Abstract
Myocardial fuel selection is a key feature of the health and function of the heart, with clear links between myocardial function and fuel selection and important impacts of fuel selection on ischemia tolerance. Radiopharmaceuticals provide uniquely valuable tools for in vivo, non-invasive assessment of these aspects of cardiac function and metabolism. Here we review the landscape of imaging probes developed to provide noninvasive assessment of myocardial fatty acid oxidation (MFAO). Also, we review the state of current knowledge that myocardial fatty acid imaging has helped establish of static and dynamic fuel selection that characterizes cardiac and cardiometabolic disease and the interplay between fuel selection and various aspects of cardiac function.
Introduction
Significance of MFAO in health and disease
Although the heart is a metabolic omnivore, fatty acids are the dominant myocardial fuel under usual circumstances in health and disease [1]. The balance between fuel types can be shifted as an externally imposed change that affects myocardial fuel selection, or as intrinsic changes that are the result of myocardial disease.
Primary sites of regulation of fuel selection and MFAO include transmembrane transport, oxidative and non-oxidative fatty acid metabolic processes, levels of regulatory intermediates, and external regulators like insulin or catecholamines. The importance of these sites as targets of regulation or sites of disease-related imbalance is beginning to be explored.
Fuel selection impacts oxidative efficiency, i.e. efficiency of energy generation per unit O2 used. This also impacts work efficiency (work produced per unit O2 used) but the importance of shifts in oxidative efficiency on MFAO, contractile function or other outcomes of importance has not yet been well explored.
Significance of MFAO imaging
Measurement of fuel selection in the heart is challenging. Ex vivo experiments on isolated hearts are extremely useful but incompletely informative, and ultimately measurements in vivo in circumstances of health and disease are needed. The traditional methods of ‘organ balance’ measurements of fuel metabolism require measurements of rates and amounts of fuel delivery and uptake, using invasive tools to make samples and measurements of analyte blood concentrations. Imaging tools provide a major advantage in animal and human studies, because a set of in vivo measurements can be made with only modest needs for blood sampling to assess metabolite concentrations. Particularly for evaluations of myocardial metabolism, tracer-based methods have been advanced that provide arterial measurements of imaging tracers, obviating the need for peripheral arterial sampling. Together with progressive advances in the design and production of radiolabeled fatty acid probes, and in the modeling approaches to extracting relevant kinetic parameters from the time-activity curves, imaging studies can provide accessible, accurate and quantitative measurements of MFAO safely and noninvasively. These tools have already provided major advances in our understanding of myocardial fatty acid metabolism, and of fuel metabolism more generally, in health and disease. In the following sections we will review the state of the art in radiopharmaceutical tracers that allow non-invasive measurement of MFAO, followed by a review of the knowledge that these approaches have provided for us in realms of human health and disease.
Radiopharmaceuticals for MFAO Imaging
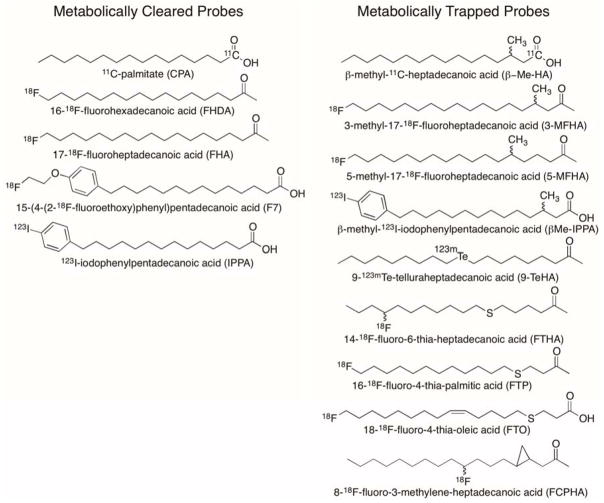
Metabolically Cleared MFAO Probes
The central role of fatty acids in energy provision to the myocardium motivated efforts to develop radiolabeled long-chain fatty acids (LCFAs) that could be imaged by PET or SPECT. As early as 1976, the synthesis of 1-11C-palmitate (CPA, T1/2 = 20 min, Figure 1) had been achieved and this radiotracer was evaluated in isolated perfused rabbit hearts and in living dogs[2]. CPA has been used extensively in cardiovascular PET research studies to monitor changes in palmitate uptake and metabolism in response to physiologic conditions and pathologies ([3–6]). Compartmental modeling of myocardial time-activity curves allows estimation of CPA uptake, esterification and oxidation [7]. However, the modeling technique has not been validated in conditions of myocardial ischemia, where enhanced backdiffusion of unoxidized CPA is confounded with metabolic clearance of β-oxidized CPA. The utility of CPA for indication of MFAO is therefore limited to conditions that exclude myocardial ischemia. Fluorine-18 labeled LCFA analogs were developed to take advantage of the longer isotopic half-life of 18F (T1/2 = 109.8 min) for radiotracer distribution and more practical clinical PET imaging logistics [8]. In mice, the odd-chain length LCFA analog, 17-18F-fluoroheptadecanoic acid (FHA, Figure 1) was found to have rapid uptake in heart similar to CPA with biphasic clearance from the myocardium. The even-chain LCFA analog, 16-18F-fluorohexadecanoic acid (FHDA, Figure 1) also showed similar biphasic clearance from the heart, but with different clearance rates, and different labeled metabolites in the heart as predicted by their different end-products of β-oxidation. Bone uptake was highest for FHA, consistent with end-stage metabolic defluorination of the putative radiolabeled metabolite, 3-18F-fluoropropionyl-CoA. In the case of FHDA, the primary putative metabolite is 2-18F-fluoroacetyl-CoA, which may undergo a variety of metabolic transformations, including defluorination. The complex metabolic handling of the 18F-labeled LCFA analogs, in addition to their in vivo defluorination, complicates the development of quantitative modeling strategies. To provide a more metabolically stable 18F-labeled LCFA analog, Tu et al. [9] recently synthesized 15-(4-(2-18F-fluoroethoxy)phenyl)pentadecanoic acid (F7, Figure 1) that showed dramatically reduced in vivo defluorination. F7 showed robust uptake in rat myocardium and a biphasic clearance pattern. Quantitative data analysis for estimation of myocardial fatty acid metabolic fluxes has yet to be shown with F7.
Figure 1.
Long-chain fatty acid metabolic probes.
The radioiodinated LCFA analog, 123I-iodophenylpentadecanoic acid (IPPA, Figure 1), was developed for SPECT imaging applications [10]. In myocytes, radioiodinated IPPA is esterified to form labeled complex lipids and metabolized by β-oxidation to the predominant metabolite, iodobenzoic acid and other short chain oxidation end-products [11, 12]. In a canine model of regional low-flow ischemia, initial uptake of IPPA was lower in ischemic regions relative to non-ischemic regions, however myocardial clearance rate was significantly slower in ischemic regions leading to relatively increased retention of radioactivity in ischemic myocardium at later intervals [13]. The longer acquisition periods required for SPECT imaging limits the utility of IPPA for determining clearance kinetics from the human myocardium. Compartmental modeling was applied to the kinetics of IPPA in isolated rat heart [14], but application of modeling strategies in humans has been limited by the complex metabolic fate of the tracer.
Metabolically Trapped MFAO Probes
To simplify the myocardial kinetics of radiolabel LCFAs, structural modifications were investigated to impede oxidation or esterification. The 3-methyl branched chain analog, β-methyl-1-11C-heptadecanoic acid (β-Me-HA, Figure 1) was developed to inhibit β-oxidation [15]. In PET imaging studies, β-Me-HA showed prolonged retention in normal and infarcted dog myocardium. Quantitative autoradiographic imaging of rats administered β-methyl-1-14C-heptadecanoic acid showed the highest heart concentration at 60 min post-injection, with myocardial concentration diminishing to 0.4% injected dose/g at 24 h [16]. Terminally 18F-labeled branched chain LCFA analogs have also been pursued. 3-Methyl-17-18F-fluoroheptadecanoic acid (3MFHA, Figure 1) and 5-methyl-17-18F-fluoroheptadecanoic acid (5MFHA, Figure 1) showed somewhat lower initial uptake than unbranched FHDA in rat heart [17]. Clearance rate from the heart was slowest for 3MFHA, while clearance of 5FMHA was similar to the unbranched analog FHDA. Metabolic defluorination was evident for both 3MFHA and 5MFHA. Further work is required to understand the differences in metabolic handling of 3MFHA and 5MFHA and the potential for quantitative modeling of their myocardial kinetics using PET. For SPECT imaging, the 123I-labeled β-methyl substituted probe β-methyl-15-123I-iodophenylpentadecanoic acid (β-MeIPPA, Figure 1) has been used extensively in animals and humans [18–21]. Metabolic studies have elucidated that BMIPP is a substrate for α-oxidation followed by β-oxidation in the myocardium [22–24]. However, since BMIPP is not accepted for mitochondrial transport by the CPT-1 dependent shuttle system [25], its early retention in myocardium reflects activation and esterification to complex lipids with slow turnover related to α-oxidation rate. Its utility as an MFAO probe is therefore very limited.
LCFA imaging probes modified by heteroatom substitution with stable or radioactive tellurium isotopes were investigated by Knapp and colleagues [26–28]. The LCFA analog 9-123mTe-telluraheptadecanoic acid (9-TeHA, Figure 1) was shown to have prolonged retention in rat and dog myocardium [26]. Although the metabolism of these analogs was not fully elucidated, their slow myocardial clearance was presumed to reflect incomplete β-oxidation caused by the tellurium heteroatom. Heteroatom substitution with sulfur was subsequently pursued by DeGrado and colleagues as a more physiologically acceptable substitution in LCFA probes [29]. Indeed, the 6-thia LCFA analog, 14-18F-fluoro-6-thia-heptadecanoic acid (FTHA, Figure 1) was shown to exhibit >100 heart:blood concentration ratio with prolonged myocardial retention. 18F-labeling at the ω-3 position was developed to minimize in vivo defluorination in rodent models, but subsequent studies in pigs [30] and humans [31] showed that terminally 18F-labeled thia fatty acids do not exhibit appreciable metabolic defluorination in these higher mammals. Inhibition of MFAO with a CPT-1 inhibitor caused an 81% reduction in murine heart uptake of FTHA, indicating specificity of uptake for imaging of MFAO [29]. Indeed, very low incorporation of 18F into complex lipids showed FTHA to have a low esterification rate. The 18F-radiolabel was found to bind to mitochondrial protein in the myocardium, presumably through a long-chain thiol β-oxidative metabolite [29]. PET studies in healthy human volunteers showed the myocardial trapping rate of FTHA to be increased with exercise but unchanged with elevated blood flow induced by dipyridamole [32]. However, a later study with FTHA in hypoxic canine myocardium showed retention in hypoxic myocardium independent of β-oxidation rate [33]. It was subsequently shown that sulfur substitution at the 4th carbon enhanced specificity of thia fatty acid analog probes for indication of MFAO [30]. Myocardial uptake of the palmitate analog, 16-18F-fluoro-4-thia-hexadecanoic acid (FTP, Figure 1), was shown to track β-oxidation rates in normal and hypoxic perfused rat heart [30]. Further quantitative validation studies for FTP were performed in isolated perfused rat heart to define the relationship of MFAO (measured using tritiated palmitate) to FTP trapping rate in myocardium under diverse conditions [34]. The concept of a “lumped constant” (LC) for FTP was invoked, as analogous to the lumped constant utilized for quantitation of glucose phosphorylation using 18F-FDG. Recently, DeGrado et al. [35, 36] have described an oleate analog of (FTO, Figure 1) which shows high specificity for MFAO imaging and enhanced myocardial retention relative to FTP in rat myocardium. Since oleate is the most prevalent LCFA in the blood, and is highly utilized as an energy-providing fatty acid [37], the oleate imaging analog, FTO, may provide higher sensitivity and specificity for MFAO imaging than palmitate analogs [36].
Incorporation of a cyclopropyl group is another structural modification employed to inhibit oxidation of LCFA imaging probes in heart. Shoup et al. [38] have developed trans-9(RS)-18F-fluoro-3,4(RS,RS)-methyleneheptadecanoic acid (18F-FCPHA, Figure 1) with the cyclopropyl group encompassing carbons 3 and 4. 18F-FCPHA showed high uptake and prolonged retention in rat heart. The metabolism of 18F-FCPHA and its specificity for imaging of MFAO have yet to be clarified.
MFAO imaging in ischemic heart disease
The application of fatty acid oxidation imaging to myocardial ischemia has been in two overall areas of interest. First is the pathophysiologic question of the shifts in fuel selection in ischemic myocardium. Second is the more clinically approachable application of using fatty acid imaging to identify and quantify regions of ischemia. We will further explore each of these topics.
The heart does not maintain a significant depot of stored fuel substrate, and in the absence of ongoing supply of fuel and oxygen myocardial cells are able to sustain metabolism for only a duration of minutes. In the region of the myocardium subjected to ischemia, impaired fuel availability and hypoxia necessarily produce local shifts in fuel selection. These shifts, and accompanying changes in blood flow rates and distribution, have been studied in part using tracer-based methodologies, including SPECT and PET imaging [39–44]. Most such studies have used radiolabeled glucose (to quantify glucose uptake) and radiolabeled acetate (to quantify blood flow or perfusion), without concurrent imaging of fatty acid kinetics. Fuel selection is reciprocal between glucose and fatty acids, and the studies that have used fatty acid tracers overall have provided complementary findings compared to those measuring glucose. Specifically, ischemic myocardium metabolizes glucose preferentially, with reduced MFAO [45–47], with some sensitivity of the magnitude of the observed response to the specific fatty acid tracers used [47]. Post-ischemic changes in metabolism in the affected zone have also been described [48], with recovery of fatty acid metabolism directly associated with recovery of perfusion. Some studies of anti-ischemia approaches have used fatty acid imaging to evaluate effectiveness of treatment as well as the specific effects on myocardial fuel selection [40, 49]. These observations demonstrate the utility of fatty acid kinetic assessment using radionuclide imaging to elucidate the pathophysiology of ischemia, and increasingly to explore the mechanisms of benefit of novel anti-ischemic treatment approaches.
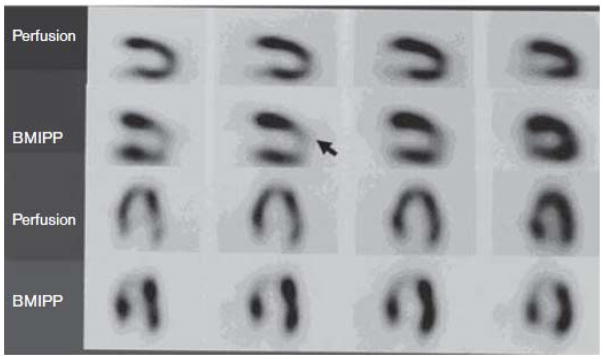
PET imaging in ischemia finds clinical utility in the estimation of infarct size, and more specifically in distinguishing viable from non-viable regions in the infarcted zone [34, 41, 42, 50–62]. PET measures of blood flow in ischemia can provide prognostic information [58, 63], and some have even argued that a PET-derived metabolic definition of infarct is superior to other imaging approaches [64]. Tracers of fatty acid uptake are similarly informative in these applications [65–70], and in some instances have been found to provide superior diagnostic and prognostic information [65, 67, 71] (Figure 2) and insights into the metabolic physiology underlying metabolic adaptation to ischemia and recovery in reperfused tissue [70, 72].
Figure 2.
SPECT imaging evaluating viable myocardium using a perfusion probe (99Tc-Tetrofosmin) and an MFAO probe (BMIPP). The arrow indicates the location of a severe stenosis of the left anterior descending coronary artery. Perfusion imaging was performed at the time of hospital admission, and BMIPP imaging was performed the following day. The authors conclude that the fatty acid uptake probe provides superior sensitivity for detecting the injured zone, and suggested that the metabolic probe could be used to assess a metabolic memory of the injury. From [122]
Despite the widespread clinical availability of PET methodology, particularly that using 18F-fluorodeoxyglucose (FDG), to date PET has not found widespread application in clinical cardiology. Nevertheless, the value of PET-based measurements of glucose or fatty acid kinetics in providing non-invasive assessment of physiology has been clearly demonstrated, and tracers of fatty acid metabolism can provide unique information about the metabolic shifts accompanying various degrees and stages of ischemic injury.
MFAO imaging in heart failure
Myocardial fuel selection is abnormal in heart failure. Imaging of MFAO has contributed to our understanding of these phenomena, and their contribution to progression or recovery of disease. In parallel, fatty acid imaging has been applied in studies of treatments targeting cardiac dysfunction including those targeting myocardial metabolism directly. These therapeutic studies have also contributed to our understanding of the contributions of metabolic dysfunction to the pathogenesis of heart failure.
In heart failure, there is abnormal fuel metabolism overall, with variably reported shifts toward glucose with reduced fatty acid uptake, or away from glucose with increased fatty acid uptake. The majority of reports include increased fatty acid uptake, whether the underlying problem is ischemia [73], a non-ischemic cardiomyopathy [74–77], or a diabetic cardiomyopathy [78, 79], but others report reduced fatty acid uptake in idiopathic cardiomyopathy [80]. It is unclear whether these various conditions produce altered fatty acid utilization via the same mechanisms, how increased fatty acid transport relates to the impairment of function, and what the developmental time course of metabolic and mechanical dysfunction, and the inter-relationships of these changes, might be. Specifically, it is possible that changes in fuel selection result directly from compensatory changes due to impaired function (which may be a shared phenomenon driving metabolic shifts across various etiologies of dysfunction). It is also possible that the metabolic changes are in response to the whole-body response to impaired cardiac function or tissue perfusion (mediated for example by myokines, cytokines, or neurologically-driven changes). Mechanisms for these potential effects have not been systematically investigated. It is known that in health the myocardium responds to acute increases in fat exposure with an acute reduction in mechanical function [81], but conversely acute reduction in myocardial fat in cardiomyopathy is also associated with impaired function [82]. An increase in fatty acid uptake directly in response to impairment of mechanical function has not been demonstrated with imaging methods, but such changes are seen with experimentally imposed pressure overload [83, 84]. The underlying abnormalities include alterations in regulatory metabolic intermediates such as malonyl-CoA, and adverse changes in mitochondrial content and function [85–87].
There have been a small number of studies using fatty acid imaging as a measurement endpoint in clinical studies of heart failure, in particular in studies of metabolic modulators targeting abnormal fatty acid metabolism rates [77, 88–91]. Overall these show the anticipated restoration of fatty acid uptake and oxidation toward normal, in association with an improvement in function. These observations confirm that the metabolic and functional aberrations are strongly interconnected, and demonstrate that the metabolic abnormalities are not a fixed feature of the dysfunctional hearts.
An interesting and relatively recent observation made using myocardial fatty acid imaging studies is that there is a sex difference in the rates of myocardial fatty acid uptake, and oxidation with higher rates observed in women under normal physiologic conditions and with dysfunction [92–97]. These differences in turn relate to sex differences in metabolic efficiency in the heart and sex-related differences in the relationships among metabolism, efficiency, and mechanical function [92, 93, 98], and in responses to treatment [95]. This set of observations may provide novel insights into the longstanding unexplained sex difference in cardiac disease [99]. The molecular phenomena underlying these sex-specific changes in fuel selection, and the clinical implications of these recent observations, are only beginning to be explored.
MFAO imaging in obesity and diabetes
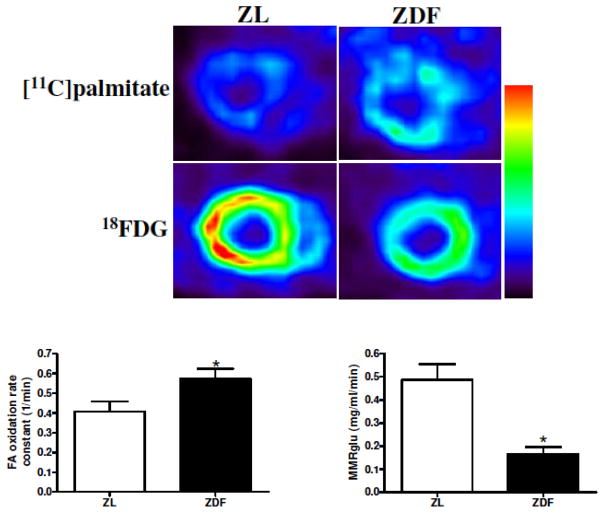
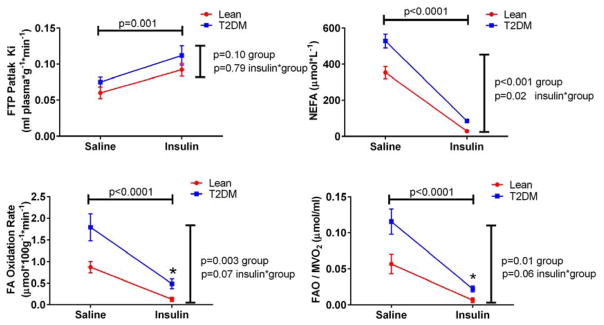
Abnormal fuel selection is a feature of skeletal muscle in obesity in type 2 diabetes [100], generally in the setting of resistance to the actions of insulin to drive glucose uptake and utilization (i.e. tissue insulin resistance). Parallel phenomena are at play in the heart, where there is now evidence that abnormally increased uptake of fatty acids, beyond the baseline preference for fatty acid, contributes to metabolic abnormalities in the heart in obesity and diabetes [78, 98, 100–112] (Figure 3). Many of these observations have been uniquely made possible by the availability of non-invasive quantitative assessments of fuel flux using radiolabeled glucose [95, 103, 113–116] and fatty acid tracers [78, 92, 95, 98, 103, 105, 109, 114, 117–121], showing augmented fatty acid uptake and utilization under fasting conditions, and importantly impaired capacity to switch among fuel sources (i.e. metabolic inflexibility)[31, 105, 113, 115, 122] (Figure 4).
Figure 3.
Increased fatty acid uptake and oxidation, and lower glucose uptake, in obese fat-fed Zucker rats (ZDF) compared to age-matched lean Zucker rats (ZL). Upper panel, color-scale matched short-axis images of the myocardium demonstrating qualitatively increased fatty acid uptake and reduced glucose uptake in obesity. Lower panel, kinetic quantification of data from the time-activity curves. From [79]
Figure 4.
Augmented fatty acid uptake and oxidation, reduced metabolic efficiency, and impaired insulin-induced fuel switching, in human Type 2 diabetes compared to age-matched lean controls. Fatty acid kinetics were measured using FTP, 18-18F-fluoro-4-thia-oleic acid. NEFA, circulating non-esterified fatty acid; FAO, fatty acid oxidation rate; MVO2, oxygen consumption rate (estimated from acetate kinetics assessed by PET). Insulin induced major shifts in all of these parameters; this effect of insulin was statistically different between the two groups only for the suppression of NEFA, but the steady state values achieved under insulin stimulation differed for both FAO and FAO/MVO2. From [31]
Although Type 1 diabetes (an insulin sensitive, insulin deficient state) and Type 2 diabetes (an insulin resistant, hyperinsulinemic state) are pathophysiologically distinct and produce different clinical heart disease risk patterns, they are both typified by abnormal increases in myocardial fatty acid uptake [104, 110, 113, 123]. This likely relates to the shared underlying myocardial preference for fatty acids, and the shared phenomenon of abnormal control of adipose lipolysis resulting in augmented fatty acid availability.
Interestingly, in the intermediate insulin resistant state of impaired glucose tolerance abnormally increased fatty acid uptake has been less consistently seen [100, 117, 118, 124], raising the possibility that effects of elevated fatty acid delivery may be sensitive to the accompanying glycemic state. However, recent observations that in obesity weight loss-associated reductions in fatty acid availability are associated with reductions in myocardial fatty acid uptake and improvements in cardiac function [117, 125] argue in favor of an adverse effect of augmented myocardial fatty acid uptake even in the non-diabetic state.
Imaging evaluation of myocardial fatty acid and glucose kinetics have been used to explore the effects of metabolically targeted therapies that alter systemic fatty acid metabolism, alter myocardial fatty acid uptake, or alter the metabolic fate of fatty acids [77, 104, 105, 113, 115, 126–128]. These disparate approaches have converged on a unified and convincing set of observations causally linking increased fatty acid delivery, uptake and MFAO to metabolic dysfunction in the heart.
The factors that drive diabetes and obesity-associated increases in myocardial fatty acid uptake and utilization, and the impaired capacity to switch among fuel sources remain incompletely understood. Also requiring further exploration are the mechanistic connection between abnormalities of fuel selection and abnormalities of myocardial function, and the previously mentioned sex differences in myocardial fatty acid utilization. Further studies applying myocardial fatty acid imaging will be needed to explore and better understand these phenomena.
Conclusions
Quantitiative imaging of myocardial fatty acid uptake and oxidation provides a uniquely valuable set of tools for clinical and research applications. The optimal fatty acid probe has not yet been defined, and work is ongoing attempting to optimize these probes by designing probes with specific metabolic fates or mitochondrial targeting, for example. The application of the probes available to date has defined abnormalities in myocardial fuel selection as a key feature of many cardiac and cardiometabolic diseases, with a small set of studies demonstrating that metabolically targeted therapies can produce improvements in myocardial function or ischemia tolerance. Future possibilities include more widespread application of MFAO imaging as a measure of cardiac injury with ischemia, with improved prognostic capabilities, and application as a research tool to explore in more detail the mechanisms and treatments of myocardial disease in obesity and diabetes.
Highlights.
Myocardial fatty acid oxidation (MFAO) imaging allows in vivo assessment of cardiac fuel utilization.
Fatty acid tracers and analysis models have been developed that allow detailed assessments of fuel kinetics in the heart.
MFAO imaging has identified abnormalities of fuel selection in cardiac disease, and in cardiometabolic disease.
MFAO imaging can be used clinically to identify regions of ischemia, and can be used in clinical trials to follow effects of therapies.
Acknowledgments
Support for research endeavors from our group presented here was provided by National Institutes of Health project grants DK071142, M01-RR00750 and TR000006.
Contributor Information
Kieren J Mather, Indiana University School of Medicine, Indianapolis IN.
Tim DeGrado, Mayo Clinic, Rochester MN.
References
- 1.Abel ED. Glucose transport in the heart. Frontiers in bioscience : a journal and virtual library. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ES, Hoffman EJ, Phelps ME, Welch MJ, Henry PD, Ter-Pogossian MM, Sobel BE. External detection and visualization of myocardial ischemia with 11C-substrates in vitro and in vivo. Circulation research. 1976;39:24–32. doi: 10.1161/01.res.39.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Lerch RA, Ambos HD, Bergmann SR, Welch MJ, Ter-Pogossian MM, Sobel BE. Localization of viable, ischemic myocardium by positron-emission tomography with 11C-palmitate. Circulation. 1981;64:689–699. doi: 10.1161/01.cir.64.4.689. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann SR. Use and limitations of metabolic tracers labeled with positron-emitting radionuclides in the identification of viable myocardium. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1994;35:15S–22S. [PubMed] [Google Scholar]
- 5.Schelbert HR, Henze E, Keen R, Schon HR, Hansen H, Selin C, Huang SC, Barrio JR, Phelps ME. C-11 palmitate for the noninvasive evaluation of regional myocardial fatty acid metabolism with positron-computed tomography. IV. In vivo evaluation of acute demand-induced ischemia in dogs. American heart journal. 1983;106:736–750. doi: 10.1016/0002-8703(83)90096-0. [DOI] [PubMed] [Google Scholar]
- 6.Rijzewijk LJ, Jonker JT, van der Meer RW, Lubberink M, de Jong HW, Romijn JA, Bax JJ, de Roos A, Heine RJ, Twisk JW, Windhorst AD, Lammertsma AA, Smit JW, Diamant M, Lamb HJ. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. Journal of the American College of Cardiology. 2010;56:225–233. doi: 10.1016/j.jacc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 8.Knust EJ, Kupfernagel C, Stocklin G. Long-chain F-18 fatty acids for the study of regional metabolism in heart and liver; odd-even effects of metabolism in mice. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1979;20:1170–1175. [PubMed] [Google Scholar]
- 9.Tu Z, Li S, Sharp TL, Herrero P, Dence CS, Gropler RJ, Mach RH. Synthesis and evaluation of 15-(4-(2-[(1)(8)F]Fluoroethoxy)phenyl)pentadecanoic acid: a potential PET tracer for studying myocardial fatty acid metabolism. Bioconjugate chemistry. 2010;21:2313–2319. doi: 10.1021/bc100343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reske SN, Sauer W, Machulla HJ, Winkler C. 15(p-[123I]Iodophenyl)pentadecanoic acid as tracer of lipid metabolism: comparison with [1-14C]palmitic acid in murine tissues. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1984;25:1335–1342. [PubMed] [Google Scholar]
- 11.Reske SN. Experimental and clinical experience with iodine 123-labeled iodophenylpentadecanoic acid in cardiology. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 1994;1:S58–64. doi: 10.1007/BF02940070. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhut M, Lehmann WD, Sutterle A. Metabolism of 15-(4′-[123I]iodophenyl)pentadecanoic acid ([123I]IPPA) in the rat heart; identification of new metabolites by high pressure liquid chromatography and fast atom bombardment-mass spectrometry. Nuclear medicine and biology. 1993;20:747–754. doi: 10.1016/0969-8051(93)90161-m. [DOI] [PubMed] [Google Scholar]
- 13.Shi CQ, Young LH, Daher E, DiBella EV, Liu YH, Heller EN, Zoghbi S, Wackers FJ, Soufer R, Sinusas AJ. Correlation of myocardial p-(123)I-iodophenylpentadecanoic acid retention with (18)F-FDG accumulation during experimental low-flow ischemia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2002;43:421–431. [PubMed] [Google Scholar]
- 14.DeGrado TR, Holden JE, Ng CK, Raffel DM, Gatley SJ. Quantitative analysis of myocardial kinetics of 15-p-[iodine-125] iodophenylpentadecanoic acid. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1989;30:1211–1218. [PubMed] [Google Scholar]
- 15.Livni E, Elmaleh DR, Levy S, Brownell GL, Strauss WH. Beta-methyl [1-11C]heptadecanoic acid: a new myocardial metabolic tracer for positron emission tomography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1982;23:169–175. [PubMed] [Google Scholar]
- 16.Elmaleh DR, Livni E, Levy S, Varnum D, Strauss HW, Brownell GL. Comparison of 11C and 14C-labeled fatty acids and their beta-methyl analogs. International journal of nuclear medicine and biology. 1983;10:181–187. doi: 10.1016/0047-0740(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Nishimura S, Ido T, Ishiwata K, Iwata R. Biological evaluation of 5-methyl-branched-chain omega-[18F]fluorofatty acid: a potential myocardial imaging tracer for positron emission tomography. Nuclear medicine and biology. 1996;23:303–308. doi: 10.1016/0969-8051(95)02084-5. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MM, Kirsch G, Knapp FF., Jr Synthesis and evaluation of radioiodinated terminal p-iodophenyl-substituted alpha- and beta-methyl-branched fatty acids. Journal of medicinal chemistry. 1984;27:390–397. doi: 10.1021/jm00369a027. [DOI] [PubMed] [Google Scholar]
- 19.Knapp FF, Jr, Kropp J, Franken PR, Visser FC, Sloof GW, Eisenhut M, Yamamichi Y, Shirakami Y, Kusuoka H, Nishimura T. Pharmacokinetics of radioiodinated fatty acid myocardial imaging agents in animal models and human studies. The quarterly journal of nuclear medicine : official publication of the Italian Association of Nuclear Medicine. 1996;40:252–269. [PubMed] [Google Scholar]
- 20.Takeishi Y, Minamihaba O, Yamauchi S, Arimoto T, Hirono O, Takahashi H, Akiyama H, Miyamoto T, Nitobe J, Nozaki N, Tachibana H, Okuyama M, Fukui A, Kubota I, Okada A, Takahashi K. Dynamic 123I-BMIPP single-photon emission computed tomography in patients with congestive heart failure: effect of angiotensin II type-1 receptor blockade. Clinical cardiology. 2004;27:204–210. doi: 10.1002/clc.4960270406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakao S, Miyauchi H, Voelkel NF, Sugiura T, Tanabe N, Kobayashi Y, Tatsumi K. Increased Right Ventricular Fatty Acid Accumulation in Chronic Thromboembolic Pulmonary Hypertension. Annals of the American Thoracic Society. 2015;12:1465–1472. doi: 10.1513/AnnalsATS.201504-236LE. [DOI] [PubMed] [Google Scholar]
- 22.Hosokawa R, Nohara R, Fujibayashi Y, Okuda K, Ogino M, Hirai T, Fujita M, Tamaki N, Konishi J, Sasayama S. Myocardial metabolism of 123I-BMIPP in a canine model with ischemia: implications of perfusion-metabolism mismatch on SPECT images in patients with ischemic heart disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1999;40:471–478. [PubMed] [Google Scholar]
- 23.Hosokawa R, Nohara R, Fujibayashi Y, Hirai T, Fujita M, Magata Y, Tadamura E, Konishi J, Sasayama S. Myocardial metabolism of 123I-BMIPP during low-flow ischaemia in an experimental model: comparison with myocardial blood flow and 18F-FDG. European journal of nuclear medicine. 2001;28:1630–1639. doi: 10.1007/s002590100617. [DOI] [PubMed] [Google Scholar]
- 24.Hosokawa R, Nohara R, Hirai T, Fujibayashi Y, Fujita M, Kambara N, Ohba M, Tadamura E, Kimura T, Kita T. Myocardial metabolism of 123I-BMIPP under low-dose dobutamine infusion: implications for clinical SPECT imaging of ischemic heart disease. European journal of nuclear medicine and molecular imaging. 2005;32:75–83. doi: 10.1007/s00259-004-1622-x. [DOI] [PubMed] [Google Scholar]
- 25.DeGrado TR, Holden JE, Ng CK, Raffel DM, Gatley SJ. beta-Methyl-15-p-iodophenylpentadecanoic acid metabolism and kinetics in the isolated rat heart. European journal of nuclear medicine. 1989;15:78–80. doi: 10.1007/BF00702623. [DOI] [PubMed] [Google Scholar]
- 26.Knapp FF, Jr, Ambrose KR, Callahan AP, Ferren LA, Grigsby RA, Irgolic KJ. Effects of chain length and tellurium position on the myocardial uptake of Te-123m fatty acids. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1981;22:988–993. [PubMed] [Google Scholar]
- 27.Goodman MM, Knapp FF, Jr, Callahan AP, Ferren LA. Synthesis and biological evaluation of 17-[131I]iodo-9-telluraheptadecanoic acid, a potential imaging agent. Journal of medicinal chemistry. 1982;25:613–618. doi: 10.1021/jm00348a001. [DOI] [PubMed] [Google Scholar]
- 28.Knapp FF, Jr, Srivastava PC, Callahan AP, Cunningham EB, Kabalka GW, Sastry KA. Effect of tellurium position on the myocardial uptake of radioiodinated 18-iodotellura-17-octadecenoic acid analogues. Journal of medicinal chemistry. 1984;27:57–63. doi: 10.1021/jm00367a011. [DOI] [PubMed] [Google Scholar]
- 29.DeGrado TR, Coenen HH, Stocklin G. 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA): evaluation in mouse of a new probe of myocardial utilization of long chain fatty acids. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1991;32:1888–1896. [PubMed] [Google Scholar]
- 30.DeGrado TR, Wang S, Holden JE, Nickles RJ, Taylor M, Stone CK. Synthesis and preliminary evaluation of (18)F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nuclear medicine and biology. 2000;27:221–231. doi: 10.1016/s0969-8051(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 31.Mather KJ, Hutchins GD, Perry K, Territo W, Chisholm R, Acton A, Glick-Wilsom B, Considine RV, Moberly S, DeGrado TR. Assessment of myocardial metabolic flexibility and work efficiency in human Type 2 diabetes using 16-18F-fluoro-4-thiapalmitate, a novel PET fatty acid tracer. American journal of physiology. Endocrinology and metabolism. 2016 doi: 10.1152/ajpendo.00437.2015. ajpendo 00437 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebert A, Herzog H, Stocklin GL, Henrich MM, DeGrado TR, Coenen HH, Feinendegen LE. Kinetics of 14(R,S)-fluorine-18-fluoro-6-thia-heptadecanoic acid in normal human hearts at rest, during exercise and after dipyridamole injection. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1994;35:51–56. [PubMed] [Google Scholar]
- 33.Renstrom B, Rommelfanger S, Stone CK, DeGrado TR, Carlson KJ, Scarbrough E, Nickles RJ, Liedtke AJ, Holden JE. Comparison of fatty acid tracers FTHA and BMIPP during myocardial ischemia and hypoxia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39:1684–1689. [PubMed] [Google Scholar]
- 34.DeGrado TR, Kitapci MT, Wang S, Ying J, Lopaschuk GD. Validation of 18F-fluoro-4-thia-palmitate as a PET probe for myocardial fatty acid oxidation: effects of hypoxia and composition of exogenous fatty acids. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:173–181. [PubMed] [Google Scholar]
- 35.DeGrado TR, Bhattacharyya F, Pandey MK, Belanger AP, Wang S. Synthesis and preliminary evaluation of 18-(18)F-fluoro-4-thia-oleate as a PET probe of fatty acid oxidation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:1310–1317. doi: 10.2967/jnumed.109.074245. [DOI] [PubMed] [Google Scholar]
- 36.Pandey MK, Belanger AP, Wang S, DeGrado TR. Structure dependence of long-chain [18F]fluorothia fatty acids as myocardial fatty acid oxidation probes. Journal of medicinal chemistry. 2012;55:10674–10684. doi: 10.1021/jm301345v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. The American journal of clinical nutrition. 2000;72:905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 38.Shoup TM, Elmaleh DR, Bonab AA, Fischman AJ. Evaluation of trans-9-18F-fluoro-3,4-Methyleneheptadecanoic acid as a PET tracer for myocardial fatty acid imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:297–304. [PubMed] [Google Scholar]
- 39.Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis. 1989;32:217–238. doi: 10.1016/0033-0620(89)90027-3. [DOI] [PubMed] [Google Scholar]
- 40.Mody FV, Singh BN, Mohiuddin IH, Coyle KB, Buxton DB, Hansen HW, Sumida R, Schelbert HR. Trimetazidine-induced enhancement of myocardial glucose utilization in normal and ischemic myocardial tissue: an evaluation by positron emission tomography. The American journal of cardiology. 1998;82:42K–49K. doi: 10.1016/s0002-9149(98)00536-0. [DOI] [PubMed] [Google Scholar]
- 41.Kofoed KF, Schoder H, Knight RJ, Buxton DB. Glucose metabolism in reperfused myocardium measured by [2-18F] 2-fluorodeoxyglucose and PET. Cardiovascular research. 2000;45:321–329. doi: 10.1016/s0008-6363(99)00278-3. [DOI] [PubMed] [Google Scholar]
- 42.Knuuti J, Tuunanen H. Metabolic imaging in myocardial ischemia and heart failure. Q J Nucl Med Mol Imaging. 2010;54:168–176. [PubMed] [Google Scholar]
- 43.Heather LC, Pates KM, Atherton HJ, Cole MA, Ball DR, Evans RD, Glatz JF, Luiken JJ, Griffin JL, Clarke K. Differential translocation of the fatty acid transporter, FAT/CD36, and the glucose transporter, GLUT4, coordinates changes in cardiac substrate metabolism during ischemia and reperfusion. Circulation. Heart failure. 2013;6:1058–1066. doi: 10.1161/CIRCHEARTFAILURE.112.000342. [DOI] [PubMed] [Google Scholar]
- 44.Povlsen JA, Lofgren B, Dalgas C, Birkler RI, Johannsen M, Stottrup NB, Botker HE. Protection against myocardial ischemia-reperfusion injury at onset of type 2 diabetes in Zucker diabetic fatty rats is associated with altered glucose oxidation. PloS one. 2013;8:e64093. doi: 10.1371/journal.pone.0064093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelbert HR. Evaluation of “metabolic fingerprints” of myocardial ischemia. The Canadian journal of cardiology. 1986;(Suppl A):121A–130A. [PubMed] [Google Scholar]
- 46.Rosamond TL, Abendschein DR, Sobel BE, Bergmann SR, Fox KA. Metabolic fate of radiolabeled palmitate in ischemic canine myocardium: implications for positron emission tomography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1987;28:1322–1329. [PubMed] [Google Scholar]
- 47.Renstrom B, Rommelfanger S, Stone CK, DeGrado TR, Carlson KJ, Scarbrough E, Nickles RJ, Liedtke AJ, Holden JE. Comparison of fatty acid tracers FTHA and BMIPP during myocardial ischemia and hypoxia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39:1684–1689. [PubMed] [Google Scholar]
- 48.Miyabe H, Ohte N, Iida A, Narita H, Yoshida T, Kimura G. Evaluation of fatty acid beta-oxidation in patients with prior myocardial infarction in relation to myocardial blood flow, total oxidative metabolism, and left ventricular wall motion. Circulation journal : official journal of the Japanese Circulation Society. 2005;69:1459–1465. doi: 10.1253/circj.69.1459. [DOI] [PubMed] [Google Scholar]
- 49.Bessi VL, Labbe SM, Huynh DN, Menard L, Jossart C, Febbraio M, Guerin B, Bentourkia M, Lecomte R, Carpentier AC, Ong H, Marleau S. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovascular research. 2012;96:99–108. doi: 10.1093/cvr/cvs225. [DOI] [PubMed] [Google Scholar]
- 50.Mariano-Goulart D, Ilonca D, Bourdon A. Diagnosis of silent myocardial ischemia during the staging of HIV-associated lymphoma with FDG PET/CT. Clin Nucl Med. 2009;34:731–733. doi: 10.1097/RLU.0b013e3181b53a46. [DOI] [PubMed] [Google Scholar]
- 51.Demirkol MO. Myocardial viability testing in patients with severe left ventricular dysfunction by SPECT and PET. Anadolu Kardiyol Derg. 2008;8(Suppl 2):60–70. [PubMed] [Google Scholar]
- 52.Steinmetz AP, Maisey MN, Hardoff R. Patterns of papillary muscle ischemia in myocardial PET. Clin Nucl Med. 2004;29:1–4. doi: 10.1097/01.rlu.0000102760.54371.b4. [DOI] [PubMed] [Google Scholar]
- 53.Schindler TH, Nitzsche EU, Magosaki N, Mix M, Facta AD, Prior JO, Solzbach U, Schelbert HR, Just H. Myocardial viability in patients with ischemic cardiomyopathy-evaluation by 3-D integration of myocardial scintigraphic data--and coronary angiographic data. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2004;6:160–171. doi: 10.1016/j.mibio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Kuhl HP, Beek AM, van der Weerdt AP, Hofman MB, Visser CA, Lammertsma AA, Heussen N, Visser FC, van Rossum AC. Myocardial viability in chronic ischemic heart disease: comparison of contrast-enhanced magnetic resonance imaging with (18)F-fluorodeoxyglucose positron emission tomography. Journal of the American College of Cardiology. 2003;41:1341–1348. doi: 10.1016/s0735-1097(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 55.Rickers C, Sasse K, Buchert R, Stern H, van den Hoff J, Lubeck M, Weil J. Myocardial viability assessed by positron emission tomography in infants and children after the arterial switch operation and suspected infarction. Journal of the American College of Cardiology. 2000;36:1676–1683. doi: 10.1016/s0735-1097(00)00891-3. [DOI] [PubMed] [Google Scholar]
- 56.Niemeyer MG, Kuijper AF, Meeder JG, Cramer MJ, Cleophas AJ, van der Wall EE. Comparison of thallium scintigraphy and positron emission tomography. Angiology. 1997;48:843–853. doi: 10.1177/000331979704801001. [DOI] [PubMed] [Google Scholar]
- 57.Schroter G, Schneider-Eicke J, Schwaiger M. Assessment of tissue viability with fluorine-18-fluoro-2-deoxyglucose (FDG) and carbon-11-acetate PET imaging. Herz. 1994;19:42–50. [PubMed] [Google Scholar]
- 58.Allman KC. Noninvasive assessment myocardial viability: current status and future directions. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:618–637. doi: 10.1007/s12350-013-9737-8. quiz 638–619. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Schindler TH, Prior JO, Sayre J, Dahlbom M, Huang SC, Schelbert HR. Blood flow, flow reserve, and glucose utilization in viable and nonviable myocardium in patients with ischemic cardiomyopathy. European journal of nuclear medicine and molecular imaging. 2013;40:532–541. doi: 10.1007/s00259-012-2311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syrota A. In vivo investigation of myocardial perfusion, metabolism and receptors by positron emission tomography. Int J Microcirc Clin Exp. 1989;8:411–422. [PubMed] [Google Scholar]
- 61.Eisenberg JD, Sobel BE, Geltman EM. Differentiation of ischemic from nonischemic cardiomyopathy with positron emission tomography. The American journal of cardiology. 1987;59:1410–1414. doi: 10.1016/0002-9149(87)90930-1. [DOI] [PubMed] [Google Scholar]
- 62.Geltman EM, Biello D, Welch MJ, Ter-Pogossian MM, Roberts R, Sobel BE. Characterization of nontransmural myocardial infarction by positron-emission tomography. Circulation. 1982;65:747–755. doi: 10.1161/01.cir.65.4.747. [DOI] [PubMed] [Google Scholar]
- 63.Wong RC, Cerqueira MD, Brunken RC. Ischemic changes on rubidium-82 positron emission tomography imaging are associated with left ventricular functional and volumetric change independent of metabolic properties and echocardiographic functional variables in ischemic cardiomyopathy. Int J Cardiovasc Imaging. 2012;28:1395–1405. doi: 10.1007/s10554-011-9973-4. [DOI] [PubMed] [Google Scholar]
- 64.Bober RM, Jahangir E. What is ischemia and how should this be defined based on modern imaging? Prog Cardiovasc Dis. 2015;57:537–554. doi: 10.1016/j.pcad.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Kawamoto M, Tamaki N, Yonekura Y, Tadamura E, Fujibayashi Y, Magata Y, Nohara R, Sasayama S, Ikekubo K, Kato H, et al. Combined study with I-123 fatty acid and thallium-201 to assess ischemic myocardium: comparison with thallium redistribution and glucose metabolism. Annals of nuclear medicine. 1994;8:47–54. doi: 10.1007/BF03164986. [DOI] [PubMed] [Google Scholar]
- 66.Kageyama H, Morita K, Katoh C, Tsukamoto T, Noriyasu K, Mabuchi M, Naya M, Kawai Y, Tamaki N. Reduced 123I-BMIPP uptake implies decreased myocardial flow reserve in patients with chronic stable angina. European journal of nuclear medicine and molecular imaging. 2006;33:6–12. doi: 10.1007/s00259-005-1863-3. [DOI] [PubMed] [Google Scholar]
- 67.Seki H, Toyama T, Higuchi K, Kasama S, Ueda T, Seki R, Hatori T, Endo K, Kurabayashi M. Prediction of functional improvement of ischemic myocardium with (123I-BMIPP SPECT and 99mTc-tetrofosmin SPECT imaging: a study of patients with large acute myocardial infarction and receiving revascularization therapy. Circulation journal : official journal of the Japanese Circulation Society. 2005;69:311–319. doi: 10.1253/circj.69.311. [DOI] [PubMed] [Google Scholar]
- 68.Tani T, Teragaki M, Watanabe H, Muro T, Yamagishi H, Akioka K, Yoshiyama M, Takeuchi K, Yoshikawa J. Detecting viable myocardium and predicting functional improvement: comparisons of positron emission tomography, rest-redistribution thallium-201 single-photon emission computed tomography (SPECT), exercise thallium-201 reinjection SPECT, I-123 BMIPP SPECT and dobutamine stress echocardiography. Circulation journal : official journal of the Japanese Circulation Society. 2004;68:950–957. doi: 10.1253/circj.68.950. [DOI] [PubMed] [Google Scholar]
- 69.Yamagishi H, Akioka K, Takagi M, Tanaka A, Takeuchi K, Yoshikawa J, Ochi H. Relation between the kinetics of thallium-201 in myocardial scintigraphy and myocardial metabolism in patients with acute myocardial infarction. Heart. 1998;80:28–34. [PMC free article] [PubMed] [Google Scholar]
- 70.Maki MT, Haaparanta MT, Luotolahti MS, Nuutila P, Voipio-Pulkki LM, Bergman JR, Solin OH, Knuuti JM. Fatty acid uptake is preserved in chronically dysfunctional but viable myocardium. The American journal of physiology. 1997;273:H2473–2480. doi: 10.1152/ajpheart.1997.273.5.H2473. [DOI] [PubMed] [Google Scholar]
- 71.Giedd KN, Bergmann SR. Fatty acid imaging of the heart. Current cardiology reports. 2011;13:121–131. doi: 10.1007/s11886-010-0163-0. [DOI] [PubMed] [Google Scholar]
- 72.Fukuoka Y, Nakano A, Uzui H, Amaya N, Ishida K, Arakawa K, Kudo T, Okazawa H, Ueda T, Lee JD, Tada H. Reverse blood flow-glucose metabolism mismatch indicates preserved oxygen metabolism in patients with revascularised myocardial infarction. European journal of nuclear medicine and molecular imaging. 2013;40:1155–1162. doi: 10.1007/s00259-013-2423-x. [DOI] [PubMed] [Google Scholar]
- 73.Ishida Y, Nagata S, Uehara T, Yasumura Y, Fukuchi K, Miyatake K. Clinical analysis of myocardial perfusion and metabolism in patients with hypertrophic cardiomyopathy by single photon emission tomography and positron emission tomography. Journal of cardiology. 2001;37(Suppl 1):121–128. [PubMed] [Google Scholar]
- 74.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2001;42:55–62. [PubMed] [Google Scholar]
- 75.Tuunanen H, Kuusisto J, Toikka J, Jaaskelainen P, Marjamaki P, Peuhkurinen K, Viljanen T, Sipola P, Stolen KQ, Hannukainen J, Nuutila P, Laakso M, Knuuti J. Myocardial perfusion, oxidative metabolism, and free fatty acid uptake in patients with hypertrophic cardiomyopathy attributable to the Asp175Asn mutation in the alpha-tropomyosin gene: a positron emission tomography study. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2007;14:354–365. doi: 10.1016/j.nuclcard.2006.12.329. [DOI] [PubMed] [Google Scholar]
- 76.Geltman EM. Metabolic imaging of patients with cardiomyopathy. Circulation. 1991;84:I265–272. [PubMed] [Google Scholar]
- 77.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 78.Menard SL, Croteau E, Sarrhini O, Gelinas R, Brassard P, Ouellet R, Bentourkia M, van Lier JE, Des Rosiers C, Lecomte R, Carpentier AC. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. American journal of physiology. Endocrinology and metabolism. 2010;298:E1049–1057. doi: 10.1152/ajpendo.00560.2009. [DOI] [PubMed] [Google Scholar]
- 79.van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovascular diabetology. 2009;8:39. doi: 10.1186/1475-2840-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. Journal of cardiac failure. 2006;12:644–652. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Haim TE, Wang W, Flagg TP, Tones MA, Bahinski A, Numann RE, Nichols CG, Nerbonne JM. Palmitate attenuates myocardial contractility through augmentation of repolarizing Kv currents. Journal of molecular and cellular cardiology. 2010;48:395–405. doi: 10.1016/j.yjmcc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 83.Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovascular research. 2013;97:676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 84.Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC. Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. American journal of physiology. Heart and circulatory physiology. 2008;294:H954–960. doi: 10.1152/ajpheart.00557.2007. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circulation. Heart failure. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 86.Zhou L, Cabrera ME, Huang H, Yuan CL, Monika DK, Sharma N, Bian F, Stanley WC. Parallel activation of mitochondrial oxidative metabolism with increased cardiac energy expenditure is not dependent on fatty acid oxidation in pigs. The Journal of physiology. 2007;579:811–821. doi: 10.1113/jphysiol.2006.123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King KL, Okere IC, Sharma N, Dyck JR, Reszko AE, McElfresh TA, Kerner J, Chandler MP, Lopaschuk GD, Stanley WC. Regulation of cardiac malonyl-CoA content and fatty acid oxidation during increased cardiac power. American journal of physiology. Heart and circulatory physiology. 2005;289:H1033–1037. doi: 10.1152/ajpheart.00210.2005. [DOI] [PubMed] [Google Scholar]
- 88.Khan RS, Chokshi A, Drosatos K, Jiang H, Yu S, Harris CR, Schulze PC, Homma S, Blaner WS, Shulman GI, Huang LS, Goldberg IJ. Fish oil selectively improves heart function in a mouse model of lipid-induced cardiomyopathy. Journal of cardiovascular pharmacology. 2013;61:345–354. doi: 10.1097/FJC.0b013e318283d845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 90.Fragasso G, Salerno A, Spoladore R, Cera M, Montanaro C, Margonato A. Effects of metabolic approach in diabetic patients with coronary artery disease. Current pharmaceutical design. 2009;15:857–862. doi: 10.2174/138161209787582093. [DOI] [PubMed] [Google Scholar]
- 91.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 92.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, DeMoss AJ, Dence CS, Gropler RJ. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity. 2012;20:802–810. doi: 10.1038/oby.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC. Cardiovascular imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kunach M, Noll C, Phoenix S, Guerin B, Baillargeon JP, Turcotte EE, Carpentier AC. Effect of Sex and Impaired Glucose Tolerance on Organ-Specific Dietary Fatty Acid Metabolism in Humans. Diabetes. 2015;64:2432–2441. doi: 10.2337/db14-1166. [DOI] [PubMed] [Google Scholar]
- 95.Lyons MR, Peterson LR, McGill JB, Herrero P, Coggan AR, Saeed IM, Recklein C, Schechtman KB, Gropler RJ. Impact of sex on the heart’s metabolic and functional responses to diabetic therapies. American journal of physiology. Heart and circulatory physiology. 2013;305:H1584–1591. doi: 10.1152/ajpheart.00420.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeong J, Kong E, Chun K, Cho I. The Impact of Energy Substrates, Hormone Level and Subject-Related Factors on Physiologic Myocardial (18)F-FDG Uptake in Normal Humans. Nuclear medicine and molecular imaging. 2013;47:225–231. doi: 10.1007/s13139-013-0230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banke NH, Yan L, Pound KM, Dhar S, Reinhardt H, De Lorenzo MS, Vatner SF, Lewandowski ED. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. Journal of molecular and cellular cardiology. 2012;52:733–740. doi: 10.1016/j.yjmcc.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2011;18:421–429. doi: 10.1007/s12350-011-9362-3. quiz 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Opie LH, Stubbs WA. Carbohydrate metabolism in cardiovascular disease. Clinics in endocrinology and metabolism. 1976;5:703–729. doi: 10.1016/s0300-595x(76)80047-3. [DOI] [PubMed] [Google Scholar]
- 100.Turpeinen AK, Takala TO, Nuutila P, Axelin T, Luotolahti M, Haaparanta M, Bergman J, Hamalainen H, Iida H, Maki M, Uusitupa MI, Knuuti J. Impaired free fatty acid uptake in skeletal muscle but not in myocardium in patients with impaired glucose tolerance: studies with PET and 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid. Diabetes. 1999;48:1245–1250. doi: 10.2337/diabetes.48.6.1245. [DOI] [PubMed] [Google Scholar]
- 101.Kota SK, Kota SK, Jammula S, Panda S, Modi KD. Effect of diabetes on alteration of metabolism in cardiac myocytes: therapeutic implications. Diabetes technology & therapeutics. 2011;13:1155–1160. doi: 10.1089/dia.2011.0120. [DOI] [PubMed] [Google Scholar]
- 102.Shoghi KI, Finck BN, Schechtman KB, Sharp T, Herrero P, Gropler RJ, Welch MJ. In vivo metabolic phenotyping of myocardial substrate metabolism in rodents: differential efficacy of metformin and rosiglitazone monotherapy. Circulation. Cardiovascular imaging. 2009;2:373–381. doi: 10.1161/CIRCIMAGING.108.843227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. Journal of the American College of Cardiology. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 104.Herrero P, McGill J, Lesniak DS, Dence CS, Scott SW, Kisrieva-Ware Z, Gropler RJ. PET detection of the impact of dobutamine on myocardial glucose metabolism in women with type 1 diabetes mellitus. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2008;15:791–799. doi: 10.1007/BF03007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lautamaki R, Airaksinen KE, Seppanen M, Toikka J, Luotolahti M, Ball E, Borra R, Harkonen R, Iozzo P, Stewart M, Knuuti J, Nuutila P. Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease: a 16-week randomized, double-blind, placebo-controlled study. Diabetes. 2005;54:2787–2794. doi: 10.2337/diabetes.54.9.2787. [DOI] [PubMed] [Google Scholar]
- 106.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 107.Linke A, Zhao G, Recchia FA, Williams J, Xu X, Hintze TH. Shift in metabolic substrate uptake by the heart during development of alloxan-induced diabetes. American journal of physiology. Heart and circulatory physiology. 2003;285:H1007–1014. doi: 10.1152/ajpheart.00528.2002. [DOI] [PubMed] [Google Scholar]
- 108.Iozzo P, Chareonthaitawee P, Rimoldi O, Betteridge DJ, Camici PG, Ferrannini E. Mismatch between insulin-mediated glucose uptake and blood flow in the heart of patients with Type II diabetes. Diabetologia. 2002;45:1404–1409. doi: 10.1007/s00125-002-0917-3. [DOI] [PubMed] [Google Scholar]
- 109.Takeishi Y, Atsumi H, Fujiwara S, Takahashi K, Tomoike H. Fatty acid metabolic imaging with 123I-BMIPP for the diagnosis of coronary artery disease: application to patients with diabetes mellitus and hyperlipidaemia. Nuclear medicine communications. 1996;17:675–680. doi: 10.1097/00006231-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Monti LD, Lucignani G, Landoni C, Moresco RM, Piatti P, Stefani I, Pozza G, Fazio F. Myocardial glucose uptake evaluated by positron emission tomography and fluorodeoxyglucose during hyperglycemic clamp in IDDM patients. Role of free fatty acid and insulin levels. Diabetes. 1995;44:537–542. doi: 10.2337/diab.44.5.537. [DOI] [PubMed] [Google Scholar]
- 111.Doria A, Nosadini R, Avogaro A, Fioretto P, Crepaldi G. Myocardial metabolism in type 1 diabetic patients without coronary artery disease. Diabetic medicine : a journal of the British Diabetic Association. 1991;8(Spec No):S104–107. doi: 10.1111/j.1464-5491.1991.tb02168.x. [DOI] [PubMed] [Google Scholar]
- 112.Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Sacca L, Toffolo G, Cobelli C, Trevisan R, et al. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. The American journal of physiology. 1990;258:E606–618. doi: 10.1152/ajpendo.1990.258.4.E606. [DOI] [PubMed] [Google Scholar]
- 113.Vitale GD, deKemp RA, Ruddy TD, Williams K, Beanlands RS. Myocardial glucose utilization and optimization of (18)F-FDG PET imaging in patients with non-insulin-dependent diabetes mellitus, coronary artery disease, and left ventricular dysfunction. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2001;42:1730–1736. [PubMed] [Google Scholar]
- 114.Peterson LR, Herrero P, Coggan AR, Kisrieva-Ware Z, Saeed I, Dence C, Koudelis D, McGill JB, Lyons MR, Novak E, Davila-Roman VG, Waggoner AD, Gropler RJ. Type 2 diabetes, obesity, and sex difference affect the fate of glucose in the human heart. American journal of physiology. Heart and circulatory physiology. 2015;308:H1510–1516. doi: 10.1152/ajpheart.00722.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hallsten K, Virtanen KA, Lonnqvist F, Janatuinen T, Turiceanu M, Ronnemaa T, Viikari J, Lehtimaki T, Knuuti J, Nuutila P. Enhancement of insulin-stimulated myocardial glucose uptake in patients with Type 2 diabetes treated with rosiglitazone. Diabetic medicine : a journal of the British Diabetic Association. 2004;21:1280–1287. doi: 10.1111/j.1464-5491.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 116.Monti LD, Landoni C, Setola E, Galluccio E, Lucotti P, Sandoli EP, Origgi A, Lucignani G, Piatti P, Fazio F. Myocardial insulin resistance associated with chronic hypertriglyceridemia and increased FFA levels in Type 2 diabetic patients. American journal of physiology. Heart and circulatory physiology. 2004;287:H1225–1231. doi: 10.1152/ajpheart.00629.2003. [DOI] [PubMed] [Google Scholar]
- 117.Labbe SM, Noll C, Grenier-Larouche T, Kunach M, Bouffard L, Phoenix S, Guerin B, Baillargeon JP, Langlois MF, Turcotte EE, Carpentier AC. Improved cardiac function and dietary fatty acid metabolism after modest weight loss in subjects with impaired glucose tolerance. American journal of physiology. Endocrinology and metabolism. 2014;306:E1388–1396. doi: 10.1152/ajpendo.00638.2013. [DOI] [PubMed] [Google Scholar]
- 118.Labbe SM, Grenier-Larouche T, Noll C, Phoenix S, Guerin B, Turcotte EE, Carpentier AC. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes. 2012;61:2701–2710. doi: 10.2337/db11-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 120.Welch MJ, Lewis JS, Kim J, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial metabolism in diabetic rats using small-animal PET: a feasibility study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:689–697. [PubMed] [Google Scholar]
- 121.Turpeinen AK, Kuikka JT, Vanninen E, Uusitupa MI. Abnormal myocardial kinetics of 123I-heptadecanoic acid in subjects with impaired glucose tolerance. Diabetologia. 1997;40:541–549. doi: 10.1007/s001250050713. [DOI] [PubMed] [Google Scholar]
- 122.Palaniswamy SS, Padma S. Cardiac fatty acid metabolism and ischemic memory imaging with nuclear medicine techniques. Nuclear medicine communications. 2011;32:672–677. doi: 10.1097/MNM.0b013e32834774e3. [DOI] [PubMed] [Google Scholar]
- 123.Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. Journal of the American College of Cardiology. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 124.Knuuti J, Takala TO, Nagren K, Sipila H, Turpeinen AK, Uusitupa MI, Nuutila P. Myocardial fatty acid oxidation in patients with impaired glucose tolerance. Diabetologia. 2001;44:184–187. doi: 10.1007/s001250051597. [DOI] [PubMed] [Google Scholar]
- 125.Viljanen AP, Karmi A, Borra R, Parkka JP, Lepomaki V, Parkkola R, Lautamaki R, Jarvisalo M, Taittonen M, Ronnemaa T, Iozzo P, Knuuti J, Nuutila P, Raitakari OT. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. The American journal of cardiology. 2009;103:1721–1726. doi: 10.1016/j.amjcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 126.Angin Y, Steinbusch LK, Simons PJ, Greulich S, Hoebers NT, Douma K, van Zandvoort MA, Coumans WA, Wijnen W, Diamant M, Ouwens DM, Glatz JF, Luiken JJ. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. The Biochemical journal. 2012;448:43–53. doi: 10.1042/BJ20120060. [DOI] [PubMed] [Google Scholar]
- 127.Nakatani K, Watabe T, Masuda D, Imaizumi M, Shimosegawa E, Kobayashi T, Sairyo M, Zhu Y, Okada T, Kawase R, Nakaoka H, Naito A, Ohama T, Koseki M, Oka T, Akazawa H, Nishida M, Komuro I, Sakata Y, Hatazawa J, Yamashita S. Myocardial energy provision is preserved by increased utilization of glucose and ketone bodies in CD36 knockout mice. Metabolism: clinical and experimental. 2015;64:1165–1174. doi: 10.1016/j.metabol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 128.Cannon MV, Sillje HH, Sijbesma JW, Khan MA, Steffensen KR, van Gilst WH, de Boer RA. LXRalpha improves myocardial glucose tolerance and reduces cardiac hypertrophy in a mouse model of obesity-induced type 2 diabetes. Diabetologia. 2015 doi: 10.1007/s00125-015-3827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]