Abstract
Aromaticity is a key concept in organic chemistry. Even though this concept has already been theoretically extrapolated to three dimensions, it usually still remains restricted to planar molecules in organic chemistry textbooks. Stacking of antiaromatic π-systems has been proposed to induce three-dimensional aromaticity as a result of strong frontier orbital interactions. However, experimental evidence to support this prediction still remains elusive so far. Here we report that close stacking of antiaromatic porphyrins diminishes their inherent antiaromaticity in the solid state as well as in solution. The antiaromatic stacking furthermore allows a delocalization of the π-electrons, which enhances the two-photon absorption cross-section values of the antiaromatic porphyrins. This feature enables the dynamic switching of the non-linear optical properties by controlling the arrangement of antiaromatic π-systems on the basis of intermolecular orbital interactions.
 It has been proposed that stacking antiaromatic molecules can build three-dimensional aromaticity, but this claim has lacked experimental validation. Here the authors report that π–π stacked antiaromatic porphyrins display significantly reduced antiaromaticity in solid state and in solution.
It has been proposed that stacking antiaromatic molecules can build three-dimensional aromaticity, but this claim has lacked experimental validation. Here the authors report that π–π stacked antiaromatic porphyrins display significantly reduced antiaromaticity in solid state and in solution.
The parallel orientation of two or more planar π-conjugated molecules is usually referred to as π–π-stacking1,2,3,4, which often controls the structures of supramolecules and liquid crystalline materials. It is also important in biological systems, where it controls in many cases the structure and functionality of DNA and proteins (tertiary structure)5. Stacked aromatic π-systems are also key components in optoelectronic organic devices, as the close contact of π-systems with a large orbital overlap offers an effective conduction pathway for charge carriers, which affords high-performance conducting materials6.
In the solid state, large planar π-conjugated molecules often adopt π–π stacked structures with interplanar distances that typically range from 3.4 to 3.6 Å, that is, the sum of van der Waals radii for sp2-hybridized carbon atoms. However, a complete overlap of two π-systems (face-centred stacking) would be difficult on account of the resulting severe electrostatic repulsion between the π-electrons. Consequently, a slipped stacked structure (offset stacking) is more commonly observed, where dispersion forces between π-electrons and peripheral protons dominate the attractive interaction between two π-systems7. However, the orbital interactions between the π-systems are substantially reduced in the offset stacking. To achieve more effective intermolecular electronic communication via a closer stacking of π-conjugated molecules, a new approach is required.
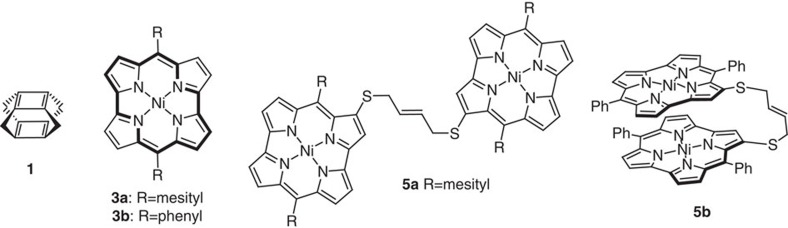
The stacking of antiaromatic compounds represents one promising strategy. Corminboeuf et al.8 have proposed that stacking of two antiaromatic systems in methano-bridged superphanes such as cyclobutadiene dimer 1 (Fig. 1) can eliminate their antiaromaticity due to the resulting three-dimensional aromaticity, which results from mutual interactions between frontier orbitals of each π-system. This intriguing proposal was further supported by a theoretical study of Bean and Fowler9 on the ring current effect in antiaromatic cyclophanes with a very short interplanar distance between the two π-systems (∼2.2 Å). In such systems, intermolecular electronic delocalization should be significantly increased. However, experimental support to substantiate this prediction still remains elusive on account of the synthetic difficulties associated with constructing superphanes from unstable antiaromatic compounds10.
Figure 1. Structures of antiaromatic superphane 1 and norcorroles.
The bold lines in 3a and 3b indicate one of their 16 π-electronic circuits.
We have recently developed an efficient synthesis of stable antiaromatic porphyrins such as dimesitylnorcorrole Ni(II) (3a), which can be obtained on a gram scale11. Norcorrole is a ring-contracted porphyrin that contains two meso-carbons fewer than a regular porphyrin12. According to the Hückel rule, norcorrole Ni(II) complexes should be characterized as distinctly antiaromatic, given their 16 π-electronic circuit and the planar structure. Subsequently, we attempted the synthesis of norcorroles with less bulky peripheral substituents in order to facilitate π–π stacking in the solid state. We discovered that diphenylnorcorrole Ni(II) (3b) adopts a triple-decker π–π stacked structure in the solid state with a very short interplanar distance (3.149 Å). Most importantly, a significant reduction of antiaromaticity of the norcorrole skeleton was observed for 3b in the solid state. Following that discovery, we constructed the tethered norcorrole dimers 5a and 5b to confirm the substantially diminished antiaromaticity of the stacked antiaromatic systems in solution. These stacked norcorroles represent the experimental evidence for the emergence of aromaticity in antiaromatic π-systems upon stacking.
Results
Synthesis and properties of diphenylnorcorrole Ni(II)
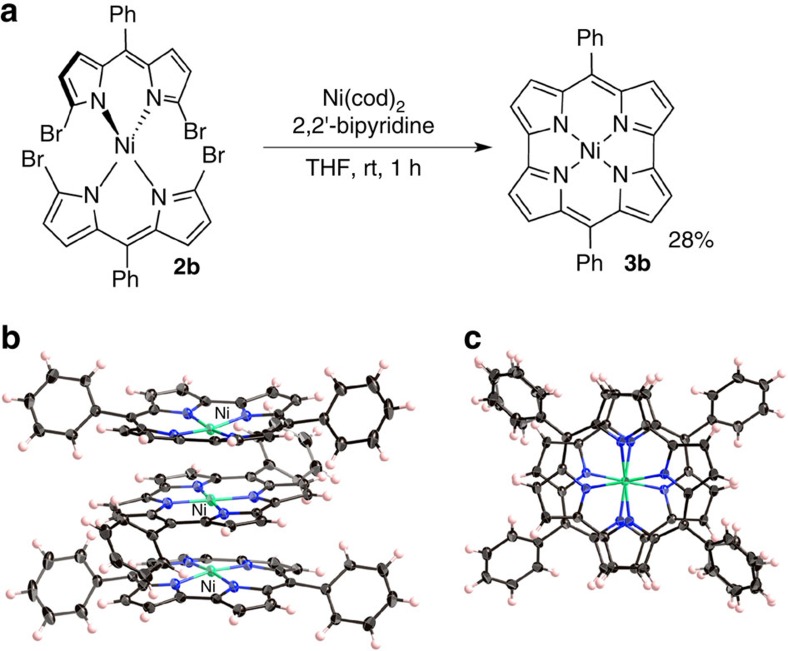
The synthetic route from dibromodipyrrin Ni(II) complex 2b to diphenylnorcorrole Ni(II) (3b) is shown in Fig. 2a. A Ni(0)-mediated intramolecular cyclization of 2b afforded 3b in 28% yield. Compound 3b was characterized by multinuclear NMR spectroscopy and high-resolution mass spectrometric analyses. The 1H NMR spectrum of 3b displayed two sets of pyrrole protons in the far upfield region (δ=1.7–2.2 p.p.m.), owing to a strong paratropic ring current effect, which clearly demonstrates distinct antiaromaticity for 3b in solution. Even though 3b is relatively stable in the solid state, slow oxidation was observed in solution; no such oxidation was observed for dimesitylnorcorrole 3a.
Figure 2. Synthesis and solid-state structure of 3b.
(a) Synthetic route to 3b, (b) oblique view of 3b and (c) top view of 3b (atomic displacement parameters set at 50% probability).
A single-crystal X-ray diffraction analysis unambiguously confirmed that 3b adopts a triple-decker stacking structure in the solid state (Fig. 2b), which is in stark contrast to the herringbone packing structure that 3a adopts. In the triple-decker structure, the Ni atom of the central molecule lies on a centre of symmetry. The distance between the two nickel centres is 2.998 Å, while the distance between the two mean planes of the four nitrogen atoms of each macrocycle is 3.149 Å (Supplementary Fig. 13), which is much shorter than typical π–π stacking distances for aromatic compounds (3.4–3.6 Å)1,6. The central norcorrole molecule is relatively planar, while the two outer ones are distorted into a bowl-shaped conformation. As shown in Fig. 2c, both outer molecules are perfectly eclipsed with respect to each other, whereas the central macrocycle is offset by 72°.
The bond length alternation (BLA) in cyclic compounds is a good indicator of aromaticity, as it allows an evaluation of the degree of effective π-electron delocalization. Interestingly, the BLA in triple-decker norcorrole 3b is significantly smaller than that in 3a, which does not adopt any π–π stacking in the crystal. For example, the C–C bond lengths around the meso-carbon atom are 1.447 and 1.403 Å in 3a, which is a characteristic feature of antiaromatic porphyrinoids. In contrast, the C–C bond lengths around the meso-carbon atom in 3b are 1.441 and 1.422 Å (central molecule), as well as 1.438 and 1.419 Å (outer molecules). The BLA is often quantified on the basis of the harmonic oscillator model of aromaticity (HOMA) values, which are close to 1 in aromatic molecules13. For antiaromatic dimesitylnorcorrole 3a, a HOMA value of 0.45 was obtained, while for stacked norcorrole 3b values of 0.58 (outer molecules) and 0.56 (central molecule) were observed (Supplementary Fig. 15). These values are quite substantial, considering that aromatic tetramesitylporphyrin exhibits a HOMA value of 0.77. Accordingly, the attenuated BLA in 3b strongly supports an increase in aromaticity through stacking of antiaromatic π-systems, as predicted by Corminboeuf et al.8 and Bean and Fowler9.
Synthesis and properties of a stacked norcorrole dimer
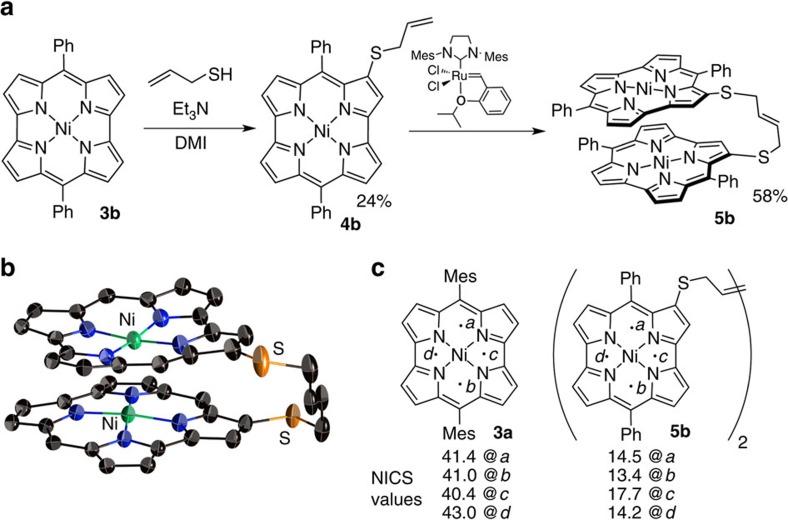
To elucidate the properties of the stacked norcorrole Ni(II) complex in solution, we synthesized and investigated tethered dimer 5b. The synthesis of 5b started with the introduction of allylthiol to 3b to provide allylthionorcorrole 4b (Fig. 3a)14. Subsequently, 4b was dimerized using a second-generation Hoveyda–Grubbs complex to afford 5b in 58% yield15. For comparison with non-stacked analogues, mesityl-substituted dimer 5a was prepared in a similar manner. A single-crystal X-ray diffraction analysis of 5b revealed a closely stacked structure with very short interplanar (3.050 Å) and Ni–Ni distances (2.849 Å) (Fig. 3b and Supplementary Fig. 14). For the norcorrole cores in 5b, HOMA values of 0.56 and 0.54 were observed, which corroborates the significant decrease of the inherent antiaromaticity of the norcorrole unit in the solid state (Supplementary Fig. 15).
Figure 3. Synthesis and aromaticity of stacked norcorrole dimer 5b.
(a) Synthetic route to 5b (DMI=1,3-dimethyl-2-imidazolidinone), (b) molecular structure of 5b (atomic displacement parameters set at 50% probability; phenyl groups and hydrogen atoms omitted for clarity) and (c) NICS values of 3a and 5b at several points (mean values of two norcorrole units).
With stacked dimer 5b in hand, we went on to explore its solution properties. Interestingly, the 1H NMR spectrum of 5b exhibited considerably down-field shifted resonances for the pyrrole protons relative to those of monomeric norcorroles 3 or non-stacked dimer 5a. The pyrrole protons of 5b were observed at 3.5–4.7 p.p.m., while those of 3a appeared at 1.57 and 1.47 p.p.m. Upon lowering the temperature, the pyrrole protons of 5b experienced a further down-field shift in the 1H NMR spectrum (Supplementary Fig. 11). This change indicates the presence of an equilibrium between stacked and non-stacked conformers of 5b, in which the former predominates at lower temperatures. The chemical shift change was analysed using the van't Hoff equation (Supplementary Fig. 12). The extrapolated chemical shifts for entirely stacked 5b should be expected at 3.8–5.1 p.p.m. Such down-field shifts were not observed for non-stacked dimer 5a.
The drastic down-field shifts of the proton signals in stacked 5b could either be ascribed to the weakened antiaromaticity of the individual norcorrole unit or to the paratropic ring current effect of the other norcorrole unit. To clarify the origin of the down-field shift, the ring currents in 3a and 5b were visualized using anisotropy of the induced current density (ACID)16 plots (Supplementary Fig. 18). These clearly demonstrated an attenuated current density in 5b and thus refute the latter possibility. The nucleus-independent chemical shift (NICS)17 (Fig. 3c) as well as two-dimensional NICS plot (Supplementary Fig. 19) of 5b revealed a considerably smaller magnetic effect of each norcorrole macrocycle compared with monomer 3a, thus supporting the weakened antiaromaticity in 5b. Taking also the bond length equalization observed in the crystal structure of 5b into account, we thus conclude that a close stacking of two antiaromatic π-systems results in a substantial decrease of their individual antiaromaticity.
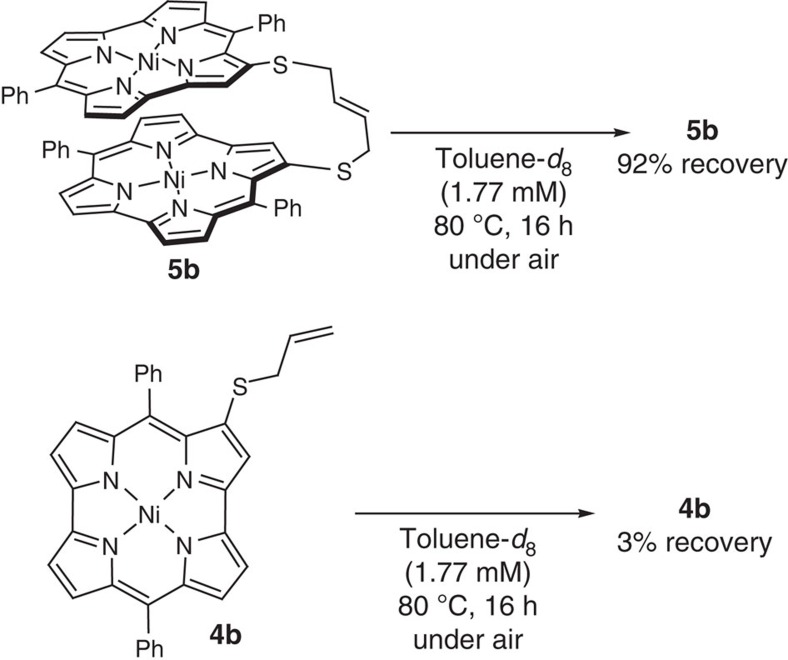
Generally, aromaticity in molecules should provide energetic stabilization by electronic delocalization. Indeed, we found that the dimer 5b is certainly more stable than monomer 4b (Fig. 4). While heating 4b in toluene-d6 to 80 °C under an atmosphere of air resulted in virtually quantitative decomposition (97%), stacked dimer 5b could be recovered in 92% under identical conditions, thus demonstrating enhanced chemical stability relative to 5b.
Figure 4. Stability of 5b and 4b.
Thermal decomposition experiments of 4b and 5b at 80 °C under air.
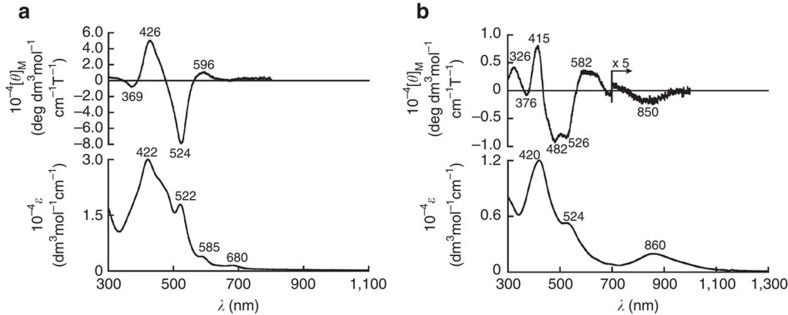
Antiaromatic porphyrinoids typically exhibit weak and broad absorption bands in the near infrared region (>800 nm). This is also the case for monomer 4b (Fig. 5a, bottom). In contrast, the absorption spectrum of dimer 5b does not display this characteristic feature of antiaromatic porphyrinoids (Fig. 5b, bottom). Interestingly, 5b showed a distinct absorption band at ∼860 nm. This low-energy absorption band increased at 77 K, which supports a shift of the equilibrium between the stacked and the non-stacked conformer towards the former at low temperatures (Supplementary Fig. 16). In contrast, 5a, in which π–π stacking is not possible on account of the bulky mesityl substituents, did not exhibit any such absorption band but only weak, broad absorption bands in the near infrared region, similar to the monomers. Time-dependent density functional theory calculations revealed that this peak should contain significant contributions of the transition from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) and from the HOMO−1 to the LUMO+1; the HOMO and HOMO−1, as well as the LUMO and LUMO+1, are virtually degenerate (Supplementary Fig. 20). Such degenerate frontier orbitals are typical for aromatic porphyrins. In addition, the molecular absorption extinction coefficient of 5b is less than half of those of 3a and 3b. This feature is intriguing, as the dimer of a chromophore should usually exhibit an almost doubled molecular absorption extinction coefficient in the absence of inter-chromophore interaction. This result thus indicates that the electronic structure of 5b should be significantly different from that of the monomer on account of the substantial spatial electronic interactions between the two norcorrole π-systems.
Figure 5. Optical properties of 4b and 5b.
(a) Absorption (bottom) and MCD (top) spectra of norcorrole monomer 4b in CH2Cl2 and (b) absorption (bottom) and MCD (top) spectra of norcorrole dimer 5b in CH2Cl2.
In order to obtain further experimental insight into the electronic structure, we measured the magnetic circular dichroism (MCD) spectrum of 5b (Fig. 5b, top). The MCD spectrum of 5b showed a weak but nevertheless distinct Faraday B term at ∼850 nm. Based on the perimeter model18,19,20,21, the lowest-energy band of norcorrole monomers should be forbidden owing to the intrashell nature. Accordingly, the absorption and MCD band observed for 5b at ∼850 nm should be ascribed to transitions between the molecular orbitals of the stacked two norcorrole units. It is reasonable to assume that the overlap between these orbitals should increase the probability of the otherwise forbidden HOMO–LUMO transition in the norcorrole monomers.
Two-photon absorption (TPA) properties of the stacked dimer
In porphyrinoids, the TPA cross-section values usually correlate with the degree of aromaticity. The TPA process is a third-order nonlinear optical phenomenon and its occurrence is largely related to π-electron delocalization, that is, TPA cross-section values can be employed to evaluate the aromaticity of porphyrinoids22,23,24. In general, aromatic porphyrinoids exhibit substantially larger TPA cross-section values than their antiaromatic counterparts, mostly owing to a more effective π-electron delocalization. The TPA cross-section value of stacked dimer 5b in toluene (1,000 GM at 1,700 nm) is significantly higher than those of monomer 3a (180 GM at 1,700 nm) and non-stacked dimer 5a (220 GM at 1,800 nm) (Supplementary Fig. 17). This result suggests effective π-electron delocalization through three-dimensionally extended π-orbital overlap, which is consistent with HOMA and NICS values, as well as with the ACID analysis. These results also demonstrate that nonlinear optical properties of antiaromatic π-systems can be dynamically tuned by controlling their supramolecular alignment.
Electronic origins of the stabilization in the stacked dimer
We observed that the antiaromatic π-system of the norcorroles easily forms close face-to-face stacking structures in order to diminish their antiaromatic nature. This phenomenon is not generally observed in aromatic compounds, for which close face-to-face π-stacking typically leads to large repulsive forces between the stacked π-electrons. To explain the difference in intermolecular interactions underlying this contrasting feature, we carried out natural bond orbital (NBO) analyses25 on antiaromatic Ni(II) norcorrole dimer 3b and on a comparable aromatic Ni(II) porphyrin dimer. In 3b, much stronger intermolecular orbital interactions were observed than in the corresponding stacked porphyrin dimer. On the basis of an NBO analysis, we thus conclude that the chemical origin of the stacking should be attributed to significantly stronger donor–acceptor interactions between the occupied orbitals on the carbon atoms of one monomer and the π* orbitals of the other monomer (Supplementary Table 2). Furthermore, a Wiberg bond order26 of <0.2 was observed for the Ni–Ni interaction in 3b at a Ni–Ni separation of 3.0 Å (Supplementary Fig. 21). These results indicate that the intermolecular orbital interactions between the norcorrole units outweigh the Ni–Ni bonding in the stacked dimer. Consequently, our results on the increased aromaticity of norcorroles upon dimerization are consistent with previous theoretical predictions for antiaromatic superphanes.
Discussion
In summary, we have presented an experimental example of closely stacked antiaromatic π-conjugated systems using norcorrole Ni(II) complexes. In the stacked norcorroles, significantly diminished antiaromaticity was observed, as well as the emergence of aromatic features such as enhanced stability, bond length equalization and effective electronic delocalization. As stacked antiaromatic compounds allow an exceptionally close alignment of π-conjugated systems and accordingly large intermolecular orbital interactions, they constitute a design principle for materials with potential applications in organic electronics. Furthermore, this study demonstrates the possibility to tune the non-linear optical properties of such antiaromatic π-systems dynamically by controlling their spatial arrangement through supramolecular interactions. Although antiaromatic compounds currently still lack practical applications, this study should provide the conceptual basis for a multitude of applications in nonlinear optical materials.
Methods
Materials and characterization
1H NMR (500 MHz) and 13C NMR (126 MHz) spectra of the compounds were recorded on a Bruker AVANCE III HD spectrometer. Chemical shifts were reported as the delta scale in p.p.m. relative to CDCl3 (δ=7.26 p.p.m.) for 1H NMR and CDCl3 (δ=77.16 p.p.m.) for 13C NMR. 1H and 13C NMR spectra are provided for compounds; see Supplementary Figs 1–10. Ultraviolet/Vis/near-infrared spectroscopy (NIR) absorption spectra were recorded on a Shimadzu UV-2550 or JASCO V670 spectrometer. Mass spectra were recorded on a Bruker microTOF using electrospray ionization (ESI)-time-of-flight method for acetonitrile solutions. Unless otherwise noted, materials obtained from commercial suppliers were used without further purification.
Synthesis of compounds
Meso-phenyl-α,α′-dibromodipyrrin Ni(II) complex 2b
To a mixture of meso-phenyl-α,α′-dibromodipyrrin (2.43 g, 6.43 mmol) and Ni(OAc)2·4H2O (808 mg, 3.23 mmol), CH2Cl2 (58 ml) and methanol (20 ml) were added. After 1 h of stirring at room temperature, the mixture was evaporated under reduced pressure and the solid residue was recrystallized from CH2Cl2/MeOH to afford meso-phenyl-α,α′-dibromodipyrrin Ni(II) complex 2b as a green solid (2.54 g, 3.15 mmol, 98%). The NMR spectra of this compound could not be observed owing to its paramagnetic nature. High-resolution mass spectrometry (HR-MS) ESI-MS): m/z=809.7650, calcd for (C30H18Br4N4Ni)+=809.7613 [(M)+].
Ni(II) meso-diphenylnorcorrole 3b
To a mixture of meso-phenyl-α,α′-dibromodipyrrin Ni(II) complex (2b, 327.8 mg, 0.40 mmol), Ni(cod)2 (277.52 mg, 10.0 mmol), and 2,2′-bipyridine (155.8 mg, 10.0 mmol), dry tetrahydrofuran (22.5 ml) was added under argon atmosphere. The solution was stirred at room temperature for 3 h in a glovebox. The solution was passed through alumina pad and evaporated under reduced pressure to provide solid residue. The residue was purified by silica gel column chromatography (hexane/CH2Cl2=3/1 as an eluent) to afford Ni(II) meso-diphenylnorcorrole 3b as a green solid (54.13 mg, 0.11 mmol, 28%). 1H NMR (500 MHz, CDCl3, 1.3 mM): δ 6.89 (t, 2H, J=7.5 Hz, Ph), 6.67 (t, 4H, J=8.0 Hz, Ph), 5.98 (dd, 4H, J=8.0, J=1.0 Hz, Ph), 2.14 (d, 4H, J=4.5 Hz, β-H), 1.78 (d, 4H, J=4.5 Hz, β-H); 13C NMR (126 MHz, CDCl3, 1.3 mM): δ 168.08, 158.93, 147.60, 131.98, 131.16, 131.02, 127.89, 120.32, 114.79; Ultraviolet-Vis-NIR (CH2Cl2): λmax (ɛ(M−1 cm−1)) 431 (46,000), 525 (17,000) nm; HR-MS (ESI-MS): m/z=492.0855, calcd for (C30H18N4Ni)+=492.0879 [(M)+].
Synthesis of 4a
To a mixture of Ni(II) meso-dimesitylnorcorrole 3a (22.92 mg, 0.04 mmol) and dry 1,3-dimethyl-2-imidazolidinone (2.0 ml) was added. Then allylthiol (16.6 μl, 0.2 mmol) under nitrogen atmosphere, triethylamine (84.0 μl, 0.6 mmol) was added to the solution slowly. The solution was stirred at room temperature for 6 h. Water and EtOAc were then added. The organic layer was separated, washed with brine and evaporated under reduced pressure to leave a solid residue. The residue was purified by silica gel column chromatography (hexane/CH2Cl2=3/1 as an eluent) to afford 3-allylthiodimesitylnorcorrole 4a as a green solid (5.99 mg, 9.2 μmol, 23%). 1H NMR (500 MHz, CDCl3): δ 6.31 (s, 2H, Mes), 6.30 (s, 2H, Mes), 5.05–5.01 (m, 1H, vinyl), 4.79 (dd, 1H, J=10 Hz, J=1.0 Hz, vinyl), 4.58 (dd, 1H, J =17 Hz, J=1.0 Hz, vinyl), 2.84 (s, 6H, ortho-Me), 2.83 (s, 6H, ortho-Me), 2.15 (d, 2H, J=6.0 Hz, CH2), 2.13 (d, 1H, J=5.0 Hz, β-H), 1.97 (d, 1H, J=4.5 Hz, β-H), 1.89 (d, 1H, J=4.0 Hz, β-H), 1.87–1.86 (m, 7H, para-Me, β-H), 1.78–1.76 (m, 2H, β-H), 1.73 (s, 1H, β-H); 13C NMR (126 MHz, CDCl3): δ 172.64, 171.38, 161.83, 159.72, 157.24, 153.97, 148.64, 147.67, 147.43, 147.16, 145.03, 137.52, 136.95, 133.85, 133.79, 132.69, 131.90, 128.30, 128.15, 126.36, 126.19, 125.54, 124.43, 117.80, 116.51. 113.94, 113.27, 109.60, 34.99, 20.89, 20.72, 17.90, 17.79; Ultraviolet-Vis-NIR (CH2Cl2): λmax (ɛ(M−1 cm−1)) 428 (28,000), 515 (24,000) nm; HR-MS (ESI-MS): m/z=648.1849, calcd for (C39H34N4NiS)+=648.1852 [(M)+].
Synthesis of 5a
To a mixture of 3-allylthiodimesitylnorcorrole 4a (7.40 mg, 11.4 μmol) and second-generation Hoveyda–Grubbs catalyst (7.25 mg, 11.6 μmol), dry dichloromethane (1.0 ml) was added under argon atmosphere in a glovebox. The solution was stirred at room temperature for 4 h. The reaction mixture was filtered through a pad of Celite and concentrated. The residue was purified by silica gel column chromatography (hexane/CH2Cl2=3/1 as an eluent) to afford dimesitylnorcorrole dimer 5a as a green solid (2.25 mg, 1.77 μmol, 31%). 1H NMR (500 MHz, CDCl3): δ 6.50 (s, 4H, Mes), 6.31 (s, 4H, Mes), 4.20–4.19 (br, 2H, vinyl), 3.07 (s, 12H, ortho-Me), 2.92 (s, 12H, ortho-Me), 2.56 (d, 2H, J=4.0 Hz, β-H), 2.20 (d, 2H, J=4.5 Hz, β-H), 2.12 (d, 2H, J=4.5 Hz, β-H), 2.08 (s, 6H, para-Me), 1.94–1.93 (m, 6H, CH2, β-H), 1.91 (d, 2H, J=4.0 Hz, β-H), 1.86 (d, 2H, J=4.5 Hz, β-H), 1.85 (s, 6H, para-Me), 1.25 (s, 2H, β-H); 13C NMR (126 MHz, CDCl3): δ 172.40, 171.18, 162.49, 160.23, 157.58, 154.22, 147.76, 147.49, 144.98, 137.68, 136.94, 133.99, 133.86, 132.55, 128.44, 128.17, 126.89, 126.43, 126.34, 126.22, 124.82, 116.39, 114.04, 109.45, 33.42, 21.21, 20.71, 18.01, 17.97. (Three sp2-carbon signals were not observed owing to the overlap); Ultraviolet-Vis-NIR (CH2Cl2): λmax (ɛ(M−1 cm−1)) 428 (43,000) and 516 (36,000) nm; HR-MS (ESI-MS): m/z=1268.3417, calcd for (C76H64N8Ni2)+=1268.3397 [(M)+].
Synthesis of 4b
To a mixture of Ni(II) meso-diphenylnorcorrole 3b (9.9 mg, 0.02 mmol) and dry 1,3-dimethyl-2-imidazolidinone (2.0 ml) under nitrogen atmosphere, triethylamine (14.0 μl, 0.2 mmol) was added. The solution was degassed through freeze–pump–thaw three times. Allylthiol (8.3 μl, 0.2 mmol) was added to the solution. The solution was stirred at room temperature for 3 h. Water and EtOAc were then added. The organic layer was separated, washed with brine quickly and evaporated under reduced pressure to leave a solid residue. The residue was purified by silica gel column chromatography (hexane/CH2Cl2=3/1 as an eluent) to afford 3-allylthiodiphenylnorcorrole 4b as a red-black solid (2.7 mg, 4.8 μmol, 24%). 1H NMR (500 MHz, CDCl3): δ 6.93–6.83 (m, 4H, Ph), 6.72 (t, 2H, J=8.0 Hz, Ph), 6.21 (d, 2H, J=8.0 Hz, Ph), 6.07–6.05 (d, 2H, J=8.0 Hz, Ph), 5.13–5.06 (m, 1H, vinyl), 4.82 (dd, 1H, J=10 Hz, J=1.0 Hz, vinyl), 4.65 (dd, 1H, J=17 Hz, J=1.0 Hz, vinyl), 2.60 (d, 1H, J=4.5 Hz, β-H), 2.35 (d, 1H, J=4.5 Hz, β-H), 2.35 (d, 2H, J=4.0 Hz, CH2), 2.19 (d, 1H, J=4.5 Hz, β-H), 2.12 (d, 1H, J=4.0 Hz, β-H), 2.09–2.07 (m, 2H, β-H), 2.05 (s, 1H, β-H); 13C NMR (126 MHz, CDCl3): δ 171.13, 169.81, 162.41, 161.18, 157.69, 154.75, 148.56, 147.62, 147.18, 146.92, 144.45, 133.47, 131.92, 131.75, 131.50, 130.73, 130.30, 129.27, 128.02, 127.92, 126.84, 123.60, 121.09, 118.07, 116.20, 114.24, 113.72, 111.65, 36.06; Ultraviolet-Vis-NIR (CH2Cl2): λmax (ɛ(M−1 cm−1)) 422 (30 000) and 519 (19,000) nm; HR-MS (ESI-MS): m/z=564.0866, calcd for (C33H22N4NiS)+=564.0913[(M)+].
Synthesis of 5b
A Schlenk tube containing 3-allylthiodiphenylnorcorrole 4b (3.46 mg, 6.14 μmol) and second-generation Hoveyda–Grubbs catalyst (3.97 mg, 6.14 μmol) was filled with argon before dry dichloromethane (0.4 ml) was added in a glovebox. The solution was stirred at room temperature for 15 min before the reaction mixture was purified by column chromatography on silica gel (eluent: hexane/CH2Cl2=3/1, v/v) to afford bridged diphenylnorcorrole dimer 5b as a black solid (1.97 mg, 3.58 μmol, 58%). 1H NMR (500 MHz, CDCl3): δ 7.08–7.03 (m, 8H, Ph), 6.89–6.92 (t, 4H, J=7.5 Hz, Ph), 6.83 (br, 8H, Ph), 4.65 (d, 2H, J=4.5 Hz, β-H), 4.50 (d, 2H, J=4.0 Hz, β-H), 4.42 (d, 2H, J=4.0 Hz, β-H), 4.00–4.02 (m, 2H, vinyl), 3.99 (d, 2H, J=4.5 Hz, β-H), 3.88 (d, 2H, J=4.0 Hz, β-H), 3.84 (d, 2H, J=4.0 Hz, β-H), 3.53 (s, 2H, β-H), 2.56–2.58 (d, 4H, J=4.5 Hz, CH2); 13C NMR (126 MHz, CDCl3): δ 165.87, 165.00, 156.88, 155.08, 150.58, 148.89, 144.98, 144.42, 142.65, 142.10, 141.20, 134.97, 134.05, 132.50, 130.37, 129.28, 128.66, 127.54, 127.36, 127.24, 126.55, 125.94, 124.63, 117.28, 116.96, 115.69, 111.47, 34.59; Ultraviolet-Vis-NIR(CH2Cl2): λmax (ɛ(M−1 cm−1)) 421 (12,000), 527 (5,500), 863 (2,200) nm; HR-MS (ESI-MS): m/z=1100.1529, calcd for (C64H40N8Ni2S2)+=1100.1519 [(M)+].
X-ray diffraction analysis
X-ray diffraction data of 3b were taken on a Bruker SMART APEX X-Ray diffractometer equipped with a large area CCD detector. X-ray diffraction data of 5b were collected on CCD (MX225HE, Rayonix) with the synchrotron radiation (λ=0.8000 Å) monochromated by the fixed exit Si (111) double crystal at the BL38B1 in the SPring-8 with approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal Nos. 2015B1397, 2016A1121). The oscillation angle, camera distance and exposure time per frame were 1°, 75 mm and 1 s, respectively. Two data sets consisting of 180 frames were integrated, scaled and merged with the programs HKL200027. The structure was solved by SHELXT28 and refined by least-squares calculations (SHELXL)29 on F2 for all reflections using the crystallographic software packages CrystalStructure30. All non-hydrogen atoms were refined with anisotropic displacement parameters and hydrogen atoms were placed in idealized positions and refined as rigid atoms with the relative isotropic displacement parameters. Crystallographic details are given in CIF files (Supplementary Data 1 and 2). The detailed crystallographic data for both the compounds are listed in Supplementary Table 1.
HOMA calculations
HOMA values of 3a, 3b, 5b and Ni(II) porphyrin were calculated using C–C and C–N bond lengths of the X-ray crystallographic structure, according to the following equations:
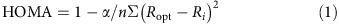 |
where n is the number of bonds taken into summation, α is an empirical constant, Ropt is an optimal bond length and Ri is a bond length of ith bond. Ropt=1.388 (C–C) and 1.334 (C–N) Å and α=257.7 (C–C) and 93.52 (C–N) were used.
TPA measurement
The TPA spectrum was measured in the NIR region using the open-aperture Z-scan method with 130 fs pulses from an optical parametric amplifier (Light Conversion, TOPAS) operating at a repetition rate of 1 kHz generated from a Ti:sapphire regenerative amplifier system (Spectra-Physics, Hurricane). After passing through a 10 cm focal length lens, the laser beam was focused and passed through a 1 mm quartz cell. As the position of the sample cell could be controlled along the laser beam direction (z axis) using the motorcontrolled delay stage, the local power density within the sample cell could be simply controlled under constant laser intensity. The transmitted laser beam from the sample cell was then detected by the same photodiode as used for reference monitoring. The on-axis peak intensity of the incident pulses at the focal point, I0, ranged from 40 to 60 GW cm−2. For a Gaussian beam profile, the nonlinear absorption coefficient can be obtained by curve fitting of the observed open-aperture traces T(z) with the following equation:
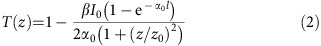 |
where α0 is the linear absorption coefficient, l is the sample length and z0 is the diffraction length of the incident beam. After the nonlinear absorption coefficient has been obtained, the TPA cross-section σ2 of one solute molecule (in units of GM, where 1 GM=10−50 cm4 s photon−1 molecule−1) can be determined by using the following relationship:
 |
where NA is the Avogadro constant, C is the concentration of the compound in solution, h is the Planck constant and ν is the frequency of the incident laser beam.
Theoretical calculations
All calculations were carried out using the Gaussian 09 program31. Geometries of 3a and 5b for NICS and ACID calculations were obtained from their X-ray structures. All calculations were performed with Becke's three-parameter hybrid exchange functional and the Lee–Yang–Parr correlation functional (B3LYP)32,33 and the 6-31G(d) basis set was used for all atoms. NBO calculations and Wiberg bond order analysis were performed with the PBE0 hybrid functional34 and LANL2DZ combination of basis set and effective core potentials35.
Data availability
Crystallographic data (CIF files) for 3b and 5b have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications. CCDC 1484882 (3b) and CCDC 1484883 (5b) contain the supplementary crystallographic data. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information.
Additional information
How to cite this article: Nozawa, R. et al. Stacked antiaromatic porphyrins. Nat. Commun. 7:13620 doi: 10.1038/ncomms13620 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures 1-21 and supplementary tables 1-2.
cif file for compound 3b.
cif file for compound 5b.
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific Research on Innovative Areas (2601): π-System Figuration (JSPS KAKENHI grant numbers JP26102003 (to H.S.), JP15H00998 (to I.H.) and JP15H01001 (to S.S.) and by the Program for Leading Graduate Schools ‘Integrative Graduate Education and Research in Green Natural Sciences' from MEXT, Japan. Crystallographic data were collected at the SPring-8 beam line BL38B1 with approval of the JASRI (proposal nos. 2015B1397 and 2016A1121). H.S. acknowledges the Asahi Glass Foundation for financial support. Research at Yonsei University was supported by the Samsung Science and Technology Foundation (SSTF-BA1402-10).
Footnotes
Author contributions H.S. designed and conducted the project and prepared the manuscript. R.N. and H.T. carried out the synthesis, characterization and determination of the optical properties. I.H. and J.-Y.S. carried out the X-ray diffraction analysis. W.-Y.C., Y.H. and D.K. measured and analysed the TPA properties. S.S. measured and analysed the MCD spectra. T.K. and S.I. conducted the theoretical analyses.
References
- Hunter C. A. & Sanders J. K. M. The nature of π–π interactions. J. Am Chem. Soc. 112, 5525–5534 (1990). [Google Scholar]
- Hunter C. A., Lawson K. R., Perkins J. & Urch C. J. Aromatic interactions. J. Chem. Soc. Perkin Trans. 2, 651–669 (2001). [Google Scholar]
- Meyer E. A., Castellano R. K. & Diederich F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 42, 1210–1250 (2003). [DOI] [PubMed] [Google Scholar]
- Hoeben F. J. M., Jonkheijm P., Meijer E. W. & Schenning A. P. H. J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 105, 1491–1546 (2005). [DOI] [PubMed] [Google Scholar]
- Kelley S. O. & Barton J. K. Electron transfer between bases in double helical DNA. Science 283, 375–381 (1999). [DOI] [PubMed] [Google Scholar]
- Salonen L. M., Ellermann M. & Diederich F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew. Chem. Int. Ed. 50, 4808–4842 (2011). [DOI] [PubMed] [Google Scholar]
- Martinez C. R. & Iverson B. L. Rethinking the term ‘pi-stacking'. Chem. Sci. 3, 2191–2201 (2012). [Google Scholar]
- Corminboeuf C., Schleyer P. V. R. & Warner P. Are antiaromatic rings stacked face-to-face aromatic? Org. Lett. 9, 3263–3266 (2007). [DOI] [PubMed] [Google Scholar]
- Bean D. E. & Fowler P. W. Stacked-ring aromaticity: an orbital model. Org. Lett. 10, 5573–5576 (2008). [DOI] [PubMed] [Google Scholar]
- Adams C. M. & Holt E. M. Synthesis of [μ-1,9,10,11-η:4,5,6,12-η-tricyclo[7.1.1.14,6]dodeca-1(11),4,6-(12),9-tetraene]bis(tricarbonyliron): the smallest cyclophane with metal-stabilized antiaromatic decks. Organometallics 9, 980–986 (1990). [Google Scholar]
- Ito T. et al. Gram-scale synthesis of nickel(II) norcorrole: the smallest antiaromatic porphyrinoid. Angew. Chem. Int. Ed. 51, 8542–8545 (2012). [DOI] [PubMed] [Google Scholar]
- Bröring M., Köhler S. & Kleeberg C. Norcorrole: observation of the smallest porphyrin variant with a N4 core. Angew. Chem. Int. Ed. 47, 5658–5660 (2008). [DOI] [PubMed] [Google Scholar]
- Krygowski T. M. & Cyranski M. K. Structural aspects of aromaticity. Chem. Rev. 101, 1385–1419 (2001). [DOI] [PubMed] [Google Scholar]
- Nozawa R., Yamamoto K., Shin J.-Y., Hiroto S. & Shinokubo H. Regioselective nucleophilic functionalization of antiaromatic nickel(II) norcorroles. Angew. Chem. Int. Ed. 54, 8454–8457 (2015). [DOI] [PubMed] [Google Scholar]
- Garber S. B., Kingsbury J. S., Gray B. L. & Hoveyda A. H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 122, 8168–8179 (2000). [Google Scholar]
- Geuenich D., Hess K., Köhler F. & Herges R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005). [DOI] [PubMed] [Google Scholar]
- Chen Z., Wannere C. S., Corminboeuf C., Puchta R. & Schleyer P. V. R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005). [DOI] [PubMed] [Google Scholar]
- Howeler U. W., Downing J., Fleischhauer J. & Michl J. MCD of non-aromatic cyclic π-electron systems. Part 1. The perimeter model for antiaromatic 4N-electron [n]annulene biradicals. J. Chem. Soc. Perkin Trans. 2 1101–1118 (1998). [Google Scholar]
- Fleischhauer J., Höweler U. & Michl J. MCD of nonaromatic cyclic π-electron systems. 3. The perimeter model for low-symmetry ‘unaromatic' and ‘ambiaromatic' molecules derived from 4N-electron [n]Annulenes. J. Phys. Chem. A 104, 7762–7775 (2000). [Google Scholar]
- Fleischhauer J., Höweler U. & Michl J. Magnetic circular dichroism of non-aromatic cyclic π-electron systems. 2. [1] The perimeter model for high-symmetry ‘unaromatic' and ‘ambiaromatic' molecules derived from 4N-electron [n]annulenes. Spetrochim. Acta. Part A 55, 585–606 (1999). [Google Scholar]
- Fleischhauer J. & Michl J. MCD of nonaromatic cyclic π-electron systems. 4. Explicit relations between molecular structure and spectra. J. Phys. Chem. A 104, 7776–7784 (2000). [Google Scholar]
- He G. S., Tan L.-S., Zheng Q. & Prasad P. N. Multiphoton absorbing materials: molecular designs, characterizations, and applications. Chem. Rev. 108, 1245–1330 (2008). [DOI] [PubMed] [Google Scholar]
- Lim J. M. et al. The photophysical properties of expanded porphyrins: relationships between aromaticity, molecular geometry and non-linear optical properties. Chem. Commun. 261–273 (2009). [DOI] [PubMed] [Google Scholar]
- Yoon Z. S. et al. Nonlinear optical properties and excited-state dynamics of highly symmetric expanded porphyrins. J. Am. Chem. Soc. 128, 14128–14134 (2006). [DOI] [PubMed] [Google Scholar]
- Reed A. E., Curtiss L. A. & Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88, 899–926 (1988). [Google Scholar]
- Wiberg K. B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968). [Google Scholar]
- Otwinowski Z. & Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr. Sect. A 64, 112–122 (2008). [DOI] [PubMed] [Google Scholar]
- CrystalStructure Version 4.2 Rigaku Corporation, Tokyo, Japan (2015). [Google Scholar]
- Frisch M. J. et al. Gaussian 09, Revision D.01 Gaussian, Inc., Wallingford, CT (2013). [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988). [DOI] [PubMed] [Google Scholar]
- Lee C., Yang W. & Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 78–789 (1988). [DOI] [PubMed] [Google Scholar]
- Adamo C. & Barone V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6169 (1999). [Google Scholar]
- Hay P. J. & Wadt W. R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures 1-21 and supplementary tables 1-2.
cif file for compound 3b.
cif file for compound 5b.
Data Availability Statement
Crystallographic data (CIF files) for 3b and 5b have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications. CCDC 1484882 (3b) and CCDC 1484883 (5b) contain the supplementary crystallographic data. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information.