total RNA was isolated from 5-d light-grown Arabidopsis seedlings, roots, leaves, stems, flowers, and siliques using the Qiagen RNAeasy isolation kit (Qiagen, Valencia, CA). The total RNA samples were used for RT-PCR analysis; ubiquitin mRNA was used as an internal control. The two gene-specific primers for amplifying AtCAND1 cDNA were as follows: 5′-GTTCGAGTGCAAGAGCTGTC-3′, 5′-CAGAGTAGTACGCCCAAGTAC-3′. The expected sizes of the amplified cDNA fragment and the genomic fragment were 503 and 1,202 bp, respectively.
Hormone Response Analysis
For auxin responses, 5-d-old seedlings grown on vertical plates of 0.5× MS were transferred to 0.5× MS plates containing various concentrations of IAA or 2,4-D acid. The locations of the root tips of all transferred seedlings were marked. The seedlings were grown on vertical plates for an additional 2 d before quantitation. Root elongations during the 2-d period were quantified using the NIH Image software (http://rsb.info.nih.gov/nih-image/Default.html).
For ethylene responses, Arabidopsis seeds were sown on 0.5× MS containing various concentrations of ACC stratified at 4°C for 2 d, and grown for exactly 72 h in the dark. Seedlings were then transferred to MS plates and hypocotyl lengths were measured using the NIH Image software.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers NM_018448 and BT010134.
Acknowledgments
We thank J. Nemhauser, J. Perry, and A. Bowers for their comments on the manuscript.
This work was supported by the National Institutes of Health (grant no. 1RO1GM68631–01 to Y. Z.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.044495.
References
- Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91: 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Wong LM, Theologis A (1993) Identification of the auxin-responsive element, AuxRE, in the primary indoleacetic acid-inducible gene, PS-IAA4/5, of pea (Pisum sativum). J Mol Biol 233: 580–596 [DOI] [PubMed] [Google Scholar]
- Blackwell HE, Zhao Y (2003) Chemical genetic approaches to plant biology. Plant Physiol 133: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cope GA, Deshaies RJ (2003) COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell 114: 663–671 [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Dharmasiri N, Hellmann H, Estelle M (2003) The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis. EMBO J 22: 1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S, Estelle M (2002) The role of regulated protein degradation in auxin response. Plant Mol Biol 49: 401–409 [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Hellmann H, Dharmasiri S, Estelle M (2002) Role of the Arabidopsis RING-H2 protein RBX1 in RUB modification and SCF function. Plant Cell 14: 2137–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Ulmasov T, Hagen G (1998) The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol Life Sci 54: 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle TJ (1985) Rapid induction of selective transcription by auxins. Mol Cell Biol 5: 1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M (2003) Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22: 3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M (2000) The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127: 23–32 [DOI] [PubMed] [Google Scholar]
- Leyser HM, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364: 161–164 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Furukawa M, Matsumoto T, Xiong Y (2002) NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases. Mol Cell 10: 1511–1518 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa K, Matsumoto M, Yada M, Kamura T, Hatakeyama S, Nakayama KI (2003) Preferential interaction of TIP120A with Cul1 that is not modified by NEDD8 and not associated with Skp1. Biochem Biophys Res Commun 303: 1209–1216 [DOI] [PubMed] [Google Scholar]
- Reed JW (2001) Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci 6: 420–425 [DOI] [PubMed] [Google Scholar]
- Rogg LE, Bartel B (2001) Auxin signaling: derepression through regulated proteolysis. Dev Cell 1: 595–604 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng XW (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292: 1379–1382 [DOI] [PubMed] [Google Scholar]
- Theologis A, Huynh TV, Davis RW (1985) Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol 183: 53–68 [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1997) ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96: 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Liu ZB, Hagen G, Guilfoyle TJ (1995) Composite structure of auxin response elements. Plant Cell 7: 1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbelauer C, Sutterluty H, Blondel M, Gstaiger M, Peter M, Reymond F, Krek W (2000) The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J 19: 5362–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A, Callis J (2000) Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J 21: 553–562 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dai X, Blackwell HE, Schreiber SL, Chory J (2003) SIR1, an upstream component in auxin signaling identified by chemical genetics. Science 301: 1107–1110 [DOI] [PubMed] [Google Scholar]
- Zheng J, Yang X, Harrell JM, Ryzhikov S, Shim EH, Lykke-Andersen K, Wei N, Sun H, Kobayashi R, Zhang H (2002) CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Mol Cell 10: 1519–1526 [DOI] [PubMed] [Google Scholar]
- Zhou P, Howley PM (1998) Ubiquitination and degradation of the substrate recognition subunits of SCF ubiquitin-protein ligases. Mol Cell 2: 571–580 [DOI] [PubMed] [Google Scholar]