Abstract
Combinations of spatially and temporally restricted transcription factors are shown to coordinate movement in nematode worms by controlling the formation of synaptic connections to and from motor neurons.
Nervous systems are staggeringly complex. To generate appropriate behavioural outputs, the countless synaptic connections that neurons form with other cells must be precisely regulated to ensure that they are arranged in the right circuits at the right time. On page 83 of this issue, Howell et al.1 show that, in the simple neuronal circuits of the nematode worm Caenorhabditis elegans, such spatio-temporal precision is achieved through transcription factors that function together to restrict the expression of a newly identified synaptic organizer protein called OIG-1.
Nematode movement involves coordinated muscle contractions that are regulated by complex interactions between motor neurons on the worm’s dorsal (upper) and ventral (lower) sides. Two classes of inhibitory D-type motor neuron2 in particular help to control this process. Dorsal D (DD) neurons make synaptic connections to muscles on the dorsal side of the worm, and are themselves innervated by excitatory motor neurons called cholinergic neurons from the animal’s ventral side. By contrast, ventral D (VD) neurons innervate ventral muscles and receive synaptic inputs from dorsal cholinergic neurons. Activation of ventral cholinergic neurons therefore contracts ventral muscles and activates DD neurons, inhibiting dorsal-muscle contraction, whereas activation of dorsal cholinergic neurons leads to contraction of dorsal, but not ventral, muscles.
DD neurons form during embryonic development, but VD neurons arise after a larva’s exoskeleton has moulted for the first time2. In fact, before VD neurons develop, early DD neurons innervate ventral muscles and receive synaptic inputs from motor neurons on the dorsal side of the animal2 (Fig. 1a). They later undergo a synaptic inversion when VD neurons arise (Fig. 1b). Howell et al. investigated the regulatory logic behind this synaptic rewiring using worms that harbour mutations in the gene unc-30, which encodes an evolutionarily conserved transcription factor, UNC-30. This protein is expressed in all D-type motor neurons throughout development, and is regarded as a ‘terminal-identity selector’ — its expression ultimately defines whether neurons will become D-type motor neurons3–5.
Figure 1. Transcriptional regulation of synaptic specificity.
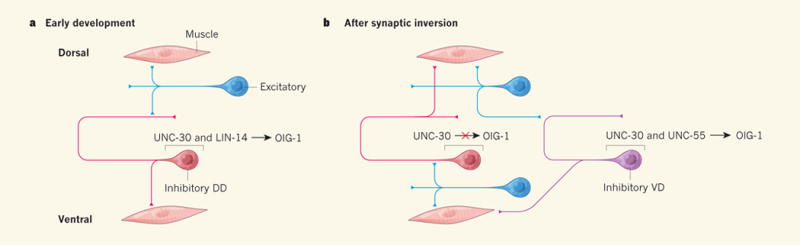
a, During early development of nematodes, excitatory motor neurons (blue) on the dorsal side of the embryo make synaptic connections with, and so excite, dorsal muscle and inhibitory dorsal D-type (DD) motor neurons (red). DD neurons innervate, and thus inhibit contraction of, ventral muscle. Howell et al.1 report that the synaptic inputs and outputs of early DD neurons are controlled by LIN-14. This transcription factor, together with the ubiquitously expressed transcription factor UNC-30, promotes expression of the protein OIG-1, which prevents the formation of synaptic outputs from DD neurons to dorsal muscle cells. b, After the larva’s first moult, LIN-14 is no longer expressed and DD neurons undergo a synaptic inversion — they become innervated by ventral excitatory neurons and themselves innervate dorsal muscle. Ventral D-type (VD) motor neurons (purple) innervate ventral muscle cells and express the transcription factor UNC-55, which, together with UNC-30, promotes OIG-1 expression and prevents VD neurons from forming inhibitory connections to dorsal muscle.
In addition to the uncoordinated locomotion for which the gene is named, the authors found that unc-30 mutant worms had defects in synaptic specificity. Although the cell bodies (the nucleus-containing regions) of D-type motor neurons are appropriately positioned in unc-30 mutants, the early DD and VD neurons fail to innervate ventral muscles and instead make abnormal synaptic connections to dorsal muscles. Moreover, VD neurons do not receive synaptic inputs from dorsal cholinergic motor neurons.
How does UNC-30 regulate synapse formation in specific spatio-temporal patterns, given its ubiquitous expression in D-type neurons throughout development? Previous studies6–9 have shown that mutations in two other genes encoding transcription factors, lin-14 and unc-55, recapitulate different aspects of the defects found in unc-30 mutants. Expression of LIN-14 is temporally restricted to early development, and mutation of lin-14 leads to the abnormal formation of dorsal synapses from early DD neurons8,9. By contrast, UNC-55 is restricted to VD neurons, where it regulates synaptic specificity to ventral muscles6,7.
Howell et al. reasoned that UNC-30 might cooperate with LIN-14 and UNC-55 to promote the expression of a molecule that blocks dorsal synaptic output. This molecule would be expected to be expressed under the control of UNC-30 acting with LIN-14 in early DD neurons, and under the control of UNC-30 acting with UNC-55 in VD neurons, inhibiting the formation of synaptic outputs on the dorsal side of the animal. But under this simple model, ventral synaptic outputs would remain normal in unc-30 mutants. Because this is not the case, additional layers of regulation must be involved.
OIG-1, a member of the immunoglobulin superfamily, whose members mediate interactions between cells, is a putative target of UNC-30, LIN-14 and UNC-55 (ref. 10). Howell and colleagues provide evidence that oig-1 is expressed in early DD neurons and is later restricted to VD neurons. Furthermore, its expression is altered by perturbations to lin-14 and unc-55 expression. The authors report that loss of oig-1 leads to the formation of dorsal synaptic outputs similar to those seen in unc-30, lin-14 and unc-55 mutants. Moreover, synaptic innervation of early DD and VD neurons by cholinergic neurons on the dorsal side of the animal is disrupted in oig-1 mutants. This suggests that OIG-1 coordinates both the inputs to and outputs of D-type motor neurons.
Howell et al. found that OIG-1 is located along the ventral side of early DD and VD neurons. Given that the protein can organize dorsal synaptic inputs and outputs, this observation suggests that it acts indirectly. The authors also show that forced expression of oig-1 could not block synaptic rewiring of late DD motor neurons to ventral muscles, indicating that other factors must cooperate with OIG-1 to regulate synaptic specificity. Identifying these cofactors, which, like oig-1, must be differentially expressed in early DD and VD neurons, will be of great interest, as will determining the molecular mechanisms by which they act with OIG-1 to regulate synaptic inputs and outputs through pre- and postsynaptic partner molecules.
Achieving appropriate synaptic specificity involves many developmental steps that act together to ensure that neurons assume the correct identity. Determinants of neuronal identity, which are regulated by terminal-identity selectors, include the production of particular neurotransmitter molecules, the guidance of nerve fibres in specific directions and the appropriate growth of branched projections called dendrites. Howell and colleagues’ work demonstrates that the formation and targeting of synapses are also traits that give neurons a particular identity, and that synapses, too, can be regulated by terminal-identity selectors such as UNC-30.
References
- 1.Howell K, White JG, Hobert O. Nature. 2015;523:83–87. doi: 10.1038/nature14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JG, Albertson DG, Anness MAR. Nature. 1978;271:764–766. doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- 3.Eastman C, Horvitz HR, Jin YJ. Neurosci. 1999;19:6225–6234. doi: 10.1523/JNEUROSCI.19-15-06225.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y, Hoskins R, Horvitz HR. Nature. 1994;372:780–783. doi: 10.1038/372780a0. [DOI] [PubMed] [Google Scholar]
- 5.Cinar H, Keles S, Jin Y. Curr Biol. 2005;15:340–346. doi: 10.1016/j.cub.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Walthall WW, Plunkett JA. J Neurosci. 1995;15:1035–1043. doi: 10.1523/JNEUROSCI.15-02-01035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedgecock EM, Culotti JG, Hall DH, Stern BD. Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- 8.Ruvkun G, Giusto J. Nature. 1989;338:313–319. doi: 10.1038/338313a0. [DOI] [PubMed] [Google Scholar]
- 9.Hallam SJ, Jin Y. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- 10.Aurelio O, Hall DH, Hobert O. Science. 2002;295:686–690. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]


