Abstract
Objective: Oral anticoagulants are widely administered to patients with atrial fibrillation in order to prevent the onset of cardiogenic embolisms. However, intracranial bleeding during anticoagulant therapy often leads to fatal outcomes. Accordingly, the use of novel oral anticoagulants (NOACs), which less frequently have intracranial bleeding as a complication, is expanding. A nationwide survey of intracranial bleeding and its prognosis in Japan reported that intracranial bleeding of advanced severity was not common after NOAC administration. In this report, two cases from our institute are presented.
Patients: Case 1 was an 85-year-old man with a right frontal lobe hemorrhage while under dabigatran therapy. Case 2 was an 81-year-old man who had cerebellar hemorrhage while under rivaroxaban therapy.
Result: In both patients, the clinical course progressed without aggravation of bleeding or neurological abnormalities once anticoagulant therapy was discontinued.
Conclusion: These observations suggest that intracranial hemorrhage during NOAC therapy is easily controlled by discontinuation of the drug. NOAC administration may therefore be appropriate despite the risk of such severe complications. Further case studies that include a subgroup analysis with respect to each NOAC or patient background will be required to establish appropriate guidelines for the prevention of cardiogenic embolisms in patients with atrial fibrillation.
Keywords: novel oral anticoagulant, intracranial hemorrhage, anticoagulant therapy
Introduction
Anticoagulants are effective in preventing cerebral embolisms that may complicate atrial fibrillation1,2,3). Conventionally, warfarin has been the only oral anticoagulant used. However, its administration is associated with problems, such as the adjustments needed to maintain an effective blood concentration. In addition, dietary restrictions are necessary, because vitamin K levels influence warfarin’s efficacy. In recent years, novel oral anticoagulants (NOACs) have been developed. Those have demonstrated encouraging outcomes, as well as effects comparable to those of warfarin4,5,6,7). Further a lower reported incidence of intracranial and other massive bleeding8,9,10). NOACs are increasingly being used for primary and secondary prevention with respect to cardiogenic embolisms. With the aging of society, elderly patients suffering from atrial fibrillation make up a growing population. As a result, the prevention of cardiogenic embolisms is becoming a major issue for social and health economics.
Unfortunately, intracranial bleeding is a serious complication of anticoagulant treatment. Surgical treatments such as hematoma removal may be required in some cases. Since warfarin has a long half-life, controlling intracranial bleeding is very difficult. There are some reports that the incidence of intracranial bleeding after NOAC administration is not as frequent as in patients treated with warfarin4,5,6,7). Other reports indicate that the clinical course following intracranial hemorrhage is better in NOAC patients than in warfarin patients11, 12). We investigated the progress and prognosis of cases at our institute in which conservative therapy was selected, with intracranial bleeding as a complication during NOAC administration.
Materials and Methods
NOACs were administered to 313 patients (dabigatran: 173, rivaroxaban: 140) at our institute between 2011 and July 2014. All patients were diagnosed with non-valvular atrial fibrillation and NOAC medication was started for the prevention of cardiogenic embolization. Patients were allocated to dabigatran or rivaroxaban on the basis of their application time, medication compliance, and other factors. Random assignment was not performed. Among these patients, intracranial bleeding occurred in 2 cases, which are described below.
Case Presentations
Case 1
An 85-year-old man with a history of high-blood pressure, diabetes, and episodic atrial fibrillation (cardiac pacemaker) was staying at a health and welfare institution for the elderly because of dementia. He was taking a hypoglycemic agent, β-inhibitor, anti-dementia drug, aspirin (100 mg per day), and dabigatran (220 mg per day). He was transported to our institute after the occurrence of left hemiparesis. On initial examination, his level of consciousness was 10 points on the Japan Coma Scale (JCS 10) and pupil diameters were equal at 2.5 mm bilaterally; however, right conjugate deviation was observed. Paralysis was observed in the left upper and lower limbs, and he scored 3/5 on a manual muscle test. His renal function indicated a creatinine clearance (Ccr) of 49.6 mL/min. An evaluation of the risk factors for cerebral stroke gave 3 points on the congestive heart failure, hypertension, age, diabetes mellitus, and stroke/TIA (CHADS2) scale13), and 4 points on the congestive heart failure, hypertension, age, diabetes mellitus, stroke/TIA, vascular disease, age and sex category (CHA2DS2-VASc) scale14), making him eligible for anticoagulant therapy. At the same time, his risk factors for bleeding during anticoagulant therapy produced a hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drug/alcohol use (HAS-BLED) 15, 16) score of 2 points. Mild midline shift was observed on a head computed tomography (CT) scan as a complication of subcortical bleeding in the right frontal lobe (Figure 1A). Anticoagulant therapy (dabigatran) and antiplatelet therapy (aspirin) were discontinued and conservative therapy was applied. The level of consciousness shifted to JCS 3 without aggravation of the cerebral hemorrhage (Figure 1B). Rehabilitation was continued with no aggravation of the central nervous system disorders. The patient was transferred to a nursing home 10 months after the onset of cerebral hemorrhage and the discontinuation of anticoagulant therapy.
Figure 1.

Case 1: the patient’s head computed tomography on admission (A) and 7 days later (B).
Case 2
An 81-year-old man with a history of cerebral infarction, diabetes, and atrial fibrillation was treated with insulin and rivaroxaban (10 mg per day). A residual disability caused by cerebral infarction left him requiring total assistance in his day-to-day living. He was admitted because of torso tilt and vomiting. On initial examination, his consciousness level was JCS 3 with no apparent immobility of the pupils, deviation, or paralysis of the four limbs. Ccr was 39.3 mL/min. Regarding risk factors for cerebral stroke, his CHADS2 score was 4 points and CHA2DS2-VASc was 5 points. At the same time, the HAS-BLED score was 2 points, with the risk of bleeding during anticoagulant therapy determined as moderate. A head CT scan indicated a left cerebellar hemorrhage (Figure 2A). Anticoagulant therapy (rivaroxaban) was discontinued and conservative therapy was applied. No increase in the amount of hematoma was observed (Figure 2B). The clinical course progressed without aggravation of the neurological abnormalities. The patient was transferred to a geriatric health services facility 3 months after the onset of cerebellar hemorrhage while still receiving no anticoagulant therapy.
Figure 2.
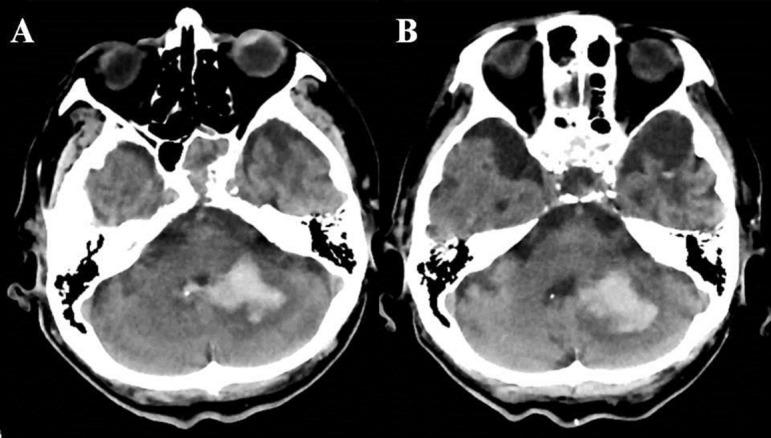
Case 2: the patient’s head computed tomography on admission (A) and 5 days later (B).
Results
Among the 313 patients treated with NOAC at our institute between 2011 and July 2014 (dabigatran: 173, rivaroxaban: 140) the average age was 74.6 years, with 96 patients aged up to 70 years and 217 aged 71 years or older. There were 220 men and 93 women. Regarding CHADS2, 54 patients scored 0–1 point, while 259 scored 2 points or more. The CHA2DS2-VASc score was 0–1 point in 24 cases and 2 points or more in 289 cases. Serious complications requiring discontinuation of anticoagulant therapy occurred in 9 cases, of which intracranial bleeding was observed in 2. In addition, recurrence of cerebral infarction was observed in 8 cases. The intracranial bleeding involved hemorrhage in the cerebral parenchyma (1 case of cerebral hemorrhage in the cerebrum and 1 case of cerebellar hemorrhage), with no cases of subdural bleeding. Conservative therapy was selected in both intracranial hemorrhage cases; no aggravation of hematoma was observed as a result of the antihypertensive therapy, the administration of a hemostatic drug, and the discontinuation of anticoagulants.
Discussion
I have described above two cases in which the administration of anticoagulants was indicated (CHADS2 and CHA2DS2-VASc scores of 2 points or more) and intracranial hemorrhage was observed as a complication during NOAC administration. The bleeding risk in both cases was moderate (HAS-BLED score 2 points). However, no increase in the quantity of hematoma or aggravation of the abnormal neurological findings were observed as a result of conservative therapy. Whereas the half-life of warfarin in serum is considered to be 20 to 60 hours, the half-lives of 12–17 hours for dabigatran and 5–9 hours for rivaroxaban mean that the anticoagulant effect of NOAC is likely to disappear within a very short period of time following withdrawal. In addition, in NOAC the mechanism of coagulation inhibition and hemostasis differs from that of warfarin, being limited to a single cause (Figure 3). Furthermore, the brain system is rich in tissue factor, which contributes to the activation of the extrinsic clotting system due to factor VII4, 8, 17,18,19,20). Unlike warfarin, NOAC never inhibit factor VII function. The microenvironment of the hemorrhaged brain system contains abundant active factor VII in patients taking NOAC. Therefore, the rapid functional restoration of the coagulation and hemostasis mechanisms occurs in parallel with the decline in blood concentration once the NOAC intake is stopped. Thus, the increase in hematoma size can be controlled by rapid withdrawal alone, even after the onset of intracranial bleeding.
Figure 3.
Mechanism of hemostasis and targets of warfarin and novel oral anticoagulants.
A comparative outcome analysis has indicated that NOAC have a lower incidence of complications such as massive bleeding or intracranial bleeding. Substantial anticoagulant effects in preventing cerebral infarction and systemic embolisms have been reported4,5,6,7, 17, 21). Although intracranial bleeding during anticoagulant therapy is likely to become serious, no sudden increase in the hematoma is observed, because of the short half-lives of NOAC in blood compared to that of warfarin. In cases with a high anticoagulant therapy recommendation score (CHADS2 and CHA2DS2-VASc), anticoagulant therapy is recommended even if the bleeding risk is high. Currently, in Japan, the suitability of drugs is limited to inhibiting the occurrence of ischemic cerebral stroke and systemic embolisms in patients with non-valvular atrial fibrillation. Based on our results and a previous report20), NOAC are more likely than warfarin to inhibit an increase in severity at the onset of hemorrhagic complications.
The HAS-BLED score is usually used for the prediction of the risk of intracranial hemorrhage during anticoagulation therapy. These two cases scored 2 points on the HAS-BLED scale. This moderate risk had been predicted before the onset of intracranial hemorrhage. Neither of these cases showed any increase in intracranial hematoma. However, a moderate HAS-BLED score has not been established as a prognostic factor. The relationship between HAS-BLED score and hematoma size or prognosis needs to be investigated by accumulating more patient cases and performing a statistical analysis. Further study would be desirable.
With the rapid aging of society, the number of elderly patients developing atrial fibrillation as underlying disease is expected to increase. Accordingly, for the purposes of primary and secondary prevention, the expansion of the range of diseases suitable for anticoagulant therapy cannot be avoided. Against such a social background, it has been predicted that cases of intracranial bleeding during anticoagulant therapy will continue to increase in the future. Although the use of NOAC may be limited by social and health economic backgrounds, along with the limitation of pharmaceutical registration, the types of disease suitable for anticoagulant treatment are likely to increase. Since NOAC have the advantage of not worsening the severity of serious complications, they may be useful in the management of patients with atrial fibrillation.
Acknowledgments
Department of Neurosurgery, Hokuto Hospital, to which Akira Tempaku belongs, received an honorarium from the Japan Cardiovascular Research Foundation for contributions to the Recurrent Embolism Lessened by rivaroxaban, an Anti-Xa agent of Early Dosing for acute ischemic stroke and transient ischemic attack with atrial fibrillation (RELAXED) study. I would like to thank Dr. Wataru Ide for research into the patients’ profile.
References
- 1.Atwood JE, Albers GW. Anticoagulation and atrial fibrillation. Herz 1993; 18: 27–38. [PubMed] [Google Scholar]
- 2.Halperin JL, Rothlauf EB. Stroke prevention in atrial fibrillation. Mt Sinai J Med 1993; 60: 289–294. [PubMed] [Google Scholar]
- 3.Singer DE. Overview of the randomized trials to prevent stroke in atrial fibrillation. Ann Epidemiol 1993; 3: 563–567. doi: 10.1016/1047-2797(93)90117-M [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S. RE-LY Steering Committee and InvestigatorsDabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S. Randomized Evaluation of Long-Term Anticoagulation Therapy InvestigatorsNewly identified events in the RE-LY trial. N Engl J Med 2010; 363: 1875–1876. doi: 10.1056/NEJMc1007378 [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J. ROCKET AF InvestigatorsRivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJ. ARISTOTLE Committees and InvestigatorsApixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 8.Hart RG, Diener HC, Yang S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012; 43: 1511–1517. doi: 10.1161/STROKEAHA.112.650614 [DOI] [PubMed] [Google Scholar]
- 9.Hagii J, Tomita H, Metoki N. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke 2014; 45: 2805–2807. doi: 10.1161/STROKEAHA.114.006661 [DOI] [PubMed] [Google Scholar]
- 10.Komori M, Yasaka M, Kokuba K. Intracranial hemorrhage during dabigatran treatment. Circ J 2014; 78: 1335–1341. doi: 10.1253/circj.CJ-13-1534 [DOI] [PubMed] [Google Scholar]
- 11.Alonso A, Bengtson LG, MacLehose RF. Intracranial hemorrhage mortality in atrial fibrillation patients treated with dabigatran or warfarin. Stroke 2014; 45: 2286–2291. doi: 10.1161/STROKEAHA.114.006016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saji N, Kimura K, Aoki J. Intracranial hemorrhage caused by non-vitamin K antagonist oral anticoagulants (NOACs) –multicenter retrospective cohort study in Japan. Circ J 2015; 79: 1018–1023. doi: 10.1253/circj.CJ-14-1209 [DOI] [PubMed] [Google Scholar]
- 13.Lip GYH, Nieuwlaat R, Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 14.Mason PK, Lake DE, DiMarco JP. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med 2012; 125: 603e1-603e6. [DOI] [PMC free article] [PubMed]
- 15.Pisters R, Lane DA, Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 16.Lip GYH, Frison L, Halperin JL. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57: 173–180. doi: 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Matsumoto M, Tanahashi N. J-ROCKET AF study investigatorsRivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study –. Circ J 2012; 76: 2104–2111. doi: 10.1253/circj.CJ-12-0454 [DOI] [PubMed] [Google Scholar]
- 18.Eikelboom JW, Wallentin L, Connolly SJ. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123: 2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747 [DOI] [PubMed] [Google Scholar]
- 19.Yasaka M. J-ROCKET AF trial increased expectation of lower-dose rivaroxaban made for Japan. Circ J 2012; 76: 2086–2087. doi: 10.1253/circj.CJ-12-0923 [DOI] [PubMed] [Google Scholar]
- 20.Takagishi S, Kameda K, Shono T. Repeated cerebellar hemorrhage related to factor Xa inhibitors, ribaroxaban and apixaban: a case report. Jpn J Stroke 2015; 37: 236–240. doi: 10.3995/jstroke.10302 [DOI] [Google Scholar]
- 21.Giugliano RP, Ruff CT, Braunwald E. ENGAGE AF-TIMI 48 InvestigatorsEdoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]



