Abstract
The presence of pesticides in human milk (HM) is of great concern due to the potential health effects for the breastfed infant. To determine the relationships between HM pesticides and infant growth and development, a longitudinal study was conducted. HM samples (n = 99) from 16 mothers were collected at 2, 5, 9 and 12 months of lactation. A validated QuEChERS method and Gas chromatography-tandem mass spectrometry (GC-MS/MS) were used for the analysis of 88 pesticides in HM. Only p,p’-DDE, p,p’-DDT and β-HCH were detected with a mean concentration (±SD) of 52.25 ± 49.88 ng/g fat, 27.67 ± 20.96 ng/g fat and 48.00 ± 22.46 ng/g fat respectively. The concentrations of the detected pesticides decreased significantly throughout the first year of lactation. No significant relationships between HM p,p’-DDE and infant growth outcomes: weight, length, head circumference and percentage fat mass were detected. The actual daily intake (ADI) of total DDTs in this cohort was 14–1000 times lower than the threshold reference and significantly lower than the estimated daily intake (EDI). Further, the ADI decreased significantly throughout the first 12 months of lactation.
Persistent organic pollutants (POPs), such as organochlorine pesticides (OCPs), organophosphate pesticides (OPPs), polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins (dioxins), are synthetic chemicals that are present in the environment1,2. When introduced into the environment, these chemicals can percolate into the soil and ground water, and can be transported over long distances via atmospheric circulation and water movement. These chemicals are absorbed by inhalation, ingestion and dermal contact3,4. As these xenobiotics enter the body, they bind to transport proteins such as human blood albumin (HBA), globulins and lipoprotein in the plasma5. The more lipophilic chemicals, such as OCPs, are then redistributed and accumulated in tissue compartments with high fat content, such as adipose tissue, the liver, kidneys, brain and breasts3. Whereas the more hydrophilic xenobiotics, such as carbamate, are more easily metabolized in the liver and then excreted6. In lactating women, these xenobiotics can be transferred from the blood to milk together with other necessary nutrients, precursors for the production of human milk (HM) components7,8. However, the mechanisms underlying the transfer of these chemicals in HM are not yet known. This paper will focus on the persistent organic pollutants in HM with respect to pesticides.
The presence of pesticides in HM is of great concern due to the potential health effects for the breastfed infant, as many of these pesticides are known to interfere with the function of normal endocrine systems2. Exposure to these xenobiotics has been associated with a wide range of adverse effects, such as delayed neurodevelopment, poor cognitive performance and growth retardation during early childhood9,10,11. High prenatal exposure to OPPs and its metabolites has been associated with attention deficit/hyperactivity disorder in children at 5 years of age12. Similarly, a longitudinal study of 329 children through to 7 years of age confirmed that high maternal OPPs concentrations were associated with poor intellectual development and cognitive performance13. In terms of infant growth or size, high levels of chlorpyrifos in maternal prenatal plasma has been associated with smaller infant birth length and weight14 and high concentrations of DDE in maternal prenatal urine with smaller infant birth weight and head circumference15.
With respect to recent study postnatal exposure to pesticides, Liu (2016) reported an association between smaller newborn head circumference and the metabolites of OPPs, such as dialkylphosphate (DAP) and diethyl phosphate (DEP) which was more pronounced in the male infant. Follow-up assessment of these infants at 2 years of age showed that prenatal exposure of the fetus to OPPs was associated with delayed adaptive skills whilst postnatal exposure to OPPs were associated with delayed social and motor development skills16. Caution must be taken in interpretation of these studies as it is difficult to differentiate between prenatal and postnatal exposure to these pesticides. Further unraveling postnatal infant exposure due to HM or other sources such as food and the environment is even more difficult due to lack of measurement of both volume of HM consumed as well as reliable dietary records. Epidemiological studies of pesticides in HM in relation to postnatal infant growth outcomes are non-existent therefore we lack understanding of the effects of the pesticides in HM on the infant growth and development despite the infant in being more vulnerable to the potential effects due to their immature biological systems and the high levels of enzymes required to detoxify these pesticides17.
Pesticides have been extensively used in Western Australia (WA) in the past to protect agricultural products, buildings and households against insects and pests18, and high concentrations of pesticides, such as DDT and its metabolites, HCB and dieldrin, have been detected in Western Australian (WA) women’s milk previously19,20,21. The most recent study was conducted in 2002/0322, however this result was based on a single pooled milk, making it a conservative estimate for the individual and not allowing for investigation of factors that might influence on HM POPs. Since then little information about these pesticides in women’s milk in WA has been reported.
The aims of this study were to describe the current pesticide concentrations in HM and changes during the first year of lactation in a longitudinal cohort. Associations between pesticide concentrations between infant growth outcomes were investigated along with maternal characteristics. The daily intake of the pesticides was calculated rather than estimated and tracked throughout the first year of lactation.
Results
Participants
The demographics and characteristics of the study participants are presented in Table 1. No significant differences were observed in maternal BMI (P > 0.96), maternal fat mass (P > 0.46) and infant fat mass measured by ultrasound skinfolds (P > 0.87) and bioimpedance spectroscopy (BIS; P > 0.24) throughout the first year of lactation. Whereas significant differences (P < 0.01) were observed in infants’ weight, body length and head circumference throughout the first year of life.
Table 1. Participant characteristics of a longitudinal study measuring the concentration of pesticides in human milk collected from women (n = 16) at 2, 5, 9 and 12 months postpartum in Western Australia.
| Mean ± SD (Range) | ||||
|---|---|---|---|---|
| Mother (n = 16) | ||||
| Age | 33.9 ± 5.1 (24–43) | |||
| Parity | 2.3 ± 1.1 (0–4) | |||
| Infant gestational age (weeks) | 39.4 ± 1.4 (38–43) | |||
| Lactation stage (Months) | 2 | 5 | 9 | 12 |
| Maternal body mass index (BMI) (kg.m−2) | 24.9 ± 4.6 (20.4–35.5) | 24.3 ± 5.3 (19.0–35.2) | 24.5 ± 5.9 (17.9–37.2) | 23.7 ± 6.5 (18.2–37.2) |
| Percentage fat mass (BIS#) | 34.4 ± 5.3 (25.7–44.7) | 32.8 ± 6.2 (25.1–47.2) | 32.6 ± 6.9 (20.0–44.3) | 30.2 ± 7.6 (19.4–44.5) |
| Infant (Male/Female = 9/7) | ||||
| Age (Months) | 2.0 ± 0.1 (1.9–2.2) | 5.1 ± 0.2 (1.8–5.4) | 9.2 ± 0.3 (8.8–9.8) | 12.2 ± 0.3 (11.6–12.7) |
| Infant weight (kg)* | 5.6 ± 0.9 (4.4–7.4) | 7.3 ± 0.8 (6.2–8.7) | 8.5 ± 0.9 (6.7–10.1) | 9.3 ± 0.7 (8.3–10.6) |
| Body length (cm)* | 57.9 ± 1.8 (54.5–60.0) | 64.5 ± 2.4 (60.5–69.5) | 70.0 ± 2.3 (66.0–74.0) | 73.6 ± 2.4 (69.0–76.3) |
| Head circumference (cm)* | 39.6 ± 1.5 (37.0–42.0) | 42.8 ± 1.9 (40.0–45.0) | 45.4 ± 1.8 (43.0–48.5) | 46.3 ± 1.5 (44.3–49.5) |
| Percentage fat mass (ultrasound skinfolds) | 25.6 ± 4.7 (20.9–37.9) | 26.1 ± 3.5 (19.7–33.2) | 25.7 ± 4.7 (15.0–34.7) | 24.8 ± 4.2 (18.2–30.9) |
| Percentage fat mass (BIS#) | 22.1 ± 2.0 (19.2–24.1) | 27.6 ± 2.8 (21.7–30.9) | 25.1 ± 5.3 (16.3–33.8) | 25.2 ± 3.8 (19.6–30.6) |
#BIS: bioimpedance spectroscopy.
*Significant difference (P < 0.05) was observed between months 2–5, 2–9, 2–12, 5–9, 5–12 and 9–12.
HM fat content and pesticide concentrations
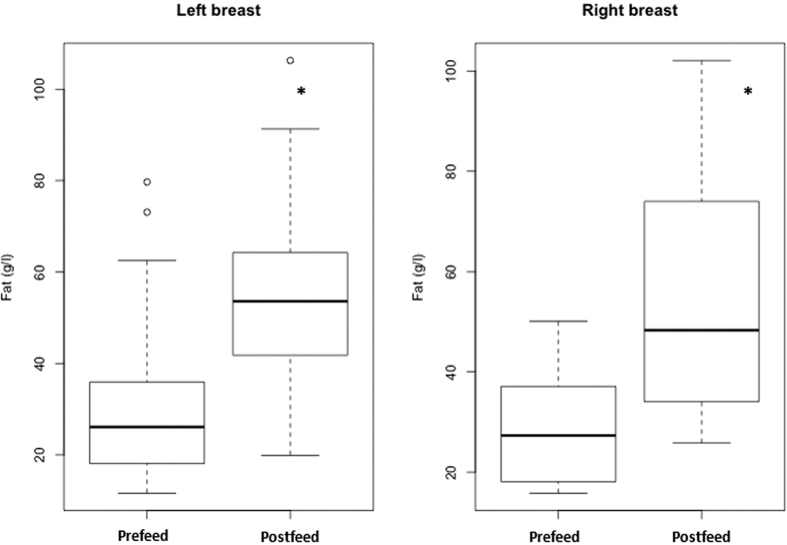
The mean fat content of all HM samples (postfeed: n = 39; prefeed: n = 60) was 38.9 ± 21.6 g/L (11.6 to 106.3 g/L), Fat content of postfeed milk (left: 55.1 ± 21.4 g/L; right: 54.9 ± 24.0 g/L) was significantly higher (P < 0.01) than prefeed milk (left: 28.8 ± 15.3 g/L; right: 27.6 ± 10.0 g/L) (Fig. 1).
Figure 1. Distribution of fat content in pre- and postfeed milk collected from each breast from 2 to 12 months (n = 99 samples from 16 mothers).
The fat contents are shown by box plots illustrating range (error bars), quartiles (box), median (indicated by bold line) and outliers (o). *Indicates significant difference (P < 0.05).
Only 3 of the 88 pesticides (3.4%), p,p’-DDE, p,p’-DDT and β-HCH, were detected (Table 2). Other pesticides, such as OPPs, fungicides, carbamates and pyrethroids, were not detected. The most frequently detected and abundant pesticide in HM was p,p’-DDE; detected in 83 of the 99 samples (83%). The mean concentration of p,p’-DDE was 1.56 ± 1.22 ng/mL (range: 0.21–6.21 ng/mL) and when normalized to the HM fat content was 52.25 ± 49.88 ng/g fat (range: 5.67–278.48 ng/g fat). The level of p,p’-DDE was significantly higher in the postfeed milk (left: 2.12 ± 1.36 ng/mL; right: 1.57 ± 0.95 ng/mL) compared to the prefeed milk (left: 1.44 ± 1.32 ng/mL; right: 1.08 ± 0.78 ng/mL) (P < 0.01; Fig. 2A). After the normalization of p,p’-DDE to HM fat content there were no significant differences between the pre- and postfeed p,p’-DDE concentrations (left: P = 0.24; right: P = 0.37; Fig. 2B).
Table 2. Summary of detected pesticides in HM samples (n = 99) collected from 16 Western Australian mothers.
| Pesticide | Frequency of detection (%) | Mean ± SD (ng/mL) (Range) | Mean (ng/g fat) (Range) |
|---|---|---|---|
| p,p’-DDE | 83 | 1.56 ± 1.22 (0.21–6.21) | 52.25 ± 49.88 (5.67–278.48) |
| p,p’-DDT | 9 | 0.70 ± 0.42 (0.26–1.55) | 27.67 ± 20.96 (6.08–69.55) |
| β-HCH | 4 | 2.17 ± 1.69 (0.59–4.55) | 48.00 ± 22.46 (14.95–65.08) |
Figure 2.
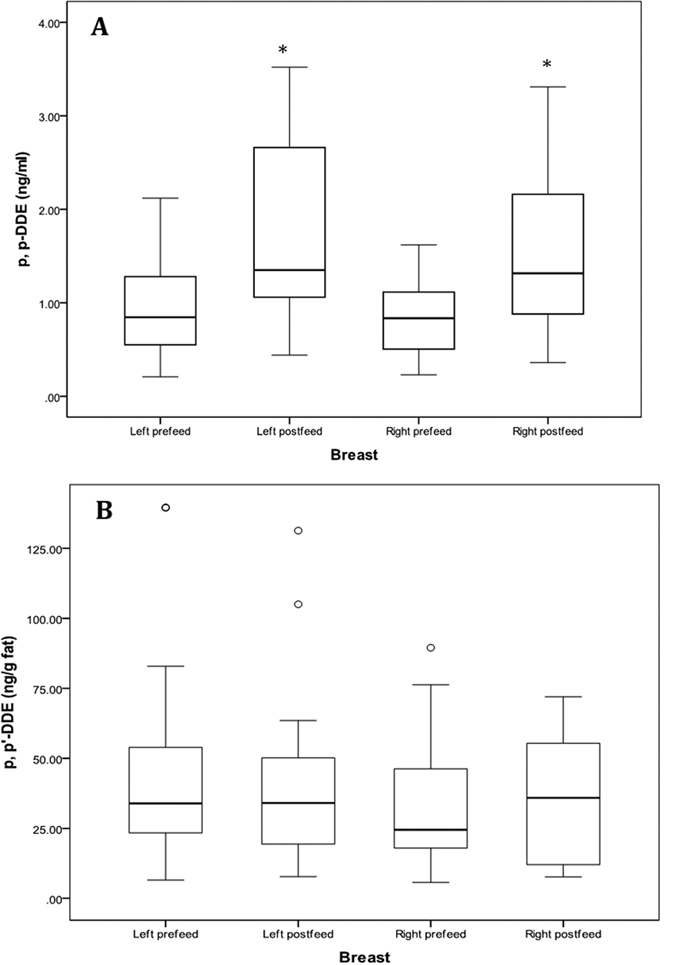
Distribution of p,p’-DDE in pre- and postfeed milk collected from each breast before (A) and after (B) normalized to fat content of HM. Values of p,p’-DDE are shown by box plots illustrating range (error bars), quartiles (box), median (indicated by bold line) and outliers (o). *Indicates significant difference (P < 0.05).
p,p’-DDT was detected in the milk of 2 mothers with a mean concentration of 27.67 ± 20.96 ng/g fat (range: 6.08–69.55 ng/g fat) (Table 2). The insecticide, β-HCH, was detected in 1 mother, with a mean concentration of 48.00 ± 22.46 ng/g fat (range: 14.95–65.08 ng/g fat) (Table 2).
Changes in pesticide concentrations during the first year of lactation
The overall HM p, p’-DDE concentrations declined from 70.90 ± 70.58 ng/g fat at 2 months to 57.54 ± 47.32 ng/g fat at 5 months, 45.16 ± 31.71 ng/g fat at 9 months and to 22.35 ± 13.96 ng/g fat at 12 months. There was an overall 68% decrease of p,p’-DDE concentrations over the first 12 months of lactation (Fig. 3). A significant difference (P = 0.03) was observed in p,p’-DDE concentrations between 2 and 12 months only. Similarly, the concentrations of p,p’-DDT and β-HCH in this study also decreased by 45% and 73% respectively over the lactation period of 2–12 months.
Figure 3. The average concentrations of p,p’-DDE (ng/g fat) in HM collected during lactation period from 2 to 12 months.
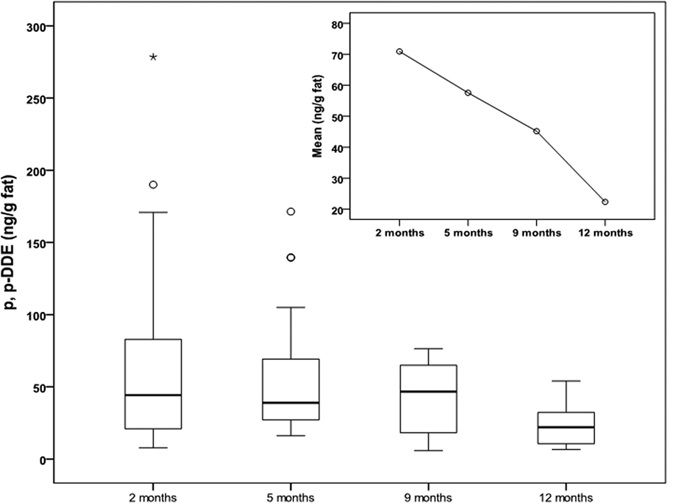
Values of p,p’-DDE are shown by box plots illustrating range (error bars), quartiles (box), median (indicated by bold line) and outliers (o). Insert is the mean plot of p,p’-DDE levels from this cohort. Significant difference (P < 0.05) is observed between 2 and 12 months.
Pesticide concentrations, maternal and infant characteristics
There were no significant relationships between HM p,p’-DDE concentrations and maternal age (P = 0.06), parity (P = 0.65), maternal body mass index (BMI) (P = 0.27) and percentage fat mass (P = 0.08). However, mothers of male infants had significantly higher (P = 0.03) concentrations of p,p’-DDE in their milk (55.18 ± 45.41 ng/g fat) compared to mothers of female infants (29.34 ± 18.90 ng/g fat) (Supplementary Fig. S1).
Significant increases in infant weight (P < 0.01), length (P < 0.01) and head circumference (P < 0.01) were observed throughout the first year of life (Supplementary Fig. S2). No significant associations were observed between p,p’-DDE and the infant growth outcomes; weight (P = 0.40), length (P = 0.13), head circumference (P = 0.07) and percentage fat mass (ultrasound skinfolds: P = 0.34; BIS: P = 0.11).
Infant exposure to HM DDT
The average calculated actual daily intake (ADI) and estimated daily intake (EDI) of DDTs (sum of DDT and its metabolites, DDE and DDD) throughout the first year of lactation were 0.16 μg/kg body wt./day and 0.23 μg/kg body wt./day, respectively (Table 3). The ADI of DDTs by the infants decreased significantly during the first year of lactation from 0.33 μg/kg body wt./day at 2 months to 0.03 μg/kg body wt./day at 12 months (P < 0.01; Fig. 4), which is in conjunction with the infants’ rapid development and also the significant decrease of the maternal bioburden (P = 0.03; Fig. 3). No significant difference was observed in the EDI of DDTs by the infants over the 12-month period. The average EDI of DDT at 2 and 5 months (0.27–0.30 μg/kg body wt./day) is comparable to that observed in ADI and drastically overestimates at 9 and 12 months (0.10–0.23 μg/kg body wt./day; P < 0.01; Table 3).
Table 3. Average calculated daily intake (ADI) and estimated daily intake (EDI) of DDTs by infants during the first year of lactation in comparison to tolerable daily intake (TDI) proposed by FAO/WHO and Health Canada.
| Lactation months | ADI (μg/kg body wt./day) (Range) | EDI (μg/kg body wt./day) (Range) | P value* | TDI (μg/kg body wt./day) (Range) |
|---|---|---|---|---|
| All data | 0.16 (0.02–0.69) | 0.23 (0.03–0.90) | P < 0.05 | 0.5a,b10.00c20.00d |
| 2 months | 0.33 (0.07–0.69) | 0.30 (0.08–0.90) | 0.45 | |
| 5 months | 0.18 (0.06–0.52) | 0.27 (0.08–0.72) | 0.08 | |
| 9 months | 0.09 (0.02–0.22) | 0.23 (0.03–0.65) | P < 0.05 | |
| 12 months | 0.03 (0.02–0.05) | 0.10 (0.03–0.25) | P < 0.05 |
Figure 4. Actual daily intake (ADI) and estimated daily intake (EDI) of DDTs by individual infants throughout the first year of lactation in WA.
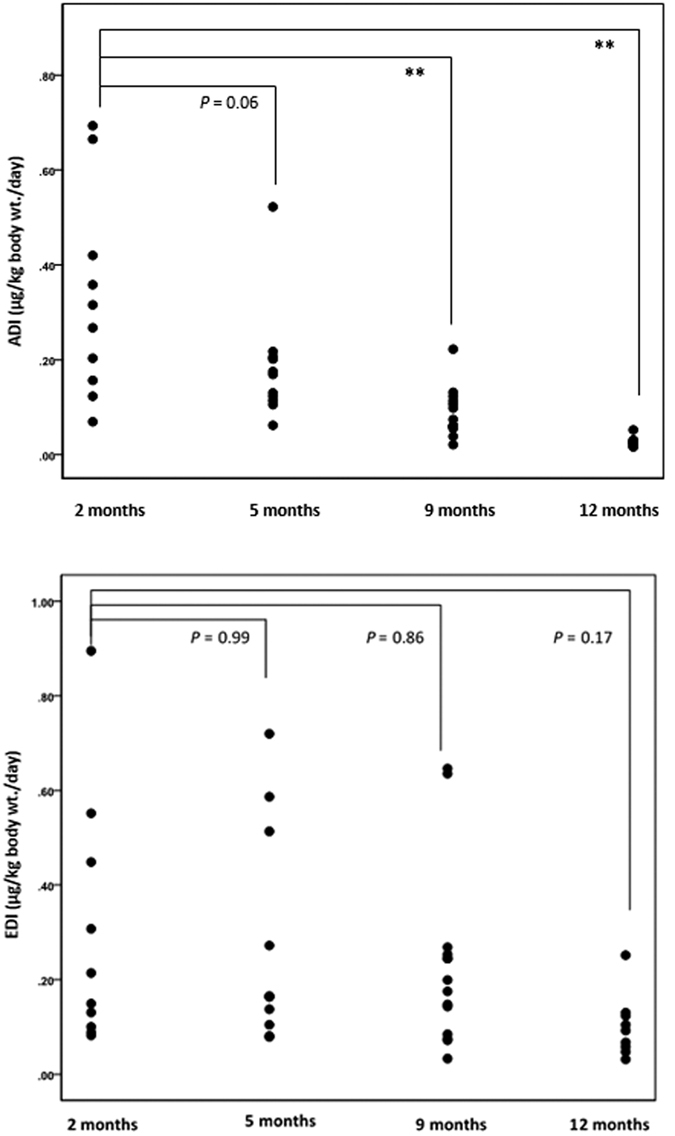
**Indicates significant difference (P < 0.01).
Discussion
This is the first longitudinal study to examine pesticide concentrations in HM and infant growth parameters over the first 12 months of lactation. Only p,p’-DDE, p,p’-DDT and β-HCH in HM were detected out of the 88 pesticides tested with a sensitive and validated QuEChERS (“Quick, Easy, Cheap, Effective, Rugged and Safe”) method. This microscale extraction method is based on the liquid-liquid partitioning of acetonitrile and dispersive solid-phase extraction (dSPE), such as MgSO4, sodium acetate, primary secondary amine (PSA), and the pesticides are partitioned into the acetonitrile layer23. After normalization of the concentrations to the fat content of HM, the levels of pesticides decreased substantially throughout the first year of lactation and p,p’-DDE levels were not related to measures of infant growth such as infant weight, length, head circumference and adiposity.
The HM samples in this cohort were analyzed for pesticides with an optimized QuEChERS methodology24. This method had excellent recoveries (70–120%) and detection limits (0.2–2.0 ng/mL) lower than that of previous studies25, which ensured we did not underestimate the levels of pesticides in HM. The GC-MS/MS method was also optimized for HM which allows the simultaneous screening of 88 different pesticides within a single injection.
The fat content of HM is the most variable component in HM and is known to be higher in postfeed milk as we have demonstrated26. HM p,p’-DDE concentrations were also significantly higher in postfeed milk than prefeed milk (Fig. 2A), suggesting an association between lipophilic pesticides (e.g. p,p’-DDE) and the fat content of HM. These results support the current hypothesis that POPs are encapsulated either in the core (triacyglycerol) or adhere to the surface (phospholipids) of HM fat during milk synthesis27,28. Thus after normalization of p,p’-DDE to the HM fat content, the significant difference of p,p’-DDE concentration between pre- and postfeed milk disappeared (Fig. 2B). Therefore, either pre- or postfeed milk can be collected for the measurement of pesticides provided the concentrations are normalized to the fat content of HM.
We detected large individual variation in HM pesticide concentrations, for example p,p’-DDE concentrations ranged from 5.67 to 278.48 ng/mL, and the types of pesticides (e.g. p,p’-DDT, p,p’-DDE and β-HCH), which likely reflect the differences in exposure, lifestyle, dietary habits, travel and metabolic activity between mothers29.
As p,p’-DDE is a metabolite of p,p’-DDT, the 2 mothers with detectable HM p,p’-DDT also had higher p,p’-DDE concentrations of 125.34 ng/g fat and 80.50 ng/g fat respectively as expected. The ratio between DDE and DDT is commonly used as an indicator of DDT exposure history, where a high DDE/DDT ratio (>5) represents historical exposure and low DDE/DDT ratio (<5) suggests recent exposure30,31. The DDE/DDT ratio of the two mothers with detected DDT was 3.4 and 8.1 respectively. Both mothers had different exposure characteristics, where one mother frequently visits a country that still has documented high levels of DDT (DDT/DDE ratio of 3.4), while the other grew up on a farm and was involved in the harvesting process during the period from 2004 to 2010 (DDT/DDE ratio of 8.1). These are possible exposure sources of DDT are however based on maternal recollection and further investigation is required including sampling of lactating women living in agricultural regions.
Hexachlorocyclohexane (HCH), is widely used in Australia for insect control18. Among its isomers (α-HCH, β-HCH, γ-HCH and δ-HCH), β-HCH is the most metabolic stable and persistent, and accounts for over 90% of the total HCH detected in HM32,33. Similar to DDE/DDT exposure history, the β-HCH/α-HCH ratio is used as an indicator of HCH exposure history34. Since α-HCH and γ-HCH were not detected in this study, suggesting that the mother in whom we detected β-HCH had historical exposure to HCH (β-HCH/α-HCH ratio: >5).
Over the first 12 months of lactation, there was an overall trend for the detected POPs in HM to steadily decline for p,p’-DDE (68%), p,p’-DDT (45%) and β-HCH (73%) suggesting a reduction in maternal body burden35,36. However, we only observed significant differences between the time points 2 and 12 months for HM p,p’-DDE concentrations. This may be attribute to the fact that 10 women showed a decline in HM POPs, while the other 6 showed fluctuations possibly due to change in diet or metabolic activity. Increasing the sample size may increase the ability to detect differences between the months. These results are consistent with previous studies that have shown reductions of pesticides concentration in HM over shorter periods of time such as a decrease in p,p’-DDE and p,p’-DDT in colostrum (day 4/5) to week 2 by 4% and 10% respectively37. Similarly a substantial decrease of p,p’-DDE between colostrum (day 3) and week 3 of 22% has been documented38,39,40,41,42. While extensive studies have been carried out on dioxins and furans43, PCBs44,45 and polybrominated diphenyl ethers (PBDEs)44. There are limited studies that have investigated pesticide levels in HM which extend to a period of more than 6 months of lactation and have a sufficient number of participants45,46. This is one of the more extensive studies which measured pesticides in HM from 16 mothers, longitudinally over 12 months, providing information relevant to the longer term development of the infant in accordance to the WHO recommendation of breastfeeding for up to 1 years and beyond47.
Many factors, such as maternal age, parity and BMI, have been associated with HM pesticide concentrations. Previous studies have reported associations between HM p,p’-DDE and maternal age48,48, suggesting increased bioaccumulation of pesticides with increasing age. Further, increased maternal parity has been associated with lower HM pesticides concentration, which is attributed to the increased excretion of body stores through multiple pregnancies and lactations22,42, whereas increased BMI has been associated with lower HM pesticides concentration. In this study we found no association between HM p,p’-DDE and maternal age (P = 0.06) or parity (P = 0.65) although maternal age is borderline. The absence of a relationship between maternal age and HM POPs may be due to the small number of participants (n = 16) in this study. As body composition measurements, such as BIS are better measures of fat mass we hypothesized that we might find a relationship between fat mass and HM pesticides. However, we were unable to find a relationship between HM p,p’-DDE and maternal BMI (P = 0.27) and maternal fat mass (P = 0.08). These results are similar to that observed by Dirinck et al. where pesticide levels in maternal serum were investigated with respect to BMI and fat mass50. A greater sample size may determine whether or not maternal adiposity is associated with HM pesticides content.
Many studies have reported the adverse effect of pesticides by associating prenatal exposures with infant growth and development39,51,52. However, none has evaluated the potential influence of these pesticides in HM on the infant growth throughout the first year of lactation. In this cohort, we saw significant increases in infant growth outcomes, such as weight, length and head circumference, over the first 12 months as expected. However, none of these measures were associated with HM levels of p,p’-DDE. Further utilizing 2 different measures of infant adiposity (ultrasound skinfolds and BIS), we found no relationship with HM p,p’-DDE. These findings may be influenced by the fact that the levels of pesticides detected in HM in this study were at trace levels and the small numbers of participants (n = 16). Whilst associative relationships do not indicate causation, it is reassuring that the low levels of pesticides detected in WA women’s milk do not appear to be of concern.
Whilst the levels of the detected pesticides in HM in this study were very low, HM is the sole food source for a breastfed infant in the first 6 months of life and contributes substantially to the infant diet in the following 6 months53, thus it is important to consider the dose of ingested pesticides. To get a more accurate measure of the dose, we measured both the fat content of the milk and the 24 hour intake of the infant at 2 to 5 months, where intake has been shown not to change during this time period40, and 9 and 12 months and substitute the estimated values with actual values in the formula for estimated daily intake41,42. This provides a more accurate calculation of the actual daily intake (ADI) by the infant as it is well documented that both the fat content of milk and the daily volume of milk vary 3-fold between breastfeeding infants54. A more accurate quantification for the daily intake of pesticides in infant is essential as studies assessing the effects of pesticides in HM are currently inaccurate and have the potential of not detecting an effect or producing an effect that is not real. Further effects of pesticide must be carefully monitored before implementing interventions to reduce both maternal and infant exposure. As expected, we found a significant difference between ADI and EDI. The EDI, which is based on constant values, underestimated the pesticide intake by 10% at 2 months and overestimated intake by 50 to 233% in the later months (Table 3). The differences are largely due to the overestimation of milk intake at 9 and 12 months of lactation (9 months: 482 ± 76 mL; 12 months: 256 ± 81 mL) for the EDI where a constant of 700 mL is used. Interestingly, the ADI decreased significantly from 2 to 12 months of lactation (Fig. 4), but EDI did not, clearly demonstrating that EDI is not an accurate measure of infant dose. The ADI for several infants exceeded the TDI55,56 (0.5 μg/kg body wt./day) at 2 and 5 months. However, as the breastfeeding progresses, the ADI at 9 and 12 months are 2 to 25 times lower than the TDI, suggesting that maternal HM pesticides in this cohort poses minimal risk to individual infants in WA. Whilst concern about the effects of pesticide exposure to infants is warranted as one must take into consideration the relatively short period of exposure via breastfeeding relative to a lifetime. Further, HM serves as an important environmental indicator of population exposure.
In order to understand the current magnitude of POPs in HM in WA, the results obtained in this study were compared with those reported in Australia as well as those reported from other countries. The total DDTs in this study have decreased by 93% and 80% as compared to previous studies conducted in WA (1990 s)21 and in Australia (2000 s)22 respectively. Our findings are also consistent with the trace pesticides levels found in biofluids (e.g. maternal blood and cord blood) and in tissues (e.g. placenta and abdominal) in WA18,57, which demonstrate the low presence of environmental pesticides and the continuing decline of human exposure to these pesticides over time in WA. As compared to other countries, the concentrations of DDTs observed in this study are similar to those reported for Norway32 and USA58, but are a few orders of magnitude lower than that observed in malaria-prone countries such as Vietnam49, Malaysia59 and India42 where DDTs was widely used to combat mosquito borne malaria and have only recently been banned in the 1990 s and 2000 s (Table 4). WHO however, have recently allowed limited use of DDT for indoor control of malaria vectors in malaria endemic countries60. Therefore, concentrations of DDTs in South Africa61 and Ethiopia62 are 122 and 330 times higher than that in WA. Concurrent with these DDTs findings, the HCHs concentrations measured in this study are 4 to 63 times lower than the concentrations recorded in countries such as India42, Malaysia59 and Iran63, where HCHs are still extensively used and are commensurate with levels observed in Vietnam49, Slovakia37 and USA61.
Table 4. Comparison of DDTs and HCHs (ng/g fat) in HM from various countries.
| Country/region | Year of sampling | Mothers (N) | HM samples | Fat content (g/L) | DDTs | HCHs | References |
|---|---|---|---|---|---|---|---|
| Iran | 2006 | 23 | 23 | 22 | 2685a | 3005A | 53 |
| Indonesia | 2003 | 15 | 15 | na | 1100b | 11A | 63 |
| Taiwan | 2000/01 | 36 | 36 | 31 | 333c | 3B | 24 |
| India | 2011 | 53 | 53 | 32 | 1914b | 199B | 48 |
| Vietnam | 2000/01 | 42 | 42 | 23 | 2100d | 58C | 36 |
| Malaysia | 2003 | 17 | 17 | 17 | 1600d | 230C | 52 |
| Philippine | 2004 | 33 | 33 | 22 | 170b | 6A | 23 |
| Slovakia | 2003 | 12 | 12 | 27 | 665d | 20C | 34 |
| Norway | 2002/06 | 377 | 377 | 36 | 53e | 5.4C | 30 |
| USA | 2004 | 38 | 38 | 22 | 65a | 19D | 38 |
| South Africa | 2001 | 28 | 28 | na | 6320c | 12A | 40 |
| Ethiopia | 2010 | 33 | 33 | na | 17170b | na | 41 |
| New Zealand | 2007/2010 | 39 | 37 | 39 | 385 f | 9A | 64 |
| Australia/WA | 2013/15 | 16 | 99 | 39 | 52e | 48C | Present study |
na: data not available.
*Values represented as median value.
aSum of p,p’-DDE + o,p’-DDE + p,p’-DDD + p,p’-DDT.
bSum of p,p’-DDE + p,p’-DDD + p,p’-DDT.
cSum of p,p’-DDE + p,p’-DDD + p,p’-DDT + o,p’-DDT.
dSum of p,p’-DDE + p,p’-DDT.
ep,p’-DDE only.
fSum of p,p’-DDE + o,p’-DDE + p,p’-DDD + o,p’-DDD + p,p’-DDT + o,p’-DDT.
ASum of α-HCH + β-HCH + γ-HCH.
BSum of β-HCH + γ-HCH.
Cβ-HCH only.
DSum of α-HCH + β-HCH + γ-HCH + δ-HCH.
The results regarding the levels of pesticides in HM from women in WA, Australia are encouraging however development of analytical methods should continue to focus on reducing detection limits thereby making associative studies more meaningful.
Conclusions
In this study, out of the 88 targeted pesticides, we have detected trace amounts of p,p’-DDE, p,p’-DDT and β-HCH in HM collected from Western Australian women, and the levels of these pesticides decreased substantially throughout the first year of lactation. This is the first longitudinal study to investigate the relationships of detected pesticides on the infant growth outcomes such as weight, length, head circumference and body composition of which we found none. Further, EDI dramatically overestimates infant dose after 2 months of lactation while the more accurate ADI show reduced dose to infants. The ADI of pesticides for individual infants in Western Australia decreased significantly during the first year of lactation, and was 2 to 17 times lower than current recommended guidelines
Materials and Methods
Chemicals and reagents
The pesticide standard solutions (100 μg/mL) at 95% or higher purity were obtained from Ultra Scientific (North Kingstown, RI, USA). Pesticide standard solutions (100 μg/mL) were mixed and diluted with acetonitrile (ACN) to prepare a stock standard solution (1 μg/mL) of all the pesticides. LC-MS grade acetonitrile (ACN) and water were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Ethylglycerol (98%), gulonolactone (95%) and D-sorbitol (≥98%) were from Sigma-Aldrich (St. Louis, MO, USA). Sodium acetate (>99.0%) and magnesium sulfate (99.5%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Octadecylsiyl (C18) and primary secondary amine (PSA) were obtained from Agilent (Little Falls, DE, USA). Isotopic labeled quality control (QC) standards, acenaphthene-D10, phenathrene-D10 and chrysene-D12 were purchased from Restek (Bellefonte, PA, USA), and the internal standard (IS), triphenylphosphate (TPP) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Ethics, sample collection and processing
This study was approved by the Human Research Ethics Committee of The University of Western Australia, and the methods were carried out in accordance with the approved guidelines. Western Australian breastfeeding mothers (n = 16) were recruited between 2013 and 2015. All participants provided informed consent and completed a questionnaire including relevant demographic data. Milk samples were collected (1–5 mL) at 2, 5, 9 and 12 months of lactation into glass containers before and after feeding from each breast. HM fat content was measured immediately using the Creamatocrit method64, and the remaining milk was stored at −20 °C.
HM samples (n = 99) were thawed at room temperature for 3 hours then homogenized with a mixer (ELMI Ltd., Riga, Latvia) for 15 seconds. 1 mL of HM was put into a 15 mL centrifuge tube and 1 mL of ACN containing 1% acetic acid and 100 ng/mL QC standards (acenaphthene-D10, phenathrene-D10 and chrysene-D12) was added, and the tube was vortexed for 1 min. A validated method based on acetate buffered QuEChERS was employed to extract the HM24,65,66. Extraction reagents (0.4 g MgSO4 and 0.1 g NaAc) were added to the mixture and shaken immediately. The tube was then placed in an ice bath to prevent thermal degradation of some pesticides during the salting out process. The extraction tube was centrifuged at 3993 g for 10 min. 0.6 mL of the supernatant was transferred into a clean 15 mL centrifuge tube and stored in a freezer (− 20 °C) for 2 hours. The supernatant was centrifuge at 3993 g (0 °C) for 10 min and 0.5 mL was transferred to a cleanup tube (157 mg MgSO4, 9 mg C18 and 9 mg PSA). The tube was vortexed for 1 min and centrifuged at 3993 g for 10 min. The final extract was transferred into a screw cap amber vial and kept at −80 °C until analysis.
Maternal and infant anthropometric measurements
All maternal and infant anthropometrics measurements were made at 2, 5, 9 and 12 months at the time of milk sampling. Maternal body weight was measured by an electronic scale (Seca, California, USA, accuracy 0.1 kg). The height, age and parity were self-reported by participants. Maternal BMI was calculated as: BMI = kg/(m2).
Infant weight was determined by weighing before breastfeeding using electronic scales (±2.0 g; Medela Electronic Baby Weigh Scales, Medela AG, Switzerland). Infant crown-heel length was measured, to the nearest 0.1 cm, on a hard surface with between a head and footpiece with non-stretch tape. Infant head circumference was measured with non-stretch tape.
Maternal and infant body composition measurements
Whole body bioimpedance (wrist to ankle) was measured with Impedimed SFB7 bioelectrical impedance analyzer (ImpediMed, Brisbane, Queensland, Australia) according to the manufacturer’s instructions however the infants’ whole bioimpedance were analyzed with settings customized for each infant according to Lingwood et al.67. Resistance at 50 kHz was used in infant percentage fat mass equations67,68.
Infants’ ultrasound skinfold measurements (triceps and subscapular) were made using Aplio XG (Toshiba, Japan) machine, PLT-1204BX 14–8 MHz transducer and Parker ultrasonic gel (Fairfield, NJ, USA). The double skinfold thickness, measured directly from images using the on screen electronic calipers, was used in percentage fat mass equations developed for skinfolds measured with skinfold calipers69.
24–hour infant milk intake
24–hour milk intake was determined by the testing weighing procedure as previously documented70. Briefly mothers weighed their infants before and after each feed from each breast for a period of 24 hours. The difference in weight in grams is considered equivalent to mL (density of milk: 1.03 g/mL). If the data was unavailable, the data from previous 24–hour milk intake study were used71.
GC-MS/MS analysis and Quality control
Chromatographic separation and determination of the pesticides were carried on a Bruker Daltonics 450 gas chromatography (GC) with a Bruker Daltonics Scion TQ triple quadrupole mass spectrometer (MS), a Bruker 1177 Split/Splitless injector (Billerica, MA, USA) and a PAL Combi autosampler (CTC Analytics AG, Switzerland). Sky 4.0 ID precision inlet liner with wool from Restek (Bellefonte, PA, USA) was used. For the GC separation, a Rtx-5MS with Integra-Guard column (10 m + 30 m × 0.25 mm × 0.25 μm) (Bellefonte, PA, USA) was used. The inlet temperature was kept constant at 250 °C. The initial oven temperature of the column was 80 °C for 3 min and ramped at 30 °C/min to 150 °C and then ramped at 10 °C/min to 300 °C, where it was held for 10 min. The total run time was 31 min. Helium (6.0 GC grade) was used as carrier gas at a constant flow of 1.1 mL/min. The injection was performed at pulsed splitless mode (head pressure; 44 psi) with an injection volume of 2 μL. The mass spectrometer was operated in electron impact (EI) mode. Transfer line and ion source temperature were 270 °C and 200 °C, respectively. Argon was used as the collision gas. Identification and quantification of 88 pesticides were carried out by tandem MS using scheduled multiple reaction monitoring (MRM) mode. The optimized MRM transitions and collision energies for each compound are listed in Supplementary Table 1. Data collection and processing were performance using Bruker MSWS 8 Software.
Working standard solutions (0.5, 1, 2, 5, 10, 20, 50 and 100 ng/mL) were prepared by appropriate dilution of the stock standard solution (1 μg/mL) with ACN. A combination of APs mixture (ethylglycerol, gulonolactone and D-sorbitol) containing internal standard TPP (IS) was added to the final extract of each HM sample and all the working standard solutions for GC-MS/MS analysis. The final concentrations of ethylglycerol, gulonolactone and D-sorbitol were 20, 2 and 2 mg/mL, respectively and the IS was 100 ng/mL. Isotopically labeled quality control (QC) standards were employed to evaluate the efficiency of the extraction and cleanup steps, while TPP (IS) was used to evaluate the performance of the instrument throughout the entire analytical procedure. In the absence of a true “blank” HM matrix, the use of APs mixture was investigated to counteract the matrix effect in HM. Similar peak response were observed between the pesticides spiked (10–100 μg/L) in APs mixture and in HM samples with APs mixture. A QC mixture containing pesticide standards was analyzed between sample batches to check for any interferences and cross-contaminations. The recoveries of majority (88.6%) of the 88 pesticides were within the range of 70–120%. Limit of detection (LOD) and limit of quantitation (LOQ) were assessed experimentally with the lowest standards spiked in APs mixture providing signal-to-noise ratio (S/N ratio) greater than 3 and 10, respectively. The LOD and LOQ of the 88 pesticides spiked in APs mixture were 0.2–2.0 ng/mL and 0.5–5.0 ng/mL respectively.
Infant daily intake
The ADI (μg/kg body wt./day) of the pesticides for each infant was calculated based on the pesticides concentration in HM (μg/g fat; Cpesticide), fat content in HM (g/mL; Cfat), average daily consumption of HM (mL; Vmilk) and body weight of infant (kg; Minfant) using the following equation:
 |
The estimated daily intake (EDI) is based on the same formula as ADI, but was calculated using constant values for Cfat (0.03 g/mL), Minfant (5 kg) and Vmilk (700 mL) based on previous studies41,42.
Statistical analyses
Statistical analyses were carried out using SPSS software (SPSS, version 19.0 for windows, SPSS, Inc, IL, USA) and R 3.2.0 using the package nlme for linear mixed models which account for intra- and inter-individual variation72. Results were expressed as mean ± SD unless stated otherwise. Pesticides that were below the LOD were considered as absent and were not included in the calculations. Detected pesticides were reported based on the HM fat (ng/g fat). Linear mixed models were used to investigate associations between HM pesticide concentrations and both maternal and infant anthropometrics. One-way ANOVA and Tukey’s all pair comparison tests73 were used to compare differences in pesticides concentration, EDI and ADI at the different lactation months. Paired samples t-test was used to compare the daily intake of the detected pesticides using the EDI and ADI. P < 0.05 was considered significant.
Additional Information
How to cite this article: Du, J. et al. Longitudinal study of pesticide residue levels in human milk from Western Australia during 12 months of lactation: Exposure assessment for infants. Sci. Rep. 6, 38355; doi: 10.1038/srep38355 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
D.T.G., M.C.L.G., C.T.L. and P.E.H. received an unrestricted research grant from Medela A.G. (Switzerland). J.D. received financial support from the International Postgraduate Research Scholarships (IPRS). J.D. and Z.G. received financial support from the Australian Postgraduate Award (APA). The authors gratefully acknowledge Bruker for the support for this project with the provision of the SCION GC/MS/MS system used for all the measurements. The authors gratefully acknowledge the support of the NCRIS scheme through Bioplatforms Australia and Metabolomics Australia for pesticide research. Many thanks to all participating mothers and infants in this study.
Footnotes
Author Contributions J.D. collected samples, conducted experiments and data analyses, interpreted data and results, wrote and reviewed the manuscript; Z.G. collected samples and reviewed the manuscript; M.C.L.G. conducted experiments, interpreted data, wrote and critically reviewed the manuscript; C.T.L. conducted statistical analyses and reviewed the manuscript; R.D.T. designed experiments and reviewed the manuscript; P.E.H. reviewed the manuscript; D.T.G. designed the study, wrote and critically reviewed the manuscript.
References
- Loganathan B. G. & Kannan K. Global Organochlorine Contamination Trends: An Overview. Ambio 23, 187–191 (1994). [Google Scholar]
- Tanabe S. Contamination and toxic effects of persistent endocrine disrupters in marine mammals and birds. Mar Pollut Bull 45, 69–77 (2002). [DOI] [PubMed] [Google Scholar]
- Needham L. L. & Wang R. Y. Analytic considerations for measuring environmental chemicals in breast milk. Environmental Health Perspectives 110, A317–A324 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oostdam J. et al. Human health implications of environmental contaminants in Arctic Canada: A review. Science of The Total Environment 351–352, 165–246, doi: 10.1016/j.scitotenv.2005.03.034 (2005). [DOI] [PubMed] [Google Scholar]
- Maliwal B. P. & Guthrie F. E. Interaction of insecticides with human plasma lipoproteins. Chemico-Biological Interactions 35, 177–188, doi: 10.1016/0009-2797(81)90141-1 (1981). [DOI] [PubMed] [Google Scholar]
- Risher J. F., Mink F. L. & Stara J. F. The toxicologic effects of the carbamate insecticide aldicarb in mammals: a review. Environ Health Perspect 72, 267–281 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky H. L. & Guthrie F. E. Binding of insecticides to human serum proteins. Toxicology and Applied Pharmacology 43, 229–235, doi: 10.1016/0041-008X(78)90002-9 (1978). [DOI] [PubMed] [Google Scholar]
- van Hoof F. & Heyndrickx A. The excretion in urine of four insecticidal carbamates and their phenolic metabolites after oral administration to rats. Arch Toxicol 34, 81–88 (1975). [DOI] [PubMed] [Google Scholar]
- Eskenazi B. et al. In Utero Exposure to Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) and Neurodevelopment Among Young Mexican American Children. Pediatrics 118, 233–241, doi: 10.1542/peds.2005-3117 (2006). [DOI] [PubMed] [Google Scholar]
- Boucher O. et al. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. NeuroToxicology 35, 162–168, doi: 10.1016/j.neuro.2013.01.007 (2013). [DOI] [PubMed] [Google Scholar]
- Eskenazi B. et al. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environmental Health Perspectives 115, 792–798, doi: 10.1289/ehp.9828 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A. R. et al. Organophosphate Pesticide Exposure and Attention in Young Mexican-American Children: The CHAMACOS Study. Environmental Health Perspectives 118, 1768–1774, doi: 10.1289/ehp.1002056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M. F. et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environmental Health Perspectives 119, 1189–1195, doi: 10.1289/ehp.1003185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F. P. et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environmental Health Perspectives 111, 201–205 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M. S. et al. Prenatal Pesticide and PCB Exposures and Birth Outcomes. Pediatr Res 61, 243–250 (2007). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Adverse Associations of both Prenatal and Postnatal Exposure to Organophosphorous Pesticides with Infant Neurodevelopment in an Agricultural Area of Jiangsu Province, China. Environ Health Perspect, doi: 10.1289/EHP196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N. et al. Paraoxonase Polymorphisms, Haplotypes, and Enzyme Activity in Latino Mothers and Newborns. Environmental Health Perspectives 114, 985–991, doi: 10.1289/ehp.8540 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A. et al. Maternal exposure to organochlorine pesticides in Western Australia. Science of The Total Environment 449, 208–213, doi: 10.1016/j.scitotenv.2013.01.067 (2013). [DOI] [PubMed] [Google Scholar]
- Stacey C. I. & Thomas B. W. Organochlorine pesticide residues in human milk, Western Australia–1970-71. Pesticides Monitoring Journal 9, 64–66 (1975). [PubMed] [Google Scholar]
- Stacey C., Stanley W. & Whitney S. Organochlorine pesticide residue levels in human milk: Western Australia, 1979–1980. Archives of Environmental Health 40, 102–108 (1985). [DOI] [PubMed] [Google Scholar]
- Stevens M. F., Ebell G. F. & Psaila-Savona P. Organochlorine pesticides in Western Australian nursing mothers. The Medical Journal of Australia 158, 238–241 (1993). [DOI] [PubMed] [Google Scholar]
- Mueller J. F., Harden F., Toms L.-M., Symons R. & Fürst P. Persistent organochlorine pesticides in human milk samples from Australia. Chemosphere 70, 712–720, doi: 10.1016/j.chemosphere.2007.06.037 (2008). [DOI] [PubMed] [Google Scholar]
- Anastassiades M., Lehotay S. J., Stajnbaher D. & Schenck F. J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86, 412–431 (2003). [PubMed] [Google Scholar]
- Du J. et al. Pesticides in human milk of Western Australian women and their influence on infant growth outcomes: A cross-sectional study. Chemosphere 167, 247–254, doi: 10.1016/j.chemosphere.2016.10.005 (2017). [DOI] [PubMed] [Google Scholar]
- Zhou P. et al. National survey of the levels of persistent organochlorine pesticides in the breast milk of mothers in China. Environmental Pollution 159, 524–531, doi: 10.1016/j.envpol.2010.10.014 (2011). [DOI] [PubMed] [Google Scholar]
- Daly Rosso, Owens & Hartmann. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Experimental Physiology 78, 741–755 (1993). [DOI] [PubMed] [Google Scholar]
- Robenek M. J. et al. Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis. The FASEB Journal 18, 866–868, doi: 10.1096/fj.03-0782fje (2004). [DOI] [PubMed] [Google Scholar]
- Farese R. V. Jr. & Walther T. C. Lipid Droplets Finally Get a Little R-E-S-P-E-C-T. Cell 139, 855–860, doi: 10.1016/j.cell.2009.11.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waliszewski M. S., Aguirre A. A., Infanzon M. R., Silva S. C. & Siliceo J. Organochlorine Pesticide Levels in Maternal Adipose Tissue, Maternal Blood Serum, Umbilical Blood Serum, and Milk from Inhabitants of Veracruz, Mexico. Archives of Environmental Contamination and Toxicology 40, 432–438, doi: 10.1007/s002440010194 (2001). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Musks and organochlorine pesticides in breast milk from Shanghai, China: Levels, temporal trends and exposure assessment. Ecotoxicology and Environmental Safety 84, 325–333, doi: 10.1016/j.ecoenv.2012.08.011 (2012). [DOI] [PubMed] [Google Scholar]
- Mishra K. & Sharma R. C. Assessment of organochlorine pesticides in human milk and risk exposure to infants from North-East India. Science of The Total Environment 409, 4939–4949, doi: 10.1016/j.scitotenv.2011.07.038 (2011). [DOI] [PubMed] [Google Scholar]
- Polder A., Skaare J. U., Skjerve E., Løken K. B. & Eggesbø M. Levels of chlorinated pesticides and polychlorinated biphenyls in Norwegian breast milk (2002–2006), and factors that may predict the level of contamination. Science of The Total Environment 407, 4584–4590, doi: 10.1016/j.scitotenv.2009.04.032 (2009). [DOI] [PubMed] [Google Scholar]
- Malarvannan G. et al. Organohalogen compounds in human breast milk from mothers living in Payatas and Malate, the Philippines: Levels, accumulation kinetics and infant health risk. Environmental Pollution 157, 1924–1932, doi: 10.1016/j.envpol.2009.01.010 (2009). [DOI] [PubMed] [Google Scholar]
- Devanathan G. et al. Persistent organochlorines in human breast milk from major metropolitan cities in India. Environmental Pollution 157, 148–154, doi: 10.1016/j.envpol.2008.07.011 (2009). [DOI] [PubMed] [Google Scholar]
- Bergkvist C. et al. Exposure to dioxin-like pollutants via different food commodities in Swedish children and young adults. Food and Chemical Toxicology 46, 3360–3367, doi: 10.1016/j.fct.2008.07.029 (2008). [DOI] [PubMed] [Google Scholar]
- Koppe J. G. Nutrition and breast-feeding. European Journal of Obstetrics & Gynecology 61, 73–78, doi: 10.1016/0028-2243(95)02156-M (1995). [DOI] [PubMed] [Google Scholar]
- Yu Z. et al. Comparison of organochlorine compound concentrations in colostrum and mature milk. Chemosphere 66, 1012–1018, doi: 10.1016/j.chemosphere.2006.07.043 (2007). [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N. et al. Breastfeeding and concentrations of HCB and p,p’-DDE at the age of 1 year. Environ. Res. 98, 8–13 (2005). [DOI] [PubMed] [Google Scholar]
- Stasinska A. et al. Polybrominated diphenyl ether (PBDE) concentrations in plasma of pregnant women from Western Australia. Science of The Total Environment 493, 554–561, doi: 10.1016/j.scitotenv.2014.06.001 (2014). [DOI] [PubMed] [Google Scholar]
- Kent J. C. et al. Longitudinal Changes in Breastfeeding Patterns from 1 to 6 Months of Lactation. Breastfeed. Med. 8, 401–407, doi: 10.1089/bfm.2012.0141 (2013). [DOI] [PubMed] [Google Scholar]
- Van Oostdam J. et al. Human health implications of environmental contaminants in Arctic Canada: a review. Science of The Total Environment 230, 1–82, doi: 10.1016/S0048-9697(99)00036-4 (1999). [DOI] [PubMed] [Google Scholar]
- Bedi J. S. et al. Pesticide residues in human breast milk: Risk assessment for infants from Punjab, India. Science of The Total Environment 463–464, 720–726, doi: 10.1016/j.scitotenv.2013.06.066 (2013). [DOI] [PubMed] [Google Scholar]
- Schecter A. et al. Decrease in milk and blood dioxin levels over two years in a mother nursing twins: estimates of decreased maternal and increased infant dioxin body burden from nursing. Chemosphere 32, 543–549 (1996). [DOI] [PubMed] [Google Scholar]
- Hooper K. et al. Depuration of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from California first-time mothers (primiparae). Environ Health Perspect 115, 1271–1275, doi: 10.1289/ehp.10166 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan W. J. et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health 76, 172–177 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A., Ryan J. J. & Papke O. Decrease in levels and body burden of dioxins, dibenzofurans, PCBS, DDE, and HCB in blood and milk in a mother nursing twins over a thirty-eight month period. Chemosphere 37, 1807–1816 (1998). [DOI] [PubMed] [Google Scholar]
- World Health Organization & UNICEF. Global strategy for infant and young child feeding. (WHO, 2003). [Google Scholar]
- Chao H.-R., Wang S.-L., Lin T.-C. & Chung X.-H. Levels of organochlorine pesticides in human milk from central Taiwan. Chemosphere 62, 1774–1785, doi: 10.1016/j.chemosphere.2005.07.036 (2006). [DOI] [PubMed] [Google Scholar]
- Minh N. H. et al. Persistent organochlorine residues in human breast milk from Hanoi and Hochiminh City, Vietnam: contamination, accumulation kinetics and risk assessment for infants. Environmental Pollution 129, 431–441, doi: 10.1016/j.envpol.2003.11.012 (2004). [DOI] [PubMed] [Google Scholar]
- Dirinck E. et al. Obesity and Persistent Organic Pollutants: Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity 19, 709–714, doi: 10.1038/oby.2010.133 (2011). [DOI] [PubMed] [Google Scholar]
- Wu K. et al. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from Guiyu, China. Environ Sci Technol 44, doi: 10.1021/es9024518 (2010). [DOI] [PubMed] [Google Scholar]
- Mazdai A., Dodder N. G., Abernathy M. P., Hites R. A. & Bigsby R. M. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect 111, doi: 10.1289/ehp.6146 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J. C., Mitoulas L., Cox D. B., Owens R. A. & Hartmann P. E. Breast volume and milk production during extended lactation in women. Exp Physiol 84, 435–447 (1999). [PubMed] [Google Scholar]
- Kent J. C. et al. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 117, e387–395, doi: 10.1542/peds.2005-1417 (2006). [DOI] [PubMed] [Google Scholar]
- RIVM. Re-Evaluationof Human-Toxicological Maximum Permissible Risk Levels. Report No. 711701025, 249-257 (National Institute of Public Health and the Environment, Bilthoven, The Netherlands, 2001).
- USEPA. Integrated Risk Information System (IRIS). DDT: Non-cancer assessment., https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=147 (1996).
- Noakes P. S., Taylor P., Wilkinson S. & Prescott S. L. The relationship between persistent organic pollutants in maternal and neonatal tissues and immune responses to allergens: A novel exploratory study. Chemosphere 63, 1304–1311, doi: 10.1016/j.chemosphere.2005.09.008 (2006). [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Addink R., Wong C., Arcaro K. & Kannan K. Polybrominated diphenyl ethers and organochlorine pesticides in human breast milk from Massachusetts, USA. Journal of Environmental Monitoring 9, 1205–1212, doi: 10.1039/B711409P (2007). [DOI] [PubMed] [Google Scholar]
- Sudaryanto A., Kunisue T., Tanabe S., Niida M. & Hashim H. Persistent Organochlorine Compounds in Human Breast Milk from Mothers Living in Penang and Kedah, Malaysia. Archives of Environmental Contamination and Toxicology 49, 429–437, doi: 10.1007/s00244-004-0208-8 (2005). [DOI] [PubMed] [Google Scholar]
- Breman J. G., Alilio M. S. & White N. J. Defining and Defeating the Intolerable Burden of Malaria III. Progress and Perspectives. The American Journal of Tropical Medicine and Hygiene 77, i–ii (2007). [Google Scholar]
- Darnerud P. O. et al. Levels of brominated flame retardants and other pesistent organic pollutants in breast milk samples from Limpopo province, South Africa. Science of The Total Environment 409, 4048–4053, doi: 10.1016/j.scitotenv.2011.05.054 (2011). [DOI] [PubMed] [Google Scholar]
- Gebremichael S., Birhanu T. & Tessema D. A. Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 90, 1652–1657, doi: 10.1016/j.chemosphere.2012.09.008 (2013). [DOI] [PubMed] [Google Scholar]
- Behrooz R. D., Sari A. E., Bahramifar N. & Ghasempouri S. M. Organochlorine pesticide and polychlorinated biphenyl residues in human milk from the Southern Coast of Caspian Sea, Iran. Chemosphere 74, 931–937, doi: 10.1016/j.chemosphere.2008.10.014 (2009). [DOI] [PubMed] [Google Scholar]
- Meier P. P. et al. Mother’s Milk Feedings in the Neonatal Intensive Care Unit: Accuracy of the Creamatocrit Technique. Journal of Perinatology 22, 646–649 (2002). [DOI] [PubMed] [Google Scholar]
- Lehotay S. J., Mastovská K. & Lightield A. R. Use of Buffering and Other Means to Improve Results of Problematic Pesticides in a Fast and Easy Method for Residue Analysis of Fruits and Vegetables. Journal of AOAC International 88, 615–629 (2005). [PubMed] [Google Scholar]
- Lehotay S. J., Mastovská K. & Yun S. J. Evaluation of Two Fast and Easy Methods for Pesticide Residue Analysis in Fatty Food Matrixes. Journal of AOAC International 88, 630–638 (2005). [PubMed] [Google Scholar]
- Lingwood B. E. et al. Prediction of fat-free mass and percentage of body fat in neonates using bioelectrical impedance analysis and anthropometric measures: validation against the PEA POD. British Journal of Nutrition 107, 1545–1552, doi: 10.1017/S0007114511004624 (2012). [DOI] [PubMed] [Google Scholar]
- Bocage C. Impedance mesurements of body composition in children. University of the West Indies (1988). [Google Scholar]
- Slaughter M. H. et al. Skinfold Equations for Estimation of Body Fatness in Children and Youth. Human Biology 60, 709–723 (1988). [PubMed] [Google Scholar]
- Arthur P. G., Hartmann P. E. & Smith M. Measurement of the milk intake of breast-fed infants. Journal of pediatric gastroenterology and nutrition 6, 758–763 (1987). [DOI] [PubMed] [Google Scholar]
- Kent J. C., Mitoulas L., Cox D. B., Owens R. A. & Hartmann P. E. Breast volume and milk production during extended lactation in women. Exp. Physiol. 84, 435–447, doi: doi:null (1999). [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S. & Sarkar D. nlme: Linear and Nonlinear Mixed Effects Models. R Package, Version 3.1-96 [computer program]. Vienna, Austria: R Foundation for Statistical Computing (2009). [Google Scholar]
- Hothorn T., Bretz F. & Westfall P. Simultaneous Inference in General Parametric Models. Biometrical Journal 50, 346–363, doi: 10.1002/bimj.200810425 (2008). [DOI] [PubMed] [Google Scholar]
- FAO/WHO. Joint FAO/WHO meeting on pesticide residue, 2000. (2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.