Abstract
We examined the acute dose-dependent effects of intracoronary glucagon-like peptide (GLP)-1 (7–36) on coronary vascular tone, cardiac contractile function and metabolism in normal and ischemic myocardium. Experiments were conducted in open chest, anesthetized dogs at coronary perfusion pressures (CPP) of 100 and 40 mmHg before and during intracoronary GLP-1 (7–36) infusion (10 pmol/L to 1 nmol/L). Isometric tension studies were also conducted in isolated coronary arteries. Cardiac and coronary expression of GLP-1 receptors (GLP-1R) was assessed by Western blot and immunohistochemical analysis. GLP-1R was present in the myocardium and the coronary vasculature. The tension of intact and endothelium-denuded coronary artery rings was unaffected by GLP-1. At normal perfusion pressure (100 mmHg), intracoronary GLP-1 (7–36) (targeting plasma concentration 10 pmol/L to 1 nmol/L) did not affect blood pressure, coronary blood flow or myocardial oxygen consumption (MVO2); however, there were modest reductions in cardiac output and stroke volume. In untreated control hearts, reducing CPP to 40 mmHg produced marked reductions in coronary blood flow (0.50 ±0.10 to 0.17 ±0.03 mL/min/g; P < 0.001) and MVO2 (27 ±2.3 to 15 ±2.7 μL O2/min/g; P < 0.001). At CPP = 40 mmHg, GLP-1 had no effect on coronary blood flow, MVO2 or regional shortening, but dose-dependently increased myocardial glucose uptake from 0.11±0.02 μmol/min/g at baseline to 0.17±0.04 μmol/min/g at 1 nmol/L GLP-1 (P < 0.001). These data indicate that acute, intracoronary administration of GLP-1 (7–36) preferentially augments glucose metabolism in ischemic myocardium, independent of effects on cardiac contractile function or coronary blood flow.
Keywords: coronary blood flow, myocardial oxygen consumption, GLP-1, canine, cardiac metabolism
Introduction
Glucagon-like peptide-1 (GLP-1) is an insulinotropic hormone released from intestinal L-cells in response to feeding. The full-length peptide GLP-1 (7–36) is a ligand for the G-protein-coupled GLP-1 receptor (GLP-1R). GLP-1 (7–36) is quickly degraded by circulating dipeptidyl peptidase 4 (DPP-4) to yield GLP-1 (9–36), which does not activate the GLP-1R, and is inactive as an insulinotropic agent.1 Endogenous plasma concentrations of GLP-1 (7–36) observed in humans range from ~5 to 30 pmol/L in a fasting state to ~30 to 40 pmol/L following a mixed meal.2–5 Current GLP-1-based therapeutics for the treatment of type 2 diabetes mellitus include GLP-1R agonists with long circulating half-lives (e.g. exenatide), and DPP-4 inhibitors (e.g. sitagliptin).6 Although these therapies have been linked with cardioprotective mechanisms, they are not currently prescribed for this purpose.5,7–18
Recent research on the cardiac effects of GLP-1 indicates that systemic infusion of the full-length (7–36) peptide influences cardiac contractile function and glucose utilization. Studies in humans and in canines demonstrate that intravenous administration of GLP-1 (7–36) (1.5–2.5 pmol/L/kg/min) for 24–72 h improves cardiac contractile performance following coronary artery occlusion,9,10 and pacing-induced heart failure.7,8,11 This improvement in cardiac function is associated with an increase in myocardial glucose utilization and occurs both independently and synergistically with insulin.8 Other studies have documented that GLP-1 (7–36) induces vasodilation of isolated aortic, femoral and pulmonary arteries in rats,19–22 and increases coronary blood flow in normal and postischemic isolated murine hearts.12,13 Systemic administration of recombinant GLP-1 (7–36) has also been shown to increase coronary blood flow in vivo in canines with pacing-induced dilated cardiomyopathy.7 However, cardiac contractile performance and myocardial oxygen consumption (MVO2) were also increased by GLP-1 in this study. Thus, it is unclear if the changes in coronary flow were the result of direct actions of GLP-1 on the coronary circulation or if they were the result of increased metabolic demand, i.e. metabolic vasodilation. While it has been demonstrated by Western blot that GLP-1R is present within canine myocardium,8 the distribution of GLP-1R within canine heart, including the possible presence within coronary microvessels, has not previously been determined. Taken together, these findings suggest that GLP-1 (7–36) could protect the heart from ischemic injury by shifting cardiac substrate metabolism toward glucose and/or by augmenting myocardial perfusion.14,23 Whether these effects of GLP-1 in vivo occur acutely (min versus h) and/or are dependent on whole-body responses to systemic exposure to GLP-1 has not been assessed.
This study tested the hypothesis that acute administration of GLP-1 (7–36) directly into the coronary circulation augments coronary blood flow, myocardial glucose metabolism and cardiac contractile function in normal and ischemic myocardium in a dose-dependent manner. Experiments were conducted in open chest anesthetized dogs at coronary perfusion pressures (CPP) of 100 and 40 mmHg before and during intracoronary GLP-1 (7–36) infusion (intracoronary concentrations of 10 pmol/L to 1 nmol/L). Coronary vascular effects of GLP-1 (7–36) (10 pmol/L to 1 nmol/L) were also assessed by isometric tension studies in isolated coronary arteries. In addition, cardiac and coronary expression of GLP-1R was determined by Western blot analysis and immunohistochemistry with confocal microscopy.
Methods
Surgical preparation
Animal procedures used for this investigation were approved by the Institutional Animal Care and Use Committee and conducted in accordance with guidelines in the Guide for the Care and Use of Laboratory Animals. Adult male mongrel dogs weighing ~20 kg were sedated with morphine (3 mg/kg, subcutaneous) and anesthetized with α-chloralose (100 mg/kg, intravenous). Following intubation, animals were ventilated with room air supplemented with oxygen, and positive end-expiratory pressure was held at ~2 cm H2O to prevent atelectasis. Aortic pressure was measured through a catheter introduced into the thoracic aorta through the left femoral artery. Another catheter, inserted into the right femoral vein, was used to maintain anesthesia, and to administer sodium bicarbonate as needed to maintain pH within normal physiological limits. The right femoral artery was also catheterized to supply blood to a pump perfusing the left anterior descending coronary artery (LAD). A left lateral thoracotomy was then performed to expose the heart. The LAD was isolated distal to its first major diagonal branch, cannulated and connected to the extracorporeal perfusion system. CPP was maintained constant at 100 or 40 mmHg during the experimental protocol by a servo-controlled roller pump. Coronary blood flow was measured within the extracorporeal perfusion circuit with an in-line flow transducer (Transonic Systems, Inc, Ithaca, NY, USA). Intravenous heparin was administered (500 U/kg) to prevent coagulation. The great cardiac vein was also cannulated to collect blood for metabolic analysis of the LAD perfusion territory. Left ventricular (LV) pressure and cardiac output (CO) were measured with a Millar® Mikro-Tip SPR-524 catheter in the LV (Millar Instruments, Inc, Houston, TX, USA), and a flow probe around the root of the aorta (Transonic Systems, Inc), respectively. Regional contractile function was determined using ultrasonic crystals (Sonometrics, Inc, London, ON, Canada) placed in myocardium of the LAD perfusion territory at a depth of ~7 mm. These data were analysed by custom-made software developed in Matlab® (Mathworks®, Natick, MA, USA). Percent segment shortening was calculated as [(end diastolic length–end systolic length)/end diastolic length]. End diastolic length was taken at the beginning of the positive deflection of LV dP/dt (rate of pressure development), and end systolic length was taken 20 ms before the peak negative deflection of dP/dt, corresponding with the dicrotic notch of the aortic pressure recording, as previously reported.23,24 All materials that were implanted into the animals or in contact with the circulation (i.e. cannulas, catheters and flow probes) were cleaned with the multi-enzyme detergent Enzyte™ (Decon Labs, Inc, King of Prussia, PA, USA) for the removal of blood, protein and other biological material, then rinsed thoroughly with deionized water and allowed to dry prior to use.
Acute dose-dependent effects of GLP-1 (7–36) on cardiovascular hemodynamics
Acute in vivo experiments were conducted in open chest, anesthetized dogs (n = 9) at CPP of 100 and then 40 mmHg before and during intracoronary GLP-1 (7–36) infusion (10 pmol/L to 1 nmol/L). Following cannulation of the LAD, hemodynamic and contractile function parameters were allowed to stabilize for ~10–15 min before acquiring baseline data. After baseline parameters were measured and blood samples obtained, an infusion of GLP-1 (7–36) (G8147; Sigma-Aldrich, St Louis, MO, USA) was initiated into the LAD perfusion line at a constant infusion rate in order to obtain coronary plasma concentrations of 10 pmol/L to 1 nmol/L. Plasma flow was calculated as [(1-hematocrit) × coronary blood flow]. After completion of the GLP-1 (7–36) infusion at CPP = 100 mmHg, a 10-min washout period was allowed before CPP was lowered to 40 mmHg. Following stabilization of hemodynamic parameters at CPP = 40 mmHg (~5–10 min), baseline data were acquired and the intracoronary infusion of GLP-1 (7–36) repeated. Data were averaged from 10 consecutive beats following ~5 min of GLP-1 (7–36) administration at each dose.
Metabolic analysis
Arterial and coronary venous blood were collected simultaneously, immediately sealed and placed on ice. The samples were analyzed for pH, PCO2, PO2, O2 content, hematocrit, glucose concentration and lactate concentration with an Instrumentation Laboratories automatic blood gas analyzer (GEM Premier 3000; Instrumentation Laboratory Company, Bedford, MA, USA) and CO-oximeter (682) system (Instrumentation Laboratory Company). LAD perfusion territory was estimated to be 30% of total heart weight, as previously described by Feigl et al.24 MVO2 (μL O2/min/g) was calculated as [coronary blood flow × (arterial O2 content–coronary venous O2 content)]. Fick principle was also used to calculate glucose and lactate uptake.
Functional assessment of isolated coronary artery rings
Isometric tension studies were conducted in both endothelium intact and denuded isolated coronary artery rings treated with the same concentrations of GLP-1 used in the in vivo studies (10 pmol/L to 1 nmol/L). For these experiments, canine hearts were excised, immediately rinsed and bathed with ice-cold saline. Epicardial LV coronary arteries were dissected from the heart, cleaned of perivascular fat and cut into ~3 mm rings. These fresh arterial rings were mounted in organ baths with warm oxygenated Krebs solution and brought to an optimal preload of ~4 g, as previously described.25 Rings were then preconstricted with the thromboxane A2 mimetic U46619 (1 μmol/L). Graded doses of GLP-1 (7–36) were then added to the baths in a cumulative manner. Presence or absence of functional endothelium was assessed by reactivity to acetylcholine (10 μmol/L). Viability of arterial smooth muscle was determined by reactivity to sodium nitroprusside (20 μmol/L).
Western blot analysis
Following excision of the hearts, transmural LV samples were quickly isolated, snap frozen in liquid N2 and stored at −80°C. These samples were later homogenized in lysis buffer, and protein was quantitated using a DC protein assay. Thirty-five micrograms of protein were loaded onto a 7.5% acrylamide gel and transferred overnight. Membranes were blocked prior to a one-hour incubation with rabbit polyclonal GLP-1R antibody (ab39072; Abcam, Cambridge, MA, USA) in blocking buffer with 0.1% Tween 20 at ambient temperature. Membranes were washed and incubated for one hour with IRDye 800 donkey anti-rabbit secondary antibody. Immunoreactivity to GLP-1R was determined by a Li-Cor Odyssey system (Li-Cor Biosciences, Lincoln, NE, USA). Band size was determined using comparison with a standard protein ladder (161-0375; Bio-Rad, Hercules, CA, USA).
Immunohistochemistry and confocal microscopy
Fresh LV cardiac samples were snap frozen in liquid N2 and stored at −80°C. Frozen LV samples were then embedded in Tissue-Tek® OCT™ (Sakura Finetek USA, Inc, Torrance, CA, USA) and sliced into 10 μmol/L sections. Slices were incubated in mouse monoclonal antibodies against cardiac troponin I (ab10231; 1:1,000), 4′,6-diamidino-2-phenylindole (DAPI; 100 ng/mL) and rabbit polyclonal antibodies raised against GLP-1R (ab39072; 1:25) for one hour. Slices were subsequently washed, and treated with anti-rabbit IgG and anti-mouse IgG secondary antibodies for 30 min, conjugated with alexa-488 and alexa-594, respectively. Slides were imaged using an Olympus2 single photon confocal microscope (Olympus America Inc, Center Valley, PA, USA).
Statistical analyses
Data are presented as mean ±SE. Statistical comparisons were made with one-way repeated-measures analysis of variance (ANOVA). For all comparisons, P < 0.05 was considered statistically significant. When significance was found with ANOVA, a Student–Newman–Keuls multiple comparison test was performed to identify differences between treatment levels and/or CPP.
Results
Cardiac and coronary expression of GLP-1R
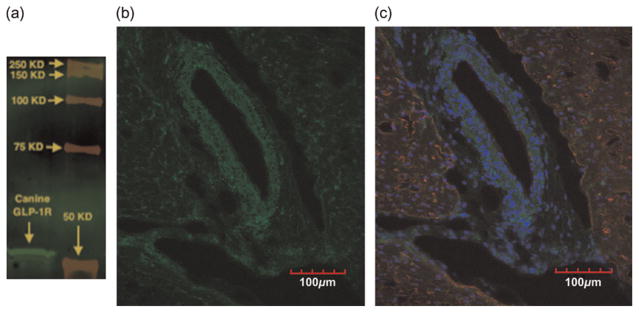
Western blot analysis demonstrated staining of the GLP-1R in canine cardiac tissue with the predicted band at ~53 kilodaltons (kDa) (Figure 1a). Immunohistochemistry with fluorescence confocal microscopy confirmed GLP-1R expression (green) in the canine coronary vasculature and myocardium (Figures 1b and c). Cardiac sections were counterstained with the nuclear stain DAPI (blue) and myocardial troponin I (red) to demonstrate cellular architecture in context to localization of GLP-1R.
Figure 1.
Cardiac and coronary expression of GLP-1R. High antibody selectivity for GLP-1R is demonstrated by Western blot analysis (a). Fluorescence confocal microscopy demonstrated GLP-1R expression (green) in both myocardium and coronary vessels (b). Counter-staining of cardiac troponin I (red) and nuclei (blue) identifies myocardial tissue and cellular architecture (c). GLP-1R, glucagon-like peptide-1 receptor
Coronary vascular effects of GLP-1 (7–36)
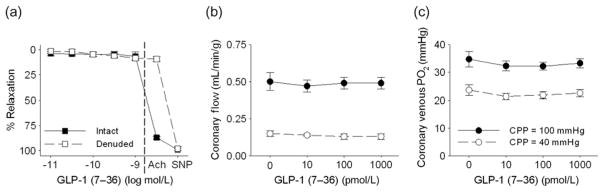
GLP-1 (10 pmol/L to 1 nmol/L) had no effect on isometric tension of isolated, endothelium intact and denuded epicardial coronary rings (n = 5) preconstricted with 1 μmol/L U46619 (Figure 2a). Endothelial denudation was confirmed by a lack of relaxation to acetylcholine (10 μmol/L) and smooth muscle viability confirmed by relaxation to sodium nitroprusside (20 μmol/L). GLP-1 also had no effect on basal tension of non-preconstricted coronary arteries (data not shown). Likewise, acute intracoronary administration of GLP-1 (7–36) (10 pmol/L to 1 nmol/L) had no effect on coronary blood flow (Figure 2b) or coronary venous PO2 (Figure 2c) at either CPP = 100 or 40 mmHg (n = 9).
Figure 2.
Direct coronary vascular effects of GLP-1 (7–36). GLP-1 (7–36) had no effect on isometric tension of intact or endothelial-denuded canine coronary artery rings preconstricted with U46619 (1 μmol/L). Denudation was confirmed by a lack of responsive to acetylcholine (Ach; 10 μmol/L), and viability confirmed by relaxation to sodium nitroprussside (SNP; 20 μmol/L) (a). Intracoronary infusion of GLP-1 (7–36), 10 pmol/L to 1 nmol/L, had no effect on coronary blood flow (b) or coronary venous PO2 (c) at CPP = 100 or 40 mmHg. GLP-1, glucagon-like peptide-1; CPP, coronary perfusion pressure
Effects of GLP-1 (7–36) on global and regional indices of cardiac function
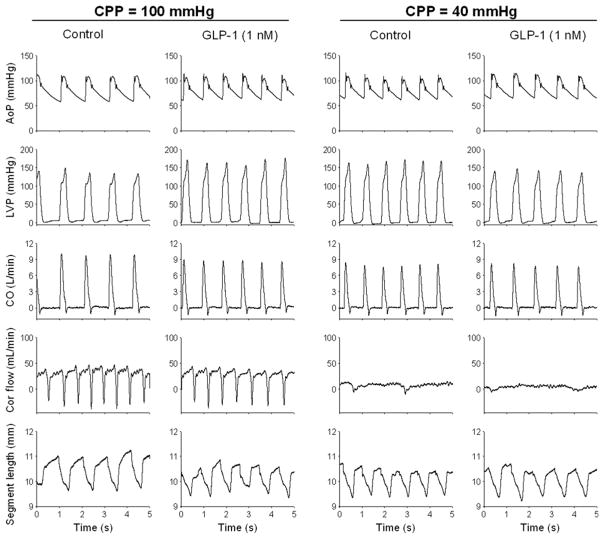
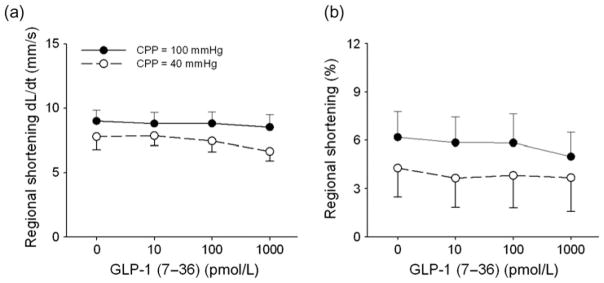
Hemodynamic data on the effects of GLP-1 in normal and ischemic hearts are provided in Table 1. A representative tracing of key hemodynamic data before and during GLP-1 (1 nmol/L) administration at CPP = 100 and 40 mmHg is shown in Figure 3. In these experiments, we used a servo-controlled roller pump system to maintain perfusion pressure constant at 100 and 40 mmHg. This servo-controlled system maintains pressure at these set values by alternating the frequency of a roller pump. Therefore, the phasic nature observed in coronary flow is not reflective of the cardiac cycle but due to the disruption of flow by the pump rollers (Figure 3). Acute intracoronary administration of GLP-1 dose-dependently decreased stroke volume and CO at CPP = 100 mmHg. Although heart rate tended to be higher (P = 0.07), mean arterial pressure and the maximal rate of LV pressure development and relaxation (+dP/dt max and −dP/dt min) were unaffected by GLP-1. Reduction of CPP to 40 mmHg decreased stroke volume and CO (P < 0.05) but did not alter blood pressure or heart rate relative to untreated control conditions at CPP = 100 mmHg. GLP-1 had no effect on blood pressure, heart rate or indices of global cardiac function at CPP = 40 mmHg. Indices of regional cardiac contractile function in the LAD perfusion territory were also unaffected by intracoronary administration of GLP-1 (7–36) at either CPP = 100 or 40 mmHg (Figure 4).
Table 1.
Dose-dependent effects of GLP-1 (7–36) on global indices of cardiac function
| [GLP-1] (pmol/L) | MBP (mmHg) | HR (bpm) | +dP/dt max (mmHg/s) | −dP/dt min (mmHg/s) | CO (L/min) | SV (mL) |
|---|---|---|---|---|---|---|
| Coronary perfusion pressure = 100 mmHg | ||||||
| Baseline | 80 ±4 | 88 ±6 | 1425 ±216 | −1102 ±197 | 1.6 ±0.2 | 18 ±2 |
| 10 | 82 ±3 | 92 ±7 | 1379 ±216 | −1187 ±194 | 1.5 ±0.2 | 16 ±2 |
| 100 | 83 ±3 | 99 ±9 | 1350 ±181 | −1161 ±168 | 1.4 ±0.1 | 14 ±1* |
| 1000 | 80 ±4 | 99 ±8 | 1310 ±196 | −1004 ±146 | 1.3 ±0.1* | 13 ±1* |
| Coronary perfusion pressure = 40 mmHg | ||||||
| Baseline | 80 ±2 | 89 ±8 | 1228 ±174 | −974 ±207 | 1.2 ±0.1† | 13 ±1† |
| 10 | 81 ±1 | 93 ±8 | 1182 ±233 | −981 ±218 | 1.2 ±0.1 | 12 ±1 |
| 100 | 84 ±2 | 94 ±7 | 1280 ±199 | −1080 ±226 | 1.2 ±0.1 | 12 ±1 |
| 1000 | 84 ±1 | 96 ±8 | 1232 ±228 | −1035 ±207 | 1.1 ±0.2 | 11 ±2 |
Data are mean ±SE from n = 9 dogs
P < 0.05 versus baseline, same CPP.
P < 0.05 versus baseline, CPP = 100 mmHg
GLP-1, glucagon-like peptide-1; MBP, mean blood pressure; dp/dt, rate of pressure development; CO, cardiac output; SV, stroke volume
Figure 3.
Example of original recordings of aortic pressure (AoP), left ventricular pressure (LVP), cardiac output (CO), coronary blood flow (Cor flow) and segment length with and without intracoronary glucagon-like peptide-1 (GLP-1) (7–36) (1 nmol/L) at coronary perfusion pressures (CPP) of 100 and 40 mmHg from a single canine
Figure 4.
Direct effects of GLP-1 (7–36) on indices of regional cardiac function. Intracoronary infusion of GLP-1 (10 pmol/L to 1 nmol/L) had no effect on the rate (a) or degree (b) of regional myocardial shortening at CPP = 100 or 40 mmHg GLP-1, glucagon-like peptide-1; CPP, coronary perfusion pressure
Effects of GLP-1 (7–36) on myocardial substrate metabolism
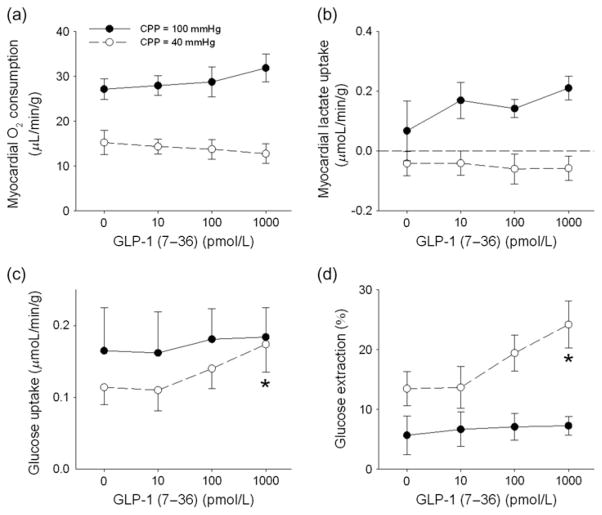
At CPP = 100 mmHg, intracoronary administration of GLP-1 (7–36) (10 pmol/L to 1 nmol/L) had no effect on MVO2, myocardial lactate uptake, glucose uptake or glucose extraction (Figure 5). In untreated hearts, reducing CPP to 40 mmHg resulted in the expected decrease in MVO2 (Figure 5a) and myocardial lactate uptake (Figure 5b). Administration of GLP-1 (7–36) dose-dependently augmented glucose uptake at CPP = 40 mmHg (Figure 5c), increasing consumption by ~55% from baseline (0.11 ±0.02 μmol/min/g) to the highest 1 nmol/L dose of GLP-1 (0.17 ±0.04 μmol/min/g). Myocardial glucose extraction was also increased ~85% at the highest dose of GLP-1 at CPP = 40 mmHg (Figure 5d). These changes in myocardial substrate metabolism induced by GLP-1 at CPP = 40 mmHg did not significantly alter MVO2 relative to untreated baseline conditions (Figure 5a).
Figure 5.
Direct dose-dependent effects of GLP-1 (7–36) on myocardial metabolism. GLP-1 did not affect myocardial oxygen consumption (a), or lactate uptake (b) at CPP = 100 or 40 mmHg. GLP-1 (7–36) dose-dependently increased myocardial glucose uptake (c) and extraction (d) at CPP = 40 mmHg, but had no effect at CPP = 100 mmHg. *P < 0.05 versus baseline at the same CPP. GLP-1, glucagon-like peptide-1; CPP, coronary perfusion pressure
Discussion
The purpose of this investigation was to evaluate the direct coronary and cardiac effects of GLP-1 (7–36) administration in vivo. We tested the hypothesis that acute, intracoronary administration of GLP-1 (7–36) augments coronary blood flow, myocardial glucose metabolism and cardiac contractile function in normal and/or ischemic hearts in a dose-dependent manner. The rationale was based on earlier studies indicating that systemic infusions of GLP-1 result in increased LV contractile function and myocardial glucose metabolism at baseline,12 and in conditions of cardiac stress (i.e. coronary artery occlusion,9,10 pacing-induced heart failure).8,11 In addition, GLP-1 has also been shown to induce endothelium-dependent vasodilation of isolated systemic arteries,21 and to increase coronary blood flow in vitro12,13 and in vivo.7 However, whether these effects of GLP-1 are mediated by direct actions on the heart and coronary circulation independent of changes in MVO2, and/or by other indirect peripheral/central effects has not been established. Novel findings of the current study were that acute, intracoronary administration of GLP-1 (7–36): (1) did not directly affect coronary blood flow in vivo or vascular tone in vitro; (2) modestly reduced stroke volume and CO at CPP = 100 mmHg without affecting arterial pressure; (3) did not influence MVO2, global or regional contractile function, or cardiac lactate uptake at CPP = 100 or 40 mmHg; and (4) significantly increased myocardial glucose uptake and extraction, but only at CPP = 40 mmHg. We also confirmed GLP-1R expression in the coronary vasculature and myocardium of canines. Taken together, these findings indicate that acute (~5–10 min), intracoronary administration of GLP-1 (7–36) preferentially augments glucose metabolism in ischemic myocardium, independent of effects on cardiac contractile function or coronary blood flow.
Coronary and cardiac expression of GLP-1R
Data from the present investigation confirm expression of GLP-1R in both the coronary vasculature and myocardium. While previous studies have demonstrated expression of GLP-1R in canine myocardium,8 mouse heart and coronary vasculature,12 and isolated human coronary artery endothelial cells,26 we are the first group to demonstrate relative GLP-1R expression in canine coronary vasculature and myocardium. We observed GLP-1R expression in all layers of coronary arterial wall, including the endothelium and adventitia. Our results indicate that GLP-1R has more uniform and ubiquitous expression in canine myocardium and coronary circulation (Figure 1b) than was previously shown in mice. Importantly, selective binding of the GLP-1R antibody used in this investigation at the specified protein band (53 kDa) was confirmed using Western blot (Figure 1a).
Coronary vascular effects of GLP-1 (7–36)
Our findings demonstrate that although GLP-1R is expressed in the coronary vasculature (Figure 1b), acute administration of GLP-1 does not directly modulate coronary vascular tone in vitro (Figure 2a) or in vivo (Figure 2b). These data are in contrast with earlier studies which documented that GLP-1 induces endothelium-dependent vasodilation in isolated arteries, increases coronary blood flow in isolated hearts under normal conditions and during reperfusion, and following pacing-induced cardiomyopathy in canines. However, such previous studies were conducted in aorta,19,20 femoral21 and pulmonary vessels,22 isolated rodent hearts,12,13 and with systemic administration of recombinant GLP-1 (7–36) in canines. It is also important to recognize that the increased coronary blood flow previously documented in vivo in canines was accompanied by increases in cardiac contractile function and MVO2,7 the primary determinants of coronary flow.27 Thus, the effects of GLP-1 on coronary blood flow in those experimental conditions could have been mediated by direct effects on the vasculature and/or by indirect effects via metabolic vasodilation secondary to increased MVO2. The reasons for the discrepant findings regarding the vascular effects of GLP-1 are unclear but are likely related to differences in specific vessels studied, species investigated and/or dose and route of GLP-1 administration. Regardless, our findings provide strong evidence that acute administration of physiological/pharmacological concentrations of GLP-1 directly into the coronary circulation does not influence coronary vasomotor tone.
Cardiometabolic effects of GLP-1 (7–36)
Earlier studies indicate that GLP-1 augments myocardial glucose metabolism under normal and postischemic conditions in isolated hearts,12,13 and following cardiac ischemia and/or dilated cardiomyopathy in vivo.7,8,11 However, we found that intracoronary administration of GLP-1 (7–36) preferentially and dose-dependently augments myocardial glucose metabolism during ischemia (CPP = 40 mmHg), but not during normal perfusion (CPP = 100 mmHg) (Figure 5). This finding is consistent with previous data indicating that GLP-1 augments myocardial glucose uptake under conditions of cardiac stress (e.g. ischemia/reperfusion injury, dilated cardiomyopathy).7,8,11 Data from the current investigation extend this finding and demonstrate that acutely increasing coronary plasma GLP-1 concentration to ~1 nmol/L increases glucose uptake of ischemic myocardium by ~55%. Importantly, this effect was concentration-dependent and differed from other studies, which found that direct coronary administration of GLP-1 increases glucose uptake under normal conditions in isolated hearts.12,13 Our results indicate that longer, systemic administration of GLP-1 is not mandatory for the insulinomimetic effect to be manifest and argue against peripheral/central actions of GLP-1 driving alterations in substrate metabolism. Whether the effect of GLP-1 (7–36) on ischemic myocardial glucose metabolism is dependent on GLP-1R activation and/or actions of the (9–36) degradation product merits further investigation.
Effects of GLP-1 (7–36) on regional and global indices of cardiac function
Several previous investigations have reported that GLP-1 increases indices of LV function (i.e. developed pressure, wall thickening, dP/dt and ejection fraction) during reperfusion, heart failure and dilated cardiomyopathy.7–13 However, we observed no direct effect of acute intracoronary GLP-1 (7–36) infusion on indices of regional (Figure 4) or global cardiac contractile function during ischemia (Table 1). This finding is in contrast to our hypothesis that increases in glucose metabolism would improve contractile function in ischemic myocardium, as glucose has a high P/O ratio (ATP produced per oxygen consumed) and earlier data support that increased myocardial glucose metabolism improves cardiac efficiency during ischemia.14,23,28–31 In particular, previous studies from our group documented that insulin-mediated increases in myocardial glucose metabolism significantly improve regional cardiac function in ischemic canine hearts (CPP = 40 mmHg).23,32 However, the increase in myocardial glucose uptake induced by insulin in these studies (~0.50 μmol/min/g) was substantially higher than that reported in the present study (~0.20 μmol/min/g).
Interestingly, we did observe a moderate dose-dependent decrease in stroke volume and CO at normal perfusion pressure (CPP = 100 mmHg), without corresponding changes in dP/dt or aortic pressure (Table 1). This reduction in CO was associated with an ~10 beats/min increase in heart rate. Although there were no changes in dP/dt in our experiments, the reductions in stroke volume and CO suggest that GLP-1 reduced contractile performance. This observation is supported by a previous study in which recombinant GLP-1 (7–36) decreased LV developed pressure and dP/dt when infused into the coronary circulation of isolated rat hearts.13 Taken together, these findings indicate that direct (intracoronary) cardiac effects of GLP-1 differ from indirect (peripheral/central) effects.
Limitations of the study
It is possible that the assessment of regional myocardial substrate uptake and contractile function can be influenced by contamination of interventricular venous drainage by blood from non-LAD perfused myocardium and/or by collateral flow to the hypoperfused LAD perfusion territory. Importantly, an earlier study by Vinten-Johansen et al.33 conducted using systemically administered radioactively-labeled red blood cells demonstrated that blood taken from the anterior interventricular vein is almost exclusively from LAD perfused myocardium, even at CPP = 40 mmHg. Our laboratory also previously demonstrated that collateral flow to the LAD at a CPP of 40 mmHg is negligible (~0.01 ±0.01 mL/min/g).28,32 This finding is supported by marked reduction of cardiac performance observed when CPP was lowered to 40 mmHg (Table 1). Thus, our measurements of myocardial substrate metabolism computed from arteriovenous differences and LAD flow were likely not significantly affected by collateral flow or venous contamination. Further, any contamination of venous drainage would have resulted in dilution of the measures, and actually served to diminish our overall treatment effect on cardiac metabolism. Therefore, if such effects were present, the ~ 55% increase in glucose uptake would be an underestimate of the effect of GLP-1 in ischemic myocardium (Figure 5c). While our studies examined the acute effects of intracoronary GLP-1 (7–36) on myocardial glucose, lactate and oxygen uptake, additional studies to also examine the effects of GLP-1 on the fates of other substrates such as fatty acids, triglycerides and myocardial glycogen stores, are needed.
GLP-1 infusions in this investigation were conducted after hemodynamic variables had stabilized at CPPs of 100 and 40 mmHg. Although it is possible that hemodynamic status could have changed over time, the relatively constant measures of coronary blood flow, cardiac function and MVO2 at each of these pressures argues for stability of the experimental preparation over time.
Our observed regional segment shortening data were lower than what is typically reported in the literature.23,34 These low values are likely related to differences in depth of crystal placement and/or local cardiac fiber orientation at the depth in which the crystals were placed. These aspects of crystal implantation environment are not controlled under the present experimental protocol. It is also possible that the low observed values for regional segment shortening are due to myocardial stunning subsequent to cannulation of the LAD. However, we carefully monitor all hemodynamic variables before and following cannulation to ensure that these parameters return to baseline values prior to beginning of the experimental protocol. These variables typically return to normal within ~2 min of reperfusion and we allowed at least a 10-min recovery period to ensure adequate recovery time. Regardless of the signal magnitude, we did observe expected reductions in regional function during ischemia, and importantly GLP-1 did not affect segment shortening at normal CPP (CPP = 100 mmHg) or during ischemia (CPP = 40 mmHg).
Conclusion
Data from this investigation indicate that acute, intracoronary infusion of GLP-1 (7–36) preferentially augments glucose metabolism in ischemic myocardium, independent of effects on coronary blood flow. These findings demonstrate that the salutary effects of GLP-1 on myocardial glucose metabolism occur dose-dependently within minutes of intracoronary administration during ischemia. Thus, acute cardiac administration of GLP-1 may be useful in modulating cardiac metabolism acutely in a setting where ischemia is anticipated, such as elective cardiac surgery. Our data also indicate that although GLP-1R is expressed in myocardium and coronary circulation, GLP-1 (7–36) does not affect coronary vasomotor tone or coronary flow when administered directly into canine coronary vasculature over an ~ 20-min time period. Further investigations to uncover direct and systemic mechanisms by which GLP-1-based treatments affect cardiac function and metabolism will be useful for determining the best strategies to optimize therapeutic utilization of these compounds.
Acknowledgments
This work was supported by NIH grant HL092245 (JDT), HL092799 (KJM) and the IU School of Medicine Medical Scientist Training Program (NIH/IU).
Footnotes
Author contributions: All authors participated in the design and performance of the experiments and were directly involved with interpretation of the studies, analysis of the data and review of the manuscript.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ, Gethmann A, Nauck MA, Gotze O, Schmitz F, Deacon CF, Gallwitz B, Schmidt WE, Holst JJ. The glucagon-like peptide-1 metabolite GLP-1-(9–36) amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am J Physiol Endocrinol Metab. 2006;290:E1118–23. doi: 10.1152/ajpendo.00576.2005. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 4.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 5.Brynes AE, Edwards CM, Ghatei MA, Bloom SR, Frost GS. Men at increased risk of coronary heart disease are not different from age- and weight-matched healthy controls in their postprandial triglyceride, nonesterified fatty acid, or incretin responses to sucrose. Metabolism. 2002;51:195–200. doi: 10.1053/meta.2002.29029. [DOI] [PubMed] [Google Scholar]
- 6.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–61. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 8.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–21. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–8. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–8. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 12.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 13.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 14.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 15.Bose AK, Mocanu MM, Carr RD, Yellon DM. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther. 2007;21:253–6. doi: 10.1007/s10557-007-6030-6. [DOI] [PubMed] [Google Scholar]
- 16.Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- 17.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 18.Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7–36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc J Afr. 2008;19:77–83. [PMC free article] [PubMed] [Google Scholar]
- 19.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478:136–42. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ozyazgan S, Kutluata N, Afsar S, Ozdas SB, Akkan AG. Effect of glucagon-like peptide-1(7–36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology. 2005;74:119–26. doi: 10.1159/000084277. [DOI] [PubMed] [Google Scholar]
- 21.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–7. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–6. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 23.Tune JD, Mallet RT, Downey HF. Insulin improves cardiac contractile function and oxygen utilization efficiency during moderate ischemia without compromising myocardial energetics. J Mol Cell Cardiol. 1998;30:2025–35. doi: 10.1006/jmcc.1998.0763. [DOI] [PubMed] [Google Scholar]
- 24.Feigl EO, Neat GW, Huang AH. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol. 1990;22:375–90. doi: 10.1016/0022-2828(90)91474-l. [DOI] [PubMed] [Google Scholar]
- 25.Knudson JD, Dincer UD, Dick GM, Shibata H, Akahane R, Saito M, Tune JD. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1038–46. doi: 10.1152/ajpheart.00244.2005. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 27.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–15. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 28.Opie LH. Heart Pysiology from Cell to Circulation. 4. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 29.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–59. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–11. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–51. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 32.Tune JD, Mallet RT, Downey HF. Insulin improves contractile function during moderate ischemia in canine left ventricle. Am J Physiol. 1998;274:H1574–81. doi: 10.1152/ajpheart.1998.274.5.H1574. [DOI] [PubMed] [Google Scholar]
- 33.Vinten-Johansen J, Johnston WE, Crystal GJ, Mills SA, Santamore WP, Cordell AR. Validation of local venous sampling within the at risk left anterior descending artery vascular bed in the canine left ventricle. Cardiovasc Res. 1987;21:646–51. doi: 10.1093/cvr/21.9.646. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Gross GJ. Glibenclamide antagonizes adenosine A1 receptor-mediated cardioprotection in stunned canine myocardium. Circulation. 1993;88:235–44. doi: 10.1161/01.cir.88.1.235. [DOI] [PubMed] [Google Scholar]