Abstract
The growth of Arabidopsis plants in chilling conditions could be related to their levels of salicylic acid (SA). Plants with the SA hydroxylase NahG transgene grew at similar rates to Col-0 wild types at 23°C, and growth of both genotypes was slowed by transfer to 5°C. However, at 5°C, NahG plants displayed relative growth rates about one-third greater than Col-0, so that by 2 months NahG plants were typically 2.7-fold larger. This resulted primarily from greater cell expansion in NahG rosette leaves. Specific leaf areas and leaf area ratios remained similar in both genotypes. Net assimilation rates were similar in both genotypes at 23°C, but higher in NahG at 5°C. Chlorophyll fluorescence measurements revealed no PSII photodamage in chilled leaves of either genotype. Col-0 shoots at 5°C accumulated SA, particularly in glucosylated form. SA in NahG shoots showed similar tendencies at 5°C, but at greatly depleted levels. Catechol was not detected as a metabolite of the NahG transgene product. We also examined growth and SA levels in SA signaling and metabolism mutants at 5°C. The partially SA-insensitive npr1 mutant displayed growth intermediate between NahG and Col-0, while the SA-deficient eds5 mutant behaved like NahG. In contrast, the cpr1 mutant at 5°C accumulated very high levels of SA and its growth was much more inhibited than wild type. At both temperatures, cpr1 was the only SA-responsive genotype in which oxidative damage (measured as thiobarbituric acid-reactive substances) was significantly different from wild type.
Salicylic acid (SA) has received much attention due to its association with economically important plant responses to disease and other stresses. Detailed evidence implicates SA in PR gene expression, systemic acquired resistance, and the hypersensitive response (Kunkel and Brooks, 2002). SA also seems to be involved in responses to abiotic stresses, such as ozone (Sharma et al., 1996; Rao and Davis, 1999; Koch et al., 2000), salt and osmotic stress (Borsani et al., 2001; Molina et al., 2002; Shim et al., 2003), UV-B (Surplus et al., 1998), drought (Senaratna et al., 2000; Nemeth et al., 2002), paraquat (Kim et al., 2003), and heat (Dat et al., 1998a, 1998b, 2000; Lopez-Delgado et al., 1998a; Senaratna et al., 2000; Larkindale and Knight, 2002; Clarke et al., 2004). Stress-influenced developmental transitions, including flowering (Hatayama and Takeno, 2003; Martinez et al., 2004), tuberization (Lopez-Delgado and Scott, 1997), and senescence (Morris et al., 2000), may also involve SA.
Cold is one of the most important limitations to crop productivity and species distribution. Freezing (subzero) or chilling (low positive) temperatures can cause injury or reduced growth depending on the cold tolerance of the species (Schneider et al., 1995; Pearce, 1999; Humphreys et al., 2003). Recent studies describe potentially valuable effects of salicylate treatment on cold tolerance in maize, rice, and wheat (Janda et al., 1999; Szalai et al., 2000; Kang and Saltveit, 2002; Tasgin et al., 2003), bean (Ding et al., 2002), cucumber (Kang and Saltveit, 2002), tomato (Senaratna et al., 2000; Ding et al., 2002), banana (Kang et al., 2003), and Persian lilac (Bernard et al., 2002).
We have recently subjected a range of SA-related Arabidopsis genotypes to different temperatures (Clarke et al., 2004) and report here novel responses during prolonged growth at 5°C. Unlike most species in the above-mentioned cold tolerance papers, Arabidopsis is chilling resistant and able to grow to maturity at 5°C. Previous genetic studies at low positive temperatures have sought abnormally chilling-sensitive mutants (Schneider et al., 1995; Tokuhisa et al., 1997; Routaboul et al., 2000). Most cold tolerance research on Arabidopsis concerns the harsher stress of subzero freezing temperatures (Thomashow, 2001).
The SA-related genotypes we tested came from pathogen defense research, but common pathways and components occur in biotic and abiotic stresses (Chen et al., 2002; Pastori and Foyer, 2002). Arabidopsis plants transformed with the bacterial SA hydroxylase gene NahG contain reduced amounts of SA and have implicated this hormone in heat (Larkindale and Knight, 2002; Clarke et al., 2004), ozone (Sharma et al., 1996; Rao and Davis, 1999), salt and osmotic stresses (Borsani et al., 2001), as well as disease (Delaney et al., 1994). There is also an extensive array of mutations in native Arabidopsis genes, which alter SA signaling or metabolism.
The npr1 mutant was characterized as a nonexpresser of SA-inducible PR genes with reduced pathogen resistance (Cao et al., 1994). It also displays reduced heat tolerance (Clarke et al., 2004). NPR1 accumulates in the nucleus in response to cellular redox changes and interacts with members of the TGA family of bZIP transcription factors (Després et al., 2000; Kinkema et al., 2000; Zhou et al., 2000; Fan and Dong, 2002; Mou et al., 2003). NPR1 is regarded as a key signal transducer functioning downstream of SA, but there are also SA-mediated NPR1-independent pathogen resistance responses (Bowling et al., 1997; Clarke et al., 2000).
One example of a signal pathway gene functioning upstream of SA is the enhanced disease susceptibility mutant eds5 (Rogers and Ausubel, 1997; Nawrath and Métraux, 1999). SA levels are low in eds5 mutants and do not increase as normal after infection or treatment with UV-C or ozone (Nawrath and Métraux, 1999). The EDS5 transcript is induced by pathogens and UV-C light, as well as by exogenous SA (Nawrath et al., 2002). The EDS5 sequence shows membrane-spanning domains and homology with transporters of the multidrug and toxin extrusion family, but its precise function is still unknown (Nawrath et al., 2002).
The cpr1 mutant, characterized as a constitutive expresser of PR genes, also seems to act upstream of SA, but, in contrast to eds5, it has elevated levels of SA (Bowling et al., 1994, 1997; Clarke et al., 2000). Germinating seeds of cpr1 show greater heat tolerance (Clarke et al., 2004). The cpr1 gene maps to a resistance gene cluster on chromosome 4 (Stokes and Richards, 2002), but information on its molecular function remains limited. Epistasis analyses by Clarke et al. (2000) showed that eds5 suppresses the SA accumulation and constitutive pathogen resistance of cpr1 mutants. On the other hand, npr1 only partially affects cpr1 pathogen resistance.
These genotypes therefore represent a range of putative points in the SA signal transduction network. We describe here how their development and SA levels at 5°C indicate that this hormone may be one contributory factor in the low-temperature inhibition of growth in Arabidopsis.
RESULTS
Growth of NahG Plants at Chilling Temperature
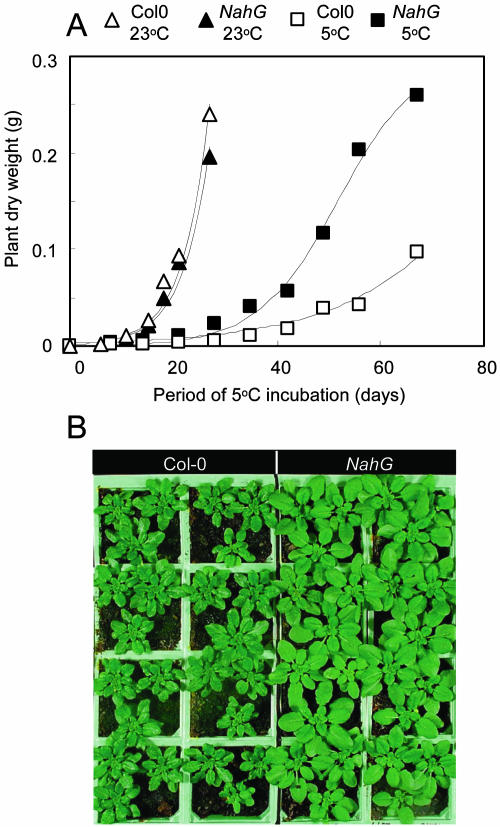
When Arabidopsis seedlings of the SA-deficient NahG and wild-type Col-0 genotypes grown in long days (16 h) were transferred to 5°C rather than incubated at 23°C, growth rates were markedly lower, as expected (Fig. 1A). Among the 5°C plants, however, a size differential in favor of NahG started to be apparent by the third week of chilling and became increasingly pronounced so that the mean biomass of NahG plants was typically 2.7-fold greater than wild type after 2 months (Fig. 1A). Relative growth rates (RGRs) were estimated as the gradient of ln (total plant biomass) over periods of exponential growth (minimum r2 of linear regression = 0.988). For the 5°C-grown plants, RGR over 8 to 35 d was significantly greater (P < 0.05) by 31% for NahG, at 0.0944 d−1 compared with 0.0719 d−1 for Col-0. RGRs for the 23°C-grown plants (over 6–21 d) were approximately threefold greater, but not significantly different between the genotypes (NahG = 0.267 d−1; Col-0 = 0.250 d−1).
Figure 1.
Temperature effects on Col-0 and NahG growth. A, Mean whole-plant biomass during incubation at 5°C or during the same period at 23°C (n = 4). B, Phenotypes of plants after 49 d at 5°C.
The phenotypic differences between NahG and Col-0 plants at 5°C, shown after 7 weeks in Figure 1B, were consistently observed in over 20 experiments. NahG plants were also significantly larger (P < 0.001) in the short-day (8 h) conditions that would be more common in the natural environment at 5°C, but growth was even slower (by >2.5-fold) than in long days (data not shown).
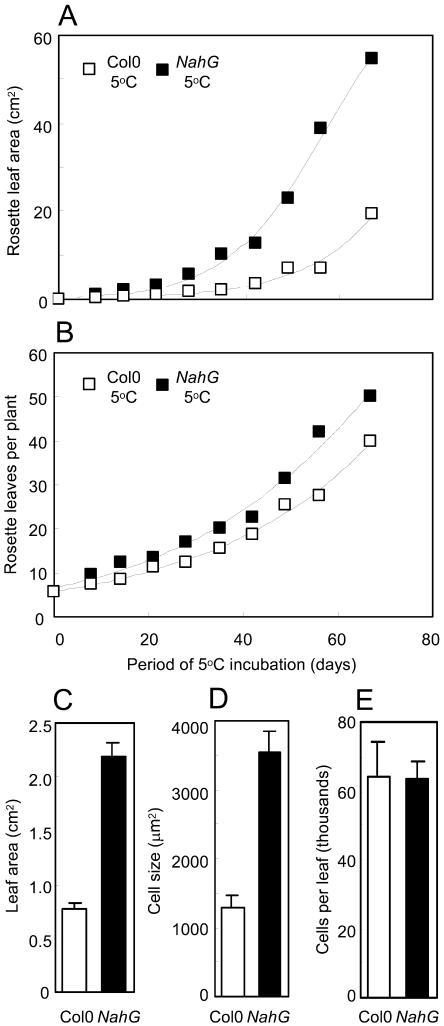
The most striking feature of NahG plants grown at 5°C was the relatively large leaf size (Fig. 1B). Total leaf area of both genotypes showed a strong linear correlation with plant biomass (minimum r2 = 0.995). The area of all rosette leaves (plants were not flowering) on NahG plants was 2.8-fold greater than wild type after 2 months of cold treatment (Fig. 2A). The number of leaves also appeared greater for NahG plants at 5°C (Fig. 2B). The ratio of NahG:Col-0 leaf numbers was, however, constant with time (regression line gradient = −0.0001) with a mean ± se of 1.31 ± 0.04 (n = 9). (For 23°C controls, the equivalent ratio was 0.96 ± 0.03.) This suggests the greater expansion of NahG leaves simply caused more leaves to reach the macroscopically countable length of 1 mm, without a changed rate of production of leaf primordia.
Figure 2.
Leaf growth in Col-0 and NahG at low temperature. A, Mean total leaf areas of rosettes. B, Mean numbers of rosette leaves per plant during incubation at 5°C (n = 4). C, Mean areas of five individual fully expanded rosette leaves from plants kept 76 d at 5°C. D, From each of these leaves, cross-sectional areas of 10 epidermal cells were averaged and overall means (n = 5) are shown. E, Mean numbers of cells per leaf were thereby calculated. Bars are se.
The size difference between NahG and wild-type leaves at 5°C could be accounted for by cell expansion. Mean areas of fully expanded NahG leaves from plants kept 76 d at 5°C were 2.8-fold greater than wild type (Fig. 2C), while mean cross-sectional areas of abaxial epidermal cells of these NahG leaves were likewise 2.8-fold greater than wild type (Fig. 2D). In consequence, no difference could be found in the numbers of abaxial epidermal cells per leaf of the two genotypes (Fig. 2E).
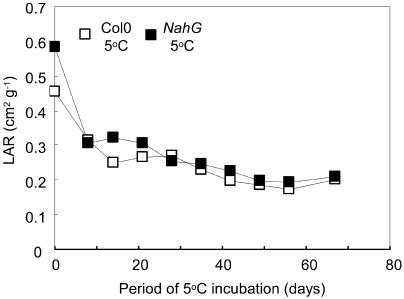
Specific leaf areas (area-to-dry matter ratio) of mature individual leaves did not differ significantly: Col-0 = 234 ± 6 cm2 g−1; NahG = 228 ± 7 cm2 g−1 (n = 9) after 46 d at 5°C. Leaf area ratio (LAR) is the ratio of leaf area to plant biomass. On chilling of NahG and Col-0 plants, LARs declined rapidly to values approximately 60% of those at 23°C (Fig. 3). However, LARs appeared similar in NahG and Col-0 (Fig. 3), consistent with a lack of significant difference found in regressions of leaf area against plant biomass in the two genotypes (not shown). This indicates that there was no gross morphological difference between chilled NahG and Col-0 plants apart from the larger size of the former.
Figure 3.
LARs during growth of Col-0 and NahG at low temperature, calculated as plant leaf area ÷ plant biomass. Values are means of four replicates.
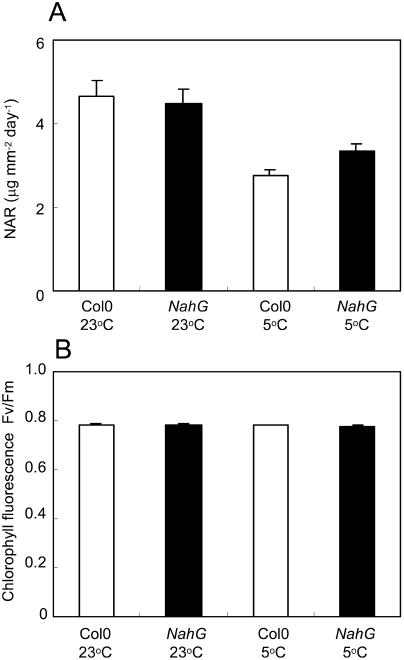
Since RGR is the product of LAR and net assimilation rate (NAR; Poorter and Remkes, 1990), the similar LARs in both genotypes suggest the greater RGR of NahG at 5°C was due to a greater NAR. Over the period used to estimate RGR, mean NAR was significantly higher (P < 0.05) in NahG than Col-0 at 5°C (Fig. 4A). NARs were higher in plants at 23°C but not significantly different between genotypes (Fig. 4A).
Figure 4.
Net assimilation rates and PSII efficiency in Col-0 and NahG plants. A, NARs during 6–21 d at 23°C, or 8–35 d at 5°C. Each value is a mean of RGR ÷ LAR at five time points in each period. B, Mean Fv/Fm ratios of mature rosette leaves from plants (n = 10) kept 12 d at 23°C or 42 d at 5°C. Bars are se.
Maximal efficiencies of PSII photochemistry were measured as the ratio of variable-to-maximum chlorophyll fluorescence (Fv/Fm), to assess the possible occurrence of PSII photodamage in chilled leaves (Maxwell and Johnson, 2000). No significant differences were found in Fv/Fm values, either between NahG and Col-0 leaves or between plants grown at 23°C or 5°C (Fig. 4B).
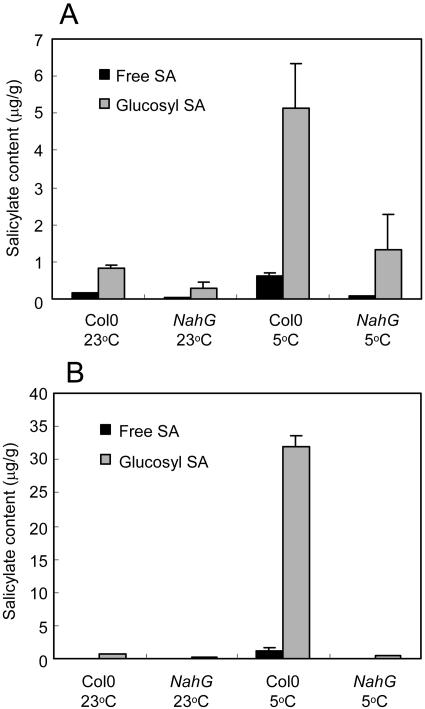
The effects of chilling on free and glucosylated SA levels in NahG and Col-0 shoots were investigated using an isotope dilution liquid chromatography-mass spectrometry (LC-MS) method. As the rates of growth at 23°C and 5°C were very different, comparisons were made both at equal ages (Fig. 5A) and at equal stages of development (Fig. 5B). By either criterion, chilling induced accumulation of free SA and, to higher levels, of glucosyl SA. In the equal-age experiment in Figure 5A, plants kept 12 d at 5°C or 23°C reached minimum growth stages of 1.06 or 5.10, respectively. In the Col-0 shoots at 5°C, free and glucosyl SA levels were, respectively, 4.1- and 6.3-fold higher than at 23°C. SA levels were depleted in NahG shoots, so that at 5°C free and glucosyl SA contents were 10% and 26% of wild type. Chilling-induced increases in the low levels of free and glucosyl SA were still observed in NahG, however, to 1.5- and 4.8-fold higher than at 23°C. In the equal-stage experiment in Figure 5B, plants were grown to stage 1.08, taking 20 d at 5°C or 5 d at 23°C. In this experiment, the chilling treatment was therefore 8 d longer than in the equal-age experiment, and SA accumulation was more pronounced. In Col-0 shoots, free and glucosyl SA levels were, respectively, 20- and 49-fold higher at 5°C than at 23°C. In NahG shoots at 5°C, free and glucosyl SA increased to only 2.3- and 1.5-fold higher than at 23°C, and were only 4.8% and 1.2% of wild type at 5°C. The substantial chilling-induced accumulation of SA in Col-0 shoots was not an early response. In the first week of chilling we were unable to detect significant increases in SA (data not shown).
Figure 5.
Salicylate accumulation in Col-0 and NahG shoots at low temperature. A, Equal ages. Mean free and glucosyl SA per g shoot fresh weight in plants kept 12 d at 23°C or 5°C. Triplicates of 36 (5°C) or 2 (23°C) shoots (approximately 0.45 g) were extracted. B, Equal stages. Mean free and glucosyl SA per g shoot fresh weight in plants grown to stage 1.08 during 5 d at 23°C or 20 d at 5°C. Six replicates of 18 shoots (approximately 0.45 g) were extracted. Bars are se.
As the NahG gene product converts SA to catechol, which may exert biological effects itself (Van Wees and Glazebrook, 2003), we monitored the catechol [M-H]− ion at m/z 109 in the same LC-MS analyses as for SA. We were not able to detect catechol above the detection limit of approximately 0.4 μg/g fresh weight in either genotype at either temperature.
Effects of Chilling on SA-Related Mutants
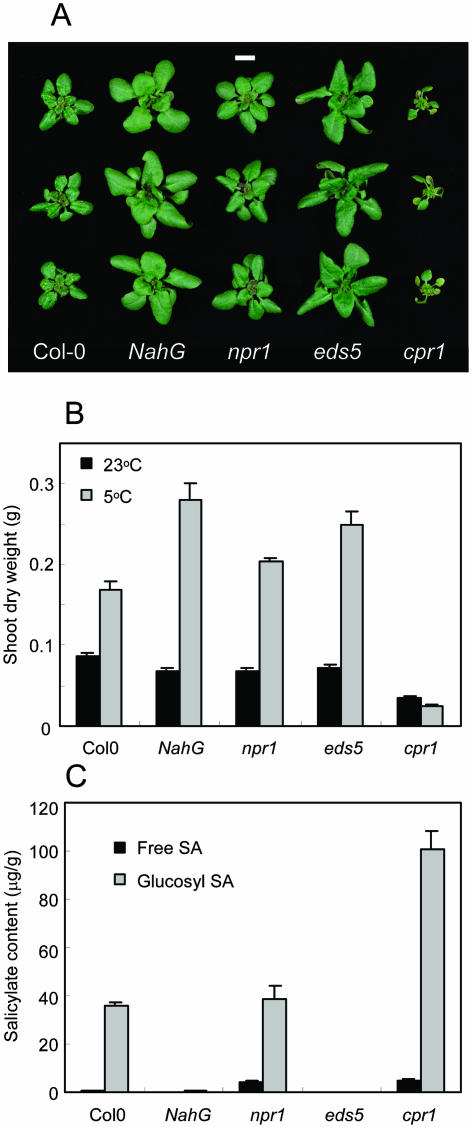
To explore further the low-temperature role of SA implied by the transgenic NahG line, we compared genotypes with mutations affecting SA signaling and metabolism: npr1, eds5, and cpr1. Like NahG, each of these mutants showed significant (P < 0.01) divergence from wild-type growth over several weeks at 5°C (Fig. 6, A and B). The npr1 mutant (which shows impaired sensitivity to SA in pathogen responses) displayed intermediate 5°C growth that was significantly different (P < 0.01) from both NahG and Col-0, reaching 120% of wild-type shoot biomass after 52 d, compared to 165% for NahG. The eds5 mutant (which exhibits greatly reduced SA accumulation on infection) showed relatively substantial 5°C growth that was significantly greater (P < 0.05) than npr1, but not significantly different (P = 0.26) from NahG. (Three comparable experiments failed to show significantly less growth in eds5 than NahG.) The enhanced low-temperature growth of NahG, npr1, and eds5 occurred despite the plants at 23°C showing slightly less growth than Col-0 (Fig. 6B). A markedly different growth pattern was observed in the SA-accumulating cpr1 mutant. This mutant has an environmentally conditioned dwarf phenotype (Stokes and Richards, 2002), which was apparent during growth at 23°C. Nevertheless, cpr1 was the only genotype in which shoots were even smaller after 52 d at 5°C than controls kept only 14 d at 23°C (Fig. 6B). While shoot biomass of the 23°C cpr1 plants was 40% of Col-0, the 5°C plants had only 14% wild-type shoot biomass and showed accelerated senescence. Development of cpr1 was therefore even more inhibited at 5°C than at 23°C.
Figure 6.
Low-temperature growth of genotypes in relation to SA levels. A, Shoot phenotypes after 36 d at 5°C. Bar = 1 cm. B, Mean shoot biomass after 14 d at 23°C or 52 d at 5°C (n = 8). Bars are se. C, Mean free and glucosyl SA per g shoot fresh weight in plants kept 42 d at 5°C. Four replicates of six shoots were extracted. Bars are se.
SA contents of the genotypes after 42 d at 5°C were consistent with growth inhibition by this hormone (Fig. 6C). SA levels in Col-0 shoots were comparable to those found after 20 d at 5°C (compare with Fig. 5B), suggesting maximal wild-type accumulation had occurred. Again, SA occurred in NahG at fractions of wild-type levels (9% free and 1.5% glucosyl SA), and SA levels in eds5 were even lower (3.6% free and 0.7% glucosyl SA). In contrast, excessive accumulation of SA occurred in cpr1, with 100 μg g−1 of glucosyl SA (2.8-fold more than wild type), and free SA even more elevated in relative terms at 5 μg g−1 (8.3-fold more than wild type). In npr1 shoots, free SA was strongly elevated at 7.3-fold more than wild type, though glucosyl SA levels were similar to Col-0. Thus, free SA in npr1 deviated from the inverse correlation between SA and shoot biomass: r2 was 0.903 for a regression of free SA against biomass in the other genotypes, but fell to 0.550 when npr1 was included.
No significant differences were found between mean Fv/Fm values for Col-0, NahG, npr1, and eds5 at either 5°C or 23°C, all values being in the range 0.777 to 0.787 (n = 10). The cpr1 plants, whose phenotype was suggestive of stress, were too small for this measurement, so another parameter of cellular damage was investigated.
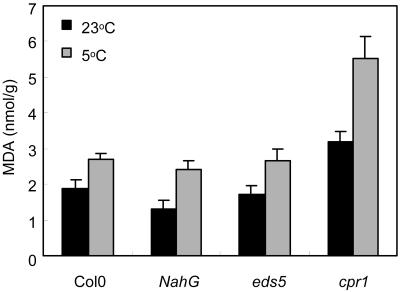
Oxidative damage measured as thiobarbituric acid-reactive substances (TBARS) is widely used as a stress indicator in biological systems, including cold-treated plants (Taulavuori et al., 2001). Malondialdehyde (MDA), which can form by decomposition of peroxidized lipids, forms an adduct with thiobarbituric acid (TBA), though other chemical species can interfere with its assay (Rice-Evans et al., 1991). We found excessive interference (Hodges et al., 1999; Taulavuori et al., 2001) when using spectrophotometry to measure TBARS in crude extracts of 5°C-grown Arabidopsis. Detection by fluorescence at 553 nm (Hodges et al., 1999) improved specificity, and the HPLC peak at the retention time of TBA-MDA represented 89 ± 0.5% (n = 30) of the TBARS detected by fluorescence in extracts of plants from 5°C or 23°C. This measurement of oxidative damage revealed no significant differences between Col-0 and the SA-deficient phenotypes NahG and eds5 (Fig. 7). However, the SA-accumulating cpr1 mutant showed significantly greater oxidative damage than Col-0 at both 5°C (P < 0.001) and 23°C (P < 0.01).
Figure 7.
Oxidative damage in genotypes at low temperature. Mean MDA yield from TBARS assays of plants kept 8 d at 23°C or 36 d at 5°C. Six replicates of three shoots were assayed. Bars are se.
DISCUSSION
Wild-type Arabidopsis shoots under chilling conditions slowly accumulated salicylate as free and glucosyl SA. Genotype comparisons indicated a strong negative correlation between growth rate and the levels or perception of SA. As our studies involved endogenous SA, production of a growth inhibitor appears to be a genuine physiological response to chilling. The growth-inhibitory properties of salicylates have already been noted without a specific explanation for a physiological role. Acetyl-SA at 100 μm retarded stem growth in potato microplants (Lopez-Delgado and Scott, 1997), such that Lopez-Delgado et al. (1998b) proposed its use as an alternative medium supplement to mannitol for slow-growth storage of potato germplasm. Growth of tobacco (Nicotiana tabacum) seedlings on 100 μm SA likewise reduced shoot biomass and leaf epidermal cell size (Dat et al., 2000). We found endogenous SA accumulation in chilled Arabidopsis plants had comparable effects: RGRs were substantially higher than wild type in SA-deficient NahG plants, whose leaves grew much larger due to greater cell expansion.
There is much current interest in cellular mechanisms of cold sensing and their transduction into physiological responses (Murata and Los, 1997; Knight and Knight, 2000; Zarka et al., 2003). The growth and morphology of cold-treated plants appear sensitive to the redox state of PSII, and it has been speculated that this is mediated by hormones (Gray et al., 1997; Huner et al., 1998; Rapacz, 2002). We propose that SA is one hormone making a significant contribution to low-temperature growth inhibition. Evidence that SA signaling in pathogen defense involves cross talk with ethylene, jasmonic acid, and fatty acid signals (Kunkel and Brooks, 2002; Kachroo et al., 2003) suggests these signal pathways also deserve attention in low-temperature growth. Another candidate is abscisic acid, which affects expression of many cold-regulated genes (Leung and Giraudat, 1998). Rapacz et al. (2003), however, found that growth rate during cold deacclimation and reacclimation in Brassica napus correlated better with GAs than abscisic acid content.
Insights into SA signaling in growth at chilling temperature were provided by SA-related mutants from pathogen defense research. The eds5 mutants had the same chilling phenotype as the similarly SA-deficient NahG transgenics, which, together with our negative catechol analyses, suggests that the NahG chilling phenotype was not due to SA degradation products, as proposed by Van Wees and Glazebrook (2003) for nonhost resistance to Pseudomonas syringae. The wild-type accumulation of SA seen in chilled shoots was absent in eds5, as found for other stresses by Nawrath et al. (2002), who suggest the substrates of the putative EDS5 metabolite transporter could be either SA precursors or other low-Mr signal cascade components. In either case, it appears that common SA-based signaling mechanisms occur in responses to chilling and pathogens as well as to UV-C and ozone stress.
Growth of npr1 at 5°C was significantly greater than wild type, though less than NahG or eds5. NPR1 is an SA-activated regulator of gene expression in SAR (Fan and Dong, 2002; Mou et al., 2003), and the growth patterns of npr1 mutants indicated that this component of SA signaling functions in chilling responses. The growth of chilled npr1 mutants was intermediate between wild-type and SA-deficient genotypes. This suggests that SA-mediated NPR1-dependent and SA-mediated NPR1-independent signaling pathways, as recognized in pathogen responses (Clarke et al., 2000), may function in the growth inhibition of chilled plants. We have found a parallel situation in heat treatments of Arabidopsis, in which npr1 responses were likewise intermediate between wild-type and NahG plants (Clarke et al., 2004). The apparent capacity of NPR1 to function in diverse responses may reflect the versatility of the TGA transcription factors with which it may interact. Seven TGA factors with differing affinities for NPR1 are known in Arabidopsis (Després et al., 2000; Zhou et al., 2000), and TGA factor binding sites occur in promoters responding to diverse stresses (Pascuzzi et al., 1998; Chen and Singh, 1999).
Clarke et al. (2000) noted that the npr1 mutation caused an increase in endogenous SA and proposed NPR1 may not only transduce the SA signal, but also reduce SA accumulation. This would explain why free SA levels in our chilled npr1 plants were over sevenfold greater than wild type.
The inverse correlation between SA and growth in chilled plants was extended with the cpr1 mutant, which had high free and glucosyl SA levels and whose development was severely restricted at 5°C. Growth of cpr1 was also restricted at 23°C, though to a less extreme extent. TBARS assays of cpr1 gave higher readings than wild type at both 5°C and 23°C, indicating a greater propensity for oxidative damage in this mutant. The phenotype of cpr1 appears to be exceptionally sensitive to environmental conditions. Stokes and Richards (2002) noted that, in long days, cpr1 plants were dwarf with twisted leaves, while in short days they had rolled leaves that became chlorotic. On the other hand, for our recent study on thermotolerance (Clarke et al., 2004), we raised young cpr1 and cpr5 plants with more normal phenotypes on nutrient medium in vitro and found that heat tolerance of cpr mutants correlated with SA content.
We assessed cellular damage using Fv/Fm and/or TBARS measurements, and cpr1 was the only genotype in which a significant difference from wild type could be demonstrated. Although differences might be revealed by other stress criteria, such as cellular redox state (Dat et al., 2000), the dramatic growth differentials between chilled Col-0 and SA-deficient NahG or eds5 plants would not seem to reflect commensurate cellular damage.
In cpr1, on the other hand, the increased oxidative damage alongside growth inhibition reaffirms the relationship between SA and cellular stress. The collective data on SA and oxidative stress have a complex pattern, consistent with the type of model proposed by Rao and Davis (1999) in which SA maintains the cellular redox state and potentiates defenses in ozone-treated plants, but excessive SA levels activate an oxidative burst and cell death. Thus, in different abiotic stresses, SA can apparently decrease (Borsani et al., 2001; Nemeth et al., 2002) or promote (Dat et al., 1998a, 2000; Lopez-Delgado et al., 1998a; Janda et al., 1999; Senaratna et al., 2000; Kang and Saltveit, 2002; Larkindale and Knight, 2002; Kang et al., 2003; Clarke et al., 2004) tolerance. Moreover, higher concentrations of SA are often superoptimal in cases where SA treatments can improve stress tolerance (Dat et al., 1998a, 2000; Lopez-Delgado et al., 1998a), including cold tolerance (Janda et al., 1999; Senaratna et al., 2000; Kang et al., 2003). Our cpr1 plants may therefore have been subject to both growth inhibition and oxidative stress due to excessive SA levels.
Previous studies of SA in low-temperature physiology have examined tolerance of treatments causing injury in the species concerned and have tended to use short treatments with exogenous SA (Janda et al., 1999; Szalai et al., 2000; Bernard et al., 2002; Kang and Saltveit, 2002; Kang et al., 2003; Tasgin et al., 2003). For the type of long-term growth studies we conducted in conditions causing no detectable wild-type injury, such pharmacological approaches would be more problematic in execution and interpretation. The role of SA suggested by our studies is likely to be distinct from that in the above-mentioned papers.
The effects of SA accumulation only partly explain the chilling-induced slowdown of growth. Temperature downshift from 23°C to 5°C caused an immediate growth reduction in both Col-0 and NahG. Indeed, no differences between these genotypes were visible for at least the first 2 weeks of chilling, and significant SA accumulation was not detectable until the second week. Numerous physiological effects of chilling have been identified, including potential disruption of all major components of photosynthesis (Allen and Ort, 2001). PSII photodamage can result when absorbed light energy exceeds the capacities of quenching or metabolic utilization, but Fv/Fm measurements revealed no photodamage in our plants. The relatively low light levels (100 μmol m−2 s−1) probably contributed to avoidance of photodamage (Yu et al., 2002). Chilling in low light or darkness may instead reduce photosynthetic performance via stromal carbon-reduction cycle activities (Kingston-Smith et al., 1997; Hutchison et al., 2000; Van Heerden et al., 2003) and possibly stomatal responses (Allen and Ort, 2001). Our growth analyses confirmed that chilling reduced NAR, a parameter reflecting the overall budget of carbon gain and loss, in both Col-0 and NahG. More interesting is our finding that maximal NAR in chilled NahG plants was significantly greater than wild type, investigation of the biochemical basis of which might provide new insights into photosynthetic regulation.
The slow development of SA-correlated growth differentials was thus superimposed on the immediate growth reduction seen upon chilling. This could reflect a hormonal role in dampening the potential for rapid reversals in growth rate under fluctuating environmental conditions, as growth rates tend to correlate inversely with frost resistance during cold acclimation, deacclimation, and reacclimation episodes (Rapacz, 2002). Another role for protracted hormonal growth inhibition might be in Arabidopsis ecotypes that adopt a winter annual habit and overwinter vegetatively (Gazzani et al., 2003). It could be interesting to monitor seasonal changes in SA levels in overwintering plants in natural environments.
The association of slow or compact plant growth patterns with adverse environments has long been recognized, but the relationships are complex (Poorter and Remkes, 1990). Arabidopsis is widely distributed in northern temperate regions and RGRs and NARs of ecotype accessions both correlate negatively with latitude of origin (Li et al., 1998). Our conclusion that NAR determined the RGR differential between Col-0 and NahG accords with the correlation between these two growth parameters in the ecotypes studied by Li et al. (1998). NAR and RGR did not correlate in interspecific comparisons made by Poorter and Remkes (1990), who proposed that the morphological LAR parameter instead determined the low RGR of species from adverse natural habitats. In Arabidopsis ecotypes, by contrast, LAR increased with latitude of origin and compensated for the negative effect of NAR on RGR (Li et al., 1998). While we found that LAR declined on chilling of Arabidopsis plants, its similarity in Col-0 and NahG suggests that SA does not affect this parameter. Thus, the genetically based differences in growth patterns exhibited by Arabidopsis ecotypes may be mediated by factors other than SA.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis ecotype Columbia (Col-0) was used as wild type and the transgenic and mutant lines were in this background. The 35S-NahG line was donated by Scott Uknes (Cropsolution, Research Triangle Park, NC). The npr1 and cpr1 mutants were from Xinnian Dong (Duke University, Durham, NC), while eds5 was from Fred Ausubel (Harvard Medical School, Boston, MA). Seeds sown in Levington Universal Extra compost (Scotts, Ipswich, UK) were vernalized for 1 week at 4°C, and then germinated in a glasshouse heated to a minimum 23°C, with a 16-h daily light period supplemented, if necessary, by 400-W sodium lamps. Experimental populations were at a mean growth stage of 1.06 ± 0.01 (Boyes et al., 2001), unless stated, at the start of temperature treatments in Fisons model 600G3/THTL controlled environment chambers (Loughborough, UK) at mean air temperatures of 5°C or 23°C. Daily light periods of 16 h (unless stated) were provided by 36-W fluorescent tubes at a maximum irradiance of 100 μmol m−2 s−1. (Higher light intensities rapidly induced purple coloration and were avoided.) Plant locations within the chambers were changed three times weekly. Plant material for biochemical analysis was fresh-frozen in liquid N2 and, if necessary, stored at −80°C.
Analysis of Growth Parameters
Dynamic growth analysis of Col-0 and NahG at 5°C and 23°C (Figs. 1–4A) was started at a mean growth stage of 1.04. Four plants, of each genotype at each temperature, were harvested at intervals, and the number of leaves >1 mm counted on each. Total leaf area per plant (A) was measured on a Delta-T Devices Area Measurement System (Cambridge, UK). Finally, oven-dried biomass of whole plants, including roots (W), was measured. Growth curves are shown in Figures 1 and 2 fitted by least-squares regression to a logistic function, W = a / (1 + be−ct), where t is time, and a, b, and c are constants. RGR was estimated as the regression line gradient of ln W against t over a period of exponential growth spanning five harvests. LAR was determined for each harvest as A/W, while NAR was derived as RGR/LAR (Poorter and Remkes, 1990).
Epidermal cell measurements were made on photomicrographs of replicas obtained by evaporating a viscous acetone solution of cellulose acetate in contact with abaxial surfaces of fully expanded rosette leaves. Cross-sectional areas of 10 cells on each of five leaves per genotype were measured using PC_Image Version 2.2 software (Foster Findlay, Aberdeen, UK). The number of abaxial epidermal cells was estimated by dividing the area of each leaf by the mean area of its cells.
Single-harvest measurements were also made of oven-dried biomass of whole shoots without roots (e.g. Fig. 6B).
Chlorophyll Fluorescence Measurements
Photochemical efficiency of PSII was measured as the ratio of variable-to-maximal chlorophyll fluorescence (Fv/Fm) in fully expanded attached leaves, following 10-min dark adaptation with leaf clips at the growth temperature. Measurements were made at a 685-nm excitation of 1000 μmol photons m−2 s−1 for 2 s with an Opti-Sciences OS-30 Continuous Source Chlorophyll Fluorometer (Tyngsboro, MA).
Analysis of SA and Catechol
Shoot tissues (up to 0.5 g) were ground in liquid N2 and extracted for at least 3 h at 5°C in 20 mL 80% methanol with addition of an internal standard of d6-SA (98 atom %; C/D/N Isotopes, Pointe-Claire, Quebec, Canada). Samples were passed through filter paper and the methanol removed by rotary evaporation at 25°C. After sample centrifugation (13,000 rpm, 3 min), half of each supernatant was incubated overnight at 37°C with an equal volume of buffer (0.2 m sodium acetate, pH 4.5) in the presence of 10 units of almond β-glucosidase EC 3.2.1.21 (NBS Biologicals, Huntingdon, UK). Glucosidase-treated and untreated fractions were each partitioned at pH 2 against equal volumes of ethylacetate. The organic phases were back-washed against H2O and reduced to dryness by rotary evaporation at 25°C. Samples were chromatographed at 30°C on a Waters Nova-Pak C18 cartridge (3.9 × 50 mm), using a 10% to 95% gradient of methanol in 2 mm formic acid over 15 min, at a flow rate of 0.5 mL min−1, on a Waters Alliance 2690 liquid chromatograph (Elstree, UK). Ten percent of the eluate was introduced into a Waters Micromass LCT electrospray ionization LC-MS in negative ion mode with a sample cone voltage of 30 V, capillary voltage of 2.0 kV, and an extraction voltage of 5 V. SA was quantified by calibration of the molar ratio between the [M-H]− ions at m/z 137 (natural SA) and m/z 141 (internal standard). SA glucoside was analyzed as the extra SA liberated in the glucosidase-treated fractions. The possible presence of catechol was also monitored in the LC-MS analyses using its m/z 109 [M-H]− ion calibrated against the d6-SA internal standard. The electrospray ionization detection was 5.6 times less sensitive for catechol than for SA, giving a detection limit of approximately 0.4 μg g−1.
TBARS Assays
TBARS were assayed by a modification of the method of Hideg et al. (2003). Up to 0.5 g shoot tissue were ground in liquid N2, and then agitated for 10 s on a vortex mixer in at least 6 volumes of 5% (w/v) trichloroacetic acid containing 0.1% (w/v) butylhydroxytoluene. The homogenates were filtered through filter paper into tubes on ice. Supernatant aliquots (0.3 mL) were mixed with 0.3 mL of 0.5% (w/v) TBA in 5% trichloroacetic acid and heated for 30 min at 95°C. TBA-MDA was quantified in the cooled assay mixtures by a modification of the HPLC method of Hodges et al. (1999). Sample aliquots were chromatographed at 26°C on a column (250 mm × 4.6 mm i.d.) of Macherey-Nagel Nucleodur C18 Gravity 5 μm (Duren, Germany). An isocratic mobile phase of 24% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid was delivered at 1 mL min−1 by a Dionex P580 pump, and eluates were monitored using Dionex RF 2000 fluorescence and PDA-100 photodiode array detectors (Camberley, UK). TBA-MDA eluted at 3.7 min and was quantified by 553-nm fluorescent emission with excitation at 532 nm. MDA standard curves were constructed using the more stable 1,1,3,3-tetraethoxypropane (Sigma-Aldrich, Poole, UK), which hydrolyzes to MDA in the assay (Hideg et al., 2003).
Statistical Treatments
Significance of differences between pairs of sample means or regression lines was estimated by two-tailed t tests. Excel was used for all statistical calculations.
Acknowledgments
We are extremely grateful to Tallulah Crow and Jim Heald for technical assistance, and to Xinnian Dong and Scott Uknes for materials donated. Valuable contributions from Mike Humphreys (Institute of Grassland and Environmental Research, UK), Steve Neill and John Hancock (University of the West of England, UK), and Rob Darby are also gratefully acknowledged.
This work was supported by the Biotechnology and Biological Sciences Research Council.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041293.
References
- Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6: 36–42 [DOI] [PubMed] [Google Scholar]
- Bernard F, Shaker-Bazarnov H, Kaviani B (2002) Effects of salicylic acid on cold preservation and cryopreservation of encapsulated embryonic axes of Persian lilac (Melia azedarach L.). Euphytica 123: 85–88 [Google Scholar]
- Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126: 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu YD, Klessig DF, Dong XN (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong XI (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired-resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong XN (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired-resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, et al (2002) Expression profile matrix of Arabidopsis transciption factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Singh KB (1999) The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J 19: 667–677 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong XN (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LAJ, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38: 432–447 [DOI] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM (1998. a) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118: 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998. b) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116: 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (2000) Effects of salicylic acid on oxidative stress and thermotolerance in tobacco. J Plant Physiol 156: 659–665 [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gutrella M, Kessmann H, Ward E, Ryals J (1994) A central role of salicylic acid in plant-disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Després C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279–290 [PMC free article] [PubMed] [Google Scholar]
- Ding CK, Wang CY, Gross KC, Smith DL (2002) Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 214: 895–901 [DOI] [PubMed] [Google Scholar]
- Fan WH, Dong XN (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzani S, Gendall AR, Lister C, Dean C (2003) Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol 132: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Chauvin LP, Sarhan F, Huner NPA (1997) Cold acclimation and freezing tolerance. A complex interaction of light and temperature. Plant Physiol 114: 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama T, Takeno K (2003) The metabolic pathway of salicylic acid rather than of chlorogenic acid is involved in the stress-induced flowering of Pharbitis nil. J Plant Physiol 160: 461–467 [DOI] [PubMed] [Google Scholar]
- Hideg E, Nagy T, Oberschall A, Dudits D, Vass I (2003) Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280-320 nm) stresses. Plant Cell Environ 26: 513–522 [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Humphreys MW, Canter PJ, Thomas HM (2003) Advances in introgression technologies for precision breeding within the Lolium-Festuca complex. Ann Appl Biol 143: 1–10 [Google Scholar]
- Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3: 224–230 [Google Scholar]
- Hutchison RS, Groom Q, Ort DR (2000) Differential effects of chilling-induced photooxidation on the redox regulation of photosynthetic enzymes. Biochemistry 39: 6679–6688 [DOI] [PubMed] [Google Scholar]
- Janda T, Szalai G, Tari I, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208: 175–180 [Google Scholar]
- Kachroo A, Lapchyk L, Fukushige H, Hildebrand D, Klessig DF, Kachroo P (2003) Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 15: 2952–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang GZ, Wang ZX, Sun GC (2003) Participation of H2O2 in enhancement of cold chilling by salicylic acid in banana seedlings. Acta Bot Sin 45: 567–573 [Google Scholar]
- Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115: 571–576 [DOI] [PubMed] [Google Scholar]
- Kim HS, Lim CJ, Han TJ, Kim JC, Jin CD (2003) Effects of salicylic acid on paraquat tolerance in Arabidopsis thaliana plants. J Plant Biol 46: 31–37 [Google Scholar]
- Kingston-Smith AH, Harbinson J, Williams J, Foyer CH (1997) Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol 114: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkema M, Fan WH, Dong XN (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12: 2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Knight MR (2000) Imaging spatial and cellular characteristics of low temperature calcium signature after cold acclimation in Arabidopsis. J Exp Bot 51: 1679–1686 [DOI] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR (2000) Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol 123: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Li B, Suzuki JI, Hara T (1998) Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 115: 293–301 [DOI] [PubMed] [Google Scholar]
- Lopez-Delgado H, Dat JF, Foyer CH, Scott IM (1998. a) Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot 49: 713–730 [Google Scholar]
- Lopez-Delgado H, Jimenez-Casas M, Scott IM (1998. b) Storage of potato microplants in vitro in the presence of acetylsalicylic acid. Plant Cell Tissue Org Cult 54: 145–152 [Google Scholar]
- Lopez-Delgado H, Scott IM (1997) Induction of in vitro tuberization of potato microplants by acetylsalicylic acid. J Plant Physiol 151: 74–78 [Google Scholar]
- Martinez C, Pons E, Prats G, León J (2004) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37: 209–217 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence - a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Molina A, Bueno P, Marin MC, Rodriguez-Rosales MP, Belver A, Venema K, Donaire JP (2002) Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol 156: 409–415 [DOI] [PubMed] [Google Scholar]
- Morris K, Mackerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J 23: 677–685 [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan WH, Dong XN (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Murata N, Los DA (1997) Membrane fluidity and temperature perception. Plant Physiol 115: 875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth M, Janda T, Horvath E, Paldi E, Szalai G (2002) Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci 162: 569–574 [Google Scholar]
- Pascuzzi P, Hamilton D, Bodily K, Arias J (1998) Auxin-induced stress potentiates trans-activation by a conserved plant basic/leucine-zipper factor. J Biol Chem 273: 26631–26637 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH (2002) Common components, networks and pathways of cross-tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol 129: 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RS (1999) Molecular analysis of acclimation to cold. Plant Growth Regul 29: 47–76 [Google Scholar]
- Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559 [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17: 603–614 [DOI] [PubMed] [Google Scholar]
- Rapacz M (2002) Regulation of frost resistance during cold de-acclimation and re-acclimation in oilseed rape. A possible role of PSII redox state. Physiol Plant 115: 236–243 [DOI] [PubMed] [Google Scholar]
- Rapacz M, Waligorski P, Janowiak F (2003) ABA and gibberellin-like substances during prehardening, cold acclimation, de- and reacclimation of oilseed rape. Acta Physiol Plant 25: 151–161 [Google Scholar]
- Rice-Evans CA, Diplock AT, Symons MCR (1991) Techniques in Free Radical Research. Elsevier, Amsterdam
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J (2000) Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol 124: 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JC, Nielsen E, Somerville C (1995) A chilling-sensitive mutant of Arabidopsis is deficient in chloroplast protein accumulation at low temperature. Plant Cell Environ 18: 23–32 [Google Scholar]
- Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul 30: 157–161 [Google Scholar]
- Sharma YK, León J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA 93: 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim IS, Momose Y, Yamamoto A, Kim DW, Usui K (2003) Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul 39: 285–292 [Google Scholar]
- Stokes TL, Richards EJ (2002) Induced instability of two Arabidopsis constitutive pathogen-response alleles. Proc Natl Acad Sci USA 99: 7792–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surplus SL, Jordan BR, Murphy AM, Carr JP, Thomas B, Mackerness SAH (1998) Ultraviolet-B-induced responses in Arabidopsis thaliana: role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant Cell Environ 21: 685–694 [Google Scholar]
- Szalai G, Tari I, Janda T, Pestenacz A, Paldi E (2000) Effects of cold acclimation and salicylic acid on changes in ACC and MACC contents in maize during chilling. Biol Plant 43: 637–640 [Google Scholar]
- Tasgin E, Atici O, Nalbantoglu B (2003) Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul 41: 231–236 [Google Scholar]
- Taulavuori E, Hellström EK, Taulavuori K, Laine K (2001) Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J Exp Bot 52: 2375–2380 [DOI] [PubMed] [Google Scholar]
- Thomashow MF (2001) So what's new in the field of plant cold acclimation? Lots! Plant Physiol 125: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhisa JG, Feldmann KA, LaBrie ST, Browse J (1997) Mutational analysis of chilling tolerance in plants. Plant Cell Environ 20: 1391–1400 [Google Scholar]
- Van Heerden PDR, Kruger GHJ, Loveland JE, Parry MAJ, Foyer CH (2003) Dark chilling imposes metabolic restrictions on photosynthesis in soybean. Plant Cell Environ 26: 323–337 [Google Scholar]
- Van Wees SCM, Glazebrook J (2003) Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J 33: 733–742 [DOI] [PubMed] [Google Scholar]
- Yu JQ, Zhou YH, Huang LF, Allen DJ (2002) Chill-induced inhibition of photosynthesis: genotypic variation within Cucumis sativus. Plant Cell Physiol 43: 1182–1188 [DOI] [PubMed] [Google Scholar]
- Zarka DG, Vogel JT, Cook D, Thomashow MF (2003) Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 133: 910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe Interact 13: 191–202 [DOI] [PubMed] [Google Scholar]