Introduction
Accumulating evidence from both behavioral and neuroimaging studies consistently suggests that performance-contingent reward enhances cognitive control, as evidenced by results from a variety of cognitive paradigms, including memory, attention, inhibition, and episodic memory (see Braver et al. (2014) for a recent literature review). This enhancing effect of rewards on cognitive control, sometimes referred to as “motivated cognitive control,” is associated with changes in activity in a number of brain regions, including the lateral prefrontal cortex (PFC) and the ventral striatum (e.g., (Boehler, Schevernels, Hopf, Stoppel, & Krebs, 2014; Hollerman, Tremblay, & Schultz, 2000; Ivanov et al., 2012; Rothkirch, Schmack, Deserno, Darmohray, & Sterzer, 2014). Much of this research has focused on cue-related or transient changes in brain activation associated with the presentation of reward cues. However, motivational incentives can also increase brain activation in a sustained fashion (e.g., (Engelmann, Damaraju, Padmala, & Pessoa, 2009; Jimura, Locke, & Braver, 2010), which has received less attention in the literature. As such, the purpose of this study is to examine both sustained and transient effects of rewards (i.e., monetary incentives) on cognitive control, with a particular focus on the relative roles of the DLPFC and the striatum. In addition, we investigated whether individual differences in a reward-related trait (i.e., anhedonia) would predict behavior or specific neural aspects of motivated cognitive control (i.e., sustained vs. transient effects).
Transient/Cue-related brain activity associated with motivational incentives
The majority of prior work on motivated cognitive control has focused on examining changes in behavioral performance and transient brain activation in response to external cues associated with reward value. A rich body of neuroimaging research has identified both cortical and subcortical brain regions (i.e., the lateral PFC, striatum) responsive to representing, predicting, and updating reward value in motivationally salient contexts during cognitive performance (e.g., (Boehler et al., 2011; Breiter & Rosen, 1999; Dixon & Christoff, 2012, 2014; Kouneiher, Charron, & Koechlin, 2009; Krebs, Boehler, Roberts, Song, & Woldorff, 2012; O’Doherty, 2004; Stoppel et al., 2011; Vassena et al., 2014), see Dixon and Christoff (2014) for a review). Single unit studies in the lateral PFC with non-human primates have shown that the majority of neurons in the lateral PFC encode the representation of value-related information (Sakagami & Watanabe, 2007). For example, when monkeys were trained to make go or no-go responses to the physical features of cue stimulus such as colors, most neurons in the lateral PFC showed differential visual responses to rewarding cues regardless of the physical features of the cue stimulus (Watanabe & Sakagami, 2007). In a similar vein, human neuroimaging and neurophysiological work provides strong evidence that reward-predicting cues exert enhancing effects on cognitive control functions, thought to be supported by regions in the lateral PFC in a variety of cognitive control paradigms such as conflict processing (Krebs, Boehler, Appelbaum, & Woldorff, 2013; Krebs, Boehler, & Woldorff, 2010), working memory (Jimura et al., 2010; Pochon et al., 2002; Taylor et al., 2004), and context processing (Chiew & Braver, 2013; Locke & Braver, 2008). Many of these regions show transient increases in activations in response to cue information indicating the potential for reward associated with upcoming performance.
These reward-predicting cues may be particularly relevant for modulating control function when there are competing stimulus dimensions, which often results in a high demand on cognitive control (e.g., (Aupperle, Melrose, Francisco, Paulus, & Stein, 2014; Padmala & Pessoa, 2011). Behavioral and neuroimaging data in humans has shown that incentive cues can enhance cognitive control by decreasing conflict processing on reward trials (Krebs et al., 2010). For example, in neuroimaging work by Padmala and Pessoa (2011), healthy individuals performed a response-conflict processing task with reward trials cued by “$20” and no-reward trials cued by “$00”. Participants were instructed that correct and fast performance on “$20” trials would be rewarded. Consistent with prior studies showing enhancing effects of rewards on cognitive control (Engelmann, Damaraju, Padmala, & Pessoa, 2009; Small et al., 2005), these researchers found that cue-related neural responses on reward trials were increased in several fronto-parietal regions, as well as in the ventral striatum and caudate. Also, these researchers found that conflict-related responses in the medial PFC on reward trials were reduced as compared to on no-reward trials.
Sustained brain activations associated with motivational incentives
Transient brain responses to cues indicating the potential for reward may not be the only mechanism that links incentive information to enhanced cognitive control. It is possible that information about potential rewards may change cognitive processing and brain activity during the entire task in a more sustained fashion. A more recent line of research has examined state-dependent reward context effects on cognitive control, providing evidence for the presence of motivation-related “state” effects on cognitive function, as evidenced by increased sustained activations across blocks of trials with incentive information (Engelmann et al., 2009; Jimura et al., 2010; Locke & Braver, 2008). For example, in a study by Engelmann et al. (2009) using a Posner-type task in which cues indicated the location of the face target stimulus, motivation was manipulated in a blocked fashion by varying the valence (e.g., winning, avoiding-loss) and the magnitude of rewards associated with task performance (e.g., winning of $1, $4, or avoid losing $2.5, and $0). They found that in several regions in a fronto-parietal attentional network (i.e., the posterior intraparietal sulcus, middle frontal gyrus, the caudate, putamen), cue-related responses were modulated by incentive values. Importantly, they also found that several regions thought to be involved in the control of attention (e.g., the intraparietal sulcus, middle frontal gyrus) showed increased sustained activations across the course of blocks with greater incentive values. These results suggest that the enhancing effects of rewards on cognitive control can be evidenced in at least two ways: 1) by increases in cue-related responses; and 2) increases in sustained responses.
In a similar vein, Locke and Braver (2008) had healthy adults perform a goal maintenance task under baseline, reward, and penalty conditions during scanning. These researchers found an increase in sustained activations during reward blocks in a network of cognitive control regions including the right lateral PFC (i.e., DLPFC, ventrolateral PFC) and the parietal cortex, as well as an improvement in task performance on trials in the reward blocks. Importantly, work by Jimura et al. (2010) extended prior findings by showing that during reward blocks, better performance was seen even on trials on which people could not earn rewards. Specifically, when healthy individuals completed a working memory task under no-reward versus reward contexts, they showed faster performance on trials for which they could earn reward as compared to trials in a baseline condition (e.g., (Locke & Braver, 2008; Padmala & Pessoa, 2011)). Interestingly, their performance was better even on trials in the reward condition for which they could not earn reward as compared to the baseline condition, an effect they referred to as a reward context effect. Importantly, this behavioral incentive context effect was associated with an increase in the right DLPFC (BA 9/46) activation that was sustained across both reward and non-reward trials in the task blocks (Jimura et al., 2010).
Incentives and the Dual Mechanisms of Control Framework
To explain these findings, Jimura et al. (2010) invoked a theoretical framework of cognitive control by Braver and his colleagues (Braver, 2012; Braver, Gray, & Burgess, 2007), referred to as the Dual Mechanisms of Control (DMC) theory. The DMC postulates that cognitive control can be supported by at least two complimentary mechanisms. The first is proactive control, which is the engagement of control mechanisms, including support for task relevant goals and tasks sets, prior to and in anticipation of the need to implement them. The second is reactive control, which is the triggering of control mechanisms, such as retrieval of task sets or goals, when conflict or difficulty in processing is encountered. This theory suggests that both the behavioral improvements seen on neutral trials in reward contexts and the increase in sustained activation in the DLPFC might reflect a role of the right lateralized DLPFC in preparatory, proactive control used to integrate reward-related information with cognitive goal representations. That is, even the knowledge that it is possible to gain rewards may enhance cognitive control, even when the current trial does not contain a specific incentive cue. This may occur by facilitating the representation of task-relevant information ((Niv, 2007), reviewed in Braver et al. (2014)), which may be reflected in enhanced sustained activity, as well as by increased cue-related responses, in regions such as the DLPFC. Interestingly, Jimura et al. (2010) also found that such increases in cue-related responses (putatively reflecting enhanced proactive control) were accompanied by reduce probe related responses in the same DLPFC region, potentially reflecting a decreased need for reactive control.
Individual difference factors relating to motivated cognitive control
Importantly, providing rewards or incentives does not lead to changes in behavioral performance and brain activity in all individuals. A growing body of research suggests that individual differences in reward-related sensitivity may modulate behavioral and neural responses to either primary (e.g., food) or secondary rewards (i.e., monetary incentives) (e.g., (Beaver et al., 2006; Cooper, Duke, Pickering, & Smillie, 2014; Jimura et al., 2010; Locke & Braver, 2008)). Specifically, several studies have reported associations between individual differences in reward-related personality traits and reward-related neural activations (e.g., (Beaver et al., 2006; Cohen, Young, Baek, Kessler, & Ranganath, 2005; Cooper et al., 2014; Jimura et al., 2010; Locke & Braver, 2008). For example, Beaver et al. (2006) found that individual differences in Behavioral Inhibition and Behavioral Approach Systems (BAS) drive scores (i.e., items asking about pursuit of goals) were significantly associated with neural responses to appetizing relative to bland foods in the ventral striatum and the orbitofrontal cortex. Cohen et al. (2005) found that when participants received immediate monetary rewards during a gambling task, people higher in extraversion showed a greater magnitude of neural response during reward receipt vs. no-rewards in the right medial orbitofrontal cortex, amygdala and right nucleus accumbens. More recent work by Jimura et al. (2010) also found positive associations between sustained DLPFC activations during reward context and reward sensitivity from the BAS. Thus, personality traits related to reward drive and sensitivity may factors in understanding individual differences in incentive effects on cognitive control.
However, another less explored individual difference factor related to reward processing is anhedonia. Anhedonia is defined as a reduction in the ability experience pleasure. Experiencing rewards as positive or pleasurable maybe a critical factor that induces approach behavior towards goals and positive emotional states (e.g., reviewed in Gorwood (2008). There are individual differences in anhedonia even in non-clinical populations (e.g.,(Franken, Rassin, & Muris, 2007; Harvey, Pruessner, Czechowska, & Lepage, 2007). Individuals who experience rewards as less pleasurable may be less “motivated” to modulate their behavior in order to enhance the likelihood of achieving such rewards. As such, individuals who self-report higher levels of anhedonia may show less improvement in cognitive control as a function of reward, and potentially less modulation of incentive-related brain activity, though it is less clear whether anhedonia would influence transient or sustained modulation of brain activity, or both.
Hypotheses of the Present Study
The purpose of this study was first to replicate prior work examining the neural mechanisms that mediate an enhancing effect of rewards on cognitive control by examining sustained as well as incentive cue-related effects on cognitive control using a mixed state-item fMRI design. We focused on the DLPFC and the striatum given prior research suggesting their involvement in mediating the influence of rewards on cognitive control. We modified a response conflict processing task originally developed by Padmala and Pessoa (2011) to use a mixed state-item design. The state-item fMRI design enabled us to examine sustained context-dependent effects and transient reward-related cue effects in the same study. Participants first performed baseline conditions without knowledge of potential for incentives in future blocks. Participants then performed additional reward blocks on which they were told that they could win money on some trials (rewarded trials) by performing fast and accurately. This variant of paradigm enabled examination of: (1) reward context effects by comparing performance and brain activity during the baseline versus the reward context and (2) reward cue effects by comparing performance during reward versus no-reward trials within reward blocks, as well as trials during the baseline blocks. We predicted that motivational states induced by reward contexts would produce greater sustained activity compared to those in non-incentive baseline blocks in the DLPFC. We also predicted that incentive cues would generate increased transient neural activity in both reward-related cortical and subcortical regions.
Secondly, we wanted to test the hypotheses that individual differences in self-reported anhedonia would moderate influence of rewards on cognitive control, with higher levels of self-reported anhedonia being associated with less of an improvement in performance as a function of reward, as well as less of an increase in the activity of the DLPFC and/or striatum in response to reward information, either in terms of sustained or transient activation (or both). To test these hypotheses, the current study included two measures of trait anhedonia- the Snaith-Hamilton Pleasure scale (Snaith et al., 1995) and Social and Physical Anhedonia scales (Chapman & Chapman, 1978; Eckblad, Chapman, Chapman, & Mishlove, 1982).
Methods
Participants
Twenty-eight healthy adults participated in the current study. One participant was not included in final analyses as the person failed to pass fMRI quality control (described below).
Thus, twenty-seven healthy adults were included in final analyses (see Table 1 for participant characteristics). Originally, we had planned to include 30 participants to achieve 80% power to detect a medium effect size (Cohen’s D= 0.5) to replicate previous findings of changes in state-related activity between task blocks (D = 0.5 in a paired t-test) and in cue-related activity (effect size f = 0.15, repeated measures ANOVA with one within-subject factor (trial types). Due to practical constraints, we were able to include a sample size of twenty-seven, which provides 75% power to detect these effect sizes. Also, this sample size provides at least 68% ~86% power to detect a correlation coefficient ranging from 0.4 to 0.5 or greater.
Table 1.
| Variables | Healthy Adults (N =27) |
|---|---|
|
| |
| Mean (SD) | |
| Age (years) | 35.56 (8.61) |
| Gender (% male) | 55.6 |
| Race (% Caucasian) | 29.6% |
| Smoking status (%Smokers) | 37.0% |
| Handedness (% right) | 92.6% |
| Highest Parental Education (years) | 14.11 (1.73) |
| Education (years) | 14.51 (1.86) |
|
| |
| Individual Difference Measures | |
| Snaith-Hamilton Pleasure Scale | 52.70 (3.27) |
| Beck Depression Inventory | 2.14 (3.55) |
| Chapman Social Anhedonia | 7.96 (6.03) |
| Chapman Physical Anhedonia | 9.81 (5.26) |
All participants had no personal or family history of psychiatric or neurological disease. All participants were recruited through the Conte Center for the Neuroscience of Mental Disorders at Washington University in St. Louis and provided written informed consent. The study protocol was approved by the Washington University Human Research Protection Office. Participants received a maximum of $20 reward money depending on their correct and fast behavioral performance in addition to money for completing the experiment ($25/hour).
Procedure
The study structure consisted of two sessions, one behavioral and one neuroimaging. In the behavioral session, participants completed several individual difference questionnaires as described below and a demographic form. During the neuroimaging session, participants performed a modified response conflict processing task inside the scanner. They had two practice sessions, one before baseline blocks and one before the reward blocks, inside the scanner, to make sure that they were familiar with the task. At the end of the neuroimaging session, participants completed a post-scan questionnaire to measure self-reported motivation and task difficulty during the task.
Stimuli and Task Paradigm
A mix of images-plus-words was used for stimuli. The images were either of a house or a building, and each image was overlaid with a word to create congruent, incongruent and neutral trials. For example, a congruent trial is one in which an image is presented with a matching word (e.g., a house picture with “HOUSE”, building picture with “BLDNG”). However, an incongruent trial is one in which an image is presented with a conflicting (e.g., a house picture with “BLDNG”, a building picture with “HOUSE”). Neutral trials are ones in which an image is presented with “XXXXX”.
The task is a variant of a previously validated reward task paradigm (Padmala & Pessoa, 2011) that revealed transient cue-related enhancing effects of monetary incentives on conflict processing in healthy individuals. We modified this task to use a mixed state-item fMRI design, allowing us to examine both sustained context-dependent effects of reward and transient cue-related effects of rewards. We used the recommendations for mixed state-item fMRI design from (Petersen & Dubis, 2012) (see Fig. 1(a)). The fMRI task paradigm consisted of two baseline and four reward runs with 54 trials per each trial-type [i.e., Baseline-Context (BCXT), Reward-Cue (RC), Reward-Context (RCXT)], resulting in a total of 162 trials. During the task, participants were instructed to focus on the image and ignore the overlaid letters, and to categorize each picture by pressing a “1” for a house image or “2” for a building image.
Fig. 1.
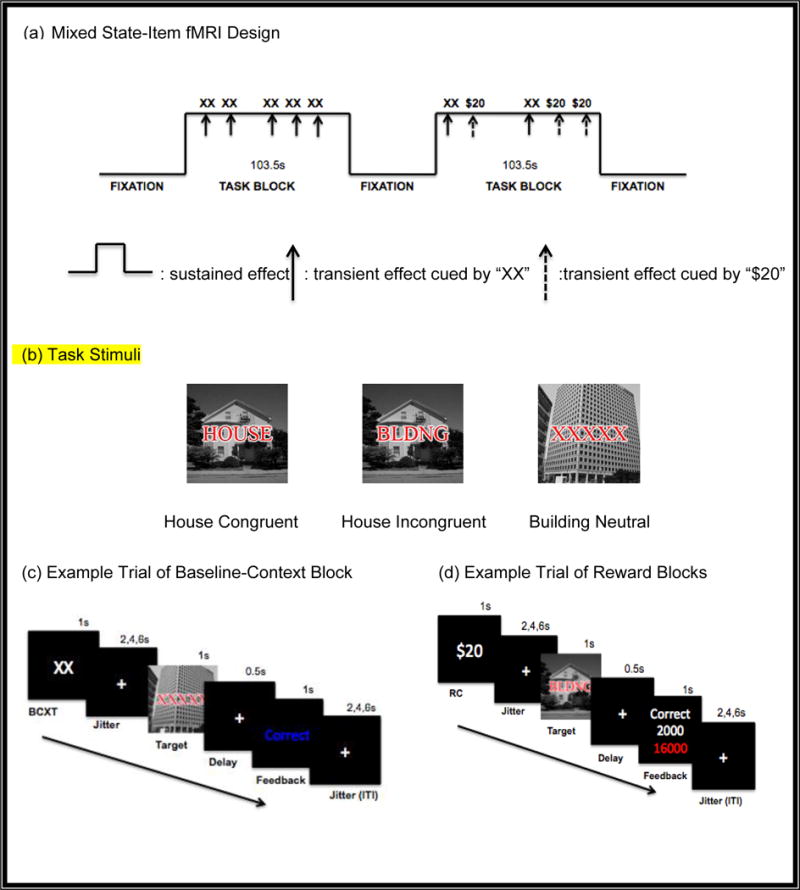
The Mixed State-Item fMRI Design of a Response Conflict Task
As presented in Fig. 1 (b), the baseline blocks consisted of two runs in which three different types of trials (i.e., congruent, incongruent, neutral) were intermixed with 18 trials per trial-type (total 54 trials). Each task block started with a start cue, “TASK” and ended with an end cue, “DONE”, each for 2 seconds. After each start cue, there was a jittered period ranging from 0 to 4 seconds. During the baseline blocks, each trial started with a “XX” cue for one second, with prior instruction to the participants indicating that these cues were not relevant to the task. Then, there was a jittered fixation ranging from 2 to 6 seconds before the onset of the stimuli, to allow for estimates of event-related responses to the cues. Then, the target stimulus was presented for one second, which was followed by a delay of 0.5 seconds, during which time the participants responded. Participants were then provided with visual feedback indicating whether performance was correct or incorrect for 1 second. Then, there was an inter-trial-interval (ITI) that varied between 2,4, and 6 seconds.
After the two baseline runs, participants performed four additional runs, for which they were instructed that they could win money on some trials for their correct and fast responses as presented in Fig. 1d. The RT threshold to determine “fast” responses was set individually based on the median correct reaction time for the second baseline run. During reward runs, ½ the trials were preceded by a “$20” cue (Reward-Cue; RC), indicating that a fast and correct response would be rewarded by 2000 points or by a “XX” cue (Reward-Context; RCXT), indicating zero points would be possible on the trial. There were 108 trials, with approximately equal numbers of congruent, incongruent, and neutral trials. After the target stimulus, participants received feedback regarding the reward points they earned on that trial, as well as their cumulative earning in points. The accumulated points were converted into real money at the end of the experiment (i.e., a maximum of $20).
fMRI data acquisition and processing
Imaging data was acquired on a 3T Siemens TM TRIO system with a 12-channel head coil. Both structural and functional images were acquired every scanning session. High-resolution MPRAGE T1 images (echo time (TE) = 2.98 ms, repetition time (TR) =2300ms, 160 slices, 1.0 × 1.0 × 1.2 mm voxels) and T2 images (TE= 84ms, TR= 7000ms, 33 slices, 2.0 × 1.0 × 4.0 mm voxels) were acquired to be registered and transformed to a standardized atlas space (Koch et al., 2010; Talairach & Tournoux, 1988), using a 12-dimensional affine transformation (Gradin et al., 2011; Woods, Cherry, & Mazziotta, 1992). Functional images were collected in six runs of 214 frames each using an asymmetric spin-echo echo-planar sequence (TR = 2000ms, TE= 27ms, field of view = 256 mm, flip = 90,° 33 slices). Functional images were acquired parallel to the anterior-posterior commissure plane with 4 mm3 isotopic voxels. Visual stimuli were projected using E-Prime software running on a Dell Inspiron laptop. Each stimulus was projected to participants with an LCD projector onto a screen located behind the scanner. A fiber optic, light-sensitive keypress interfaced with the E-prime button box was used to record participants’ behavioral performance inside the scanner.
All imaging data were preprocessed and analyzed using in-house Washington University software, (FIDL analysis package, www.nil.wustl.edu/~fidl/). The first four images of each run were discarded to allow for signal stabilization. Functional imaging data preprocessing included; (1) correction for slice-dependent time shifts, (2) removal of the first four images of each run, (3) elimination of odd/even slice intensity differences due to interpolated acquisition, (4) realignment of the data to compensate for rigid body motion, (5) normalization of image intensity to a whole-brain mode value of 1000, (6) registration of the 3-D structural volume (T1) to the atlas template in the Talairach coordinate system, using a 12-parameter affine transform and resampling to a 1-mm cubic representation ((Buckner et al., 2004; Ojemann et al., 1997); (7) coregistration of the 3-D fMRI volume to the T2, and the T2 to structural image; (8) transformation of the fMRI data to a 3 × 3 × 3 mm voxel atlas space using a single affine 12-parameter transform; and (9) spatial smoothing using a 6-mm full-width at half-maximum Gaussian filter. We assessed head movement during scanning using the output of the rigid-body rotation and translation algorithm. The translations and rotations in the x, y, and z planes across frames and total root mean square (RMS) linear and angular precision measures were calculated for each run. If standard deviation of the RMS movement exceeded 20, the BOLD runs were not included in the analysis [RMS/frame (mean, SD): 27 participants =0.15 (0.07)].
Self-reports of Anhedonia
Participants completed the Snaith-Hamilton Pleasure Scale (SHPS) (Snaith et al., 1995), which consists of 14 items to assess hedonic tone with a 4-point Likert scale (1 = definitely disagree, 4 = definitely agree). Thus, low scores represent the absence of hedonic tone (i.e., anhedonia). The SHPS has demonstrated high internal consistency, good test-retest reliability, and good convergent validity, and discriminant validity (Franken, et al., 2007). Participants also completed the Revised Social Anhedonia Scale (Eckblad et al., 1982) and the Revised Physical Anhedonia Scale (Chapman & Chapman, 1978). These scales measure self-reported ability to experience pleasure from either social or physical stimuli using true/false questions. High scores represent high levels of anhedonia. Both the Social and Physical Anhedonia scale have shown good internal consistency and test-retest reliability (Chapman, Chapman, & Kwapil, 1995).
Data Analysis
Behavioral Data
Repeated measures ANOVAs were conducted on median reaction times (RT) for correct trials and accuracy with within-subject factors of Reward (BCXT, RC, RCXT) and Trial-type (congruent, incongruent, and neutral trials). Post-hoc paired t-tests were followed used to determine the source of significant interactions. A behavioral index of reward context effects was estimated by subtracting the RT on RCXT trials cued by “XX” from the BCXT trials with the same cue, “XX.” An index of reward cue effects was computed by subtracting the RT on RC trials cued by “$20” from the RCXT trials cued by “XX” within the same reward conditions. These behavioral indices of reward context and cue effects were used in Pearson correlation analysis to examine the associations between individual differences in anhedonia trait and behavioral enhancements of rewards on cognitive tasks.
fMRI Data
We used the recommendations for mixed state-item fMRI design from Petersen and Dubis (2012) (see Fig. 1a). According to recommendations from Petersen and Dubis (2012), we simultaneously coded both sustained and transient cues, and target-related brain activity within the same GLM. Specifically, in terms of modeling transient events, we used unassumed hemodynamic responses with 8 time points considering evidence that any deviation from the assumed shape using canonical waveforms can be misattributed to the sustained activation component (Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000; Visscher et al., 2003). The unassumed GLMs enabled us to obtain one parameter for every time point following stimulation in each event type modeled (Manoach, Greve, Lindgren, & Dale, 2003; Ollinger, Corbetta, & Shulman, 2001a; Ollinger, Shulman, & Corbetta, 2001b). Thus, we can test differences between event types of interest by focusing on regions showing interactions with time point, due to our use of unassumed GLMs. As reviewed by Petersen and Dubis (2012), the mixed state-item design enables one to dissociate sustained and transient effects with the assumption that event-related trial-by-trial effect should decay back to baseline during the ITI whereas the state effects should remain sustained during the entire task block. Thus, the sustained effects (i.e., baseline and reward blocks) were modeled by box-car functions lasting the length of the task block using an assumption of a fixed-shape response of long duration (Fischl et al., 2002). Event-related cue and target effects for each trial type were separately analyzed by estimating the values of eight time point regressors (starting at trial onset) within the hemodynamic response epoch leading to a 16 second estimate (TR: 2 seconds, 8 scanning frames) using unassumed hemodynamic response shapes. Thus, the model included two regressors for the sustained effects (baseline and reward context), three regressors for the cue types (BCXT BXX,ˆ RC B$20,ˆ and RCXT BXXˆ), and two regressors for the start and end cues, respectively, during the cue phase. Considering prior research suggesting that the effect of rewards on cognitive control can be reflected in changes to the cues, either reward or no reward in reward contexts (e.g., Jimura et al., 2010), and not only in sustained activity, we used the same cue, BXX,ˆ in the baseline and reward contexts and modeled the regressor for BCXT in order to test this possibility. During the target phase, nine regressors for the combination of each cue type with each trial type (congruent, incongruent, and neutral) were included.
We used voxel-wise paired t-tests to analyze the sustained estimates, with Reward (baseline, reward) as the within-subject factor. For cue-related activity, we conducted a voxel-wise repeated measures ANOVA with a Reward (BCXT, RCXT, RC) and Time point (the 8 time frame estimates for the hemodynamic response) as within-subject factors. We focused on regions showing interactions with time point due to our use of unassumed GLMs. Post-hoc ANOVAs and t-tests were performed within all significant regions identified by the ANOVAs described above. For these post-hoc analyses, we extracted mean percent signal change across each region for each time point out of the eight estimated time points to visualize the pattern of activity. For statistical analyses, we focused on the time point 4 as this time point encompassed 7–8 seconds after stimulus onset, which corresponded to the initial peak in a stereotyped hemodynamic responses unconfounded by sustained activity. This was done for each applicable Cue-type and Trial Type effects. We then conducted post-hoc paired t-test to compare the three trial-types to parse the significant cue-related and Condition-related effects.
For target/receipt related activation, we conducted two separate repeated measures ANOVAs. The first ANOVA ignored trial type (i.e., congruent, neutral, incongruent) and compared activation in the target phase across trials that could or could not earn reward in either the baseline blocks or reward blocks (i.e., Reward: BCXT, RCXT or RC). Post-hoc paired t-tests at time point 4 [i.e., BCXT-RC, RC- RCXT, BCXT- RCXT] were conducted to follow-up on any significant effects. To attempt to replicate previous findings from prior work by Padmala and Pessoa (2011), another repeated ANOVA also included trial type (i.e., congruent, neutral, incongruent trials) and compared activation in the target phase across trials with the reward context for which participants could or could not earn reward, with Reward Context (i.e., BCXT, RC), Trial Type (i.e., congruent, neutral, incongruent trials) and time point as within-subject factors.
DLPFC and BG A Priori Masks (see Supplemental Fig. 1)
We constrained our analyses to a priori masks within the DLPFC and Basal Ganglia (BG) given the involvement of these regions in reward processing (e.g., (Jimura et al., 2010; Locke & Braver, 2008). We used anatomically defined masks of voxels within the DLPFC (Rajkowska & Goldman-Rakic, 1995) and BG(Wang et al., 2008). Voxel-by-voxel neuroimaging analyses were conducted within these masks. All statistical activation maps from these masks were corrected for multiple comparisons using combined p-value and cluster thresholds determined by the AlphaSim program in the AFNI software package. The DLPFC mask (Broadmann’s areas 9 and 46) included both left and right middle and superior frontal gyri according to anatomical landmarks (Rajkowska & Goldman-Rakic, 1995). For the DLPFC mask, we used a z-value of 2.05 and a 13 voxels. The BG mask was based on Wang et al. (2008) and generated by combining the caudate, nucleus accumbus, putamen, and globus pallidus together. We applied a z-value of 2.05 and a 14 voxels for the BG mask. Then we used information about the centroid of activation in these identified sub-regions to label them as a specific area. For any follow-up analyses to identify the source of significant effects and further correlations analyses, as described above, we extracted average of the BOLD responses values of the voxels within the identified sub-region within the mask regions and imported them into SPSS.
Individual Difference Analyses
We examined whether individual differences in anhedonia predicted behavior and/or the degree of brain activations as a function of rewards in the regions of interest (ROIs) that showed sustained reward and transient cue effects within each DLPFC and BG mask. We examined whether either of the following individual-difference measures predicted behavior, the magnitude of sustained brain activation during reward versus baseline contexts, or transient activation during RC versus RCXT trials: (1) total SHPS scores and (2) a composite score of the Chapman social and physical anhedonia scores (z-scored and then combined into one composite score). The brain–personality trait correlations were corrected by using the same small-volume procedures described above.
Results
Behavioral Data
Reaction Time (Fig. 2a)
Fig. 2.
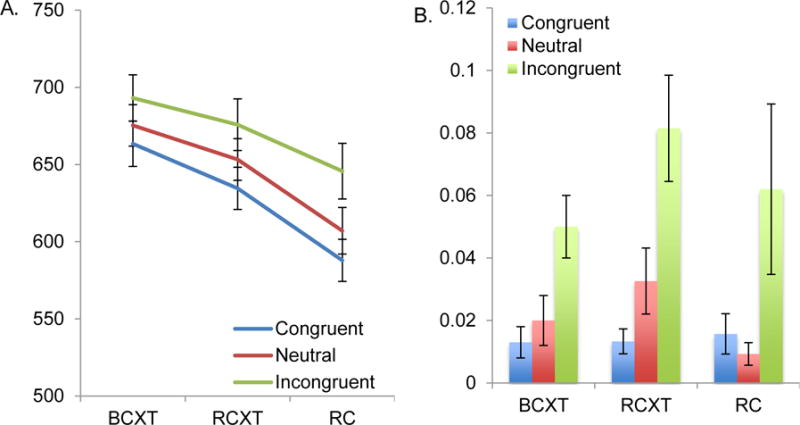
Behavioral Data: Reaction Times and Error Data
The repeated measure ANOVA indicated significant main effects of Reward [F (2,52) = 18.20, p < .001, η2p= .41] and Trial type [F (2,52) = 21.00, p < .001, η2p= .45] but no significant Reward × Trial type interaction [F (4,104) = 1.36, p = .25, η2p= .05]. The main effect of Reward (BCXT, RCXT, RC) reflected faster performance on RCXT trials compared to BCXT [F (1,26) = =4.09, p = .05, η2p= .14], as well as faster performance on RC compared to RCXT trials [F (1,26) =26.55, p < .001, η2p= .50]. The main effect of Trial type indicated slower responses on incongruent compared to congruent trials [F (1,26)= 28.92, p < .001, η2p= .53] and neutral trials [F (1,26)= 12.75, p < .002, η2p= .33] and slower RTs on neutral compared with congruent trials [F (1,26)=17.41, p < .001, η2p= .40].
Figure 2a seemingly shows that the magnitude of interference effects might be greater in the RC condition, even though the overall RTs were faster there. We conducted a paired t-test comparing the interference effect (i.e., incongruent– neutral) on the RC as compared to the BCXT blocks. The interference effects were not significantly different during RC (mean = 38.64, SEM = 12.64) than during BCXT (mean = 17.79, SEM = 8.96) blocks [t(26) = 1.58, p = .13].
Accuracy (see Fig. 2(b)
The analogous ANOVA on error data indicated a significant main effect of Trial type [F (2,52) = 13.91, p < .001, η2p= .35], reflecting more errors on incongruent compared to congruent trials [F (1,26) = 16.36, p < .001, η2p= .38]. There was no significant main effect of Reward [F (2,52) = 1.20, p = .31, η2p= .04] and no significant interaction of Reward and Trial type [F (4,104) = .83, p = .51, η2p = .03]. Given that there was no significant main effect of Reward on error data, further analyses were focused on RT data.
Behavioral Measures
Behavioral measures Neither the behavioral indices of reward context nor those of the reward cues were significantly correlated with individual differences in the anhedonia measures.
Neuroimaging Results
Sustained Effects of Reward
As presented in Table 2 and Fig. 3, the analyses revealed a significant main effect of Reward in bilateral DLPFC regions as well as the dorsal striatum, with greater activity during the reward as compared to the baseline blocks.
Table 2.
| Analysis | BA | Region of Activation | Cluster size (voxels) | Talairach Coordinates
|
Z | Activation Patterna | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DLPFC | 46 | Middle Frontal Gyrus | 18 | 43 | 29 | 21 | 2.81 | R >B |
| 9 | Middle Frontal Gyrus | 149 | 39 | 14 | 31 | 3.20 | R >B | |
| 9 | Precentral Gyrus | 60 | −38 | 10 | 30 | 2.95 | R >B | |
| Basal Ganglia | Right dorsal striatum | 57 | 23 | 7 | 4 | 3.21 | R >B | |
| Left dorsal striatum | 19 | −23 | 7 | 5 | 2.67 | R >B | ||
| Left dorsal striatum | 19 | −18 | 2 | 16 | 2.79 | R >B | ||
Fig. 3.
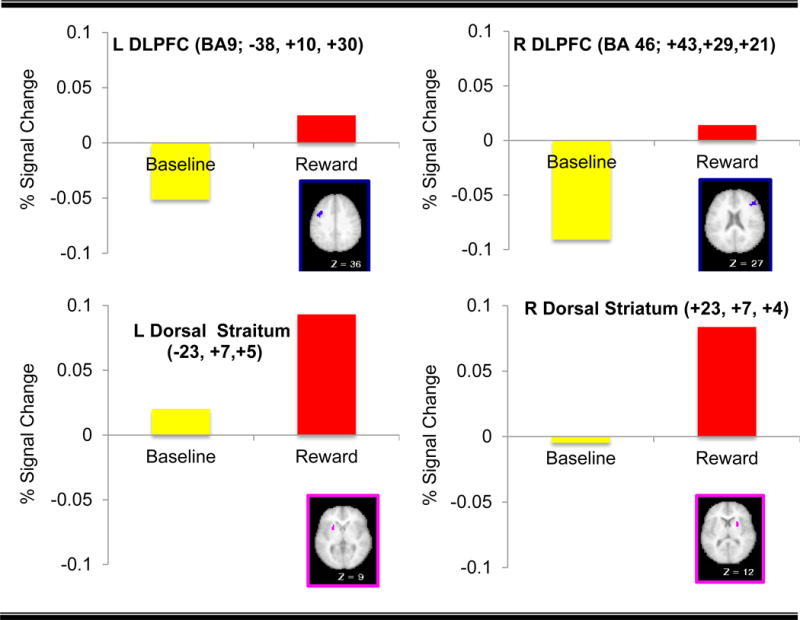
Regions Displaying Sustained Effect of Reward
Relationship between Sustained Effects of Reward and Anhedonia
Individual differences in responses to the Chapman scales and SHPS were not significantly associated with the magnitude of sustained activation in the comparison of Reward to Baseline in the ROIs described above showing significant sustained activation (all ps>.10).
Cue-related Effects of Reward
Regions in the bilateral DLPFC, lateral globus pallidus and caudate body displayed significant interactions between Reward (RC, RCXT, BCXT trials) and time point (see Table 3). Post-hoc paired t-tests at time point 4 [i.e., RC-RCXT, RC-BCXT, RCXT-BCXT] were conducted to determine source of the effect of Reward. Consistent with prior work showing the involvement of the DLPFC in reward-related effects (e.g., (Jimura et al., 2010; Pochon et al., 2002; Watanabe, 2007), bilateral DLPFC showed increased cue-related activation on RC relative to both RCXT and BCXT trials. Similarly, several regions in the left lateral globus pallidus and left caudate body and head also showed increased cue-related activity during RC trials compared to RCXT and BCXT trials [right lateral globus pallidus (see Fig. 4 for example time courses). However, we did not see any regions in DLPFC or striatum that showed greater cue-related activation on RCXT compared to BCXT trials (all ps > .05).
Table 3.
| Analysis | BA | Region of Activation | Cluster size (voxels) | Talairach Coordinates
|
Z | Activation Patterna | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DLPFC | 9 | Middle Frontal Gyrus | 380 | 37 | 25 | 28 | 4.42 | RC > RCXT = BCXT |
| 9 | Middle Frontal Gyrus | 294 | −35 | 25 | 28 | 4.40 | RC > RCXT = BCXT | |
| Basal Ganglia | Lateral Globus Pallidus | 282 | 17 | 0 | 7 | 4.76 | RC > RCXT = BCXT | |
| Lateral Globus Pallidus | 143 | −20 | −5 | 2 | 3.63 | RC > RCXT = BCXT | ||
| Caudate head | 14 | −7 | 3 | 4 | 2.80 | RC > RCXT = BCXT | ||
| Caudate Body | 50 | −16 | −5 | 17 | 3.72 | RC > RCXT = BCXT | ||
Fig. 4.
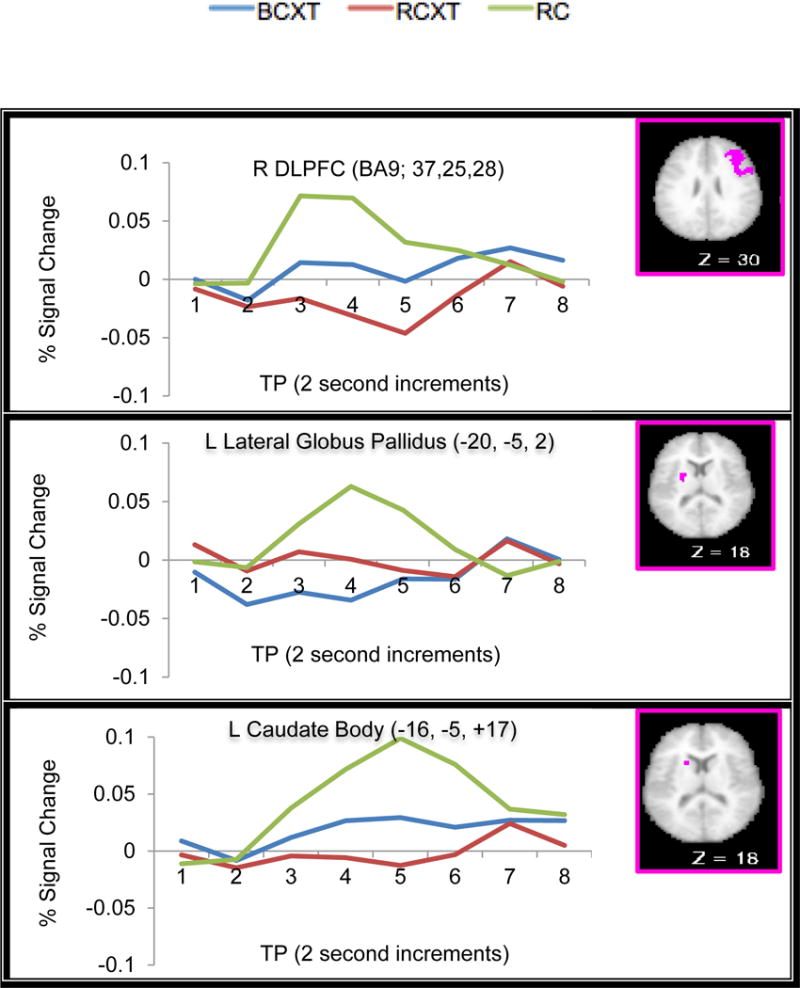
Example Time Courses From ROI Regions Displaying Significant Interactions of Reward and Time Point
Relationship between cue-related effects of reward and anhedonia
Individuals reporting greater anhedonia on the Chapman scales showed less cue-related activation as a function of reward-predicting cues in the lateral globus pallidus (r = −.54, p = .003; see Fig. 5A for scatter plot), an effect that passed Bonferroni correct (.05/6). We saw a similar relationship to self-report of hedonic tone on the SHPS in this region, with greater hedonic tone associated with greater cue-related activation as a function of reward-predicting cues in the lateral globus pallidus (r = .45, p = .02; see Fig.5B), though this correlation did not pass Bonferroni correction.
Fig. 5.
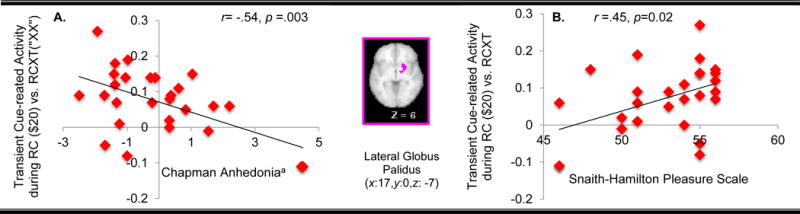
Significant Association between Transient Components of Motivated Cognitive Control and Hedonic tone
Effects of Reward Context on Target-Related Activation
Regions in the DLPFC and the BG displaying a significant Reward Context effect showed two patterns of results (see Table 4. At the top panel of the Table 4, there were regions in the DLPFC and the BG showing reduced target-related activation on RC relative to RCXT or BCXT. For example, in the medial portion of the right DLPFC (BA9; x=26, y=37, z=29), target-related activation on the RC and RCXT trials was significantly reduced relative to BCXT trials. Interestingly, in several subcortical regions, such as the lateral globus pallidus, target-related activity at time point 4 among reward-related types did not differ. However, the source of significance effect was observed at later time point 7 with a greater degree of deactivation on RC trials relative to BCXT and RCXT trials (see Fig. 6 for time courses from DLPFC and BG regions displaying Reward Context × time point effects in the target phase). At the bottom panel of the same Table, a different set of larger, more lateral and more posterior regions in the DLPFC showed greater activation on RC relative to BCXT (see Supplemental Fig.2 for time courses of each region in the DLPFC).
Table 4.
| Analysis | BA | Region of Activation | Cluster size (voxels) | Talairach Coordinates
|
Z | Activation Patterna | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DLPFC | 9 | Superior Frontal Gyrus | 36 | 26 | 37 | 29 | 3.39 | BCXT > RC = RCXT |
| 9 | Middle Frontal Gyrus | 40 | −33 | 32 | 33 | 3.33 | BCXT = RCXT > RC | |
| Basal Ganglia | Lateral Globus Pallidus | 55 | −17 | 2 | −3 | 4.71 | BCXT = RCXT > RCc | |
| Medial Globus Pallidus | 26 | 16 | −1 | −4 | 3.82 | BCXT = RCXT > RCc | ||
|
| ||||||||
| DLPFC | 9 | Middle Frontal Gyrus | 316 | 42 | 18 | 29 | 6.40 | RC, RCXT > BCXT |
| 9 | Middle Frontal Gyrus | 137 | −42 | 11 | 30 | 4.24 | RC > BCXT, RCXTb | |
Fig. 6.
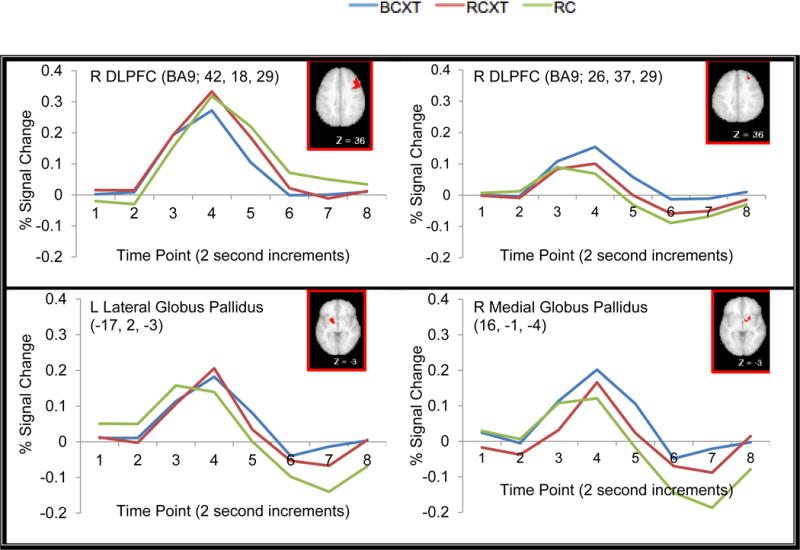
Example Time Courses from the ROI Regions Displaying Reward Context × Time Point Interactions in the Target Phase
Effects of Trial Type on Target-Related Activation
There were three regions that showed interactions between Trial Type (Incongruent, Neutral and Congruent) and time point, one left DLPFC region, one left Putamen region, and one left caudate region. According to post-hoc paired t-tests at time point 4, these regions displayed greater activation in the target phase on incongruent trials compared to congruent or neutral trials (see Table 5 for exact coordinates of each region and Fig. 7 for example time courses for each region).
Table 5.
| Analysis | BA | Region of Activation | Cluster size (voxels) | Talairach Coordinates
|
Z | Activation Patterna | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DLPFC | 9 | Middle Frontal Gyrus | 213 | −42 | 13 | 29 | 4.12 | I > C=N |
|
| ||||||||
| Basal Ganglia | Putamen | 37 | −20 | 2 | 5 | 3.34 | I = C > N+ | |
| Caudate Body | 26 | −11 | 0 | 11 | 2.67 | I > C = N | ||
Fig. 7.
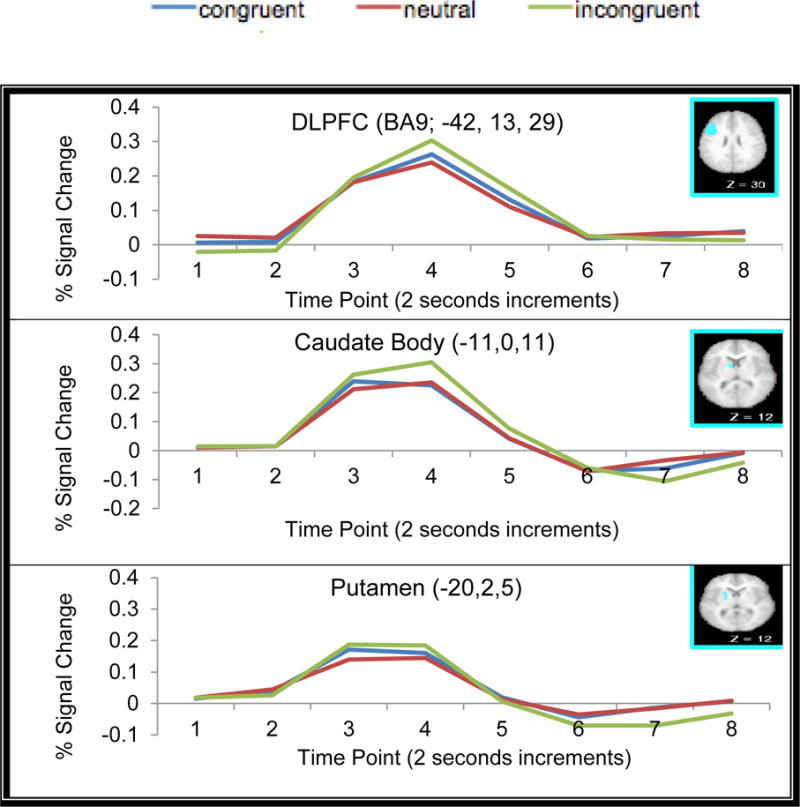
Time Courses from the ROI Regions Displaying Trial-type × Time Point Interactions in the Target Phase
Interactions of Reward Context and Trial Type
We were also interested in whether Reward Context interacted with Trial Type (incongruent, neutral, congruent) as an indicator of whether reward context modulated conflict related brain activity in DLPFC or striatum. No region in the DLPFC or BG mask regions displayed significant Reward Context × Trial type interaction.
Discussion
The goals of the current study were to: 1) replicate previous findings regarding sustained context-dependent and transient cue-driven effects of reward on cognitive control in healthy adults especially by focusing on the involvement of the DLPFC and the striatum; and 2) extend this line of research by examining the influence of individual differences in anhedonia. We found sustained increases in brain activations in the bilateral DLPFC and the putamen and caudate as a function of reward context. Further, consistent with prior work, several regions in the bilateral DLPFC and other sub-cortical regions including the lateral globus pallidus and the caudate showed transient increases of brain activations as a function of reward cues. Importantly, individual differences in anhedonia predicted transient neural responses to reward-predicting cues in the lateral globus pallidus. Each of these findings is discussed in more detail below.
Behavioral Reward Context and Cue Effects
The accuracy data showed a main effect of Trial type reflecting more errors on incongruent compared to congruent and neutral trials. However, we did not find main effect of Reward or Reward × Trial Type interaction in the accuracy data, meaning that we did find that incentives modulated accuracy. However, consistent with previous studies showing enhancement of rewards on cognitive function (Chiew & Braver, 2013; Gilbert & Fiez, 2004; Hughes, Mathan, & Yeung, 2013; Locke & Braver, 2008; Strang & Pollak, 2014; Vuillier, Whitebread, & Szucs, 2015), we did find a main effect of Reward in the RT data, with faster responses on reward cue trials compared to no-reward cue trials in reward block (incentive cue effect) as well as faster responses on no-reward cue trials in the reward block as compared to the baseline block (incentive context effect). These enhancing effects of reward on RT suggest that knowledge of potential rewards enhanced participants’ behavioral performance, potentially through facilitated representation of reward value within reward contexts. However, these were all main effects that did not further interact with trial type.
These results are somewhat in contrast with those of Padmala and Pessoa (2011) from which we modified the same task paradigm for the use of mixed state-item fMRI design. Padmala and Pessoa (2011) found an interaction between reward and congruency, suggesting reduced interference effect (incongruent vs. neutral) on reward compared to the no-reward condition. The discrepancy in the magnitude of the Reward × Trial type interaction effect between current and previous study (Padmala & Pessoa, 2011) might reflect the different of populations in each study. In current study, our participants were from the community (mean age: 35.56 ± 8.61years old) while those in Padmala and Pessoa’s work (2011) were college students (mean age: 22 ± 5 years old). Although the reward value were similar in the two studies ($18 in Padmala and Pessoa (2011)’s work, $20 in the current work), it is possible that the subjective reward value of these amounts might not have generated the same level of motivation among older community populations as it did among college students (Padmala & Pessoa, 2011). Although participants in the current study clearly sped their responses as a function of reward, it is possible that reward value among community populations in current study was not as salient enough actually reduce the magnitude of the conflict effect (Padmala & Pessoa, 2011). Furthermore, it is possible that the task difficulty in the current study was not sufficiently challenging to leave room for modulation of the conflict effect. Specifically, the accuracy level was somewhat higher in the current study (mean error rates ~3%) as compared to Padmala and Pessoa (2011) (mean error rates ~6%). Nonetheless, the fact that participants in the current study were able to speed their responses without sacrificing accuracy suggests that both reward cues and reward context were able to enhance task processing, though not to the point of reducing the magnitude of the conflict effect.
Transient Cue-related Effects of Reward-modulated Cognitive Control
In the present study, activation was increased in response to incentive cues vs. no-incentive cues in the bilateral DLPFC and several reward-related subcortical regions such as the lateral globus pallidus and caudate. These results are in line with prior work (e.g., (Padmala & Pessoa, 2011; Vassena et al., 2014), which also found transient increases in a distributed network of several regions including the lateral PFC and parietal cortex that have been thought to be engaged in cognitive control (Owen, McMillan, Laird, & Bullmore, 2005; Wager & Smith, 2003).
The DLPFC has been traditionally considered as a core component of cognitive control, as described in the Introduction. Recently, accumulated evidence suggests that the DLPFC represents reward-related value information as well as “cold” information about task goals (see Dixon and Christoff (2014) for a recent review). Further, anatomical evidence shows the DLPFC is strongly connected with key regions involved in value representations such as the OFC and the ACC (Pandya, Van Hoesen, & Mesulam, 1981; Petrides & Pandya, 1999, 2007). Also, the DLPFC (BA9 /10) projects to the basal ganglia, including the caudate nucleus and the globus pallidus. Projections from these striatal regions terminate in the thalamus, which in turn projects back to the DLPFC, premotor and motor cortices (Joel & Weiner, 1994, 1997). Considering this circuit of anatomical connections, increased DLPFC and striatal activations in response to incentive cues might reflect enhanced neural representations of reward value through a top-down regulation of activations in this circuit.
In addition to the modulation of cue-related responses as a function of incentives, we also found modulation of target related responses. As expected based on prior literature, we found that neural activation on the incongruent trials was greater relative to the congruent and neutral activations in several regions of the DLPFC, as well as in both a left putamen and a left caudate region. Interestingly, there were small bilateral relatively medial and anterior regions of the DLPFC that showed decreased target-related activations in the reward trials as compared to baseline trials, while more lateral portions of the DLPFC showed the opposite pattern of greater target-related activations during the RC trials. The more lateral portions of the DLPFC are those more typically associated with cognitive control. In the Jimura et al. (2010) study, activations in a similar region on the right showed reduced activations at the time of the target compared to the cue. These researchers interpreted this effect as potentially reflecting decreased use of reactive control though activations did not differ during the target phase for reward trials versus non-reward trials. As such, our results might suggest that in middle-aged community controls, reward cues encourage enhanced activations in cognitive control related regions even during task execution. However, we are less clear what the functional significance may be of the moderated activation in the more medial DLPFC regions, and thus further research will be needed to elucidate the implications of these activation differences.
Sustained Context-dependent effects of motivated cognitive control
As discussed in the introduction, information about incentives can modulate brain activations in both a transient cue-related fashion and in a more sustained fashion. Consistent with these previous findings, our results showed that a number of regions in bilateral DLPFC as well as the bilateral putamen showed greater sustained activations during reward blocks compared to the non-reward, baseline blocks. According to the DCM framework proposed by Braver and his colleagues (Braver, 2012; Braver, Paxton, Locke, & Barch, 2009), cognitive control is thought to involve two types of control – preparatory, proactive control and reactive control. In prior work, Braver and colleagues have argued that incentive cues promote a shift to proactive control, thought to be reflected in both enhanced cue-related activation as well as sustained neural activation in the DLPFC throughout the entire task context. This kind of sustained activation may reflect active maintenance of task-relevant context information supported by the DLPFC. On the other hand, reactive control is thought to be represented as transient activations supported by a wider brain network including the DLPFC, parietal cortex and other reward-related subcortical regions based on cognitive demand. In line with this hypothesis, increased neural activation supported by the DLPFC during reward contexts in current study may reflect actively maintained reward-related context information potentially reflecting enhanced proactive control.
Several regions in the basal ganglia also showed greater sustained activity during reward contexts. These results extend prior work by suggesting that regions in a fronto-parietal network can show sustained increases in regard to reward information. For example, both Jimura et al. (2010) and Locke and Braver (2008) found sustained increase in the parietal cortex as well as the lateral PFC during reward contexts but did not find effects in the basal ganglia. However, the current study conducted hypothesis-driven analysis using an a priori ROI approach while the previous studies used voxel-wise whole brain analysis. It is possible that this ROI approach offered greater power to detect sustained effects in both prefrontal and striatal regions (Nieto-Castanon, Ghosh, Tourville, & Guenther, 2003). This sustained activity may be reflective of the basal ganglia hypothesized role in reward-based learning and goal-directed behavior (Dasgupta, Worgotter, & Manoonpong, 2014; Schultz, Tremblay, & Hollerman, 1998). Especially, the dorsal striatum is known to receive extensive projections from the DLPFC as well as other frontal regions. Thus, increased sustained activity in the dorsal striatum during reward context may represent greater effort to maintain reward-related context information, which may facilitate preparatory responses throughout reward contexts.
Implications for the Dual Mechanisms of Control (DCM) Framework in Reward Context
As we described in introduction, according to the dual-mechanisms-of-control model, cognitive control operates via proactive and reactive control modes in different timescales, along with specific task demands. Proactive control refers to sustained and anticipatory processing of control, whereas reactive control refers to stimulus-driven, transient adjustment of control. Therefore, we hypothesized that proactive control would operate during both periods transient updating processing—during the preparatory, anticipatory period prior to stimulus onset, as well as throughout the entire task condition, due to stable task maintenance. On the other hand, reactive control should operate relatively late in the trial and should be more apparent at the time of the response to the probe. In the present study, as we present in the supplementary information, several regions in bilateral DLPFC displayed both sustained activations during reward contexts and transient cue-related activations. We interpret these overlapping regions as a reflection of a proactive control mode for stable and preparatory maintenance of reward-related context information in reward contexts. Also, we found that more lateral portions of the DLPFC showed greater activation on RC than on BCXT trials during the target phase, which may reflect transient reactive control. During the target phase, reactive control may re-access task-related goal information on a trial-by-trial basis. These results are consistent with previous work suggesting reward-dependent temporal shifts in DLPFC activations from a transient to a tonic mode during reward contexts (Jimura et al., 2010; Soutschek, Stelzel, Paschke, Walter, & Schubert, 2015; Strang & Pollak, 2014; Wilk, Ezekiel, & Morton, 2012). For example, Jimura et al. (2010) found the involvement of the DLPFC (BA46/9) during both sustained effects through the entire reward conditions, and early-trial components of transient responses at cue presentation. Interestingly, increased sustained activations in the DLPFC during reward versus no-reward contexts lead to a reduction of transient activation during the target phase, potentially suggesting a distinction between proactive mechanisms throughout the entire task block and reactive control mechanisms operating transiently during the later target phase. Consistent with these data, in other work Wilk et al. (2012) asked healthy participants to perform a size congruency task in which two digits that differed in physical and numerical magnitude were presented, and the job of this task was to select the numerically larger digit. Consistent with the present findings, Wilk et al. (2012) found transient, moment-to-moment adjustments of reactive control in bilateral DLPFC and anterior cingulate cortex during target responses, and increased sustained, stable task-set maintenance in several regions in the superior frontal gyrus. Taken together, our findings suggest that motivational incentives influence cognitive control function via sustained and anticipatory proactive control, thought to enhance reward value representations, and also via transient adjustments of reactive control, potentially by updating task-relevant goal information on a trial-by-trial basis for successful response selection. These proactive and reactive control modes appear to activate in distinct temporal modes, but at least partially overlap anatomically in the DLPFC and BG (van Belle, Vink, Durston, & Zandbelt, 2014).
Relationships to Anhedonia
In addition to group effects of reward-modulated cognitive control, the present study revealed that individual variations in anhedonia were associated with transient reward cue-related activations in the lateral globus pallidus, but not with sustained context-dependent activation in the DLPFC or basal ganglia. The lateral globus pallidus is the final output site of the basal ganglia (Joel & Weiner, 1994). Studies in non-human monkeys have revealed that neural activations in the basal ganglia is modulated by expected rewards (Hikosaka, Nakamura, & Nakahara, 2006). For example, in Hong and Hikosaka (2008), monkeys performed a saccade task in which a visual target was presented randomly on the left or right. One direction was associated with getting rewards while the other predicted no-reward outcomes. When they measured the firing activity in the globus pallidus and lateral habenula, they found reward-dependent modulations of neural activity in the globus pallidus as evidenced by increased activity in response to the reward-predicting target and decreased responses to the no-reward predicting target.
In the present study, we found that people reporting greater trait anhedonia showed less neural activation as a function of reward-predicting cues, but that there was no significant association between anhedonia and sustained context-dependent activation during reward contexts. These results are consistent with the hypothesis that anhedonia, or reductions in the ability to experience pleasure, may influence the degree to which the experience of rewards (or Blikingˆ) influences modulations of brain activation or behavior in response to the explicit presentation of reward cues, as occurred on RC trials. However, these results do not support the idea that anhedonia influences more global effects of incentives that are reflected in either the sustained aspects of brain activation or the behavioral effects of reward context. As we discussed in the introduction, without having positive experiences/expectations of rewards, it may be hard to exert the effort to pursue goal-directed behavior, potentially providing one mechanism by which individual differences in anhedonia may influence behavior.
Limitations and Future Directions
Although this study provides crucial insight into one potential neural mechanism underlying anhedonia, it has several limitations that may be answered in future studies. One limitation to this study is that we did not find significant reductions in conflict effects in either accuracy or RT, though we did find a speeding of RT as a function of reward, without a sacrifice in accuracy. Consistent with these behavioral findings, we did not find significant regions displaying interaction effects of Reward Context and Trial Type in the target phase, though our analyses were focused on the DLPFC and the basal ganglia. As discussed above, we cannot exclude a possibility that the difficulty level of current task may not have been optimally adjusted to tap cognitive control among community populations. Future research varying the difficulty level of task performance is needed to understand how engagement of the DLPFC to modulate cognitive control in reward contexts is moderated by the challenge level of the tasks. In summary, current findings shows both sustained context-dependent and transient cue-driven effects of reward on cognitive control thought to be supported by the DLPFC and the basal ganglia. Importantly, self-reported anhedonia trait in healthy adults were associated with transient neural activity during reward predictions implicated in the lateral globus pallidus, but not with sustained DLPFC activity.
Relevant to the present findings of no interaction between reward and trial type in the behavioral data, there is a possibility that our smaller sample size (n = 27) than in previous work by Padmala and Pessoa (2011; n = 45), whose task paradigm we modified and used in the present study, may have resulted in a failure to replicate some aspects of their work. However, an N of 27 still provided a reasonable power to detect transient and sustained effects of interest in the present study and the correlation coefficients ranging from .4 to .5 that are used for individual-difference analyses. Importantly, when we computed effect sizes for the enhancement of rewards on RT performance, the present study yielded an effect size for RT data (d = 0.33) similar to that from Padmala and Pessoa (d = 0.37).
Another major limitation is that, due to the fixed-order presentation of the baseline and reward conditions, the sustained context-dependent effect might have reflected practice-related effects. However, our differences between reward conditions are unlikely to have been due to practice effects, given the previous empirical evidence against this possibility. For example, Chiew and Braver (2013) examined the effect of reward incentives on cognitive control, as measured by the AX-continuous performance task (AX-CPT). Like us, they utilized a mixed block/event design. In the AX-CPT, in which participants perform baseline (no incentive offered) and reward blocks, within the reward blocks, nonincentive trials are randomly intermixed with the incentive trials. In Chiew and Braver’s supplementary analysis, they broke down each block into four 50-trial epochs, and found that potential practice effects disappeared after the first epoch, whereas incentive effects remained throughout. Furthermore, the differences between our RCXT and RC trials cannot be due to practice effects, since these trials were interleaved. The present mixed state-item design allowed us to start to dissociate sustained versus transient effects of rewards. However, additional approaches could be used in future studies to further dissociate these effects. In particular, our design did not provide an estimate of the effects of sustained rewards in the absence of transient trial-by-trial effects. Thus, an alternative future design would be to include conditions in which participants were informed that there would be a bonus at the end of a block of trials for overall improved performance, but no trial-specific reward cues. With such a manipulation, one could create a crossed design in which there either was or was not an overall block-wise manipulation of reward bonus (e.g., context effect: high vs. low sustained reward motivation) versus the presence or absence of trial-by-trial cues about the potentially of additional rewards (transient effect: reward cue vs. no-reward cue trials).
In summary, the current findings replicated previous findings showing both sustained context-dependent and transient cue-driven effects modulation of activation in DLPFC and basal ganglia regions as a function of reward, thought to reflect modulation of cognitive control. Further, we extended priori research by showing that individual differences in self-reported anhedonia were associated with individual differences in cue-related functional brain activation on reward trials in the lateral globus pallidus, but not with sustained DLPFC activation. These individual difference relationships may reflect the degree to which the hedonic experience of reward influences motivated behavior.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health Grant, R01-MH066031. We thank the members of the Cognitive Control and Psychopathology Laboratory, and all participants in this study who provided time and effort to make this study possible.
Footnotes
All authors have no financial interests or potential conflicts of interest.
Contributor Information
Yu Sun Chung, Department of Psychology, Washington University, St. Louis, MO, USA.
Deanna Barch, Departments of Psychology, Psychiatry, and Radiology, Washington University, St. Louis, MO, USA.
References
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Human Brain Mapping. 2014 doi: 10.1002/hbm.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26(19):5160–5166. doi: 10.1523/jneurosci.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Hopf JM, Krebs RM, Stoppel CM, Schoenfeld MA, Heinze HJ, Noesselt T. Task-load-dependent activation of dopaminergic midbrain areas in the absence of reward. Journal of Neuroscience. 2011;31(13):4955–4961. doi: 10.1523/jneurosci.4845-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Schevernels H, Hopf JM, Stoppel CM, Krebs RM. Reward prospect rapidly speeds up response inhibition via reactive control. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):593–609. doi: 10.3758/s13415-014-0251-5. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. doi: S1364-6613(11)00261-0 [pii]10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane MJ, Miyake A, Towse J, editors. Variation in working memory. Oxford: Oxford UP; 2007. pp. 76–106. [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, Somerville LH. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7351–7356. doi: 10.1073/pnas.0808187106. doi: 0808187106 [pii]10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Annuals of the New York Academy of Sciences. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. doi: S1053811904003271 [pii]10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine T, Lencz T, Mednick S, editors. Schizotypal personality. New York, NY: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- Chapman LJ, Chapman JP. The revised physical anhedonia scale. University of Wisconsin; Madison: 1978. [Google Scholar]
- Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology. 2013;4:15. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Research Cognitive Brain Research. 2005;25(3):851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Duke E, Pickering AD, Smillie LD. Individual differences in reward prediction error: contrasting relations between feedback-related negativity and trait measures of reward sensitivity, impulsivity and extraversion. Frontiers in Human Neuroscience. 2014;8:248. doi: 10.3389/fnhum.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Worgotter F, Manoonpong P. Neuromodulatory adaptive combination of correlation-based learning in cerebellum and reward-based learning in basal ganglia for goal-directed behavior control. Frontiers in Neural Circuits. 2014;8:126. doi: 10.3389/fncir.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Christoff K. The decision to engage cognitive control is driven by expected reward-value: neural and behavioral evidence. PLoS One. 2012;7(12):e51637. doi: 10.1371/journal.pone.0051637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Christoff K. The lateral prefrontal cortex and complex value-based learning and decision making. Neuroscience & Biobehavioral Reviews. 2014;45c:9–18. doi: 10.1016/j.neubiorev.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The revised social anhedonia scale. University of Wisconsin; Madison: 1982. [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. doi: S0165-0327(06)00358-2 [pii]10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gilbert AM, Fiez JA. Integrating rewards and cognition in the frontal cortex. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(4):540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(Pt 6):1751–1764. doi: 10.1093/brain/awr059. doi: awr059 [pii]10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Molecular Psychiatry. 2007;12(8):703, 767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95(2):567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Progress in Brain Research. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. doi: S0079-6123(00)26015-9 [pii]10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60(4):720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G, Mathan S, Yeung N. EEG indices of reward motivation and target detectability in a rapid visual detection task. Neuroimage. 2013;64:590–600. doi: 10.1016/j.neuroimage.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Liu X, Clerkin S, Schulz K, Friston K, Newcorn JH, Fan J. Effects of motivation on reward and attentional networks: an fMRI study. Brain and Behavior. 2012;2(6):741–753. doi: 10.1002/brb3.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63(2):363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Research Brain Research Reviews. 1997;23(1–2):62–78. doi: 10.1016/s0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Koch K, Schachtzabel C, Wagner G, Schikora J, Schultz C, Reichenbach JR, Schlosser RG. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage. 2010;50(1):223–232. doi: 10.1016/j.neuroimage.2009.12.031. doi: S1053-8119(09)01320-2 [pii]10.1016/j.neuroimage.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Appelbaum LG, Woldorff MG. Reward associations reduce behavioral interference by changing the temporal dynamics of conflict processing. PLoS One. 2013;8(1):e53894. doi: 10.1371/journal.pone.0053894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Roberts KC, Song AW, Woldorff MG. The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex. 2012;22(3):607–615. doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Woldorff MG. The influence of reward associations on conflict processing in the Stroop task. Cognition. 2010;117(3):341–347. doi: 10.1016/j.cognition.2010.08.018. doi: S0010-0277(10)00198-8 [pii]10.1016/j.cognition.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. Neuroimage. 2003;20(3):1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage. 2003;19(4):1303–1316. doi: 10.1016/s1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Niv Y. Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Annuals of the New York Academy of Sciences. 2007;1104:357–376. doi: 10.1196/annals.1390.018. doi: annals.1390.018 [pii]10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Resesarch. 1981;42(3–4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage. 2012;62(2):1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. doi: S1053-8119(11)01160-8 [pii]10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27(43):11573–11586. doi: 10.1523/jneurosci.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex. 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Deserno L, Darmohray D, Sterzer P. Attentional modulation of reward processing in the human brain. Human Brain Mapping. 2014;35(7):3036–3051. doi: 10.1002/hbm.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Annuals of the New York Academy of Sciences. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37(4–5):421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex. 2005;15(12):1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Soutschek A, Stelzel C, Paschke L, Walter H, Schubert T. Dissociable effects of motivation and expectancy on conflict processing: an FMRI study. Journal of Cognitive Neuroscience. 2015;27(2):409–423. doi: 10.1162/jocn_a_00712. [DOI] [PubMed] [Google Scholar]
- Stoppel CM, Boehler CN, Strumpf H, Heinze HJ, Hopf JM, Schoenfeld MA. Neural processing of reward magnitude under varying attentional demands. Brain Research. 2011;1383:218–229. doi: 10.1016/j.brainres.2011.01.095. [DOI] [PubMed] [Google Scholar]
- Strang NM, Pollak SD. Developmental continuity in reward-related enhancement of cognitive control. Developmental Cognitive Neuroscience. 2014;10c:34–43. doi: 10.1016/j.dcn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21(3):1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- van Belle J, Vink M, Durston S, Zandbelt BB. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage. 2014;103:65–74. doi: 10.1016/j.neuroimage.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Vassena E, Silvetti M, Boehler CN, Achten E, Fias W, Verguts T. Overlapping neural systems represent cognitive effort and reward anticipation. PLoS One. 2014;9(3):e91008. doi: 10.1371/journal.pone.0091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vuillier L, Whitebread D, Szucs D. ERP evidence of cognitive strategy change in motivational conditions with varying level of difficulty. Neuropsychologia. 2015;70:126–133. doi: 10.1016/j.neuropsychologia.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biological Psychiatry. 2008;64(12):1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Role of anticipated reward in cognitive behavioral control. Current Opinion in Neurobiology. 2007;17(2):213–219. doi: 10.1016/j.conb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i101–109. doi: 10.1093/cercor/bhm067. (1) [DOI] [PubMed] [Google Scholar]
- Wilk HA, Ezekiel F, Morton JB. Brain regions associated with moment-to-moment adjustments in control and stable task-set maintenance. Neuroimage. 2012;59(2):1960–1967. doi: 10.1016/j.neuroimage.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


