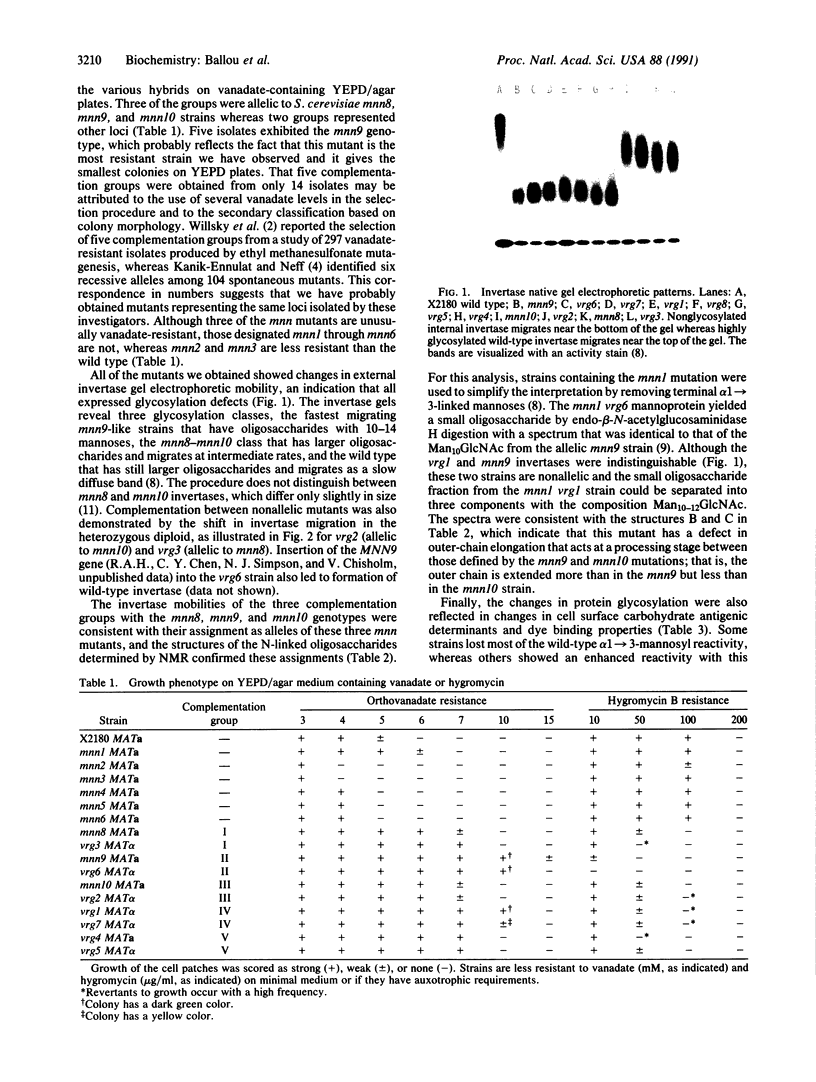
Abstract
Spontaneous recessive orthovanadate-resistant mutants of Saccharomyces cerevisiae were obtained in five complementation groups, and all show defects in protein glycosylation that mimic the previously isolated mnn mutants. Three of the groups are allelic to the known mnn8, mnn9, and mnn10 mutants, whereas the other two groups show other glycosylation defects. The vanadate-resistant phenotype was associated with enhanced hygromycin B sensitivity. The glycosylation phenotypes of the mutants are all reflections of defects in glycoprotein trafficking, and the easy isolation of vanadate-resistant or hygromycin B-sensitive mutants should facilitate the study of this process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado E., Ballou L., Hernandez L. M., Ballou C. E. Localization of alpha 1----3-linked mannoses in the N-linked oligosaccharides of Saccharomyces cerevisiae mnn mutants. Biochemistry. 1990 Mar 13;29(10):2471–2482. doi: 10.1021/bi00462a006. [DOI] [PubMed] [Google Scholar]
- Ballou C. E. A study of the immunochemistry of three yeast mannans. J Biol Chem. 1970 Mar 10;245(5):1197–1203. [PubMed] [Google Scholar]
- Ballou C. E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. doi: 10.1016/0076-6879(90)85038-p. [DOI] [PubMed] [Google Scholar]
- Ballou L., Alvarado E., Tsai P. K., Dell A., Ballou C. E. Protein glycosylation defects in the Saccharomyces cerevisiae mnn7 mutant class. Support for the stop signal proposed for regulation of outer chain elongation. J Biol Chem. 1989 Jul 15;264(20):11857–11864. doi: 10.1016/S0021-9258(18)80145-4. [DOI] [PubMed] [Google Scholar]
- Ballou L., Cohen R. E., Ballou C. E. Saccharomyces cerevisiae mutants that make mannoproteins with a truncated carbohydrate outer chain. J Biol Chem. 1980 Jun 25;255(12):5986–5991. [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Esmon B., Esmon P. C., Schekman R. Early steps in processing of yeast glycoproteins. J Biol Chem. 1984 Aug 25;259(16):10322–10327. [PubMed] [Google Scholar]
- González A., Jiménez A., Vázquez D., Davies J. E., Schindler D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim Biophys Acta. 1978 Dec 21;521(2):459–469. doi: 10.1016/0005-2787(78)90287-3. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Lewis M. J., Semenza J., Dean N., Pelham H. R. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 1990 Mar;9(3):623–630. doi: 10.1002/j.1460-2075.1990.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. M., Ballou L., Ballou C. E. Separation of yeast asparagine-linked oligosaccharides by high-performance anion-exchange chromatography. Carbohydr Res. 1990 Aug 1;203(1):1–11. doi: 10.1016/0008-6215(90)80040-a. [DOI] [PubMed] [Google Scholar]
- Kanik-Ennulat C., Neff N. Vanadate-resistant mutants of Saccharomyces cerevisiae show alterations in protein phosphorylation and growth control. Mol Cell Biol. 1990 Mar;10(3):898–909. doi: 10.1128/mcb.10.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Perlin D. S., Haber J. E. Pleiotropic plasma membrane ATPase mutations of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):4082–4088. doi: 10.1128/mcb.7.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi L. A., Chang A., Willsky G. R. Plasma membrane-stimulated vanadate-dependent NADH oxidation is not the primary mediator of vanadate toxicity in Saccharomyces cerevisiae. J Biol Chem. 1990 Sep 5;265(25):14907–14910. [PubMed] [Google Scholar]
- Penningroth S. M. Erythro-9-[3-(2-hydroxynonyl)]adenine and vanadate as probes for microtubule-based cytoskeletal mechanochemistry. Methods Enzymol. 1986;134:477–487. doi: 10.1016/0076-6879(86)34114-4. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Brown C. L., Haber J. E. Membrane potential defect in hygromycin B-resistant pma1 mutants of Saccharomyces cerevisiae. J Biol Chem. 1988 Dec 5;263(34):18118–18122. [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Leung J. O., Offermann P. V., Jr, Plotnick E. K., Dosch S. F. Isolation and characterization of vanadate-resistant mutants of Saccharomyces cerevisiae. J Bacteriol. 1985 Nov;164(2):611–617. doi: 10.1128/jb.164.2.611-617.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., White D. A., McCabe B. C. Metabolism of added orthovanadate to vanadyl and high-molecular-weight vanadates by Saccharomyces cerevisiae. J Biol Chem. 1984 Nov 10;259(21):13273–13281. [PubMed] [Google Scholar]